Abstract
Background
Body composition prediction equations using skinfolds are useful alternatives to advanced techniques, but their utility across diverse pediatric populations is unknown.
Aim
To evaluate published and new prediction equations across diverse samples of children with health conditions affecting growth and body composition.
Subjects and Methods
Anthropometric and dual-energy x-ray absorptiometry (DXA) body composition measures were obtained in children with Down syndrome (n=59), Crohn disease (n=128), steroid-sensitive nephrotic syndrome (n=67), and a healthy reference group (n=835). Published body composition equations were evaluated. New equations were developed for ages 3 to 21y using the healthy reference sample and validated in other groups and national survey data.
Results
Fat mass [FM], fat-free mass [FFM] and percent body fat [%BF]) from published equations were highly correlated with DXA-derived measures (r=0.71 to 0.98), but with poor agreement (mean difference: 2.4kg, −1.9kg, and 6.3% for FM, FFM and %BF). New equations produced similar correlations (r=0.85 to 1.0) with improved agreement for the reference group (0.2kg, 0.4kg, and 0.0% for FM, FFM and %BF, respectively), and in sub-groups.
Conclusions
New body composition prediction equations show excellent agreement with DXA, and improve body composition estimation in healthy children and those with selected conditions affecting growth.
Keywords: Fat mass, fat-free mass, percent body fat, Crohn disease, Down syndrome
Introduction
Skinfold thickness measures are used to estimate body composition, and have the advantage of being relatively accurate, reproducible, mobile, inexpensive, and safe (Durnin and Womersley, 1974, Durnin and Rahaman, 1967, Slaughter et al., 1988, Zemel et al., 1997, Stomfai et al., 2011, Freedman et al., 2013b). A set of prediction equations, published in the 1960’s and 1970’s used four skinfolds (biceps, triceps, subscapular, and suprailiac skinfolds) to estimate percent body fat (%BF), fat mass (FM) and fat-free mass (FFM) for children ≥ 1y of age. Brook (Brook, 1971) and Durnin et al. (Durnin and Rahaman, 1967, Durnin and Womersley, 1974) developed the equations using sample sizes of 23 and 191 respectively, stratified by sex. Subsequently, Slaughter and colleagues (Slaughter et al., 1988) developed other prediction equations that used 2-skinfolds (triceps and subscapular) and developed prediction equations that incorporated pubertal development and population ancestry based on a sample size of 242 subjects, for ages 8 to18 years. Both sets of prediction equations were developed with underwater weighing or stable isotope techniques as reference methods and with samples of healthy children with typical growth. Only a few studies with small sample sizes have evaluated equation validity in children with health conditions that affect growth and body composition (Wells et al., 2008, Gurka et al., 2010).
Presently, dual-energy x-ray absorptiometry (DXA) is widely used in research for assessing %BF, FM and FFM in children and adults (Kendler et al., 2013). DXA methodology for body composition assessment varies by manufacturer and software version, but it does not require training in anthropometric technique and may be less subject to inter-observer and intra-observer variability. Moreover, DXA measures form the basis of national reference data for body composition and adiposity in the U.S. (Weber et al., 2013, Ogden et al., 2011). Here we use DXA-based body composition to evaluate published prediction equations, to develop new equations based on a large sample of healthy reference children, and to test the validity of these new equations using a strategic random sample of children from the U.S. with data from the National Health and Nutrition Examination Survey (NHANES), and in children with Down syndrome, Crohn disease, and steroid-sensitive nephrotic syndrome. These three conditions typify pediatric conditions that often affect growth and body composition through genetic factors, delayed maturation, disease processes (such as malabsorption and inflammation) or treatment effects (such as glucocorticoids) (FitzSimmons et al., 1990, Styles et al., 2002, Anneren et al., 1990, Arnell et al., 1996).
We hypothesised that published body composition prediction equations have a strong correlation, but poor agreement with DXA measures of FFM, FM and %BF, and that newly developed equations using DXA as the reference method would accurately estimate body composition in the U.S. population and in children with Down syndrome, Crohn disease, and nephrotic syndrome.
Subjects and Methods
Design and Participants
Data were used from multiple prospective observational studies conducted at The Children’s Hospital of Philadelphia (CHOP). These included two studies of children with Down syndrome, a large study of healthy children, and studies of children with Crohn disease and nephrotic syndrome, as described in multiple publications (Lee et al., 2015, Tsampalieros et al., 2013a, Wetzsteon et al., 2009, Tsampalieros et al., 2013b, Thayu et al., 2010, Dubner et al., 2009, Thayu et al., 2007, Hill et al., 2013, Leonard et al., 2010, O’Neill et al., 2005) and summarised in Table 1. Healthy, typically developing children from the Reference Project on Skeletal Development and healthy sibling participants from the Energy Balance in Children with Down Syndrome Study were combined to form the healthy reference group (n=835). All studies were conducted in the CHOP Clinical and Translational Research Center Nutrition Core Laboratory using the same methods.
Table 1.
Contributing study characteristics
| Study | Participants/Data Collection | Inclusion | Exclusion | Pubertal Staging |
|---|---|---|---|---|
| Energy Balance in Non-obese Children with Down Syndrome | Down syndrome n = 24 Typically developing n = 33 Age 3–13 years Longitudinal data collected at visit 0, 12, 24, and 36 months. Multiple observations per subject. 2001 to 2005 |
At least two prepubertal children per family at enrollment.
|
Other significant chronic conditions affecting growth or energy balance | Prepubertal status confirmed at baseline via parental assisted, self-assessment questionnaire(Morris and Udry, 1980) |
| Down Syndrome Metabolic Health Study | Down syndrome n = 35 Age 10–20 years Single visit 2013 |
Down syndrome | Major organ system illness (except type 2 diabetes mellitus) Cyanotic congenital heart disease Pulmonary hypertension Pregnancy Genetic syndrome known to affect glucose tolerance Familial hypercholesterolemia |
Physical exam by pediatric endocrinologist |
| Reference Project on Skeletal Development | n = 802 Age 5–19 years Single visit 2000 to 2007 |
Healthy, typically developing child Age ≥ 5 years |
History of illness or medication use that may affect growth, nutritional status, or pubertal development | Pubertal status confirmed at baseline via validated self-assessment questionnaire(Morris and Udry, 1980) |
| Glucocorticoid Induced Osteopenia in Children | Nephrotic syndrome n = 67 Crohn disease n =128 Longitudinal data collected at visit 0, 6, and 12 months. Multiple observations per subject. 2001–2005 |
Age 5 – 21 years Steroid sensitive nephrotic syndrome according to ISKDC criteria, treated at CHOP. Newly diagnosed or prevalent biopsy-proven CD |
Decreased renal function Any history of a condition or treatment affecting bone health, growth and nutrition, unrelated to nephrotic syndrome or Crohn disease |
Pubertal status confirmed at baseline via validated self-assessment questionnaire(Morris and Udry, 1980) |
n, number of participants CHOP, The Children’s Hospital of Philadelphia
Publically available data from NHANES (National Center for Health Statistics, 2008b) were used as an independent sample for validation purposes. Between 1999 and 2004 NHANES collected whole body DXA data on children and adolescents, ages 8 and above, using a strategic random sampling strategy that oversampled non-Hispanic “blacks”, Mexican Americans and low-income “whites”.1 Whole body scans were acquired using a Hologic QDR 4500A densitometer (Hologic Inc). (National Center for Health Statistics, 2008a) and analysed centrally by the University of California, San Francisco, Radiology department using Hologic Discovery Software, version 12.1 (Hologic Inc). The NHANES data set used a multiple imputation algorithm for missing values to address the potential biases of nonrandom missing DXA data (10% of males and 13.5% of females). The average of the imputed values was used in this analysis. Full details of the methods and rationale for multiple imputation are described in the NHANES DXA technical documentation files (National Center for Health Statistics, 2008b). Participants with weights greater than 130kg were excluded from DXA assessment due to the weight limit of the DXA device. Triceps and subscapular skinfold thicknesses were also acquired as part of the physical examination (National Center for Health Statistics, 2007).
Data Collection Methods
Height was measured using a wall-mounted stadiometer (Holtain, Crymych, UK) without shoes, weight was measured with an electronic scale (Scaletronix, White Plains, NY), and skinfold thickness measurements were collected in triplicate using Holtain skinfold calipers (Holtain, Crymych UK) by research anthropometrists and averaged. All anthropometrists were trained by an expert anthropometrist (BSZ) and with established inter-rater reliability. The triceps and biceps skinfold measurements were obtained at the mid-point of the right upper arm, halfway between the acromion and the olecranon. The subscapular skinfold was measured at a 45° angle just below the inferior angle of the scapula. The suprailiac measurement was obtained approximately 2 cm anterior and medial to the anterior superior iliac spine.
Four skinfolds were measured in the Down syndrome and healthy reference samples. Only triceps and subscapular skinfolds were collected in the children with Crohn disease and nephrotic syndrome. Age- and sex-specific z-scores for height, weight and BMI were generated based on CDC 2000 growth charts (Kuczmarski et al., 2000). Pubertal stage was also assessed for all participants using a validated self-assessment questionnaire (Morris and Udry, 1980, Schall et al., 2002) or by physician exam (see Table 1). Tanner’s stages of breast development for girls and genital stage for boys were used to categorise pubertal stage (Tanner, 1962). Whole body DXA scans were acquired (Hologic 4500/Delphi/Discovery densitometers, Hologic Inc., Bedford, MA) and analysed using software versions 12.3, 12.4 or 13.3 without the “NHANES body composition” option. The DXA software body composition algorithm was the same across all software versions. The combined DXA body composition data were subsequently corrected for overestimation of lean body mass (Schoeller et al., 2005), as was done for the National Health and Nutrition Examination Survey (NHANES) (Ogden et al., 2011).
Whole body FM, FFM, and %BF were estimated with previously published equations. Four skinfolds (triceps, biceps, subscapular and suprailiac) were used for the Brook equations for ages 1 to 11y (Brook, 1971), Durnin and Rahaman for ages 12 to 16y (Durnin and Rahaman, 1967), and Durnin and Wormsley for ages 16 to 21y (Durnin and Womersley, 1974). Two skinfolds (triceps and subscapular) were used for the Slaughter et al. equations for ages 8–18y (Slaughter et al., 1988). The resulting values were compared to DXA body composition measurements as the reference method.
Data Analysis
Body composition estimates based on two sets of published prediction equations (Brook, 1971, Durnin and Rahaman, 1967, Durnin and Womersley, 1974, Slaughter et al., 1988) were calculated. For the NHANES data set, Tanner stage was not collected, but is required for estimating body composition in males using the equation of Slaughter. Consistent with another publication (Golec et al., 2014), males were categorised as prepubertal for age<12 years, peripubertal for ages 12 to 14 years, and pubertal for age>14 years.
Body composition estimated from skinfold prediction equations and DXA were compared by Spearman correlation (due to non-normal distributions) and a Bland-Altman agreement analysis (Bland and Altman, 1986) that compares the difference between DXA and skinfold body composition measures to the mean of both methods. Results were reported as the mean difference between methods, and the limits of agreement (mean difference ± 1.96*SD) (Bland and Altman, 1986). Differences between study groups in growth z-scores were compared by one-way ANOVA using the Scheffe post-hoc test of significance.
New prediction equations were developed using the healthy reference sample to predict DXA %BF, because %BF was the outcome measure predicted in previous equations. First, we conducted exploratory analysis using all possible subsets regression analysis (“allpossible” option in Stata 13.0) similar to the approach used by Sun et al. (Sun et al., 2003), which permits rapid comparison of R-square values for combinations of variables (Cox, 2002). The variables in the all possible subset analysis included those used in previous equations: skinfold thickness, sex, age, pubertal stage, and African American ancestry. We also included height, hypothesising that height will improve body composition estimation in children with atypical growth patterns. Consistent with previous equations of Brook and Durnin et al., we used the natural log of the sum of skinfolds to reduce skewness and non-linearity of associations; the log of height was also used. Based on these results, we compared the simpler models (i.e., fewer independent variables) to more complex models using the likelihood ratio test to assess whether the model was significantly improved by the addition of more variables. We repeated the final regression models using bootstrapping with 100 repetitions of random samples with replacement drawn from the subsets of healthy males and females (separately) to obtain robust estimates of the final models. Two- and three-way interaction terms with African American ancestry and pubertal stage were tested. We also estimated simpler prediction models that did not require determination of pubertal stage.
Equations predicting %BF were created using 2- or 4-skinfolds. FM and FFM were calculated as follows:
The new prediction equations were then tested using Spearman correlations and Bland Altman agreement analysis in independent data sets; the two skinfold method was used for the NHANES, nephrotic syndrome and Crohn disease samples for whom we only had two skinfolds, and the two- and four-skinfold equations for the Down syndrome sample. We had 80% power (p=0.05) to detect a significant mean difference in %BF between DXA measured vs skinfold predicted values of 0.86±3.2 in our smallest group (children with Down syndrome, n=59) and 0.245±2.6 in our largest group (healthy reference sample, n=835).
Results
Sample characteristics
Descriptive statistics for three of the four studies have been published previously (Lee et al., 2015, Tsampalieros et al., 2013a, Wetzsteon et al., 2009, Tsampalieros et al., 2013b, Thayu et al., 2010, Dubner et al., 2009, Thayu et al., 2007, Hill et al., 2013, Leonard et al., 2010, O’Neill et al., 2005) and are presented for all children in Table 2. The healthy reference group was comprised primarily of children of European or African ancestry. Consistent with contemporary children in the U.S., the healthy reference group was close to the median height for U.S. children, and had weight and BMI Z-scores that reflect the overweight of U.S. children. The distribution of height, weight and BMI Z-scores for the Down syndrome, Crohn disease and nephrotic syndrome groups reflect the expected trends in growth and adiposity for these health conditions. The NHANES, Down syndrome, Crohn disease, and nephrotic syndrome groups all had lower height Z-scores than the healthy reference group (p<0.001). The NHANES, Down syndrome and nephrotic syndrome groups had higher BMI Z-scores (p<0.001) and the Crohn disease group had lower BMI Z-scores (p<0.001) than the reference group, as previously described. All pubertal stages were represented in all study groups. Four skinfold (triceps, biceps, subscapular and suprailiac) measurements were collected for 59 children with Down syndrome, ages 3 to 21 years, and 835 healthy reference children, ages 3 to 19 years. Triceps and subscapular skinfold thickness measurements were collected for 128 children with Crohn disease, 67 with nephrotic syndrome, ages 5 to 22 years, and 9,826 children in the NHANES dataset. The NHANES sample included 32% “non-Hispanic Black”, 27% “non-Hispanic White”, 33% Mexican American, and 8% “other”.
Table 2.
Descriptive Statistics1
| Healthy Reference (n = 835) | Down syndrome (n= 59)* | Crohn disease (n= 128)* | Nephrotic syndrome (n= 67)* | NHANES (n=9826 ) | |
|---|---|---|---|---|---|
| Age, y | 11.0 (8.2, 14.2) | 12.7 (7.0, 17.7) | 13.1 (11.1, 15.1) | 8.8 (6.9, 13.7) | 14.8 (12.1, 17.5) |
| Sex, % female | 52 | 53 | 41 | 34 | 42 |
| Ancestry, % African American | 42 | 12 | 7 | 25 | 32 |
| Pubertal Stage, % | |||||
| 1 | 40 | 44 | 24 | 57 | |
| 2–3 | 27 | 5 | 47 | 27 | |
| 4–5 | 33 | 51 | 29 | 16 | |
| Weight z-score | 0.4 (−0.3, 1.1) | 0.1 (−0.7, 1.1)** | −0.5 (−1.2, 0.3)** | 0.7 (−0.1, 1.3) | 0.5 (−0.3, 1.2) |
| Height z-score | 0.2 (−0.4, 0.9) | −2.4 (−3.2, −1.5)** | −0.4 (−0.9, 0.3)** | −0.2 (−0.8, 0.4)** | 0.1 (−0.7, 0.8)** |
| BMI z-score | 0.4 (−0.4, 1.1) | 1.4 (0.5, 2.1)** | −0.4 (−1.1, 0.3)** | 0.8 (0.2, 1.7)** | 0.5 (−0.3, 1.3)** |
| Skinfolds, mm | |||||
| Triceps | 10.5 (8.0, 15.3) | 13.1 (10.7, 20.0) | 9.6 (7.8, 11.9) | 10.4 (7.8, 17.23) | 13.2 (9, 19.5) |
| Biceps | 5.7 (4.3, 8.3) | 6.8 (4.1, 9.4) | |||
| Subscapular | 7.9 (6.1, 11.9) | 15.2 (10.0, 26.0) | 6.2 (5.2, 7.9) | 7.5 (5.6, 13.3) | 10.8 (7.8, 16.9) |
| Suprailiac | 7.6 (5.1, 12.1) | 15.8 (8.4, 21.5) | |||
| FMDXA, kg | 10.1 (7.0, 15.4) | 11.8 (8.1, 17.4) | 11.0 (8.3, 14.4) | 10.0 (6.1, 15.2) | 14.4 (10.1, 20.9) |
| FFMDXA, kg | 28.5 (20.4, 39.7) | 18.8 (15.1, 30.0) | 32.4 (26.3, 39.9) | 24.8 (19.6, 39.2) | 39.8 (31.4, 50.1) |
| %BFDXA, % | 26.8 (22.0, 31.9) | 37.3 (32.1, 41.3) | 25.7 (21.9, 29.8) | 27.1 (21.7, 34.1) | 27.4 (21.0, 33.8) |
| FMnewSF4, kg | 10.1 (7.0, 15.2) | 11.0 (7.6, 16.9) | |||
| FFMnewSF4, kg | 28.1 (20.0, 39.5) | 19.1 (14.4, 30.7) | |||
| %BFnewSF4, % | 27.2 (22.8, 31.6) | 35.6 (31.3, 40.5) | |||
| FMnewSF2, kg | 10.2 (7.1, 15.3) | 11.2 (7.4, 17.2) | 10.7 (8.0, 13.9) | 10.0 (6.4, 15.9) | 15.1 (10.6, 21.1) |
| FFMnewSF2, kg | 28.1 (20.0, 39.3) | 19.2 (14.5, 30.7) | 32.6 (25.9, 40.0) | 24.1 (19.4, 38.9) | 39.8 (31.7, 49.5) |
| %BFnewSF2, % | 27.4 (22.9, 31.8) | 36.3 (32.0, 40.1) | 25.4 (21.8, 28.7) | 27.8 (23.2, 34.6) | 28.4 (22.2, 34.1) |
median (IQR) or %
Multiple observations per subject
Significantly different from healthy controls, P<0.05
DXA, dual-energy x-ray absorptiometry; newSF4, proposed 4-skinfold prediction equation; new SF2, proposed 2-skinfold prediction equation
Comparison of published prediction equations with DXA body composition
Body composition (FM, FFM, and %BF) was calculated for all groups from published equations using 2- and, when available, 4-skinfolds (SF2 and SF4). There was excellent correlation with values obtained from DXA for %BF (Figure 1), FM (Figure 2) and FFM (Figure 3). There was a large difference between measures (DXA – skinfold) and wide limits of agreement using the published equations (Figure 4 for 2-skinfold analysis and Figure 5 for 4-skinfold analysis). DXA measures of FM and %BF were significantly greater (p<0.001) than that estimated by published body composition prediction equations.
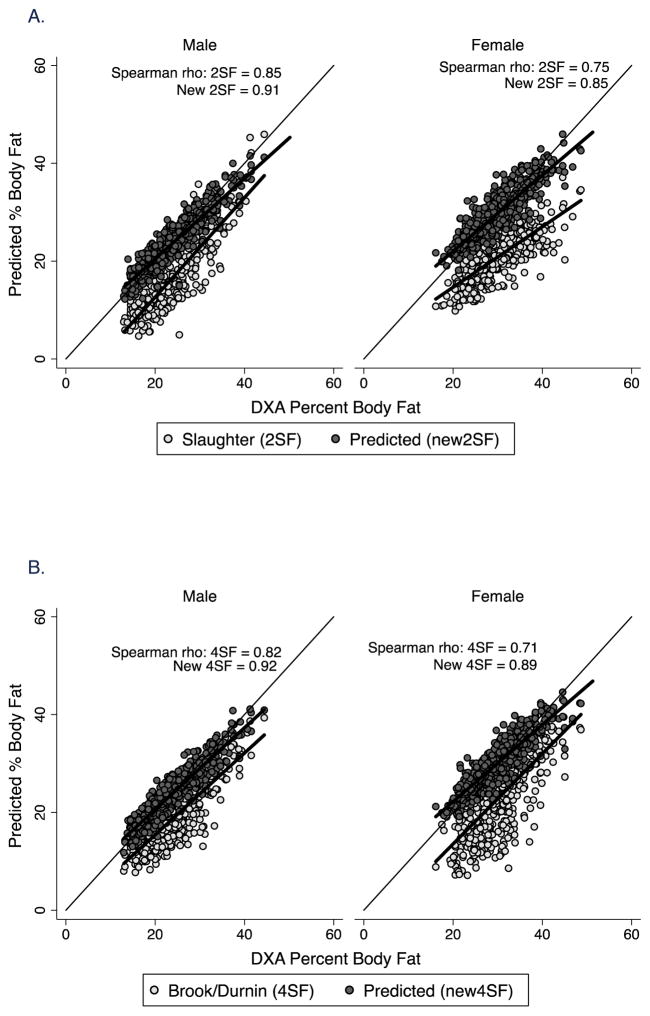
Figure 1.
Correlation plot of percent body fat by body composition prediction equations vs. DXA in males and females in the healthy reference sample. The line of identity is included. A. 2-skinfold equations of Slaughter et al (1988) (2SF) and new prediction equations (New 2SF). The Spearman correlation coefficient (rho) for both is shown; B. 4-skinfold equations from published sources (Brook, 1971, Durnin and Rahaman, 1967, Durnin and Womersley, 1974) (4SF) and new prediction equations (New 4SF). The Spearman correlation coefficient (rho) for both is shown.
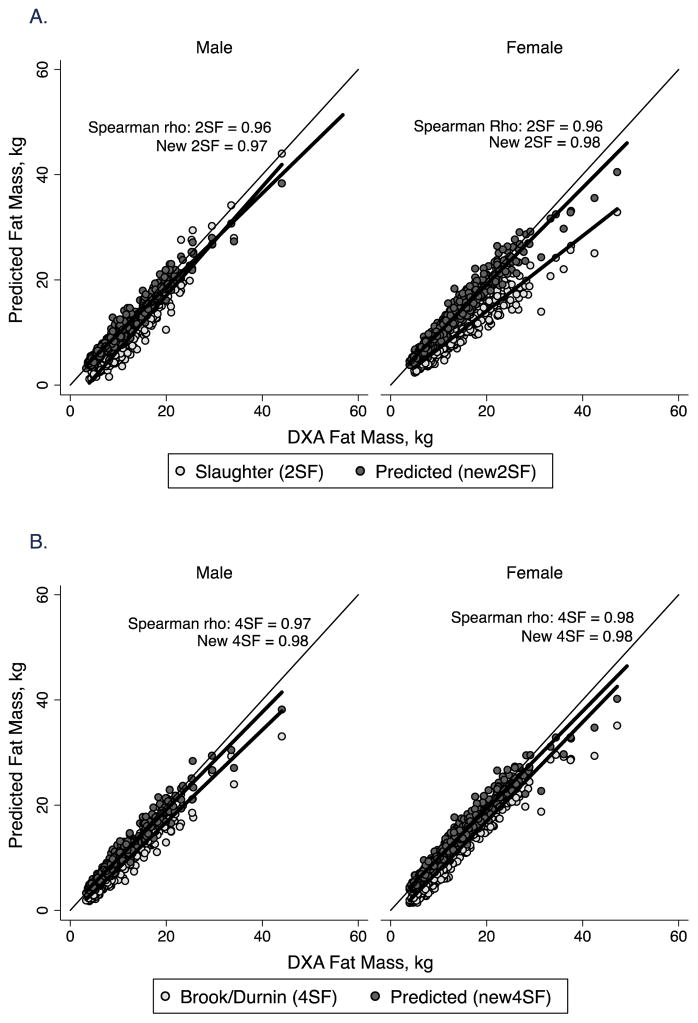
Figure 2.
Correlation plot of fat mass by body composition prediction equations vs. DXA in males and females in the healthy reference sample. The line of identity is included. A. 2-skinfold equations of Slaughter et al (1988) (2SF) and new prediction equations (New 2SF). The Spearman correlation coefficient (rho) for both is shown; B. 4-skinfold equations from published sources (Brook, 1971, Durnin and Rahaman, 1967, Durnin and Womersley, 1974) (4SF) and new prediction equations (New 4SF). The Spearman correlation coefficient (rho) for both is shown.
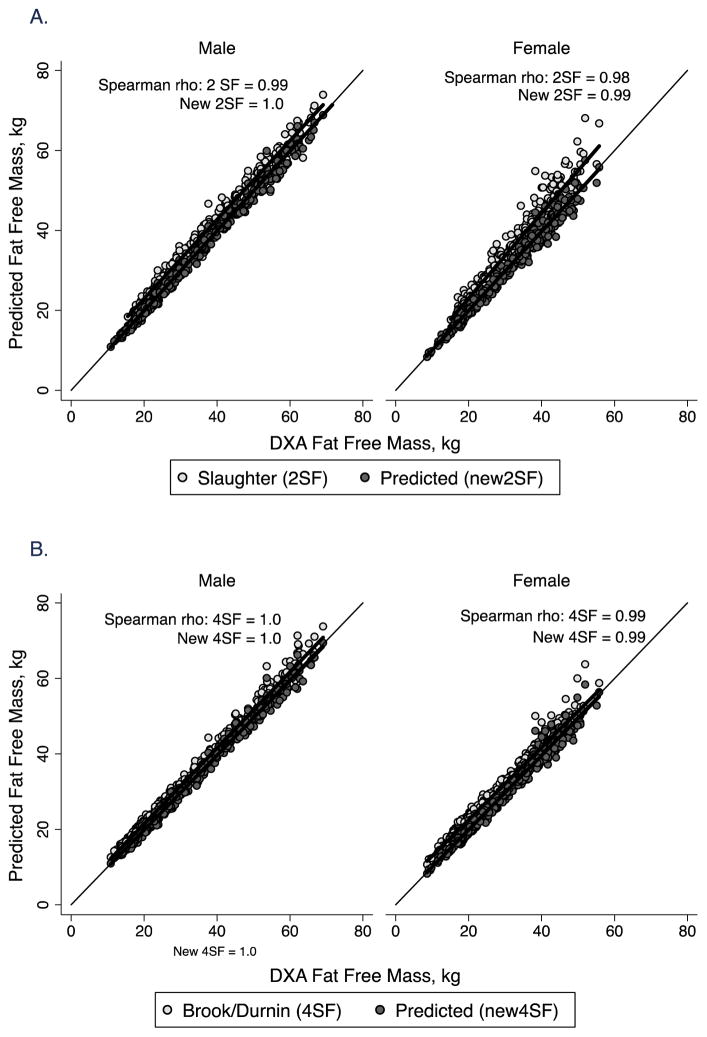
Figure 3.
Correlation plot of fat free mass by body composition prediction equations vs. DXA in males and females in the healthy reference sample. The line of identity is included. A. 2-skinfold equations of Slaughter et al (1988) (2SF) and new prediction equations (New 2SF). The Spearman correlation coefficient (rho) for both is shown; B. 4-skinfold equations from published sources (Brook, 1971, Durnin and Rahaman, 1967, Durnin and Womersley, 1974) (4SF) and new prediction equations (New 4SF). The Spearman correlation coefficient (rho) for both is shown.
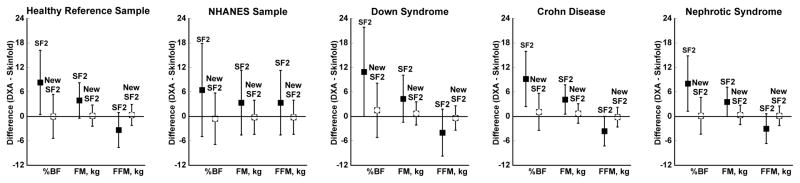
Figure 4.
Agreement (DXA – 2 skinfold prediction equations) for percent body fat, fat mass, and fat-free mass in the healthy reference sample, the NHANES dataset, and in children with Down syndrome, Crohn disease, or steroid-sensitive nephrotic syndrome. Box represents mean difference between measures and error bars show the limits of agreement (1.96*SD). %BF, percent body fat; FFM, fat-free mass; FM, fat mass; SF2, published equations (Slaughter et al., 1988); New SF2, proposed equations.
Figure 5.
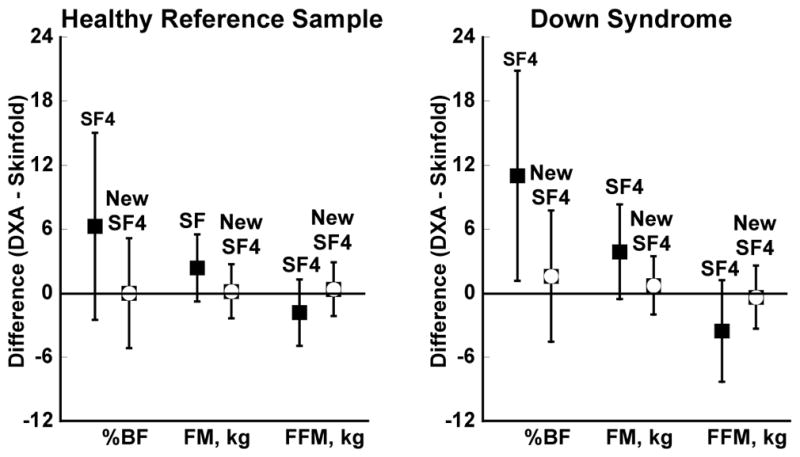
Agreement (DXA – 4 skinfold prediction equations) for percent body fat, fat mass, and fat-free mass in the healthy reference sample and in children with Down syndrome. Box represents mean difference between measures and error bars show the limits of agreement (1.96*SD). %BF, % body fat; FFM, fat-free mass; FM, fat mass; SF4, published equations (Brook, 1971, Durnin and Rahaman, 1967, Durnin and Womersley, 1974); New SF4, proposed equations.
Equation development and validation
We developed separate sets of equations to predict DXA %BF with the sum of 2-skinfolds (triceps and subscapular) and the sum of 4-skinfolds (triceps, biceps, subscapular and suprailiac) using data from the healthy reference group, stratified by sex. The all possible subsets analysis showed that after including the natural log of the sum of skinfolds, height was the most significant factor to add to the model, improving the R-squared value by 22 to 39%.
Interactions of African American ancestry and pubertal stage were tested in separate regression analyses for males and females; final models using bootstrapping with 100 replications were used to derive the prediction equations. For the new 2-skinfold prediction equation for males, there was a significant three-way interaction between African American ancestry, pubertal stage (Tanner stages 4 and 5, vs Tanner stages 1 to 3) and the log of height (p=0.035), so separate equations were tested for African American vs. Non-African American males. Pubertal stage and the interaction of pubertal stage and height remained significant (p<0.05) for African American males only. In other words, the negative effect of height on prediction of percent body fat for a boy in early puberty was less than the effect of height on percent body fat for a boy in later puberty. For the new 4-skinfold prediction equation for males, the two-way interaction of African American ancestry and the sum of 4 skinfolds was significant (p=0.006). The full prediction models, as well as simplified models that do not require pubertal stage are shown in Table 3. These new prediction equations for males explained 81 to 86 percent of the variability in percent body fat. User-friendly equations are provided in Table 4.
Table 3.
Two and Four Skinfold Prediction Models
| Variable | Coefficient | Std. Err. | p | 95% Confidence Interval | adj R2 | RMSE | |
|---|---|---|---|---|---|---|---|
| 2 Skinfold Prediction Equations | |||||||
|
| |||||||
| Non-African American Males | Log Sum 2 SF | 13.12 | 0.38 | <0.001 | (12.37, 13.87) | 0.81 | 2.45 |
| Log Height | −15.46 | 1.80 | <0.001 | (−18.98, −11.94) | |||
| Tanner 4–5 | −13.66 | 33.92 | 0.69 | (−80.13, 52.82) | |||
| Tanner 4–5 x Log Height | 2.19 | 6.61 | 0.74 | (−10.77, 15.15) | |||
| Intercept | 64.58 | 8.67 | <0.001 | (47.59, 81.57) | |||
|
| |||||||
| African American Males | Log Sum 2 SF | 14.73 | 0.55 | <0.001 | (13.66, 15.80) | 0.86 | 2.56 |
| Log Height | −10.55 | 2.13 | <0.001 | (−14.73, −6.37) | |||
| Tanner 4–5 | 75.72 | 37.37 | 0.043 | (2.48, 148.96) | |||
| Tanner 4–5 x Log Height | −15.40 | 7.32 | 0.035 | (−29.73, −1.06) | |||
| Intercept | 34.82 | 9.98 | <0.001 | (15.25, 54.38) | |||
|
| |||||||
| All Males - Simplified Equation (no Tanner Stage required) | Log Sum 2 SF | 14.30 | 0.55 | 0.0000 | (13.21, 15.38) | 0.82 | 2.64 |
| Log Height | −20.66 | 1.45 | 0.0000 | (−23.5, −17.81) | |||
| African American | −1.09 | 0.41 | 0.0070 | (−1.89, −0.30) | |||
| Intercept | 86.44 | 6.95 | 0.0000 | (72.81, 100.06) | |||
|
| |||||||
| Females | Log Sum 2 SF | 13.95 | 0.63 | <0.001 | (12.72, 15.18) | 0.74 | 3.00 |
| Log Height | −18.09 | 1.80 | <0.001 | (−21.61, −14.57) | |||
| African American | −1.77 | 0.41 | <0.001 | (−2.56, −0.97) | |||
| Intercept | 77.17 | 8.12 | <0.001 | (61.26, 93.08) | |||
|
| |||||||
| 4 Skinfold Prediction Equations | |||||||
|
| |||||||
| Males | Log Sum 4 SF | 12.41 | 0.49 | <0.001 | (11.45, 13.38) | 0.85 | 2.41 |
| Log Height | −16.90 | 2.05 | <0.001 | (−20.93, −12.88) | |||
| Tanner 4–5 | −1.84 | 0.64 | 0.004 | (−3.09, −0.59) | |||
| African American | −8.46 | 2.74 | 0.002 | (−13.83, −3.10) | |||
| Afr Amer X Log Sum 4SF | 2.27 | 0.83 | 0.006 | (0.65, 3.89) | |||
| Intercept | 66.78 | 9.81 | <0.001 | (47.56, 86.00) | |||
|
| |||||||
| Males - Simplified Equation (no Tanner Stage required) | Log Sum 4 SF | 12.74 | 0.51 | <0.001 | (11.74, 13.74) | 0.84 | 2.47 |
| Log Height | −21.47 | 0.99 | <0.001 | (−23.41, −19.53) | |||
| African American | −7.90 | 2.84 | 0.005 | (−13.47, −2.34) | |||
| Afr Amer X Log Sum 4SF | 2.09 | 0.86 | 0.015 | (0.41, 3.77) | |||
| Intercept | 87.82 | 4.75 | <0.001 | (78.51, 97.14) | |||
|
| |||||||
| Females | Log Sum 4 SF | 13.99 | 0.49 | <0.001 | (13.03, 14.96) | 0.76 | 2.83 |
| Log Height | −21.42 | 1.78 | <0.001 | (−24.92, −17.92) | |||
| African American | −34.61 | 14.25 | 0.015 | (−62.54, −6.67) | |||
| Afr Amer X Log Height | 6.73 | 2.88 | 0.02 | (1.08, 12.37) | |||
| Intercept | 85.65 | 8.75 | <0.001 | (68.50, 102.80) | |||
Table 4.
New Body Composition Prediction Equations for Percent Body Fat
| 2 Skinfold Prediction Equations | |||
|---|---|---|---|
| Non-African American | African American | ||
| Males | Tanner 1–3 | (13.12*log Sum 2SF) − (15.46*Log Height) +64.58 | (14.73*log Sum 2SF) − (10.55*Log Height) +34.82 |
| Tanner 4–5 | (13.12*log Sum 2SF) − (13.27*Log Height) +50.92 | (14.73*log Sum 2SF) − (25.95*Log Height) +110.54 | |
| All1 | (14.28*log Sum 2SF) − (21.50*Log Height) +90.69 | (14.28*log Sum 2SF) − (19.23*Log Height) +78.29 | |
| Females | All | (13.95*log Sum 2SF) − (18.09*Log Height) +77.17 | (13.95*log Sum 2SF) − (18.09*Log Height) +75.40 |
| 4 Skinfold Prediction Equations | |||
| Non-African American | African American | ||
| Males | Tanner 1–3 | (12.41*log Sum 4SF) − (16.90*Log Height) +66.78 | (14.68*log Sum 4SF) − (16.90*Log Height) +58.32 |
| Tanner 4–5 | (12.41*log Sum 4SF) − (16.90*Log Height) +64.94 | (14.68*log Sum 4SF) − (16.90*Log Height) +56.48 | |
| All1 | (12.74*log Sum 4SF) − (21.47*Log Height) +87.82 | (14.83*log Sum 4SF) − (21.47*Log Height) +79.92 | |
| Females | All | (13.99*log Sum 4SF) − (21.42*Log Height) +85.65 | (13.99*log Sum 4SF) − (14.69*Log Height) +51.04 |
Simplified equation that does not require Tanner Stage
For females, height, African American ancestry and the sum of skinfolds were significant predictors of percent body fat for both the 2-skinfold and 4-skinfold models. For the new 4-skinfold model, there was a significant interaction (p=0.02) between African American ancestry and height, such that the negative effect of height on percent body fat was less for African American as compared to non-African American females. The full prediction models, as well as simplified models that do not require pubertal stage are shown in Table 3. These new prediction equations for females explained 74 to 85 percent of the variability in percent body fat. User-friendly equations are provided in Table 4.
Application of new prediction equations in children with atypical growth
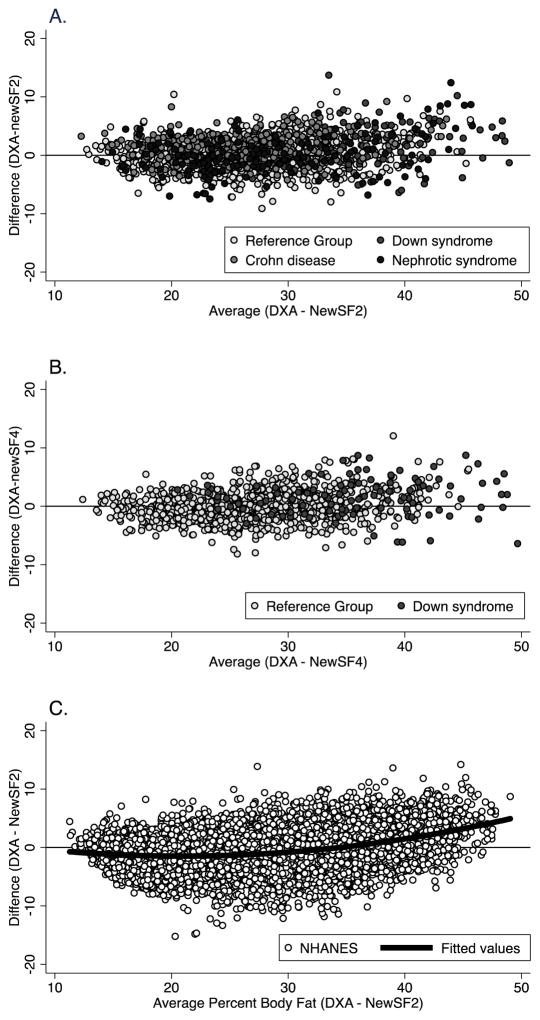
The new prediction equations produced body composition estimates that correlated highly with DXA data without the bias evident with the published equations (Figures 1 – 3). For the NHANES, Down syndrome, Crohn disease and nephrotic syndrome groups, the difference between DXA and new skinfold predicted measures was greatly reduced and the limits of agreement improved compared to previous equations (Figure 4 and 5). Results were similar for the new equations using 2- and 4-skinfolds. Bland Altman plots for percent body fat (difference between DXA vs predicted percent body fat compared to the average of DXA and predicted percent body fat) show that at highest levels of body fat, the values from the new prediction equations are less than the values measured by DXA (Figure 6).
Figure 6.
Percent body fat agreement plot showing: A. the difference between DXA and 2 skinfold body composition prediction of percent body fat compared to the mean of DXA and predicted percent body fat in the healthy reference sample and in children with Down syndrome, Crohn disease, and steroid-sensitive nephrotic syndrome; B. the difference between DXA and 4 skinfold body composition prediction of percent body fat compared to the mean of DXA and predicted percent body fat in the healthy reference sample and in children with Down syndrome; C. the difference between DXA and 2 skinfold body composition prediction of percent body fat compared to the mean of DXA and predicted percent body fat in the NHANES data set.
Discussion
Accurate body composition measurements are important in the clinical and research evaluation of children’s health status, and of even more importance in children with altered growth patterns due to genetic syndromes or medical conditions. As a measure of adiposity, skinfold thickness is a valuable tool in both the clinical and research settings. Here we showed poor agreement in body composition outcomes between published prediction equations and DXA measurements in healthy children, as well as in children with health conditions that frequently affect growth and body composition. To address this concern, new equations using a large, diverse sample of healthy children across a large age range were developed, and the generalisability of these equations was demonstrated among children with altered growth patterns, as well as in an independent sample of children measured in a national survey.
DXA is used to measure body composition in adults and children in the research setting. It is more widely available, time and cost efficient, and feasible than other methods of body composition assessment. However, DXA results vary by manufacturer and by model and software version within the same manufacturer (Toombs et al., 2012). The gold standard four-compartment body composition method has been used to evaluate DXA body composition measures from GE Lunar and Hologic devices (see review in Toombs et al., 2012). For example, for the older Lunar DPX-L model, DXA overestimated percent body fat for lean adults and underestimated percent body fat with increasing adiposity (Van Der Ploeg et al., 2003). Among children, the Lunar Prodigy overestimated fat mass in children; the bias was relatively small (0.86kg), but the limits of agreement increased with increasing fatness (Wells et al., 2010a). Similarly, Williams et al. (Williams et al., 2006) showed that the Lunar Prodigy DXA underestimated percent body fat in obese boys (1.41±2.59) and girls (1.03±3.50) and underestimated percent body fat in non-obese boys (−1.74±3.52), but did not differ for children with cystic fibrosis or glycogen storage disease. Schoeller et al. (Schoeller et al., 2005) combined data on 1195 subjects, ages 19 to 82 years, from 7 studies that used the Hologic QDR4500A device. They showed DXA overestimated fat-free mass compared to the four-compartment model. Accordingly, they recommended a reduction in fat free mass estimate of 5% and a corresponding increase in fat mass by that same amount. This correction was applied to all DXA body composition data from NHANES and is now the standard in contemporary Hologic software (as was used in this study). Presently, there are no published studies validating this new adjustment factor in DXA body composition assessment in children. Nevertheless, compared to other methods such as isotope dilution and underwater weighing, DXA has the advantage of measuring bone mass and density of soft tissue, so it is less prone to errors due to assumptions of the composition of fat-free mass. In addition, DXA is a widely accepted frame of reference because of the published NHANES reference ranges for percent body fat, lean mass index, and fat mass index for children ages 8 to 20 years (Ogden et al., 2011, Weber et al., 2013).
The new skinfold prediction equations presented here show improved agreement with DXA body composition compared to previous prediction equations. Numerous studies have also shown inadequacies in published prediction equations. In particular, the 2 skinfold prediction equation of Slaughter et al. (1988) underestimated percent body fat in Chilean children (Aguirre et al., 2015) and in 12 year old children in the U.S. (Bray et al., 2002) compared to multicompartment models. Compared to the GE Lunar DPX and DPX-L models, the Slaughter 2 skinfold prediction equations overestimated body fat, especially among those with high skinfold thicknesses (Freedman et al., 2013a).
We found that the inclusion of height in the skinfold prediction models significantly improved the explained variance. The importance of height in body composition assessment was recognised by Van Itallie et al. (VanItallie et al., 1990), and led to the wider use of lean mass index and fat mass index as nutritional status indicators. Our prediction equations also included African American ancestry and pubertal status variables. Pubertal stage is important, as it is well-recognised that body composition changes as a child progresses through puberty (Forbes, 1964, Rolland-Cachera, 1993). There are also differences in the timing of pubertal development between African American and non-African American children which may account for differences in body composition between the two groups (Ramnitz and Lodish, 2013). All prediction equations were stratified by sex because of the known sex differences in body composition even in early infancy (Butte et al., 2000). Body composition prediction equations that are stratified by sex and include height are particularly important in the assessment of children with altered patterns of growth or delayed pubertal development. We found the effect of pubertal status on body composition varied according to sex and African American ancestry. However, our simplified models provide estimates that did not include pubertal status and also had good explanatory power.
The equations of Slaughter et al. (Slaughter et al., 1988) were only validated for children ages 8–18 years, so do not meet the needs of studies that include younger children, such as ours. Additionally, reference methods for the previously published equations used underwater weighing to determine body density or the isotope dilution method to determine total body water. Both of these methods involve assumptions about the hydration of and bone mineral content in FFM. These characteristics of FFM change as children age, and may be different for children with chronic diseases that also affect growth, maturation and body composition (Wells et al., 2010b, Bianchi et al., 2014, Wells et al., 2006).
The new prediction equations performed well in children with Down syndrome, Crohn disease and nephrotic syndrome, conditions that can affect growth and body composition. Individuals with Down syndrome are shorter than their peers in the general population and commonly overweight, especially during adolescence (FitzSimmons et al., 1990, Styles et al., 2002, Arnell et al., 1996). Crohn disease, an inflammatory bowel disease, is associated with malabsorption, poor growth in height and weight, and delayed maturation (Sentongo et al., 2000). Children with steroid-sensitive nephrotic syndrome, a kidney disease with urinary protein loss, treated with prolonged, high dose glucocorticoids, typically have excess weight gain and are short for their degree of obesity (Foster et al., 2004). These conditions were selected for investigation because of their contrasting effects on growth and body composition. Our findings suggest that the new equations adequately predict body composition, even in these contrasting conditions, and thus make the development of disease specific equations unnecessary. Moreover, development of disease-specific body composition prediction equations for each group would require large numbers of subjects to encompass the age, sex, pubertal maturation and population ancestry effects on body composition.
Our study had several limitations. First, while the new equations show improved correlation with DXA body composition measures and reduced bias, some bias remains among those children with extremely high %BF. This effect likely arises secondary to reduced precision, accuracy and feasibility of skinfold thickness measurements in children with severe obesity and is an unavoidable limitation of the skinfold prediction method. The precision of DXA for measuring percent body fat ranges from 0.8 to 2.7% (Toombs et al., 2012), but agreement of DXA with the 4 compartment model “gold standard” worsens with increasing body fat (Van Der Ploeg et al., 2003, Wells et al., 2010a). Second, it is not possible to measure skinfold thicknesses in very obese individuals, and those with weight above the allowable limit for DXA devices would not be included in this analysis. Therefore, there is inherent bias in the sample due to the non-randomness of missing data. Third, the skinfold measurements were completed by research anthropometrists with excellent inter-observer reliability; the reproducibility of results outside the research setting is unknown. Our analysis of an independent data set from NHANES, that had multiple anthropometrists, showed a similar mean difference between DXA and predicted percent body fat, but larger limits of agreement than that found in our healthy reference sample. Importantly, the limits of agreement for the new skinfold prediction equations for percent body fat, fat mass and fat free mass were smaller than those for the published prediction equations in all groups evaluated in this study. Nevertheless, the limits of agreement are such that it is preferable to use the new prediction equations for group comparisons rather than body composition determination for an individual. Finally, the reference method used was DXA which has not been validated across all diseases and age ranges (Williams et al., 2006).
Our study also had numerous strengths. The prediction equations were developed on a large, multi-ethnic sample of healthy children ages 3 to 21 years, using standardised procedures. The equations using 2-skinfolds performed nearly as well as with 4-skinfolds, and both are presented. While the best prediction was achieved using equations that included African American ancestry and puberty, we present additional simplified equations that include only height and skinfolds which can be used when pubertal stage or ancestry information is unavailable.
In conclusion, this study demonstrates that the newly developed prediction equations can be used for both children with typical growth patterns as well as those with altered growth patterns such as children with Down syndrome, Crohn disease, and nephrotic syndrome. Future studies are needed to validate these equations in children with other diagnoses who are at-risk for growth faltering and abnormal body composition.
Acknowledgments
This study was supported by NIH grants K23 RR16073, K24-DK076808 (MBL), R01 HD071981, UL1TR000003, and the Nutrition Center and Research Institute of the Children’s Hospital of Philadelphia. The authors greatly appreciate the contributions of the families and children who participated in this study, and the staff of the CHOP Clinical and Translational Research Center who collected the data with meticulous care.
Abbreviations
- DXA
dual-energy x-ray absorptiometry
- FM
fat mass
- FFM
fat-free mass
- %BF
percent body fat
- IQR
interquartile range
- CHOP
The Children’s Hospital of Philadelphia
- SF4
previously published equations using 4-skinfolds
- SF2
previously published equations using 2-skinfolds
- New SF2
proposed equations using 2-skinfolds
- New SF4
proposed equations using 4-skinfolds
- Ht
height
- NHANES
National Health and Nutrition Examination Survey
Footnotes
NHANES sample weights were not used in the analyses because the study focused on correspondence in body composition estimated by different methods rather than accurate representation of body composition in the U.S. population.
Declaration of interest
The authors have no declarations of interest to report.
The authors’ responsibilities were as follows – DW and BZ: designed the study; DW: completed final analysis and wrote the final manuscript; BZ, NS, SM, AK, VS, MP: provided essential databases. All authors read and approved the final manuscript. None of the authors had a conflict of interest.
Contributor Information
Danielle Wendel, Seattle Children’s Hospital, Seattle, WA.
David Weber, Golisano Children’s Hospital, School of Medicine and Dentistry, University of Rochester, Rochester, NY.
Mary B. Leonard, Stanford University School of Medicine, Palo Alto, CA.
Sheela N. Magge, Children’s National Health System, Washington, D.C.
Andrea Kelly, The Children’s Hospital of Philadelphia, Philadelphia, PA.
Virginia A. Stallings, The Children’s Hospital of Philadelphia, Philadelphia, PA.
Mary Pipan, The Children’s Hospital of Philadelphia, Philadelphia, PA.
Nicolas Stettler, The Lewin Group, Falls Church, VA.
Babette S. Zemel, The Children’s Hospital of Philadelphia, Philadelphia, PA.
References
- AGUIRRE CA, SALAZAR GD, LOPEZ DE ROMANA DV, KAIN JA, CORVALAN CL, UAUY RE. Evaluation of simple body composition methods: assessment of validity in prepubertal Chilean children. Eur J Clin Nutr. 2015;69:269–73. doi: 10.1038/ejcn.2014.144. [DOI] [PubMed] [Google Scholar]
- ANNEREN G, GUSTAVSON KH, SARA VR, TUVEMO T. Growth retardation in Down syndrome in relation to insulin-like growth factors and growth hormone. Am J Med Genet Suppl. 1990;7:59–62. doi: 10.1002/ajmg.1320370710. [DOI] [PubMed] [Google Scholar]
- ARNELL H, GUSTAFSSON J, IV, ARSSON SA, ANNEREN G. Growth and pubertal development in Down syndrome. Acta Paediatr. 1996;85:1102–6. doi: 10.1111/j.1651-2227.1996.tb14225.x. [DOI] [PubMed] [Google Scholar]
- BIANCHI ML, LEONARD MB, BECHTOLD S, HOGLER W, MUGHAL MZ, SCHONAU E, SYLVESTER FA, VOGIATZI M, VAN DEN HEUVEL-EIBRINK MM, WARD L. Bone Health in Children and Adolescents With Chronic Diseases That May Affect the Skeleton: The 2013 ISCD Pediatric Official Positions. J Clin Densitom. 2014;17:281–94. doi: 10.1016/j.jocd.2014.01.005. [DOI] [PubMed] [Google Scholar]
- BLAND JM, ALTMAN DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- BRAY GA, DELANY JP, VOLAUFOVA J, HARSHA DW, CHAMPAGNE C. Prediction of body fat in 12-y-old African American and white children: evaluation of methods. Am J Clin Nutr. 2002;76:980–90. doi: 10.1093/ajcn/76.5.980. [DOI] [PubMed] [Google Scholar]
- BROOK CG. Determination of body composition of children from skinfold measurements. Arch Dis Child. 1971;46:182–4. doi: 10.1136/adc.46.246.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTTE NF, HOPKINSON JM, WONG WW, SMITH EO, ELLIS KJ. Body composition during the first 2 years of life: an updated reference. Pediatr Res. 2000;47:578–85. doi: 10.1203/00006450-200005000-00004. [DOI] [PubMed] [Google Scholar]
- COX N. [Accessed March 15 2015];ALLPOSSIBLE: Stata module to fit all possible models with subsets of predictors [Online] 2002 Available: http://EconPapers.repec.org/RePEc:boc:bocode:s427901.
- DUBNER SE, SHULTS J, BALDASSANO RN, ZEMEL BS, THAYU M, BURNHAM JM, HERSKOVITZ RM, HOWARD KM, LEONARD MB. Longitudinal assessment of bone density and structure in an incident cohort of children with Crohn’s disease. Gastroenterology. 2009;136:123–30. doi: 10.1053/j.gastro.2008.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DURNIN JV, RAHAMAN MM. The assessment of the amount of fat in the human body from measurements of skinfold thickness. Br J Nutr. 1967;21:681–9. doi: 10.1079/bjn19670070. [DOI] [PubMed] [Google Scholar]
- DURNIN JV, WOMERSLEY J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32:77–97. doi: 10.1079/bjn19740060. [DOI] [PubMed] [Google Scholar]
- FITZSIMMONS J, DROSTE S, SHEPARD TH, PASCOE-MASON J, FANTEL A. Growth failure in second-trimester fetuses with trisomy 21. Teratology. 1990;42:337–45. doi: 10.1002/tera.1420420403. [DOI] [PubMed] [Google Scholar]
- FORBES GB. GROWTH OF THE LEAN BODY MASS DURING CHILDHOOD AND ADOLESCENCE. J Pediatr. 1964;64:822–7. doi: 10.1016/s0022-3476(64)80640-5. [DOI] [PubMed] [Google Scholar]
- FOSTER BJ, SHULTS J, ZEMEL BS, LEONARD MB. Interactions between growth and body composition in children treated with high-dose chronic glucocorticoids. Am J Clin Nutr. 2004;80:1334–41. doi: 10.1093/ajcn/80.5.1334. [DOI] [PubMed] [Google Scholar]
- FREEDMAN DS, HORLICK M, BERENSON GS. A comparison of the Slaughter skinfold-thickness equations and BMI in predicting body fatness and cardiovascular disease risk factor levels in children. Am J Clin Nutr. 2013a;98:1417–24. doi: 10.3945/ajcn.113.065961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREEDMAN DS, OGDEN CL, BLANCK HM, BORRUD LG, DIETZ WH. The abilities of body mass index and skinfold thicknesses to identify children with low or elevated levels of dual-energy X-ray absorptiometry-determined body fatness. J Pediatr. 2013b;163:160–6. e1. doi: 10.1016/j.jpeds.2012.12.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLEC J, KMIOTEK EK, CZECHOWSKA D, SZCZYGIEL E, MASLON A, TOMASZEWSKI KA, GOLEC EB. Analysis of body composition among children and adolescents - a cross-sectional study of the Polish population and comparison of body fat measurement methods. J Pediatr Endocrinol Metab. 2014;27:603–9. doi: 10.1515/jpem-2013-0427. [DOI] [PubMed] [Google Scholar]
- GURKA MJ, KUPERMINC MN, BUSBY MG, BENNIS JA, GROSSBERG RI, HOULIHAN CM, STEVENSON RD, HENDERSON RC. Assessment and correction of skinfold thickness equations in estimating body fat in children with cerebral palsy. Dev Med Child Neurol. 2010;52:e35–41. doi: 10.1111/j.1469-8749.2009.03474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL DL, PARKS EP, ZEMEL BS, SHULTS J, STALLINGS VA, STETTLER N. Resting energy expenditure and adiposity accretion among children with Down syndrome: a 3-year prospective study. Eur J Clin Nutr. 2013;67:1087–91. doi: 10.1038/ejcn.2013.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENDLER DL, BORGES JL, FIELDING RA, ITABASHI A, KRUEGER D, MULLIGAN K, CAMARGOS BM, SABOWITZ B, WU CH, YU EW, SHEPHERD J. The Official Positions of the International Society for Clinical Densitometry: Indications of Use and Reporting of DXA for Body Composition. J Clin Densitom. 2013;16:496–507. doi: 10.1016/j.jocd.2013.08.020. [DOI] [PubMed] [Google Scholar]
- KUCZMARSKI RJ, OGDEN CL, GRUMMER-STRAWN LM, FLEGAL KM, GUO SS, WEI R, MEI Z, CURTIN LR, ROCHE AF, JOHNSON CL. CDC growth charts: United States. Adv Data. 2000:1–27. [PubMed] [Google Scholar]
- LEE DY, WETZSTEON RJ, ZEMEL BS, SHULTS J, ORGAN JM, FOSTER BJ, HERSKOVITZ RM, FOERSTER DL, LEONARD MB. Muscle torque relative to cross-sectional area and the functional muscle-bone unit in children and adolescents with chronic disease. J Bone Miner Res. 2015;30:575–83. doi: 10.1002/jbmr.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEONARD MB, ELMI A, MOSTOUFI-MOAB S, SHULTS J, BURNHAM JM, THAYU M, KIBE L, WETZSTEON RJ, ZEMEL BS. Effects of sex, race, and puberty on cortical bone and the functional muscle bone unit in children, adolescents, and young adults. J Clin Endocrinol Metab. 2010;95:1681–9. doi: 10.1210/jc.2009-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORRIS NM, UDRY JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980;9:271–80. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- NATIONAL CENTER FOR HEALTH STATISTICS. National Health and Nutrition Examination Survey Anthropometry Procedures Manual. 2007 Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_an.pdf(cited 12 December 2014) [Online]
- NATIONAL CENTER FOR HEALTH STATISTICS. National Health and Nutrition Examination Survey: body composition procedures manual. 2008a Available from: http://www.cdc.gov/nchs/data/nhanes/bc.pdf(cited 1 August 2012) [Online]
- NATIONAL CENTER FOR HEALTH STATISTICS. National Health and Nutrition Examination Survey: technical documentation for the 1999–2004 dual energy X-ray absorptiometry (DXA) multiple imputation data files. 2008b Available from: http://www.cdc.gov/nchs/nhanes/dxx/dxa.htm(cited 1 August 2012) [Online]
- O’NEILL KL, SHULTS J, STALLINGS VA, STETTLER N. Child-feeding practices in children with down syndrome and their siblings. J Pediatr. 2005;146:234–8. doi: 10.1016/j.jpeds.2004.10.045. [DOI] [PubMed] [Google Scholar]
- OGDEN CL, LI Y, FREEDMAN DS, BORRUD LG, FLEGAL KM. Smoothed percentage body fat percentiles for U.S. children and adolescents, 1999–2004. Natl Health Stat Report. 2011:1–7. [PubMed] [Google Scholar]
- RAMNITZ MS, LODISH MB. Racial disparities in pubertal development. Semin Reprod Med. 2013;31:333–9. doi: 10.1055/s-0033-1348891. [DOI] [PubMed] [Google Scholar]
- ROLLAND-CACHERA MF. Body composition during adolescence: methods, limitations and determinants. Horm Res. 1993;39(Suppl 3):25–40. doi: 10.1159/000182782. [DOI] [PubMed] [Google Scholar]
- SCHALL JI, SEMEAO EJ, STALLINGS VA, ZEMEL BS. Self-assessment of sexual maturity status in children with Crohn’s disease. J Pediatr. 2002;141:223–9. doi: 10.1067/mpd.2002.125907. [DOI] [PubMed] [Google Scholar]
- SCHOELLER DA, TYLAVSKY FA, BAER DJ, CHUMLEA WC, EARTHMAN CP, FUERST T, HARRIS TB, HEYMSFIELD SB, HORLICK M, LOHMAN TG, LUKASKI HC, SHEPHERD J, SIERVOGEL RM, BORRUD LG. QDR 4500A dual-energy X-ray absorptiometer underestimates fat mass in comparison with criterion methods in adults. Am J Clin Nutr. 2005;81:1018–25. doi: 10.1093/ajcn/81.5.1018. [DOI] [PubMed] [Google Scholar]
- SENTONGO TA, SEMEAO EJ, PICCOLI DA, STALLINGS VA, ZEMEL BS. Growth, body composition, and nutritional status in children and adolescents with Crohn’s disease. J Pediatr Gastroenterol Nutr. 2000;31:33–40. doi: 10.1097/00005176-200007000-00009. [DOI] [PubMed] [Google Scholar]
- SLAUGHTER MH, LOHMAN TG, BOILEAU RA, HORSWILL CA, STILLMAN RJ, VAN LOAN MD, BEMBEN DA. Skinfold equations for estimation of body fatness in children and youth. Hum Biol. 1988;60:709–23. [PubMed] [Google Scholar]
- STOMFAI S, AHRENS W, BAMMANN K, KOVACS E, MARILD S, MICHELS N, MORENO LA, POHLABELN H, SIANI A, TORNARITIS M, VEIDEBAUM T, MOLNAR D. Intra- and inter-observer reliability in anthropometric measurements in children. Int J Obes (Lond) 2011;35(Suppl 1):S45–51. doi: 10.1038/ijo.2011.34. [DOI] [PubMed] [Google Scholar]
- STYLES ME, COLE TJ, DENNIS J, PREECE MA. New cross sectional stature, weight, and head circumference references for Down’s syndrome in the UK and Republic of Ireland. Arch Dis Child. 2002;87:104–8. doi: 10.1136/adc.87.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUN SS, CHUMLEA WC, HEYMSFIELD SB, LUKASKI HC, SCHOELLER D, FRIEDL K, KUCZMARSKI RJ, FLEGAL KM, JOHNSON CL, HUBBARD VS. Development of bioelectrical impedance analysis prediction equations for body composition with the use of a multicomponent model for use in epidemiologic surveys. Am J Clin Nutr. 2003;77:331–40. doi: 10.1093/ajcn/77.2.331. [DOI] [PubMed] [Google Scholar]
- TANNER JM. Growth at adolescence; with a general consideration of the effects of hereditary and environmental factors upon growth and maturation from birth to maturity. Oxford: Blackwell Scientific Publications; 1962. [Google Scholar]
- THAYU M, DENSON LA, SHULTS J, ZEMEL BS, BURNHAM JM, BALDASSANO RN, HOWARD KM, RYAN A, LEONARD MB. Determinants of changes in linear growth and body composition in incident pediatric Crohn’s disease. Gastroenterology. 2010;139:430–8. doi: 10.1053/j.gastro.2010.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THAYU M, SHULTS J, BURNHAM JM, ZEMEL BS, BALDASSANO RN, LEONARD MB. Gender differences in body composition deficits at diagnosis in children and adolescents with Crohn’s disease. Inflamm Bowel Dis. 2007;13:1121–8. doi: 10.1002/ibd.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOOMBS RJ, DUCHER G, SHEPHERD JA, DE SOUZA MJ. The impact of recent technological advances on the trueness and precision of DXA to assess body composition. Obesity (Silver Spring) 2012;20:30–9. doi: 10.1038/oby.2011.211. [DOI] [PubMed] [Google Scholar]
- TSAMPALIEROS A, GUPTA P, DENBURG MR, SHULTS J, ZEMEL BS, MOSTOUFI-MOAB S, WETZSTEON RJ, HERSKOVITZ RM, WHITEHEAD KM, LEONARD MB. Glucocorticoid effects on changes in bone mineral density and cortical structure in childhood nephrotic syndrome. J Bone Miner Res. 2013a;28:480–8. doi: 10.1002/jbmr.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSAMPALIEROS A, LAM CK, SPENCER JC, THAYU M, SHULTS J, ZEMEL BS, HERSKOVITZ RM, BALDASSANO RN, LEONARD MB. Long-term inflammation and glucocorticoid therapy impair skeletal modeling during growth in childhood Crohn disease. J Clin Endocrinol Metab. 2013b;98:3438–45. doi: 10.1210/jc.2013-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DER PLOEG GE, WITHERS RT, LAFORGIA J. Percent body fat via DEXA: comparison with a four-compartment model. J Appl Physiol (1985) 2003;94:499–506. doi: 10.1152/japplphysiol.00436.2002. [DOI] [PubMed] [Google Scholar]
- VANITALLIE TB, YANG MU, HEYMSFIELD SB, FUNK RC, BOILEAU RA. Height-normalized indices of the body’s fat-free mass and fat mass: potentially useful indicators of nutritional status. Am J Clin Nutr. 1990;52:953–9. doi: 10.1093/ajcn/52.6.953. [DOI] [PubMed] [Google Scholar]
- WEBER DR, MOORE RH, LEONARD MB, ZEMEL BS. Fat and lean BMI reference curves in children and adolescents and their utility in identifying excess adiposity compared with BMI and percentage body fat. Am J Clin Nutr. 2013;98:49–56. doi: 10.3945/ajcn.112.053611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELLS GD, HEALE L, SCHNEIDERMAN JE, WILKES DL, ATENAFU E, COATES AL, RATJEN F. Assessment of body composition in pediatric patients with cystic fibrosis. Pediatr Pulmonol. 2008;43:1025–32. doi: 10.1002/ppul.20913. [DOI] [PubMed] [Google Scholar]
- WELLS JC, FEWTRELL MS, WILLIAMS JE, HAROUN D, LAWSON MS, COLE TJ. Body composition in normal weight, overweight and obese children: matched case-control analyses of total and regional tissue masses, and body composition trends in relation to relative weight. Int J Obes (Lond) 2006;30:1506–13. doi: 10.1038/sj.ijo.0803402. [DOI] [PubMed] [Google Scholar]
- WELLS JC, HAROUN D, WILLIAMS JE, WILSON C, DARCH T, VINER RM, EATON S, FEWTRELL MS. Evaluation of DXA against the four-component model of body composition in obese children and adolescents aged 5–21 years. Int J Obes (Lond) 2010a;34:649–55. doi: 10.1038/ijo.2009.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELLS JC, WILLIAMS JE, CHOMTHO S, DARCH T, GRIJALVA-ETERNOD C, KENNEDY K, HAROUN D, WILSON C, COLE TJ, FEWTRELL MS. Pediatric reference data for lean tissue properties: density and hydration from age 5 to 20 y. Am J Clin Nutr. 2010b;91:610–8. doi: 10.3945/ajcn.2009.28428. [DOI] [PubMed] [Google Scholar]
- WETZSTEON RJ, SHULTS J, ZEMEL BS, GUPTA PU, BURNHAM JM, HERSKOVITZ RM, HOWARD KM, LEONARD MB. Divergent effects of glucocorticoids on cortical and trabecular compartment BMD in childhood nephrotic syndrome. J Bone Miner Res. 2009;24:503–13. doi: 10.1359/JBMR.081101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS JE, WELLS JC, WILSON CM, HAROUN D, LUCAS A, FEWTRELL MS. Evaluation of Lunar Prodigy dual-energy X-ray absorptiometry for assessing body composition in healthy persons and patients by comparison with the criterion 4-component model. Am J Clin Nutr. 2006;83:1047–54. doi: 10.1093/ajcn/83.5.1047. [DOI] [PubMed] [Google Scholar]
- ZEMEL BS, RILEY EM, STALLINGS VA. Evaluation of methodology for nutritional assessment in children: anthropometry, body composition, and energy expenditure. Annu Rev Nutr. 1997;17:211–35. doi: 10.1146/annurev.nutr.17.1.211. [DOI] [PubMed] [Google Scholar]