Abstract
Glycans play important biological roles in cell-to-cell interactions, protection against pathogens, as well as in proper protein folding and stability, and are thus interesting targets for scientists. Although their mechanisms of action have been widely investigated and hypothesized, their biological functions are not well understood due to the lack of deglycosylation methods for large-scale isolation of these compounds. Isolation of glycans in their native state is crucial for the investigation of their biological functions. However, current enzymatic and chemical deglycosylation techniques require harsh pretreatment and reaction conditions (high temperature and use of detergents) that hinder the isolation of native glycan structures. Indeed, the recent isolation of new endoglycosidases that are able to cleave a wider variety of linkages and efficiently hydrolyze native proteins has opened up the opportunity to elucidate the biological roles of a higher variety of glycans in their native state. As an example, our research group recently isolated a novel Endo-β-N-acetylglucosaminidase from Bifidobacterium longum subsp. infantis ATCC 15697 (EndoBI-1) that cleaves N-N′-diacetyl chitobiose moieties found in the N-linked glycan (N-glycan) core of high mannose, hybrid, and complex N-glycans. This enzyme is also active on native proteins, which enables native glycan isolation, a key advantage when evaluating their biological activities. Efficient, stable, and economically viable enzymatic release of N-glycans requires the selection of appropriate immobilization strategies. In this review, we discuss the state-of-the-art of various immobilization techniques (physical adsorption, covalent binding, aggregation, and entrapment) for glycosidases, as well as their potential substrates and matrices.
Keywords: glycosidases, immobilization, N-glycans, nutrition
Introduction
Enzymes catalyze metabolic reactions in living organisms and/or substrate conversions in various chemical reactions.1 Recombinant enzymes are used in a variety of industries, such as biofuel production, medicine, waste water treatment, drug metabolism, and the food industry.2 The production of recombinant enzymes is often expensive, with the enzymes being generally unstable and sensitive to changes in process conditions such as pH, temperature, and substrate concentrations.3,4 These limitations can be minimized or even eliminated with enzyme immobilization.5 Enzyme immobilization can be defined as the attachment of the enzyme to various support materials resulting in loss of mobility of the enzyme.6 The selection of the optimal immobilization method is based on the physicochemical characteristics of the enzyme, support material and substrate matrix.7 Various enzyme immobilization techniques have been developed since Nelson and Griffin first demonstrated in 1916 that invertase retains its activity on sucrose when adsorbed to charcoal.8
There are several industrial applications of immobilized enzymes in the food industry. β-D-galactosidase is used to hydrolyze lactose into glucose and galactose for lactose-free milk as well as to produce prebiotic galacto-oligosaccharides via transgalactosylation.9 Glucose isomerase is used to isomerize glucose into fructose to enhance the sweetening capabilities of corn syrup, and lipases have been used for enzymatic interesterification of fats and oils to improve physicochemical properties of triglycerides.10 While α-amylase has not been adapted for immobilization at industrial scale due to the low cost of bulk enzyme,11 the enzyme has been investigated for starch liquefaction using a variety of immobilization techniques, including entrapment in a synthetic matrix, adsorption onto a silica-based mineral, and covalently binding to polystyrene, carboxymethylcellulose, microcrystalline cellulose, polyacrylamide, among others12–18 with a broad range of results. Industrial enzymes have a clear outlet for immobilization, whether for food, chemical, or other applications. However, novel and potentially promising enzymes lack the necessary research for understanding the immobilization process. As an example, glycans linked to glycoproteins are routinely analyzed by proteomics, but the necessary enzymes to cleave those compounds are costly and often inefficient. The purpose of this review is to discuss various immobilization strategies that are compatible with glycosidases to isolate a new class of compounds; N-glycans. In addition to the importance of enzyme and immobilization method synergy, the compatibility of the immobilization chemistry and matrix with whey proteins is also addressed to maximize accessibility of glycosidases to substrates and diffusion of the products through the immobilization matrix.
Glycans and Their Biological Functions
Glycans are oligosaccharides that can be attached to proteins through O- or N-glycosidic bonds. O-linked glycans (O-glycans) are linked to the polypeptide backbone via N-acetylgalactosamine to a hydroxyl group of a serine (Ser) or threonine (Thr) residue. N-linked glycans (N-glycans) are linked via N-acetylglucosamines (GlcNAc) to an asparagine residue of proteins (Asn) in the specific amino acid sequence Asn-X-Ser/Thr (where X can be any amino acid except pro-line).19 Glycans are very attractive compounds for scientists due to their physical and biological functions. They are involved in cell-to-cell and cell-to-microbe interactions, proper protein folding, protection, stabilization and structural support.20 Additionally, glycan moieties exhibit a broad range of functionalities. Sialylated glycans protect against rotavirus infection that cause infant diarrhea, while other studies suggest that sialic acid is essential for cognitive development and learning ability.21 Sialylated glycans can also reduce the binding of leukocytes to endothelial cells while the neutral glycans do not exert any effect.22 Despite the documented benefits of glycans, the industrial production of those compounds for investigation in humans and for potential commercialization is virtually nonexistent. Accordingly, there has been interest recently in utilizing a variety of glycoprotein sources from food production; in particular, whey proteins have been of great interest.
Major sources of glycans: whey as a case study
Whey from cheese manufacture represents a major dairy industry co-product, and is a rich source of glycoproteins containing both N-linked and O-linked glycans. With annual global production surpassing 200 million tons annually, finding effective outlets and high value applications for whey has been a challenge for the dairy industry for over half a century.23 The major components of whey are water (94%), lactose (4.5%), and protein (0.6%), which translates to an annual global whey protein production of over 1 million tons of protein, wherein only about half of the protein is isolated and further utilized commercially.24 The glycoproteins present in whey include immunoglobulins, α-lactalbumin, lactoferrin, transferrin, osteopontin, as well as glycomacropeptide (GMP) liberated from κ-casein during enzymatic coagulation of caseins.25,26 The above mentioned glycoproteins possess a variety of biological activities, such as antibacterial properties (lactoferrin and transferrin) as well as general bifidogenic properties in the infant gut.25,27,28 As substrates for N-glycosidases, whey proteins may exhibit many properties due to varying extents of glycosylation and phosphorylation, molecular weight, conformation in solution, as well as isoelectric point (Table 1).
Table 1.
Characteristics of Various Glycoproteins
| Protein | Molecular Weight (kDa) | Concentration in Milk (g/kg) | Type of Glycosylation | Denaturation Temperature (°C) |
|---|---|---|---|---|
| GMP | 7 | 1.5 g/L in whey27 | O-linked | |
| Lactoferrin | 86 | 0.129 | N-linked | 6730 |
| Transferrin | 76 | 0.01–0.0326 | N-linked | 6431 |
| IgG* | 150 | 1.832 | N-linked | 7033 |
| IgA* | 385 | 0.434 | N-linked | 72.935 |
| IgM* | 900 | 0.234 | N-linked | 7235 |
| Osteopontin | 60 | 0.018 g/L28 | O-linked | |
| α-lactalbumin | 14.2 | 1.236 | N-linked | 58.737 |
Adapted from Walstra.26
Ig stands for immunoglobulin.
Current status of processing strategies to release N-and O-glycans
Glycan release is based on various enzymatic and chemical methods. Although chemical methods (hydrazinolysis, beta-elimination, and trifluoromethanesulfonic acid) are rapid and simple, they often result in total destruction of glycans and the proteins themselves that hinders further investigations on these compounds.38–40 The detergents used during chemical deglycosylation and the high reaction temperatures required to maximize process efficiency do not sufficiently preserve the native structure of the protein or glycan moiety for subsequent elucidation of their biological functions. Non-destructive large-scale methods to isolate both glycans and deglycosylated proteins will facilitate investigations into the biological mechanisms of glycoproteins vis-à-vis their polypeptide chains and attached glycans.
Enzymatic release of N- and O-glycans at laboratory-scale
Peptide-N4-(N-acetyl-b-D-glucosaminyl) asparagine amidases (PNGases) are commonly-used enzymes for deglycosylation of glycoproteins. PNGases cleave asparagine-linked glycans by hydrolyzing the amide side chain.41,42 Although PNGases are active on several types of N-glycans, glycan release is limited if the proteins are in their native state, which would be of great interest when assessing the biological functions of the protein moiety. Additionally, PNGase efficiencies are also affected by the composition and complexity of glycans. As an example, if the glycans contain fucose α-1,3-linked to N-acetlyglucosamine, the N-glycans are resistant to hydrolysis by PNGase F.43 Endoglycosidases F1, F2 and F3 show more activity on native proteins compared to PNGase F, but they have a limited activity on tri- and tetra-antennary glycans. We recently showed that a new endo-β-N-acetylglucosaminidase (EndoBI-1) isolated from Bifidobacterium longum subsp. infantis ATCC 15697 has the ability to cleave the N-N′-diacetyl chitobiose moiety found in the N-glycan core of high mannose, hybrid and complex N-glycans and is not affected by fucosylation of the N-glycan core.44–48 Enzymatic removal of O-glycans is limited compared to N-glycans due to the lack of commercially available O-glycosidases and their limited activity on O-glycan cores.49,50 As a result of the lack of commercial O-glycosidases, and the general smaller body of research concerning O-glycans, this review will focus primarily on N-glycans. Various glycosidases and their specificities on the N-glycan core are shown in Figure 1.
Figure 1.
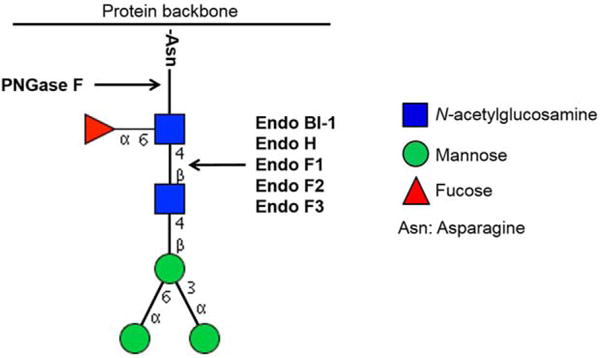
Various glycosidases and their specificities on the N-glycan core.
Challenges associated with the development of large-scale processes to produce N-glycans
Increasing the scale of glycan isolation is a prerequisite step for further investigation of their biological functions and potential commercialization. The major challenge associated with the large-scale production of glycans is the development of an effective glycosidase immobilization approach to reduce process costs and to obtain consistent and complete release of N-glycans from a variety of glycoproteins. As of this writing, all experiments concerning immobilization of N-glycosidases have been conducted at milliliter scale or smaller. While small substrates can easily reach the enzyme’s active site, larger substrates such as DNA, RNA, proteins, and polysaccharides may face diffusional limitations upon immobilization within or upon a given matrix. Appropriate choice of immobilization method and support will have a considerable impact on the kinetics and overall performance of the enzyme, and therefore on the process economics.51 In this review, we discuss a multitude of enzyme immobilization strategies and their compatibility with endoglycosidases, support materials and glycoproteins.
Immobilization Methods and Their Applications to N-Glycosidases
Enzyme immobilization methods are classified as irreversible immobilization (covalent binding, entrapment, and aggregation) and reversible immobilization (adsorption, ionic binding, affinity binding, and metal binding). In general, reversible immobilization techniques result in leaching of the enzyme that hinders the reuse of the enzyme and represents economic loss. However, when the reversibly immobilized enzyme activity is decreased, the support material can be reloaded with fresh enzyme. Irreversible immobilization may minimize enzyme loss via strong binding of support material and enzyme, but enzyme activity may decrease due to active site occlusion and inherent diffusion issues. Various immobilization strategies and their general characteristics are listed in Figure 2.
Figure 2.
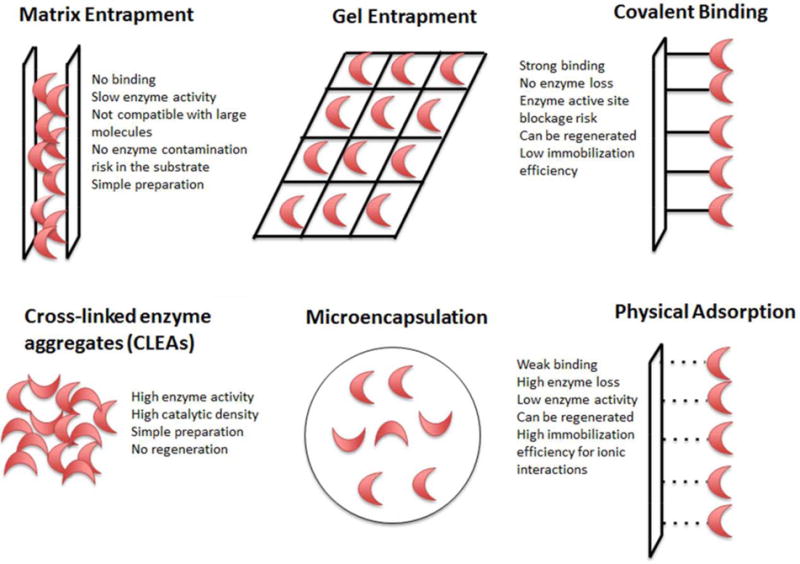
Covalent binding, entrapment, and physical adsorption immobilization techniques.
Covalent binding
Covalent binding is a common method of enzyme immobilization, and is based on the attachment of enzymes to water insoluble carriers via covalent bonds. The functional groups that participate in this binding are amino, thiol, carboxyl, imidazole, phenolic, indole, and sulfhydryl groups.52–54 These groups themselves are not necessarily essential for the catalytic activity of enzyme, but covalent binding is achieved via the binding of these functional groups to the support material.6,55 Successful binding requires the activation of the support material by reactive compounds and the subsequent modification of the polymer backbone for the matrix activation. The strong binding of the enzyme to the carrier prevents shedding of the enzyme.56,57 However, the primary drawback of covalent binding is decreased enzyme activity after immobilization.11,58 Direct linkage between the amino acid residues of the enzyme and support material limits the enzyme mobility, which may result in low enzyme activity.2,59 This drawback can be eliminated by the use of a spacer arm to prevent steric hindrance of enzyme and provides higher mobility to the enzymes.60 Immobilization of enzyme on carriers with a flexible spacer arm can be achieved with functional reagents such as glutaraldehyde or isocyanate.61,62 Various covalent binding strategies and their performance in terms of activity stability and reusability are described in Table 2.
Table 2.
Comparison of Different Support Materials used for Covalent Binding
| Support Material | Activity | Stability | Reusability |
|---|---|---|---|
| CNBr activated Sepharose 4B beads | Medium | High | NR |
| Glycidyl methacrylate-co-ethylene dimethycrylate | High | Medium | NR |
| Nanodiamond | Very high | High | High |
| Graphene oxide | High | Very high | Very high |
One of the first uses of immobilized N-glycosidases was reported in 1987 with cyanogen bromide (CNBr) activated Sepharose 4B beads, wherein PNGase F was used to deglycosylate human chorionic gonadotrophin for oligosaccharide analysis.63 Monolithic and particulate supports made of a variety of materials have also been used in either stirred reactors or integrated into a column for glycan release. Fused silica tubing can be activated with Bind-Silane and acrylamide, after which the enzyme to be immobilized can be added, and the interior of the tubing will have support-bound immobilized enzyme. Using PNGase F, this flow-through reactor configuration was shown to reduce the required reaction time from 18 h to 3.5 min, while the reactors themselves were stable for 8 weeks of storage and reuse.64 Other monolithic supports using silica capillaries for flow-through deglycosylation using PNGase F have relied on the activation and immobilization mechanism of poly (glycidyl methacrylate-co-ethylene dimethycrylate), which has itself been activated using various chemistries. This method is reported to have better overall enzyme utilization due to the lack of enzyme entrapment in acrylamide in the method described above.65
Another monolithic support used in N-glycan characterization is detonation nanodiamond, which has a remarkably high surface area and functional group density. There was a notable decrease in reaction time from over 10 h to 10 min using the immobilized enzyme for deglycosylation.66 Similarly, functionalized graphene oxide has been used as a support for PNGase F, which has yielded similar results in terms of drastically reduced reaction times due to high enzyme packing on the multitude of functional groups on the graphene oxide surface and excellent reusability and stability over the course of several months.67
Further efforts to optimize flow-through reactor designs include a recombinant PNGase F in order to facilitate directional immobilization on the monolithic support. This allows for better substrate access to the enzyme’s active site, improved kinetics and an overall faster reaction. With this configuration, deglycosylation times below a minute were achieved for bovine ribonuclease B (RNase B).65 The vast majority of covalent binding immobilization techniques implement random binding with the functionalized support, wherein any primary amine group on the enzyme will covalently bind to the support in a random fashion. Oriented immobilization, however, aims to direct the active site away from the support and towards the bulk substrate to minimize diffusional and steric hindrances. While this has primarily been investigated for proteomic and microarray-based approaches as well as for other biologically active proteins such as antibodies, industrial-scale directional immobilization could yield substantial kinetic and therefore operational benefits for enzymatic reactions.68,69 For example, glutathione-S transferase and PNGase F fusion proteins have been expressed heterologously in E. coli, initially as a method to facilitate enzyme removal for subsequent protein crystallization.70 This same fusion protein has been adapted for improved immobilization of PNGase F on a monolithic support with quite favorable results.65 The typical overnight reaction time with free PNGase F was decreased to several minutes with nonoriented immobilization in a silica capillary, and further reduced to 15 s with oriented immobilization. Excellent reactor stability and reusability was also observed with cold storage.34
The only reported immobilization of an endo-β-N-acetylglucosaminidase (EndoF1) for protein deglycosylation has been using activated cellulose beads, wherein the aforementioned enzyme from Chryseobacterium meningosepticum was coimmobilized with PNGase F. Optimal conditions for the enzyme immobilization were shown to be pH 6, 1 h incubation at 4°C. Similar enzyme activities were observed between free and immobilized enzymes in regards to the extent of deglycosylation within one hour as measured by SDS-PAGE. Additionally, the enzymes showed similar glycan release after immobilization, and 1 month of storage in buffer at 4°C had no impact on the deglycosylation time of RNase B.71
More generally, when considering industrial immobilization of enzymes, the hydrolysis of macromolecules and polymers can be challenging due to steric or diffusional limitations.10 That said, pectin hydrolysis for fruit juice clarification has been proposed using an alginate-immobilized pectin lyase, as well as that same enzyme immobilized on a variety of other supports.12 Combined microfiltration and pectin hydrolysis using a membrane-immobilized enzyme has been demonstrated.72 Membrane-immobilized enzymes typically exhibit increased Michaelis constants compared to their free counterparts. Despite the increased Michaelis constants indicating reduced substrate affinity, membrane-immobilized enzymes could be advantageous for use with N-glycosidases when considering the possibility of fractionating glycan and protein moieties. Relative size differences between the glycoproteins and their released glycan moieties along with a series of molecular weight cutoff membranes could be exploited to achieve simultaneous release and fractionation of glycan moieties73
Other Proposed Strategies
Entrapment
Entrapment immobilization is achieved by capturing the enzyme in a matrix, such as a gel or microencapsulation.5 Unlike other techniques, most entrapment methods do not require chemical interactions between enzyme and support materials. This technique allows the substrates and the products to diffuse through the membrane or matrix, while enzymes are captured in the network.74 Entrapment prevents the direct interaction of enzyme with the environment and its primary advantages include its low costs, rapid immobilization times, and high retained activity after immobilization. However, mass transfer can be extremely slow and enzyme loading capacity is limited.75 Therefore, entrapment may not be appropriate for higher molecular weight molecules that cannot easily diffuse through the gel matrix.76
Entrapment of N-glycosidases has yet to be investigated, likely due to the diffusional limitations of the large substrates. However, other endoglycosidases and enzymes with macromolecular substrates have been entrapped in a variety of matrices. Pectin lyase has been immobilized on various calcium alginate beads for the clarification of fruit juices, with promising results. However, challenges with inhibition and substrate access have hindered further development, with a substantial decrease in the maximum enzymatic reaction rate (Vmax) and little effect on the Michaelis constant upon alginate entrapment.77
For food-industry applications, calcium alginate gels have been investigated due to their compatibility in many food matrices for a variety of enzymes, including cheese ripening enzymes, glucose isomerase, and β-D-galactosidase.78–80 That said, there has been limited investigation into the efficacy of alginate as an entrapment matrix for α-amylase, whose substrate (starch, a polymer of α-1,4 linked glucose monomers) is substantially larger than the aforementioned enzymes.81 With proper supplementation of the alginate with silica gel, improved immobilization efficiency, and reusability was observed with the immobilized enzyme compared to without added silica. Additionally, pectinase has been immobilized within alginate beads to clarify fruit juices, with improved heat stability and reusability.82 Additional commonly used matrices for entrapment of food enzymes include silica sol-gels, proprietary polyvinyl-alcohol gels, carrageenan, gelatin, and gellan.11
Adsorption
Enzyme immobilization by adsorption is based on the attachment of the enzyme on carrier surface via weak forces (electrostatics, hydrogen bonds, hydrophobic interactions, and Van der Waals forces).83 Immobilization of enzymes by physical adsorption typically requires limited reagents and activation steps that make this strategy remarkably simple. The native structure of the enzyme is not drastically affected during immobilization, helping the enzyme maintain its activity. However, the amount of enzyme actually bound to the support as well as the reuse of physically adsorbed enzymes can be limited because of leaching of enzyme due to the inherently weak binding forces in nonionic interactions. A wide range of compounds can be used as carriers, such as inorganic compounds including silica, metal oxides, and cordierite, or organic carriers, such as chitin, starch, collagen and soluble dextrans. Enzymes adsorbed to a carrier can be eluted from the support material under gentle conditions.
Research regarding the adsorption of enzymes for immobilization in food applications is limited, although there are promising applications. It is worth noting that the first noted application of immobilized enzymes involved adsorption of the catalyst onto a solid support. Although the preparation can be difficult, the costs can be quite high, and the immobilized enzyme activity can be greatly diminished depending upon the support or matrix, there are multiple simple and effective adsorption methods for enzyme immobilization.11
A variety of enzymes in diverse reaction matrices have been investigated. However, no instances of immobilization of N-glycosidases via adsorption have been reported in the literature. As an example of enzymes with macromolecular substrates, excellent enzyme efficiency was obtained with high reusability by maintaining a consistent pH and ionic strength during catalysis with a pectinolytic enzyme cocktail adsorbed to an anion exchange resin. While the extent of enzyme leaching was not reported, the consistently high enzyme activities upon reuse suggest minimal enzyme loss.84 Other investigators have found that optimum pH for reuse and physical stability of pectinase, in regards to adsorption of the enzyme via electrostatic interactions, can also be used to choose reaction matrices. Retained activity of over 85% after multiple reuse cycles, along with tunable adsorption, indicate promising immobilization of pectinase through electrostatic adsorption.85 For α-amylase, it was found that while covalent binding retained not only high enzyme activity but also limited enzyme leaching, adsorption of the enzyme onto montmorillonite yielded extensive leaching. Importantly, the degree of leaching with adsorbed enzyme was a function of enzyme loading as well as reaction temperature.13 A thermostable β-D-galactosidase was immobilized to Sepabeads functionalized with polyethylenimine to add anion-exchange properties to the bead surface. The immobilized enzyme exhibited improved thermal stability compared to the soluble counterpart, and also had minimal leaching after multiple reuse cycles.86 In contrast, simple adsorption of β-D-galactosidase onto celite yielded vastly diminished enzyme activity when compared with covalent bonding and enzyme aggregation, yielding unfavorable results.87
Crosslinked enzyme aggregates (CLEAs)
Crosslinking enzymes into aggregates (CLEAs) for immobilization purposes is a promising method for enzyme immobilization first developed in the 1960s for protein crosslinking.88,89 This technology was applied to enzymes in the late 1990s, and was patented shortly thereafter. Glutaraldehyde, a bifunctional organic molecule, has historically been used for enzyme and protein crosslinking, but recently polyfunctional molecules with a higher molecular weight have gained popularity due to the limited occlusion of the enzyme active sites in crosslinking compared with glutaraldehyde.90 While CLEAs have as of yet had limited applications in food processing, they show great promise for many industries due to its high catalyst productivity. In comparison with other immobilization methods wherein inert or otherwise noncatalytic volume represents up to 99% of the added immobilized enzyme, the entirety of the mass of CLEAs is catalytic.91 Although CLEAs of enzymes relevant to the food industry have been examined, they have targeted primarily low molecular weight substrates, such as the enzymes levansucrase, β-D-galactosidase, and glucoamylase, all of which exhibited improved thermal stability and high retained activity.87,92,93 Additionally, a commercial blend of pectinases, xyalanases, and cellulases were treated with glutaraldehyde to form CLEAs, with results suggesting improved thermal stability, reusability, as well as an increased Vmax.94 As a novel approach to carrier-free enzyme immobilization, it has shown great promise for future applications. To date, no CLEAs application of N-glycosidases has been reported in the literature.
Affinity immobilization
Affinity immobilization is achieved by precoupling the enzyme to an affinity ligand for the target enzyme or the enzyme is attached to an entity that provides affinity toward the matrix. This so-called immunoaffinity immobilization provides consistent enzyme stabilization, with a high proportion of activity retained upon binding. Additionally, the reversible nature of this method offers many benefits, such as the ability to reload the ligands (typically antibodies) with new enzyme and recover enzyme for purification purposes.95 Glutathione-S-transferase-tagged PNGase F was used to deglycosylate three model glycoproteins (fetuin, RNase B, IgG), with improved reaction times compared to the free enzyme.96 While affinity immobilization has not been extensively investigated at pilot-scale, lab scale experiments using β-D-galactosidase and cellulose-bound antibodies for β-D-galactosidase harvested from rabbits have yielded promising results. This method allows for simultaneous purification and immobilization for reactions, and over 96% retained activity compared to soluble enzyme.97 Additionally, α-amylase has been immobilized using Cibacron Blue F3G-A dye as affinity ligand to a cellulose membrane via a chitosan spacer arm. Reduced concentration polarization at the membrane surface was observed due to the hydrolytic activity of the enzyme on starch in the boundary layer.98
Immobilization Carriers
Selection of immobilization carrier materials is a crucial step for successful immobilization and product quality, and a thorough guide determining appropriate carriers and protocols for various applications has yet to be developed.99 Carrier materials must be compatible with enzyme, insoluble, nontoxic, inexpensive, possess high stability and diffusivity, and enable a simple procedure.6,100 Important parameters to consider when choosing an immobilization carrier for a particular application include the hydrophobicity, porosity, functionality, charge on the surface, effective surface area, particle size, chemical and physical stability, composition (organic vs. inorganic), and its status as food grade or not.101,102 Additionally, the carrier should be inert and compatible with the substrate matrix and enzyme itself.
While the so-called monolithic supports, which are characterized by superior hydrodynamic properties and mass transfer to traditional porous supports, have been validated for use in small-scale, flow-through microreactors, large scale immobilized enzyme reactors may not be appropriate for this application.64,65,103 Commonly used support materials are functionalized cellulose, starch, ion exchange resins, silica, clay/ceramics, agarose, acrylamide, porous, and coated glass. However, practically any solid or gel-like material above described would possess appropriate functionalities and attributes to be considered a suitable support.86,104–108
Conclusions
When considering enzymatic reactions at any scale, from microreactor to industrial applications, immobilization of the biocatalyst can be a crucial step towards making a more stable, simpler, and more economically viable bioprocess. Unfortunately, research on the immobilization of enzymes with large substrates (i.e., polysaccharides, proteins) is quite limited for industrial applications. With a growing interest in recombinantly produced enzymes including glycosidases, this research could prove invaluable for both basic research to elucidate biochemical roles of glycans and for improving large-scale isolation of those compounds. Additionally, understanding the economic implications, whether positive or negative, for a given process utilizing immobilized enzymes would help promote its future development. For glycosidases used in the proteomics and food industries, multiple immobilization protocols have been developed using methods that can be characterized as entrapment, adsorption, covalent binding, as well as others including immunoaffinity binding and enzyme aggregation. Moreover, numerous support materials have been utilized for all of these applications, with a variety of resulting enzyme stabilities and activities. For any given immobilized enzyme reactor, the substrate and matrix, enzyme, reactor, and carrier properties must be considered before developing an appropriate method. While diffusional limitations of macromolecular substrates in certain immobilization methods have been discussed herein, there is a lack of research involving suitable alternatives involving spacer arms or other factors such as directional immobilization to facilitate catalysis. Mechanistic understanding of factors such as spacer arms to improve substrate access and how it will affect heterogeneous catalysis will improve the development of enzyme immobilization for large substrates. Future efforts should be put towards improving the scalability of immobilization, as well as the development of immobilization protocols that focus on enzymes with high molecular weight substrates.
Acknowledgments
This research was supported in part by funding from the National Institutes of Health (Bethesda, MD) awards R01AT007079 and R01AT008759, the UC Davis Sustainable AgTech Innovation Center (Davis, CA), Center for Advanced Processing and Packaging Studies (CAPPS) and the Peter J. Shields Endowed Chair in Dairy Food Science (Davis, CA). This research was partially supported by an industry/campus supported fellowship under the Training Program in Biomolecular Technology (T32-GM008799) at the University of California, Davis.
Contributor Information
Sercan Karav, Department of Molecular Biology and Genetics, Canakkale 18 Mart University, Canakkale, Turkey.
Joshua L. Cohen, Department of Food Science and Technology, University of California, One Shields Avenue, Davis, CA 95616.
Daniela Barile, Department of Food Science and Technology, University of California, One Shields Avenue, Davis, CA 95616Foods for Health Institute, University of California, One Shields Avenue, Davis, CA 95616.
Juliana Maria Leite Nobrega de Moura Bell, Department of Food Science and Technology, University of California, One Shields Avenue, Davis, CA 95616.
Literature Cited
- 1.Cooper GM. The central role of enzymes as biological catalysts. 2000;2:115–135. [Google Scholar]
- 2.Khan AA, Alzohairy MA. Recent advances and applications of immobilized enzyme technologies: a review. Res J Biol Sci. 2010;5:565–575. [Google Scholar]
- 3.Tischer W, Kasche V. Immobilized enzymes: crystals or carriers? Trends Biotechnol. 1999;17:326–335. doi: 10.1016/s0167-7799(99)01322-0. [DOI] [PubMed] [Google Scholar]
- 4.Mateo C, Palomo JM, Fernandez-Lorente G, Guisan JM, Fernandez-Lafuente R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb Technol. 2007;40:1451–1463. [Google Scholar]
- 5.Sheldon RA. Enzyme immobilization: the quest for optimum performance. Adv Synth Catal. 2007;349:1289–1307. [Google Scholar]
- 6.Datta S, Christena LR, Rajaram YRS. Enzyme immobilization: an overview on techniques and support materials. Biotechnology. 2013;3:1–9. doi: 10.1007/s13205-012-0071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nisha S, Arun Karthick S, Gobi NA. A review on methods, application and properties of immobilized enzyme. Chem Sci Rev Lett. 2012;1:148–155. [Google Scholar]
- 8.Nelson J, Griffin EG. Adsorption of invertase. J Am Chem Soc. 1916;38:1109–1115. [Google Scholar]
- 9.Panesar PS, Panesar R, Singh RS, Kennedy JF, Kumar H. Microbial production, immobilization and applications of β-d-galactosidase. J Chem Technol Biotechnol. 2006;81:530–543. [Google Scholar]
- 10.DiCosimo R, McAuliffe J, Poulose AJ, Bohlmann G. Industrial use of immobilized enzymes. Chem Soc Rev. 2013;42:6437–6474. doi: 10.1039/c3cs35506c. [DOI] [PubMed] [Google Scholar]
- 11.Fernandes P. Enzymes in food processing: a condensed overview on strategies for better biocatalysts. Enzyme Res. 2010:1–19. doi: 10.4061/2010/862537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linko YY, Saarinen P, Linko M. Starch conversion by soluble and immobilized α-amylase. Biotechnol Bioeng. 1975;17:153–165. [Google Scholar]
- 13.Gopinath S, Sugunan S. Leaching studies over immobilized a-amylase. Importance of the nature of enzyme attachment. React Kinet Catal Lett. 2004;83:79–83. [Google Scholar]
- 14.Manecke G. Reactive polymers and their use for the preparation of antibody and enzyme resins. Pure Appl Chem. 1962;4:507–520. [Google Scholar]
- 15.Bernfeld P, Wan J. Antigens and enzymes made insoluble by entrapping them into lattices of synthetic polymers. Science (New York, NY) 1963;142:678–679. doi: 10.1126/science.142.3593.678. [DOI] [PubMed] [Google Scholar]
- 16.Fukushi T, Isemura T. Regeneration of the native three-dimensional structure of Bacillus subtilis α-amylase and its formation in biological systems. J Biochem. 1968;64:283–292. doi: 10.1093/oxfordjournals.jbchem.a128894. [DOI] [PubMed] [Google Scholar]
- 17.Barker S, Somers P, Epton R, McLaren J. Cross-linked poly-acrylamide derivatives (Enzacryls) as water-insoluble carriers of amylolytic enzymes. Carbohydr Res. 1970;14:287–296. [Google Scholar]
- 18.Barker S, Somers P, Epton R. Preparation and properties of α-amylase chemically coupled to microcrystalline cellulose. Carbohydr Res. 1968;8:491–497. [Google Scholar]
- 19.Essentials of Glycobiology. 2nd. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2009. [PubMed] [Google Scholar]
- 20.Varki A, Lowe JB. Biological roles of glycans. 2009;18:747–749. [PubMed] [Google Scholar]
- 21.Morgan BL, Winick M. Effects of administration of N-acetylneuraminic acid (NANA) on brain NANA content and behavior. J Nutr. 1980;110:416–424. doi: 10.1093/jn/110.3.416. [DOI] [PubMed] [Google Scholar]
- 22.Bode L, Kunz C, Muhly-Reinholz M, Mayer K, Seeger W, Rudloff S. Inhibition of monocyte, lymphocyte, and neutrophil adhesion to endothelial cells by human milk oligosaccharides. Thromb Haemost. 2004;92:1402–1410. doi: 10.1160/TH04-01-0055. [DOI] [PubMed] [Google Scholar]
- 23.Mollea C, Bosco F, Marmo L. Valorisation of Cheese Whey, a By-Product from the Dairy Industry. INTECH Open Access Publisher; 2013. [Google Scholar]
- 24.De Wit J. Lecturer’s Handbook on Whey and Whey Products. Bruksela, Belgia: European Whey Products Association; 2001. [Google Scholar]
- 25.Horowitz M. The Glycoconjugates: Mammalian Glycoproteins and Glycolipids. Amsterdam: Elsevier; 2012. [Google Scholar]
- 26.Walstra P, Walstra P, Wouters JT, Geurts TJ. Dairy Science and Technology. Boca Raton: CRC Press; 2005. [Google Scholar]
- 27.Manso M, Lopez-Fandino R. κ-Casein macropeptides from cheese whey: Physicochemical, biological, nutritional, and technological features for possible uses. Food Rev Int. 2004;20:329–355. [Google Scholar]
- 28.Schack L, Lange A, Kelsen J, Agnholt J, Christensen B, Petersen TE, Sørensen ES. Considerable variation in the concentration of osteopontin in human milk, bovine milk, and infant formulas. J Dairy Sci. 2009;92:5378–5385. doi: 10.3168/jds.2009-2360. [DOI] [PubMed] [Google Scholar]
- 29.Recio I, Moreno F, López-Fandiño R. Glycosylated dairy components: their roles in nature and ways to make use of their biofunctionality in dairy products. In: Corredig M, editor. Dairy-Derived Ingredients: Food and Nutraceutical Uses. Vol. 2009. Woodhead, UK: Woodhead Publishing; pp. 170–211. [Google Scholar]
- 30.Mata L, Sánchez L, Headon DR, Calvo M. Thermal denaturation of human lactoferrin and its effect on the ability to bind iron. J Agric Food Chem. 1998;46:3964–3970. [Google Scholar]
- 31.Evans RW, Williams J, Moreton K. A variant of human transferrin with abnormal properties. Biochem J. 1982;201:19–26. doi: 10.1042/bj2010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Riordan N, Kane M, Joshi L, Hickey RM. Structural and functional characteristics of bovine milk protein glycosylation. Glycobiology. 2014;24:220–236. doi: 10.1093/glycob/cwt162. [DOI] [PubMed] [Google Scholar]
- 33.Vermeer AW, Norde W. The thermal stability of immunoglobulin: unfolding and aggregation of a multi-domain protein. Biophys J. 2000;78:394–404. doi: 10.1016/S0006-3495(00)76602-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuquay JW, Fox PF, McSweeney PL. Four-Volume Set. 2nd. New York: Academic Press; 2011. Encyclopedia of Dairy Sciences. [Google Scholar]
- 35.Mulvihill D, Donovan M. Whey proteins and their thermal denaturation—a review. Irish J Food Sci Technol. 1987;11:43–75. [Google Scholar]
- 36.Hart GW, Brew K, Grant GA, Bradshaw RA, Lennarz W. Primary structural requirements for the enzymatic formation of the N-glycosidic bond in glycoproteins. J Biol Chem. 1979;254:9747–9753. [PubMed] [Google Scholar]
- 37.Paulsson M, Dejmek P. Thermal denaturation of whey proteins in mixtures with caseins studied by differential scanning calorimetry. J Dairy Sci. 1990;73:590–600. [Google Scholar]
- 38.Nakakita S-I, Sumiyoshi W, Miyanishi N, Hirabayashi J. A practical approach to N-glycan production by hydrazinolysis using hydrazine monohydrate. Biochem Biophys Res Commun. 2007;362:639–645. doi: 10.1016/j.bbrc.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 39.Zauner G, Koeleman CA, Deelder AM, Wuhrer M. Mass spectrometric O-glycan analysis after combined O-glycan release by beta-elimination and 1-phenyl-3-methyl-5-pyrazolone labeling. Biochim Biophys Acta. 2012;1820:1420–1428. doi: 10.1016/j.bbagen.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Tams JW, Welinder KG. Glycosylation and thermodynamic versus kinetic stability of horseradish peroxidase. FEBS Lett. 1998;421:234–236. doi: 10.1016/s0014-5793(97)01573-1. [DOI] [PubMed] [Google Scholar]
- 41.Nuck R, Zimmermann M, Sauvageot D, Josi D, Reutter W. Optimized deglycosylation of glycoproteins by peptide-N4-(N-acetyl-beta-glucosaminyl)-asparagine amidase from Flavobacterium meningosepticum. Glycoconjug J. 1990;7:279–286. doi: 10.1007/BF01073372. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi N. Demonstration of a new amidase acting on glycopeptides. Biochem Biophys Res Commun. 1977;76:1194–1201. doi: 10.1016/0006-291x(77)90982-2. [DOI] [PubMed] [Google Scholar]
- 43.Tretter V, Altmann F, Marz L. Peptide-N4-(N-acetyl-beta-glucosaminyl)asparagine amidase F cannot release glycans with fucose attached alpha 1–3 to the asparagine-linked N-acetylglucosamine residue. Eur J Biochem. 1991;199:647–652. doi: 10.1111/j.1432-1033.1991.tb16166.x. [DOI] [PubMed] [Google Scholar]
- 44.Garrido D, Nwosu C, Ruiz-Moyano S, et al. Endo-beta N-acetylglucosaminidases from infant gut-associated bifidobacteria release complex N-glycans from human milk glycoproteins. Mol Cell Proteomics. 2012;11:775–785. doi: 10.1074/mcp.M112.018119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karav S, de Moura Bell JLMN, Parc AL, Liu Y, Mills DA, Block DE, Barile D. Characterizing the release of bioactive N-glycans from dairy products by a novel endo-b-N-acetylglucosaminidase. Biotechnol Prog. 2015;31:1331–1339. doi: 10.1002/btpr.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karav S, Le Parc A, de Moura Bell JMLN, Frese SA, Kirmiz N, Block DE, Mills DA. Oligosaccharides released from milk glycoproteins are selective growth substrates for infant-associated bifidobacteria. Appl Environ Microbiol. 2016;82:3622–3630. doi: 10.1128/AEM.00547-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karav S, Parc AL, de Moura Bell JMLN, Rouquie C, Mills DA, Barile D, Block DE. Kinetic characterization of a novel endo-beta-N-acetylglucosaminidase on concentrated bovine colostrum whey to release bioactive glycans. Enzym Microb Technol. 2015;77:46–53. doi: 10.1016/j.enzmictec.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parc AL, Karav S, de Moura Bell JLMN, Frese SA, Liu Y, Mills DA, Barile D. A novel endo-b-N-acetylglucosaminidase releases specific N-glycans depending on different reaction conditions. Biotechnol Prog. 2015;31:1323–1330. doi: 10.1002/btpr.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.2008 Meeting of the Society for Glycobiology, November 12–15, 2008 Fort Worth, Texas, USA. Glycobiology. 2008;18:940–940. [Google Scholar]
- 50.Koutsioulis D, Landry D, Guthrie EP. Novel endo-α-N-acetylgalactosaminidases with broader substrate specificity. Glycobiology. 2008;18:799–805. doi: 10.1093/glycob/cwn069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guisán JM, Penzol G, Armisen P, Bastida A, Blanco RM, Fernandez-Lafuente R, García-Junceda E. Immobilization of Enzymes and Cells. New York: Springer; 1997. Immobilization of enzymes acting on macromolecular substrates; pp. 261–275. [Google Scholar]
- 52.Jal P, Patel S, Mishra B. Chemical modification of silica surface by immobilization of functional groups for extractive concentration of metal ions. Talanta. 2004;62:1005–1028. doi: 10.1016/j.talanta.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 53.Karyakin AA, Presnova GV, Rubtsova MY, Egorov AM. Oriented immobilization of antibodies onto the gold surfaces via their native thiol groups. Anal Chem. 2000;72:3805–3811. doi: 10.1021/ac9907890. [DOI] [PubMed] [Google Scholar]
- 54.Schuster M, Meyer WH, Wegner G, Herz HG, Ise M, Kreuer KD, Maier J. Proton mobility in oligomer-bound proton solvents: imidazole immobilization via flexible spacers. Solid State Ionics. 2001;145:85–92. [Google Scholar]
- 55.Kim D, Herr AE. Protein immobilization techniques for microfluidic assays. Biomicrofluidics. 2013;7:041501. doi: 10.1063/1.4816934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang P, Dai S, Waezsada S, Tsao AY, Davison BH. Enzyme stabilization by covalent binding in nanoporous sol-gel glass for nonaqueous biocatalysis. Biotechnol Bioeng. 2001;74:249–255. doi: 10.1002/bit.1114. [DOI] [PubMed] [Google Scholar]
- 57.Johansson A-C, Mosbach K. Acrylic copolymers as matrices for the immobilization of enzymes: I. Covalent binding or entrapping of various enzymes to bead-formed acrylic copolymers. Biochim Biophys Enzymol. 1974;370:339–347. doi: 10.1016/0005-2744(74)90094-1. [DOI] [PubMed] [Google Scholar]
- 58.Tran DN, Balkus JR. KJ. Perspective of recent progress in immobilization of enzymes. ACS Catal. 2011;1:956–968. [Google Scholar]
- 59.Singh RK, Tiwari MK, Singh R, Lee J-K. From protein engineering to immobilization: promising strategies for the upgrade of industrial enzymes. Int J Mol Sci. 2013;14:1232–1277. doi: 10.3390/ijms14011232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang D-H, Yuwen L-X, Peng LJ. Parameters affecting the performance of immobilized enzyme. J Chem. 2013:1–7. [Google Scholar]
- 61.Alptekin Ӧ, Tükel SS, Yildirim D, Alagöz D. Covalent immobilization of catalase onto spacer-arm attached modified florisil: characterization and application to batch and plug-flow type reactor systems. Enzyme Microb Technol. 2011;49:547–554. doi: 10.1016/j.enzmictec.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 62.Noiset O, Schneider YJ, Marchand–Brynaert J. Surface modification of poly (aryl ether ether ketone)(PEEK) film by covalent coupling of amines and amino acids through a spacer arm. J Polym Sci A: Polym Chem. 1997;35:3779–3790. [Google Scholar]
- 63.Damm JB, Kamerling JP, van Dedem GW, Vliegenthart JF. A general strategy for the isolation of carbohydrate chains from N-,O-glycoproteins and its application to human chorionic gonadotrophin. Glycoconjug J. 1987;4:129–144. [Google Scholar]
- 64.Palm AK, Novotny MV. A monolithic PNGase F enzyme microreactor enabling glycan mass mapping of glycoproteins by mass spectrometry. Rapid Commun Mass Spectrom. 2005;19:1730–1738. doi: 10.1002/rcm.1979. [DOI] [PubMed] [Google Scholar]
- 65.Krenkova J, Lacher NA, Svec F. Multidimensional system enabling deglycosylation of proteins using a capillary reactor with peptide-N-glycosidase F immobilized on a porous polymer monolith and hydrophilic interaction liquid chromatography–mass spectrometry of glycans. J Chromatogr A. 2009;1216:3252–3259. doi: 10.1016/j.chroma.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 66.Wei L, Zhang W, Lu H, Yang P. Immobilization of enzyme on detonation nanodiamond for highly efficient proteolysis. Talanta. 2010;80:1298–1304. doi: 10.1016/j.talanta.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 67.Ren X, Bai H, Pan Y, et al. A graphene oxide-based immobilized PNGase F reagent for highly efficient N-glycan release and MALDI-TOF MS profiling. Anal Methods. 2014;6:2518–2525. [Google Scholar]
- 68.Turkova J. Oriented immobilization of biologically active proteins as a tool for revealing protein interactions and function. J Chromatogr B: Biomed Sci Appl. 1999;722:11–31. doi: 10.1016/s0378-4347(98)00434-4. [DOI] [PubMed] [Google Scholar]
- 69.Seong SyChoi Cy Current status of protein chip development in terms of fabrication and application. Proteomics. 2003;3:2176–2189. doi: 10.1002/pmic.200300609. [DOI] [PubMed] [Google Scholar]
- 70.Grueninger-Leitch F, D’Arcy A, D’Arcy B, Chène C. Deglycosylation of proteins for crystallization using recombinant fusion protein glycosidases. Protein Sci. 1996;5:2617. doi: 10.1002/pro.5560051224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kwan EM, Boraston AB, McLean BW, Kilburn DG, Warren RAJN. Glycosidase–carbohydrate-binding module fusion proteins as immobilized enzymes for protein deglycosylation. Protein Eng Des Select. 2005;18:497–501. doi: 10.1093/protein/gzi055. [DOI] [PubMed] [Google Scholar]
- 72.Szaniawski AR, Spencer HG. Effects of immobilized pectinase on the microfiltration of dilute pectin solutions by macroporous titania membranes: resistance model interpretation. J Membr Sci. 1997;127:69–76. [Google Scholar]
- 73.Wang H, Thomas RL, Szaniawski AR, Spencer HG. Enzymes immobilized on formed in place membranes for food processing: procedures and properties. J Food Process Eng. 1994;17:365–381. [Google Scholar]
- 74.Sassolas A, Hayat A, Marty JL. Enzyme Immobilization by Entrapment Within a Gel Network Immobilization of Enzymes and Cells. New York: Springer; 2013. pp. 229–239. [DOI] [PubMed] [Google Scholar]
- 75.Brady D, Jordaan J. Advances in enzyme immobilisation. Biotechnol Lett. 2009;31:1639–1650. doi: 10.1007/s10529-009-0076-4. [DOI] [PubMed] [Google Scholar]
- 76.Tanaka H, Matsumura M, Veliky I. Diffusion characteristics of substrates in Ca-alginate gel beads. Biotechnol Bioeng. 1984;26:53–58. doi: 10.1002/bit.260260111. [DOI] [PubMed] [Google Scholar]
- 77.Busto M, Garcia-Tramontin K, Ortega N, Perez-Mateos M. Preparation and properties of an immobilized pectinlyase for the treatment of fruit juices. Bioresour Technol. 2006;97:1477–1483. doi: 10.1016/j.biortech.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 78.Anjani K, Kailasapathy K, Phillips M. Microencapsulation of enzymes for potential application in acceleration of cheese ripening. Int Dairy J. 2007;17:79–86. [Google Scholar]
- 79.Tumturk H, Demirel G, Altinok H, Aksoy S, Hasirci N. Immobilization of glucose isomerase in surface-modified alginate gel beads. J Food Biochem. 2008;32:234–246. [Google Scholar]
- 80.Dashevsky A. Protein loss by the microencapsulation of an enzyme (lactase) in alginate beads. Int J Pharm. 1998;161:1–5. [Google Scholar]
- 81.Rajagopalan G, Krishnan C. Immobilization of malto-oligosaccharide forming α-amylase from Bacillus subtilis KCC103: properties and application in starch hydrolysis. J Chem Technol Biotechnol. 2008;83:1511–1517. [Google Scholar]
- 82.Rehman HU, Aman A, Silipo A, Qader SAU, Molinaro A, Ansari A. Degradation of complex carbohydrate: immobilization of pectinase from Bacillus licheniformis KIBGE-IB21 using calcium alginate as a support. Food Chem. 2013;139:1081–1086. doi: 10.1016/j.foodchem.2013.01.069. [DOI] [PubMed] [Google Scholar]
- 83.Jegannathan KR, Abang S, Poncelet D, Chan ES, Ravindra P. Production of biodiesel using immobilized lipase—a critical review. Crit Rev Biotechnol. 2008;28:253–264. doi: 10.1080/07388550802428392. [DOI] [PubMed] [Google Scholar]
- 84.Demir N, Acar J, Sarıoğlu K, Mutlu M. The use of commercial pectinase in fruit juice industry. Part 3: Immobilized pectinase for mash treatment. J Food Eng. 2001;47:275–280. [Google Scholar]
- 85.Bahrami A, Hejazi P. Electrostatic immobilization of pectinase on negatively charged AOT-Fe 3 O 4 nanoparticles. J Mol Catal B: Enzym. 2013;93:1–7. [Google Scholar]
- 86.Pessela BC, Fernández-Lafuente R, Fuentes M, et al. Reversible immobilization of a thermophilic β-galactosidase via ionic adsorption on PEI-coated sepabeads. Enzyme Microb Technol. 2003;32:369–374. [Google Scholar]
- 87.Gaur R, Pant H, Jain R, Khare S. Galacto-oligosaccharide synthesis by immobilized Aspergillus oryzae β-galactosidase. Food Chem. 2006;97:426–430. [Google Scholar]
- 88.Richards F, Knowles J. Glutaraldehyde as a protein cross-linking reagent. J Mol Biol. 1968;37:231–233. doi: 10.1016/0022-2836(68)90086-7. [DOI] [PubMed] [Google Scholar]
- 89.Schoevaart R, Wolbers M, Golubovic M, et al. Preparation, optimization, and structures of cross-linked enzyme aggregates (CLEAs) Biotechnol Bioeng. 2004;87:754–762. doi: 10.1002/bit.20184. [DOI] [PubMed] [Google Scholar]
- 90.Mateo C, Palomo JM, Van Langen LM, Van Rantwijk F, Sheldon RA. A new, mild cross-linking methodology to prepare cross-linked enzyme aggregates. Biotechnol Bioeng. 2004;86:273–276. doi: 10.1002/bit.20033. [DOI] [PubMed] [Google Scholar]
- 91.Sheldon R. Cross-linked enzyme aggregates (CLEAs): stable and recyclable biocatalysts. Biochem Soc Trans. 2007;35:2007. doi: 10.1042/BST0351583. [DOI] [PubMed] [Google Scholar]
- 92.Tatsumoto K, Oh KK, Baker JO, Himmel ME. Enhanced stability of glucoamylase through chemical crosslinking. Appl Biochem Biotechnol. 1989;20:293–308. [Google Scholar]
- 93.Ortiz-Soto ME, Rudiño-Piñera E, Rodriguez-Alegria ME, Munguia AL. Evaluation of cross-linked aggregates from purified Bacillus subtilis levansucrase mutants for transfructosylation reactions. BMC Biotechnol. 2009;9:68. doi: 10.1186/1472-6750-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dalal S, Sharma A, Gupta MN. A multipurpose immobilized biocatalyst with pectinase, xylanase and cellulase activities. Chem Central J. 2007;1:16. doi: 10.1186/1752-153X-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saleemuddin M. Thermal Biosensors, Bioactivity, Bioaffinitty. New York: Springer; 1999. Bioaffinity based immobilization of enzymes; pp. 203–226. [DOI] [PubMed] [Google Scholar]
- 96.Szigeti M, Bondar J, Gjerde D, Keresztessy Z, Szekrényes Á, Guttman A. Rapid N-glycan release from glycoproteins using immobilized PNGase F microcolumns. J Chromatogr B. 2016;1032:139–143. doi: 10.1016/j.jchromb.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 97.Haider T, Husain Q. Immobilization of β galactosidase from Aspergillus oryzae via immunoaffinity support. Biochem Eng J. 2009;43:307–314. [Google Scholar]
- 98.Konovalova V, Guzikevich K, Burban A, Kujawski W, Jarzynka K, Kujawa J. Enhanced starch hydrolysis using α-amylase immobilized on cellulose ultrafiltration affinity membrane. Carbohydr Polym. 2016;152:710–717. doi: 10.1016/j.carbpol.2016.07.065. [DOI] [PubMed] [Google Scholar]
- 99.Boller T, Meier C, Menzler S. Eupergit oxirane acrylic beads: how to make enzymes fit for biocatalysis. Org Process Res Dev. 2002;6:509–519. [Google Scholar]
- 100.Leenen EJ, Dos Santos VA, Grolle KC, Tramper J, Wijffels R. Characteristics of and selection criteria for support materials for cell immobilization in wastewater treatment. Water Res. 1996;30:2985–2996. [Google Scholar]
- 101.Hanefeld U, Gardossi L, Magner E. Understanding enzyme immobilisation. Chem Soc Rev. 2009;38:453–468. doi: 10.1039/b711564b. [DOI] [PubMed] [Google Scholar]
- 102.Kourkoutas Y, Bekatorou A, Banat IM, Marchant R, Koutinas A. Immobilization technologies and support materials suitable in alcohol beverages production: a review. Food Microbiol. 2004;21:377–397. [Google Scholar]
- 103.Josic D, Buchacher A, Jungbauer A. Monoliths as stationary phases for separation of proteins and polynucleotides and enzymatic conversion. J Chromatogr B: Biomed Sci Appl. 2001;752:191–205. doi: 10.1016/s0378-4347(00)00499-0. [DOI] [PubMed] [Google Scholar]
- 104.Katchalski-Katzir E, Kraemer DM. Eupergit® C, a carrier for immobilization of enzymes of industrial potential. J Mol Catal B: Enzym. 2000;10:157–176. [Google Scholar]
- 105.Sugiura M, Isobe M. Purification of microbial lipases by glass beads coated with hydrophobic materials. Chem Pharm Bull. 1977;23:1221–1225. doi: 10.1248/cpb.25.1987. [DOI] [PubMed] [Google Scholar]
- 106.Turner MB, Spear SK, Holbrey JD, Daly DT, Rogers RD. Ionic liquid-reconstituted cellulose composites as solid support matrices for biocatalyst immobilization. Biomacromolecules. 2005;6:2497–2502. doi: 10.1021/bm050199d. [DOI] [PubMed] [Google Scholar]
- 107.Benoit MR, Kohler JT. An evaluation of a ceramic monolith as an enzyme support material. Biotechnol Bioeng. 1975;17:1617–1626. [Google Scholar]
- 108.Luckarift HR, Spain JC, Naik RR, Stone MO. Enzyme immobilization in a biomimetic silica support. Nat Biotechnol. 2004;22:211–213. doi: 10.1038/nbt931. [DOI] [PubMed] [Google Scholar]


