Abstract
Nanoparticle albumin-bound (nab)-paclitaxel, is paclitaxel linked to albumin nanoparticles which makes it soluble and is an example of application of nanotechnology to cancer treatment. The development of nanotechnology as a delivery system for nab-paclitaxel has improved the pharmacokinetics and pharmacodynamics of paclitaxel, in part by decreasing its hydrophobicity. Nab-paclitaxel in combination with gemcitabine has slightly improved survival in pancreatic cancer, compared to gemcitabine alone, as demonstrated in Phase III clinical trials. Cell-cycle-phase specific drugs, such as nab-paclitaxel, which targets cells in the G2/M phase of the cell cycle, can only have limited efficacy since the vast majority of cells in a tumor are quiescent in G1/G0 phase. Recent advances in our laboratory on how to decoy cancer cells to cycle and then trap them in a sensitive phase of the cell cycle, can, in the hopefully near future, allow drugs such as nab-paclitaxel to have high efficacy, even in a treatment-resistant cancer such as pancreatic cancer.
Keywords: Paclitaxel, nano-particle albumin-bound paclitaxel, NAB, pancreatic cancer, efficacy, survival, cell cycle decoy
Pancreatic cancer is the most lethal cancer, with a survival rate of approximately 5% at five years. Pancreatic cancer is the fourth most common cause of cancer-related death. Over half of the patients have metastatic disease at diagnosis, with an approximate median survival of six months [1]. Gemcitabine is first-line treatment for locally advanced and metastatic pancreatic cancer, with slight or no increase in survival of the treated patients [2, 3].
Nanoparticle albumin-bound paclitaxel (nab-paclitaxel [Abraxane®], Celgene, Summit, NJ) is a microtubule inhibitor, as is paxlitaxel. Nab-paclitaxel was developed to avoid the toxicities of polyoxyethylated castor oil solvent (Cremophor) which is used for paclitaxel because of poor aqueous solubility [4]. Pharmacokinetics of Cremophor may result in hypersensitivity reactions, neutropenia, peripheral neuropathy and liver toxicity [5–7], red blood cells lysis and microvesicle formation [8]. Nab-paclitaxel is now used in the US as first-line combination therapy with gemcitabine for metastatic adenocarcinoma of the pancreas [4].
The use of nab-paclitaxel, in combination with gemcitabine, was based on molecular profiling of pancreatic cancer tumors, which identified overexpression of secreted protein acidic and rich in cysteine (SPARC), an albumin-binding protein [2].
In pancreatic cancer patient-derived xenografts (PDX), the gemcitabine nab-paclitaxel combination resulted in more tumor regression than either drug alone [2]. Pancreatic cancer PDX mice treated with vehicle or gemcitabine had highly desmoplastic stroma. In contrast, nab-paclitaxel treatment depleted the desmoplastic stroma which contained dilated blood vessels vascularizing the tumor. The depleted stroma and the increased vascularization increased delivery of gemcitabine to tumors by 2.8-fold in the gemcitabine - nab-paclitaxel treated tumors compared with gemcitabine-alone treated mice [2].
In operable pancreatic cancer, neoadjuvant nab-paclitaxel increased the rate of R0 surgery of the patients [8, 9].
In a Phase III clinical trial, pancreatic cancer patients with advanced-disease were randomly assigned to nab-paclitaxel - gemcitabine (431 patients) or gemcitabine-alone (430). In the nab-paclitaxel-gemcitabine group the median overall survival was 8.5 months compared to 6.7 months in the gemcitabine-alone group (hazard ratio for death, 0.72; 95% confidence interval [CI], 0.62 to 0.83; P<0.001). The median progression-free survival was 5.5 months in the nab-paclitaxel-gemcitabine group, compared to 3.7 months in the gemcitabine-alone group (hazard ratio for disease progression or death, 0.69; 95% CI, 0.58 to 0.82; P<0.001). Neutropenia and neuropathy were the common toxicities [10]. A case of acute exacerbation of congestive heart failure in gemcitabine - nab-paclitaxel treated pancreatic cancer patients has been observed [3, 11].
Pancreatic ductal adenocarcinomas is characterized by an extensive fibrosis. Provenzano et al. [12] showed that the desmoplastic reaction of pancreatic cancer generates very high interstitial fluid pressures (IFP), inducing vascular collapse, inhibiting perfusion of small molecule therapeutics. These authors showed that systemic administration of the enzyme agent PEGPH20 can decrease stromal hyaluronic acid (HA) in mouse models of pancreatic cancer, reducing IFP and re-expanding the microvasculature. In combination with gemcitabine, PEGPH20 remodeled the tumor microenvironment and increased overall survival of mouse models with pancreatic cancer.
Based in part on the preclinical results described above, there is an ongoing clinical trial that compares the efficacy of PEGPH20 combined with nab-paclitaxel and gemcitabine (PAG) to nab-paclitaxel and gemcitabine (AG) in patients with stage IV pancreatic cancer (https://clinicaltrials.gov/ct2/show/study/NCT01839487).
Other current clinical trials include gemcitabine + nab-paclitaxel with LDE-225 (Hedgehog inhibitor) as neoadjuvant therapy for pancreatic adenocarcinoma (https://clinicaltrials.gov/ct2/show/NCT01431794?term=nab-paclitaxel&rank=1).
There is another clinical trial in which patients with metastatic pancreatic cancer are treated with nab-paclitaxel and gemcitabine in combination with necuparanib (M402), derived from heparin, a blood thinner. Blood thinners have been shown in previous animal and human studies to have anti-cancer effects. Necuparanib is derived from heparin and has lower blood thinning activity. The trial is testing whether necuparanib administered in combination with nab-paclitaxel and gemcitabine is more effective than nab-paclitaxel and gemcitabine (https://clinicaltrials.gov/ct2/show/NCT01621243?term=nab-paclitaxel&rank=2).
Positive results from such clinical trials as described above should increase the use of nab-paclitaxel in combination chemotherapy in pancreatic cancer. The combination could be used in the adjuvant and neo-adjuvant settings as well as for inoperable stage IV pancreatic cancer. Patient selection will depend in part on performance status since these combinations are potentially toxic.
EXPERT OPINION
Nab-paclitaxel is a microtubule-stabilizing agent which enhances microtubule polymerization resulting in arrest in the G2/M phases of the cell cycle. Rapidly-dividing cells are the main targets for this drug [8].
The problem
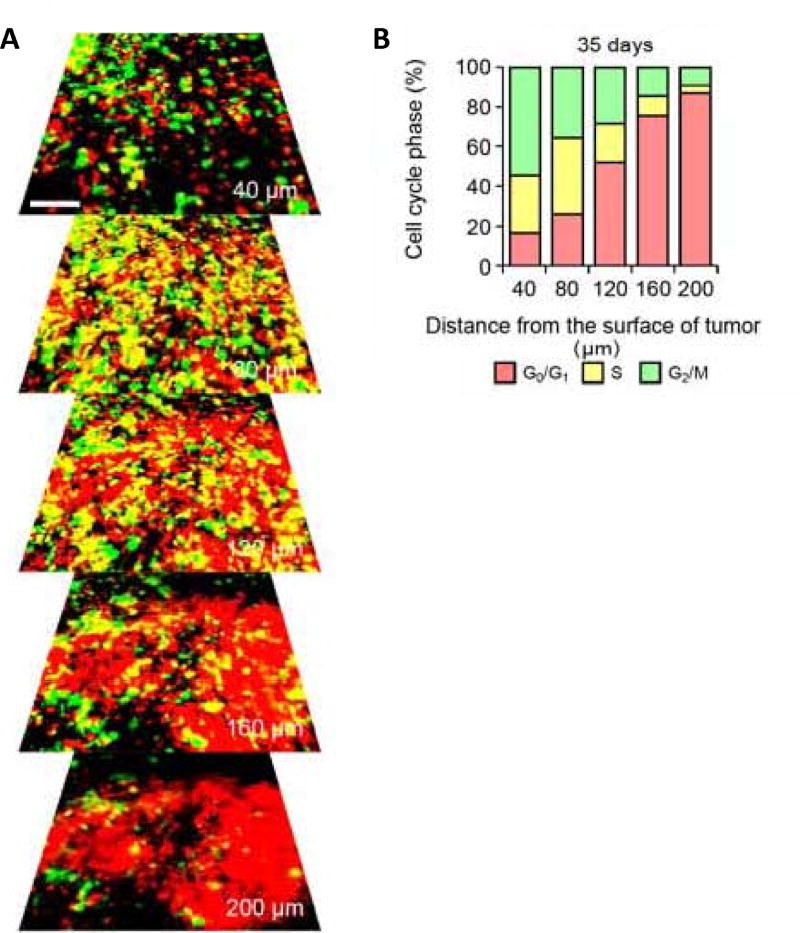
The phase of the cell cycle can determine whether a cancer cell within a tumor can respond to a given drug. We previously monitored real-time cell cycle dynamics of cancer cells throughout live tumors intravitally growing in mice using a fluorescence ubiquitination-based cell cycle indicator (FUCCI) before, during, and after chemotherapy. Approximately 90% of cancer cells in the center and 80% of total cells of an established tumor are in G0/G1 phase (Figure 1). Longitudinal real-time imaging demonstrated that cytotoxic agents, such as paclitaxel, killed only proliferating cancer cells at the surface and, in contrast, had little effect on quiescent cancer cells, which are the vast majority (Figure 2). Moreover, resistant quiescent cancer cells restarted cycling after the cessation of chemotherapy. These results suggest why most drugs currently in clinical use, which target cancer cells in S/G2/M including nab-paclitaxel, are mostly ineffective on solid tumors [13]. Nab-paclitaxel is an M-phase drug being used to treat pancreatic cancer where M-phase cells are most-probably a small minority in a mixture tumor.
Figure 1. Cell cycle phase distribution of cancer cells at the tumor surface and center.
(A) FUCCI-expressing MKN45 cells were implanted directly in the liver of nude mice and imaged at day 35. (B) Histogram show the cell cycle distribution in the tumor at day 35 after implantation. Histogram shows the distribution of FUCCI-expressing cells at different distances from the center. Reproduced with permission from [13].
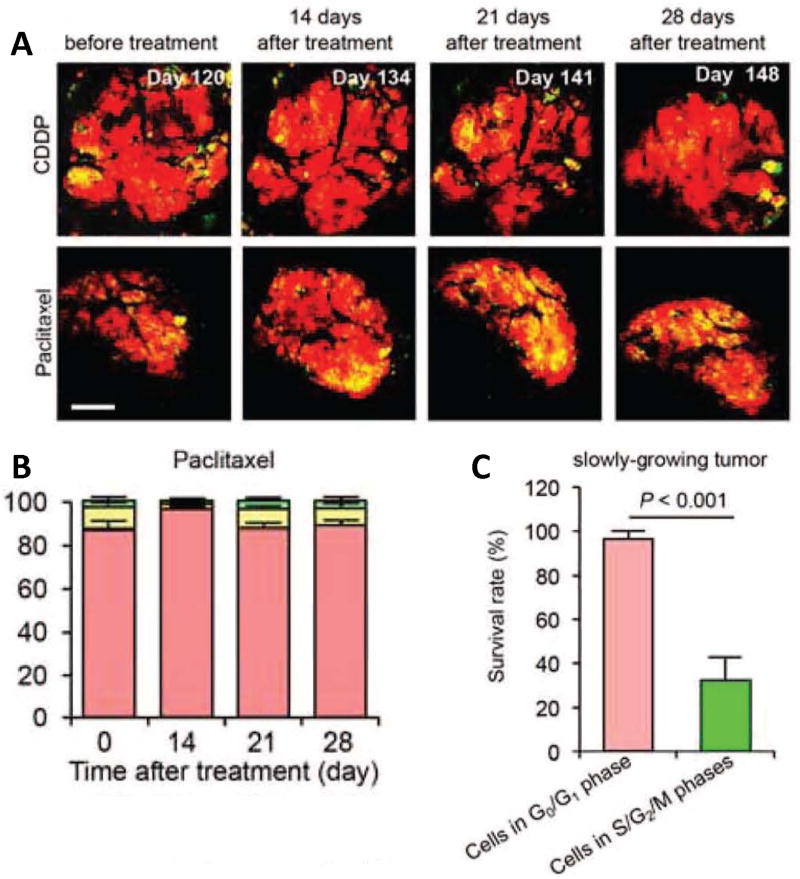
Figure 2. The efficacy of chemotherapy depends on the cell cycle phase distribution within the tumor.
(A) Representative images of slowly growing FUCCI-expressing tumor in the liver before and after CDDP or paclitaxel treatment. (B) Histogram show the cell-cycle phase distribution within the tumor at the indicated time points. (C) Histogram show the survival rate of the cancer cells in G0/G1 phase or in S/G2/M phases after chemotherapy. Scale bars = 500 µm Reproduced with permission from [13].
A solution
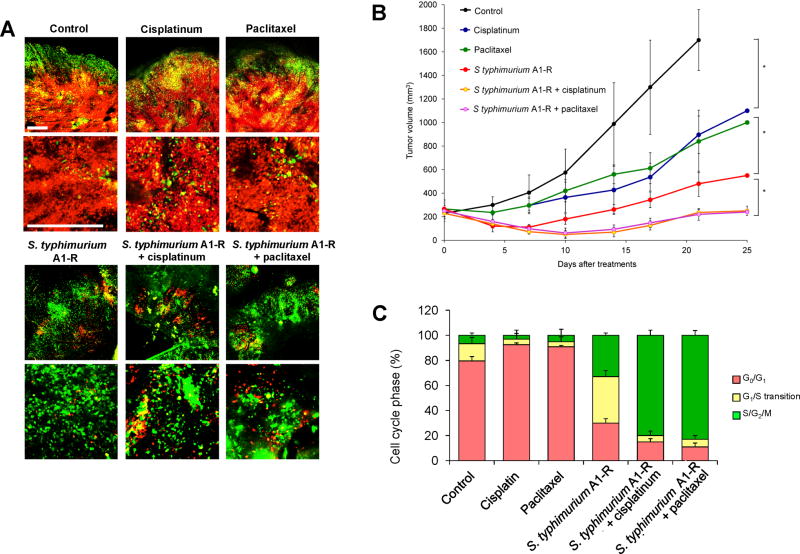
Time-lapse FUCCI imaging demonstrated that tumor-targeting Salmonella typhimurium A1-R decoyed FUCCI-expressing cancer cells in tumors growing in nude mice to cycle from G0/G1 to S/G2/M, thereby making them sensitive to cytotoxic agents. The combination of Salmonella typhimurium A1-R and paclitaxel reduced tumor size compared with A1-R monotherapy or paclitaxel alone (Figure 3). These results suggest a new paradigm of decoy chemotherapy of cancer [14].
Figure 3. S. typhimuium A1-R-decoyed tumors became sensitive to chemotherapy.
FUCCI-expressing MKN45 cells (5 × 106 cells/mouse) were injected subcutaneously into the left flank of nude mouse. When the tumors reached approximately 8 mm in diameter (tumor volume, 300 mm3), mice were administered iv S. typhimuium A1-R alone, or with cisplatinum (4 mg/kg ip) or paclitaxel (5 mg/kg ip) for five cycles every 3 days. (A) Representative images of cross-sections of FUCCI-expressing MKN45 subcutaneous tumors, untreated control, S. typhimuium A1-R-treated, cisplatinum-treated, paclitaxel-treated, or treated with the combination of S. typhimuium A1-R and either cisplainum or paclitaxel. (B) Growth curves of tumors derived from FUCCI-expressing MKN45 cells after treatment with chemotherapy, S. typhimuium A1-R or the combination of S. typhimuium A1-R and chemotherapy. The difference between control and cisplatinum-treated, p<0.01; the difference between control and paclitaxel-treated, p<0.05; the difference between control and S. typhimuium A1-R, p<0.05; the difference between control and the combination of S. typhimuium A1-R and cisplatinum, p<0.01; the difference between control and the combination of S. typhimuium A1-R and paclitaxel, p<0.01. (C) Histogram shows cell cycle phase of FUCCI-expressing MKN45 subcutaneous tumors, untreated control, S. typhimuium A1-R-treated, cisplatinum-treated, paclitaxel-treated, or the combination of S. typhimuium A1-R and either cisplatinum or paclitaxel. Scale bars; 500 µm. Reproduced with permission from [14].
Thus far, nab-paclitaxel has shown a limited benefit. If tumors can be cell-cycle decoyed by an agent, it is possible that nab-paclitaxel will have more efficacy against pancreatic cancer. It is possible that different agents can act as cell decoys on tumors. Currently, in addition to tumor-targeting Salmonella typhimurium A1-R, the tumor-targeting adenovirus OBP-401 has been shown to be a tumor cell-cycle decoy agent [15].
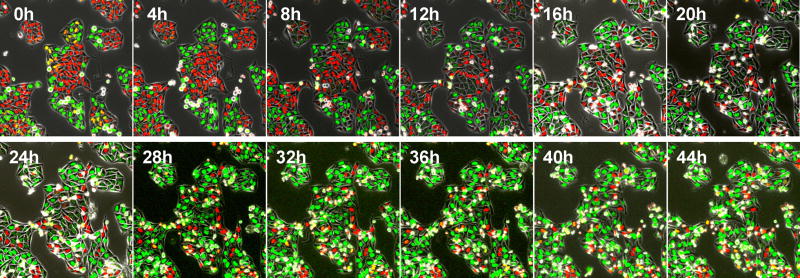
Cancer cells can also be selectively trapped in S/G2 by methionine depletion, such as by recombinant methioninase (rMETase) [16, 17] (Figure 4). With this new paradigm of treatment, the decoyed-trapped cancer cells should become highly sensitive to nab-paclitaxel.
Figure 4. Time-lapse imaging of FUCCI-expressing HeLa cells treated with rMETase being trapped in S/G2 phase.
After seeding on 35 mm glass dishes and culture over night, HeLa cells were treated with rMETase at a dose of 1.0 unit/ml. All images were acquired with the FV1000 confocal microscope (Olympus, Tokyo, Japan). The cells in G0/G1, S, or G2/M phases appear red, yellow, or green, respectively. Scale bar: 50 µmn . Reproduced from [17].
Acknowledgments
The work related to this paper done in the authors laboratory was supported in part by National Cancer Institute grant CA132791.
Footnotes
Financial and competing interests disclosure:
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- 1.Tarver T. Cancer Facts & Figures 2012. American Cancer Society (ACS) Journal of Consumer Health On the Internet. 2012;16(3):366–367. [Google Scholar]
- 2.Von Hoff D, Ramanathan R, Borad M, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: A Phase I/II Trial. J Clin Oncol. 2011;29:4548–54. doi: 10.1200/JCO.2011.36.5742. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.John P, Butler H, Saif MW. Congestive heart failure secondary to gemcitabine nab-paclitaxel in patients with pancreatic cancer. Anticancer Res. 2014;34:7267–70. [PubMed] [Google Scholar]
- 4.Hoy SM. Albumin-bound paclitaxel: a review of its use for the first-line combination treatment of metastatic pancreatic cancer. Drugs. 2014;74:1757–68. doi: 10.1007/s40265-014-0291-8. [DOI] [PubMed] [Google Scholar]
- 5.Authier N, Gillet JP, Fialip J, et al. Assessment of neurotoxicity following repeated cremophor/ethanol injections in rats. Neurotox Res. 2001;3:301–6. doi: 10.1007/BF03033269. [DOI] [PubMed] [Google Scholar]
- 6.Postma TJ, Vermorken JB, Liefting AJM, et al. Paclitaxel-induced neuropathy. Ann Oncol. 2004;6:484–94. doi: 10.1093/oxfordjournals.annonc.a059220. [DOI] [PubMed] [Google Scholar]
- 7.Brat DJ, Windebank AJ, Brimijoin S. Emulsifier for intravenous cyclosporine inhibits neurite outgrowth, causes deficits in rapid axonal transport and leads to structural abnormalities in differentiating N1E.115 neuroblastoma. J Pharmacol Exp Ther. 1992;261:803–10. [PubMed] [Google Scholar]
- 8.Viúdez A, Ramírez N, Hernández-García I, et al. Nab-paclitaxel: A flattering facelift. Crit Rev Oncol Hematol. 2014;92:166–80. doi: 10.1016/j.critrevonc.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez R, Musteanu M, Garcia-Garcia E, et al. Stromal disrupting effects of nab-paclitaxel in pancreatic cancer. Br J Cancer. 2013;109:926–33. doi: 10.1038/bjc.2013.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Senkus E, Jassem J. Cardiovascular effects of systemic cancer treatment. Cancer Treat Rev. 2011;37:300–11. doi: 10.1016/j.ctrv.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Provenzano PP, Cuevas C, Chang AE, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–29. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yano S, Zhang Y, Miwa S, et al. Spatial-temporal FUCCI imaging of each cell in a tumor demonstrates locational dependence of cell cycle dynamics and chemoresponsiveness. Cell Cycle. 2014;13:2110–19. doi: 10.4161/cc.29156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yano S, Zhang Y, Zhao M, et al. Tumor-targeting Salmonella typhimurium A1-R decoys quiescent cancer cells to cycle as visualized by FUCCI imaging and become sensitive to chemotherapy. Cell Cycle. 2014;13:3683–88. doi: 10.4161/15384101.2014.964115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yano S, Tazawa H, Hashimoto Y, et al. A genetically engineered oncolytic adenovirus decoys and lethally traps quiescent cancer stem-like cells into S/G2/M phases. Clin Cancer Res. 2013;19:6495–505. doi: 10.1158/1078-0432.CCR-13-0742. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman RM, Jacobsen SJ. Reversible growth arrest in simian virus 40-transformed human fibroblasts. Proc Natl Acad Sci USA. 1980;77:7306–10. doi: 10.1073/pnas.77.12.7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yano S, Li S, Han Q, et al. Selective methioninase-induced trap of cancer cells in S/G2 phase visualized by FUCCI imaging confers chemosensitivity. Oncotarget. 2014;5:8729–36. doi: 10.18632/oncotarget.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]