Abstract
The Fukushima Daiichi Nuclear Power Plant (FNPP) accident, the largest nuclear incident since the 1986 Chernobyl disaster, occurred when the plant was hit by a tsunami triggered by the Great East Japan Earthquake on March 11, 2011. The subsequent uncontrolled release of radioactive substances resulted in massive evacuations in a 20-km zone. To better understand the biological consequences of the FNPP accident, we have been measuring DNA damage levels in cattle in the evacuation zone. DNA damage was evaluated by assessing the levels of DNA double-strand breaks in peripheral blood lymphocytes by immunocyto-fluorescence-based quantification of γ-H2AX foci. A greater than two-fold increase in the fraction of damaged lymphocytes was observed in all animal cohorts within the evacuation zone, and the levels of DNA damage decreased slightly over the 700-day sample collection period. While the extent of damage appeared to be independent of the distance from the accident site and the estimated radiation dose from radiocesium, we observed age-dependent accumulation of DNA damage. Thus, this study, which was the first to evaluate the biological impact of the FNPP accident utilizing the γ-H2AX assays, indicated the causal relation between high levels of DNA damage in animals living in the evacuation zone and the FNPP accident.
Introduction
The Fukushima Daiichi Nuclear Power Plant (FNPP) accident was one of the worst nuclear disasters in human history (1) and resulted in widespread radiation contamination by various radionuclides such as tellurium-132 (132Te), iodine-131 (131I), iodine-132 (132I), cesium-134 (134Cs), cesium-136 (136Cs) and cesium-137 (137Cs), over large habitable areas (2–4). The release of these radionuclides caused the Japanese government to set a 20-km-radius evacuation zone from the accident site on April 22, 2011 and evacuees left behind a large number of livestock, such as cattle, pigs, and chickens (1). The Japanese government ordered the governor of Fukushima prefecture to euthanize abandoned livestock within the zone on May 12, 2011. Since April 2012, rearrangements of restricted areas, including the evacuation zone, have been performed. Thus, the original 20-km-radius evacuation zone will be referred to as the ex-evacuation zone.
Several studies have documented the impact of the FNPP accident in the evacuation zone by measuring the accumulation of radionuclides in soil samples (2–4), animals (5), the marine biota (6–8), freshwater fishes (9), plants (10–12) and microorganisms (13). The specific activity of radiocesium in abandoned cattle is dependent on the type of organ examined (5). This evidence of radionuclide accumulation in the organs of animals living in the evacuation zone is a major concern and may have harmful biological effects. For example, one key consequence of ionizing radiation is the induction of DNA damage (14, 15). Among the various DNA lesions induced by radiation, DNA double-strand breaks (DSBs) are the most dangerous lesions, leading to massive loss of genetic information and cancer (16). Radiation-induced DNA DSBs can be recognized using a sensitive quantitative assay based on the detection of phosphorylated H2AX (γ-H2AX) foci at the DNA break site, which can be visualized by both immunocytochemistry and immunohistochemistry (17, 18). Because of its sensitivity (the γ-H2AX assay can detect responses to 1.2 mGy irradiation) (19), detection of γ-H2AX foci has been widely used for radiation biodosimetry in both basic and clinical studies (20–22). Although various tissues have been used for γ-H2AX foci-based radiation biodosimetry, the analysis of lymphocytes is the most preferred technique to examine radiation-induced γ-H2AX formation in vivo (23).
Thus, in this study, we aimed to assess the biological impact of the FNPP accident by measuring γ-H2AX foci in lymphocytes from cattle grazing in the ex-evacuation zone.
Materials and Methods
Ethics
This study is a part of the national projects associated with the Great East Japan Earthquake and supported by the Japanese government through the Ministry of Education, Culture, Sports, Science and Technology, Japan, as described previously (5, 24, 25). Cattle in the ex-evacuation zone were sacrificed by veterinarians according to the Regulation for Animal Experiments and Related Activates, Tohoku University (regulation number: 122) (5, 24, 25). This study was approved by the Institutional Animal Care and Use Committee of the Tohoku University Environmental and Safety Committee.
Biosampling
A total of 70 cattle, with at least 10 cows from each area of the ex-evacuation zone, were analyzed. Peripheral blood was collected from the jugular vein and was stored on ice for less than 48 h until analysis. Blood samples (n =8) from control cows not affected by the FNPP accident were obtained from Rakuno Gakuen University, Ebetsu City, Hokkaido and a commercial farm near Tokyo, which were located more than 600 km north and 240 km southwest from FNPP, respectively (Fig. 1A). There were no significant differences in γ-H2AX levels between control cows from Hokkaido and Tokyo (data not shown). Detailed information on sample collection and ethical treatment of animals was described previously (5, 24, 25).
Fig. 1.
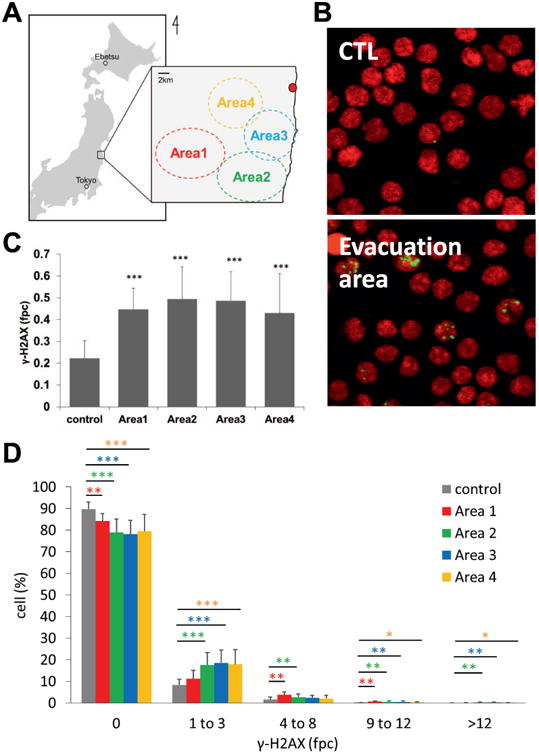
γ-H2AX foci per lymphocyte from cattle living in the control and ex-evacuation zones (A) Location of cattle sampled in this study. The red spot indicates Fukushima Daiichi Nuclear Power Plant. Control cows were obtained from Hokkaido and Tokyo. (B) Representative images of γ-H2AX immunostaining. Green, γ-H2AX, red; DNA stained by propidium iodide. (C) The mean number of γ-H2AX foci per cell from cohorts in each area (control, n = 8; Area 1, n= 12; Area 2, n= 15; Area 3, n= 25; and Area 4, n= 18). Error bars signify standard deviations. γ-H2AX foci were counted in at least 1,000 cells taken from each animal. Asterisks denote the statistical significance of the difference between cohorts from the control and ex-evacuation zones (***P < 0.001 by t-tests). (D) Distribution of foci in the ex-evacuation zone cohort (red bars, Area 1; green bars, Area 2; blue bars, Area 3; orange bars, Area 4) compared in different damage categories with the control cohorts (gray bars). γ-H2AX foci were counted in at least 1,000 cells from each animal. Error bars denote standard deviations (control, n = 8; Area 1, n = 12; Area 2, n = 15; Area 3, n = 25; and Area 4, n = 18). Asterisks denote significant differences between the ex-evacuation cohort and controls (*P < 0.05, **P < 0.01, and ***P < 0.001, Student's t test).
Ex-Vivo Radiation Exposure
Peripheral blood samples from control cattle were irradiated with 70 kV X-ray of radiation using a soft X-ray generator (OM-B205; OHMiC, Japan) at Ibaraki University.
Lymphocyte Isolation and Immunocytochemistry
Blood samples were diluted with the same volume of phosphate-buffered saline (PBS) and layered into LeucoSep tubes (Greiner Bio-One, Frickenhausen, Germany), which contained one-half volume of Ficoll-Paque (GE Healthcare, Piscataway, NJ). For γ-H2AX immunocytochemistry, isolated lymphocytes were fixed in 2% paraformal-dehyde for 20 min at room temperature and spotted on slides using a cytospin centrifuge (Thermo Fisher Scientific, Waltham, MA). For CD3 immunocytochemistry, isolated lymphocytes were fixed in methanol for 30 min at 4°C before cytospin preparation. Anti-γ-H2AX mouse monoclonal antibodies (ab18311, currently discontinued) were purchased from Abcam (Cambridge, UK). Anti-CD3 rabbit polyclonal antibodies (NB100-2000) were from Novus (Littleton, CO). All Alexa488- or Alexa555-conjugated secondary antibodies were from Life Technologies (Carlsbad, CA). γ-H2AX detection was performed as previously described (20, 21). Fluorescence was detected using a Nikon Eclipse E600 microscope (Tokyo, Japan). γ-H2AX foci were counted by eye in a blinded fashion in 1,000 randomly chosen cells.
Statistical Analysis
Differences in the number of γ-H2AX foci between the control cohort and ex-evacuation cohorts were analyzed by Student's t test. Fisher's exact test was applied to analyze significant differences in γ-H2AX responses between T- and B-lymphocytes.
Results and Discussion
Bovine blood samples were collected between November 8, 2011 (242 days after the accident) and February 21, 2013 (713 days after the accident) in four areas within the ex-evacuation zone (Fig. 1A). The distance from FNPP to each area was approximately 20 km (Area 1), 15 km (Area 2), 10 km (Area 3) and 8 km (Area 4; Fig. 1A). Compared with the control cohort, the cattle grazing in the evacuation zone exhibited a higher fraction of lymphocytes with γ-H2AX foci (Fig. 1B). The mean numbers of foci per cell (fpc) in cattle from all four areas were significantly higher than that of the control cohort (Fig. 1C). Further analysis showed that most γ-H2AX-positive lymphocytes contained 1–3 γ-H2AX fpc, with a few containing 4–8 fpc. The four ex-evacuation zone cohorts contained greater fractions of damaged lymphocytes (Fig. 1D). These data clearly demonstrated that DNA DSB levels were increased in cattle living in the ex-evacuation zone. Interestingly, DNA damage levels were independent of the distance from FNPP in this study. The similar levels of DNA damage may indicate that exposure levels in the area were similar, despite the distance from the FNPP. Indeed, several reports have demonstrated the variations in dose levels around FNPP, with the northwest areas of the accident showing the highest levels of contamination while the southwest areas in which the cattle were grazing showing relatively lower and more homogeneous dose levels (26).
To estimate the doses received by the cattle within the ex-evacuation zone based on their DNA damage levels, we generated a γ-H2AX radiation calibration curve by irradiating control blood samples ex vivo (Fig. 2A). The curve followed the function y = 12.85x + 0.20, where y = foci yield per lymphocyte and x = dose in Grays. The foci-based dose estimates for Areas 1–4 were 19.05, 22.69, 22.10 and 17.69 mGy, respectively. Previous studies have demonstrated that γ-H2AX analysis enables an accurate assessment of radiation dose when the exposure was acute and within 48 h postirradiation (20, 27). However, in the case of the FNPP accident, the cattle had been chronically irradiated. Therefore, it is challenging to estimate the initial radiation dose merely by γ-H2AX levels. As shown in Fig. 2B, γ-H2AX levels were not correlated with the estimated external- and internal-exposure doses from radiocesium, revealing there was no accumulation of DNA damage in cattle lymphocytes in a radiation dose-dependent manner. Similar results were obtained from the relationship between γ-H2AX levels and the estimated external- or internal-exposure dose rate (μGy/day; Fig. 2C). The estimated exposure dose from radiocesium (134Cs and 137Cs) was described elsewhere (25). Interestingly, a slight decrease in DNA damage levels in cattle lymphocytes could be observed during the 700-day sample collection period (Fig. 2D). These time-dependent decreases in DNA damage may be partly attributable to decreased dose levels around FNPP over the last 2 years (28).
Fig. 2.
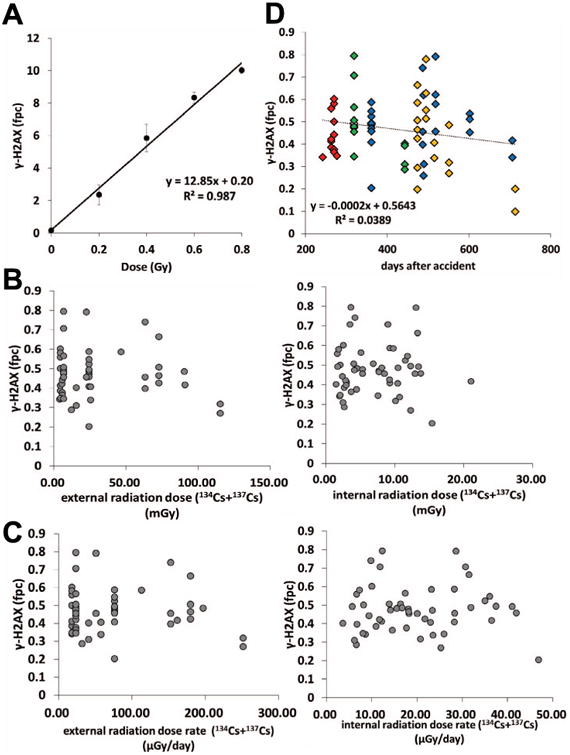
Correlation analysis of γ-H2AX levels in lymphocytes from cattle with the radiation calibration curve, estimated exposure dose, estimated radiation dose rate, and time period after the accident. (A) Estimation of radiation doses equivalent to γ-H2AX levels in lymphocytes of cattle from the ex-evacuation zone. Induction of γ-H2AX foci in control bovine blood samples irradiated ex vivo. Blood samples were X-ray irradiated following incubation at 37°C for 30 min, then processed for γ-H2AX assay. Error bars denote standard deviations in individual animals (n = 3). (B) Relationship between the numbers of γ-H2AX foci per cell in each animal and estimated radiation exposure dose from 134Cs and 137Cs (right: internal-exposure dose, left: external-exposure dose). The radiation doses were inferred from a previous study (25). (C) Relationship between the numbers of γ-H2AX foci per cell in each animal and estimated radiation dose rate from 134Cs and 137Cs (right: internal-radiation dose rate, left: external-radiation dose rate). The radiation dose rates were inferred from a previous study (25). (D) Temporal patterns of foci per cell (fpc) values in lymphocytes. Each mark indicates the numbers of γ-H2AX foci per cell in each animal. The sampling site is indicated by color (red, Area 1; green, Area 2; blue, Area 3; orange, Area 4).
While statistically significant increases in the fraction of damaged lymphocytes were detected, the majority of lymphocytes did not contain γ-H2AX foci (Fig. 1B and D). To analyze whether γ-H2AX expression after irradiation was dependent on the lymphocyte subpopulation, T cells were identified using anti-CD3 antibodies. Both the fraction of γ-H2AX-positive lymphocytes and the number of γ-H2AX fpc were not different between CD3-positive and -negative lymphocytes (Fig. 3A), suggesting that both T- and B-lymphocyte populations showed similar γ-H2AX responses to the radiation fallout. These data are consistent with another previous observation in humans that the differences in the expression of γ-H2AX between lymphocyte subsets are minimal (29). However, there is considerable evidence of the altered DNA damage responses in hematopoietic stem and progenitor cells (30). Thus, analysis of γ-H2AX responses to the FNPP accident in these specific cell types would be interesting.
Fig. 3.
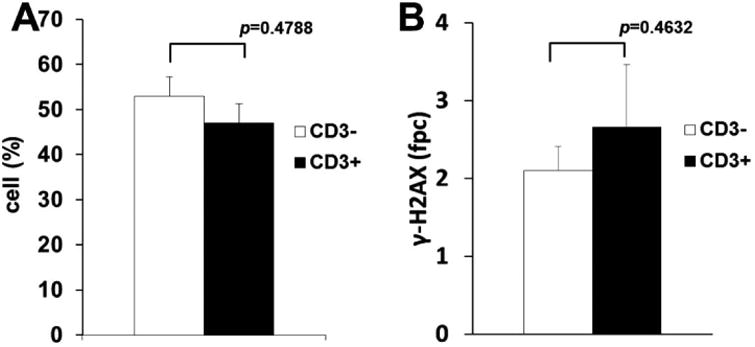
Frequencies of γ-H2AX-positive T and B lymphocytes (A) Population of γ-H2AX-positive cells in CD3-positive T lymphocytes (black bars) and CD3-negative B lymphocytes (white bars) in cattle from the ex-evacuation zone. (B) The mean numbers of γ-H2AX foci per cell in positive T lymphocytes (black bars) and positive B lymphocytes (white bars) in cattle from the Fukushima evacuation zone. Error bars denote standard deviations in individual animals (n = 2). For statistical analysis, Fisher's exact test was carried out.
Previous studies have found that the concentration of radioactive cesium in infant organs is higher than that in maternal organs (5), suggesting the age-dependent deposition of radioactive cesium in organs. Therefore, we next analyzed whether the age of the cattle was related to DNA damage levels in lymphocytes. Age could be determined in 39 out of 70 cattle, and 19 were infant calves. We divided these 58 cattle into three groups according age at the date of blood sampling, as follows: infant group (n = 19), young adult group (1–5 years old, n = 27) and adult group (over 5 years old, n = 12). Interestingly, as shown in Fig 4, γ-H2AX levels were slightly increased in lymphocytes of adult cattle, although there were no significant differences.
Fig. 4.
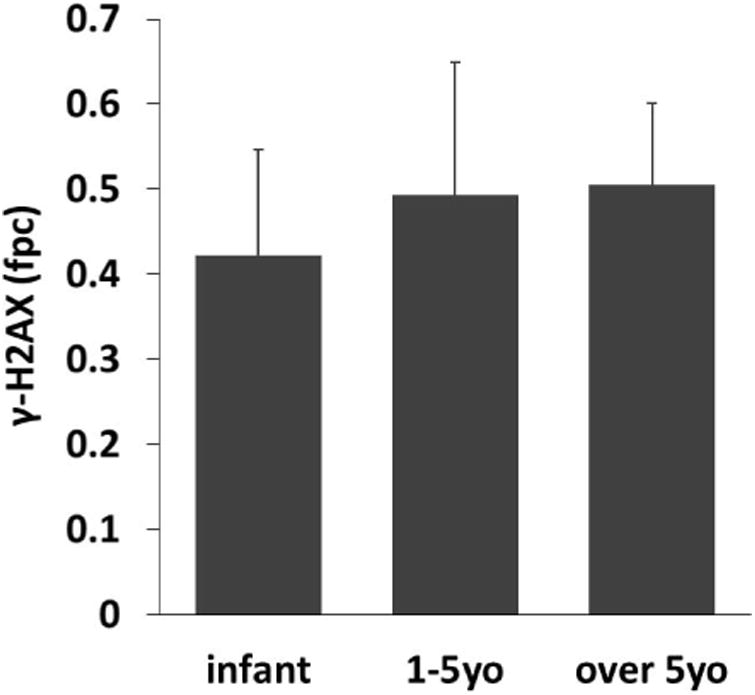
Effects of age on DNA damage levels in lymphocytes in cattle from the ex-evacuation zone The mean number of γ-H2AX foci per cell from each age group (infant group: under 1 year old, n =; 19; young adult group: 1–5 years old, n = 27; adult group: over 5 years old, n = 12). Error bars denote standard deviations.
DNA damage is known to accumulate during both cellular senescence and organismal aging (31, 32). Additionally, strong evidence has shown that DNA damage repair capacity, particularly that of DNA DSB repair, is decreased during aging (33). Garm et al. showed that DNA DSB repair capacity and γ-H2AX response decreases in lymphocytes from older individuals after irradiation. These data suggest that irreparable DNA DSBs (as measured by γ-H2AX foci) in bovine lymphocytes may be related to DNA damage repair capacity rather than the dose of radiation.
In conclusion, we used γ-H2AX assays and showed that DNA DSBs were significantly increased in cattle living in the FNPP evacuation zone; our results suggest that this assay could be used to assess biological impact of low-dose and chronic radiation exposure. However, estimation of the exposed radiation dose using only γ-H2AX assays does not provide reliable results. Because nearly 90% of ionizing radiation-induced DNA DSBs are repaired within 24 h postirradiation (34), γ-H2AX foci detected in lymphocytes from cattle may not reflect the total DNA damage. DNA DSB levels were slightly correlated with cattle age, suggesting that DNA damage repair capacity affected the level of spontaneous DNA DSBs under conditions of chronic radiation exposure. While many markers (chromosomal aberrations, mutations and thyroid dysfunction, among others) should be considered when assessing the biological effects of the FNPP accident, our results are the first to show the extent of DNA DSB accumulation in animals living in the ex-evacuation area and show the causal link between DNA damage induction and the FNPP accident. We believe that these data provide valuable information regarding the effects of the Fukushima nuclear accident on human health.
Acknowledgments
We thank H. Kurose, M. Nakanishi, and the all staff of the Department of Anatomy and Cell Biology group at Osaka Medical College for their contributions. We are grateful to Dr. F. Kawahara for his technical help. We also thank T. Sekine, Y. Kino and Y. Urushihara (Tohoku University) for their commitment to this project. This study was supported by an Emergency Budget for the Reconstruction of Northeastern Japan, MEXT, Japan; the Cooperative Research Project Program of Joint Usage/Research Center at the Institute of Development, Aging and Cancer, Tohoku University; Discretionary Expense of the President of Tohoku University; National Cancer Center Research and Development Fund, Japan; JSPS KAKENHI (JP24700955 to AJN and JP26253022 to MF), the Intramural Research Program of the National Cancer Institute, Center of Cancer Research, NIH (to REC and WMB); and the Programme for Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry, Japan (to EI and MF).
References
- 1.Dauer LT, Zanzonico P, Tuttle RM, Quinn DM, Strauss HW. The Japanese tsunami and resulting nuclear emergency at the Fukushima Daiichi power facility: technical, radiologic, and response perspectives. J Nucl Med. 2011;52:1423–32. doi: 10.2967/jnumed.111.091413. [DOI] [PubMed] [Google Scholar]
- 2.Kinoshita N, Sueki K, Sasa K, Kitagawa J, Ikarashi S, Nishimura T, et al. Assessment of individual radionuclide distributions from the Fukushima nuclear accident covering central-east Japan. Proc Natl Acad Sci U S A. 2011;108:19526–9. doi: 10.1073/pnas.1111724108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yasunari TJ, Stohl A, Hayano RS, Burkhart JF, Eckhardt S, Yasunari T. Cesium-137 deposition and contamination of Japanese soils due to the Fukushima nuclear accident. Proc Natl Acad Sci U S A. 2011;108:19530–4. doi: 10.1073/pnas.1112058108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Endo S, Kimura S, Takatsuji T, Nanasawa K, Imanaka T, Shizuma K. Measurement of soil contamination by radionuclides due to the Fukushima Dai-ichi Nuclear Power Plant accident and associated estimated cumulative external dose estimation. J Envir Radioactivity. 2012;111:18–27. doi: 10.1016/j.jenvrad.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Fukuda T, Kino Y, Abe Y, Yamashiro H, Kuwahara Y, Nihei H, et al. Distribution of artificial radionuclides in abandoned cattle in the evacuation zone of the Fukushima Daiichi nuclear power plant. PloS one. 2013;8:e54312. doi: 10.1371/journal.pone.0054312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keum DK, Kim BH, Lim KM, Choi YH. Radiation exposure to Marine biota around the Fukushima Daiichi NPP. Environ Monit Assess. 2014;186:2949–56. doi: 10.1007/s10661-013-3592-6. [DOI] [PubMed] [Google Scholar]
- 7.Chen J. Evaluation of radioactivity concentrations from the Fukushima nuclear accident in fish products and associated risk to fish consumers. Radiat Prot Dosimetry. 2013;157:1–5. doi: 10.1093/rpd/nct239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwata K, Tagami K, Uchida S. Ecological Half-Lives of Radio-cesium in 16 Species in Marine Biota after the TEPCO's Fukushima Daiichi Nuclear Power Plant Accident. Environmental science & technology. 2013;47:7696–703. doi: 10.1021/es400491b. [DOI] [PubMed] [Google Scholar]
- 9.Mizuno T, Kubo H. Overview of active cesium contamination of freshwater fish in Fukushima and Eastern Japan. Sci Rep. 2013;3 doi: 10.1038/srep01742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terashima I, Shiyomi M, Fukuda H. (134)Cs and (137)Cs levels in a grassland, 32 km northwest of the Fukushima 1 Nuclear Power Plant, measured for two seasons after the fallout. J plant research. 2014;127:43–50. doi: 10.1007/s10265-013-0608-9. [DOI] [PubMed] [Google Scholar]
- 11.Mimura T, Mimura M, Kobayashi D, Komiyama C, Sekimoto H, Miyamoto M, et al. Radioactive pollution and accumulation of radionuclides in wild plants in Fukushima. J plant research. 2014;127:5–10. doi: 10.1007/s10265-013-0599-6. [DOI] [PubMed] [Google Scholar]
- 12.Kuroda K, Kagawa A, Tonosaki M. Radiocesium concentrations in the bark, sapwood and heartwood of three tree species collected at Fukushima forests half a year after the Fukushima Dai-ichi nuclear accident. J environmental radioactivity. 2013;122:37–42. doi: 10.1016/j.jenvrad.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki H, Shirato S, Tahara T, Sato K, Takenaka H. Accumulation of Radioactive Cesium Released from Fukushima Daiichi Nuclear Power Plant in Terrestrial Cyanobacteria Nostoc commune. Microbes and environments /JSME. 2013;28:466–9. doi: 10.1264/jsme2.ME13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou BB, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433–9. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
- 15.Kavanagh JN, Redmond KM, Schettino G, Prise KM. DNA double strand break repair: a radiation perspective. Antioxidants & redox signaling. 2013;18:2458–72. doi: 10.1089/ars.2012.5151. [DOI] [PubMed] [Google Scholar]
- 16.Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S, et al. GammaH2AX and cancer. Nature reviews Cancer. 2008;8:957–67. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. The J biological chemistry. 1998;273:5858–68. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura AJ, Rao VA, Pommier Y, Bonner WM. The complexity of phosphorylated H2AX foci formation and DNA repair assembly at DNA double-strand breaks. Cell cycle. 2010;9:389–97. doi: 10.4161/cc.9.2.10475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothkamm K, Lobrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc Natl Acad Sci U S A. 2003;100:5057–62. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redon CE, Nakamura AJ, Gouliaeva K, Rahman A, Blakely WF, Bonner WM. The use of gamma-H2AX as a biodosimeter for total-body radiation exposure in non-human primates. PloS one. 2010;5:e15544. doi: 10.1371/journal.pone.0015544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redon CE, Nakamura AJ, Sordet O, Dickey JS, Gouliaeva K, Tabb B, et al. gamma-H2AX detection in peripheral blood lymphocytes, splenocytes, bone marrow, xenografts, and skin. Methods in molecular biology. 2011;682:249–70. doi: 10.1007/978-1-60327-409-8_18. [DOI] [PubMed] [Google Scholar]
- 22.Redon CE, Nakamura AJ, Zhang YW, Ji JJ, Bonner WM, Kinders RJ, et al. Histone gammaH2AX and poly(ADP-ribose) as clinical pharmacodynamic biomarkers. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:4532–42. doi: 10.1158/1078-0432.CCR-10-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Redon CE, Weyemi U, Parekh PR, Huang D, Burrell AS, Bonner WM. gamma-H2AX and other histone post-translational modifications in the clinic. Biochimica et biophysica acta. 2012;1819:743–56. doi: 10.1016/j.bbagrm.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi S, Inoue K, Suzuki M, Urushihara Y, Kuwahara Y, Hayashi G, et al. A comprehensive dose evaluation project concerning animals affected by the Fukushima Daiichi Nuclear Power Plant accident: its set-up and progress. J Radiat Res. 2015;56(Suppl 1):i36–i41. doi: 10.1093/jrr/rrv069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urushihara Y, Kawasumi K, Endo S, Tanaka K, Hirakawa Y, Hayashi G, et al. Analysis of Plasma Protein Concentrations and Enzyme Activities in Cattle within the Ex-Evacuation Zone of the Fukushima Daiichi Nuclear Plant Accident. PloS one. 2016;11:e0155069. doi: 10.1371/journal.pone.0155069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torii T, Sugita T, Okada CE, Reed MS, Blumenthal DJ. Enhanced analysis methods to derive the spatial distribution of 131I deposition on the ground by airborne surveys at an early stage after the Fukushima Daiichi nuclear power plant accident. Health physics. 2013;105:192–200. doi: 10.1097/HP.0b013e318294444e. [DOI] [PubMed] [Google Scholar]
- 27.Horn S, Barnard S, Rothkamm K. Gamma-H2AX-based dose estimation for whole and partial body radiation exposure. PloS one. 2011;6:e25113. doi: 10.1371/journal.pone.0025113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kakamu T, Kanda H, Tsuji M, Kobayashi D, Miyake M, Hayakawa T, et al. Differences in rates of decrease of environmental radiation dose rates by ground surface property in Fukushima City after the Fukushima Daiichi nuclear power plant accident. Health physics. 2013;104:102–7. doi: 10.1097/HP.0b013e31826ab94c. [DOI] [PubMed] [Google Scholar]
- 29.Andrievski A, Wilkins RC. The response of gamma-H2AX in human lymphocytes and lymphocytes subsets measured in whole blood cultures. International journal of radiation biology. 2009;85:369–76. doi: 10.1080/09553000902781147. [DOI] [PubMed] [Google Scholar]
- 30.Rube CE, Fricke A, Widmann TA, Furst T, Madry H, Pfreundschuh M, et al. Accumulation of DNA damage in hematopoietic stem and progenitor cells during human aging. PloS one. 2011;6:e17487. doi: 10.1371/journal.pone.0017487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sedelnikova OA, Horikawa I, Zimonjic DB, Popescu NC, Bonner WM, Barrett JC. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nature cell biology. 2004;6:168–70. doi: 10.1038/ncb1095. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura AJ, Chiang YJ, Hathcock KS, Horikawa I, Sedelnikova OA, Hodes RJ, et al. Both telomeric and non-telomeric DNA damage are determinants of mammalian cellular senescence. Epigenetics & chromatin. 2008;1:6. doi: 10.1186/1756-8935-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garm C, Moreno-Villanueva M, Burkle A, Petersen I, Bohr VA, Christensen K, et al. Age and gender effects on DNA strand break repair in peripheral blood mononuclear cells. Aging cell. 2013;12:58–66. doi: 10.1111/acel.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Redon CE, Dickey JS, Bonner WM, Sedelnikova OA. gamma-H2AX as a biomarker of DNA damage induced by ionizing radiation in human peripheral blood lymphocytes and artificial skin. Advances in space research : the official journal of the Committee on Space Research. 2009;43:1171–8. doi: 10.1016/j.asr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


