Abstract
Prenatal alcohol exposure can result in a range of physical, neuropathological, and behavioral alterations, collectively termed fetal alcohol spectrum disorders (FASD). We have shown that supplementation with the nutrient choline reduces the severity of developmental alcohol-associated deficits in hippocampal-dependent behaviors and normalizes some aspects of hippocampal cholinergic development and DNA methylation patterns. Alcohol’s developmental effects may also be mediated, in part, by altering microRNAs (miRNAs) that serve as negative regulators of gene translation. To determine whether choline supplementation alters ethanol’s long-lasting effects on miRNAs, Sprague-Dawley rats were exposed to 5.25 g/kg/day ethanol from postnatal days (PD) 4–9 via intubation; controls received sham intubations. Subjects were treated with choline chloride (100 mg/kg/day) or saline vehicle subcutaneously (s.c.) from PD 4–21. On PD 22, subjects were sacrificed, and RNA isolated from the hippocampus. MiRNA expression was assessed with TaqMan Human MicroRNA Panel Low-Density Arrays. Ethanol significantly increased miRNA expression variance, an effect that was normalized with choline supplementation. Cluster analysis of stably expressed miRNAs that exceeded an ANOVA p<0.05 criterion indicated that for both male and female offspring, control and ethanol-exposed groups were most dissimilar from each other, with choline-supplemented groups in between. MiRNAs that expressed an average 2-fold change due to ethanol exposure were further analyzed to identify which ethanol-sensitive miRNAs were protected by choline supplementation. We found that at a false discovery rate (FDR)-adjusted criterion of p<0.05, miR-200c was induced by ethanol exposure and that choline prevented this effect. Collectively, our data show that choline supplementation can normalize disturbances in miRNA expression following developmental alcohol exposure and can protect specific miRNAs from induction by ethanol. These findings have important implications for the mechanisms by which choline may serve as a potential treatment for FASD.
Keywords: Fetal alcohol, intervention, genetic, choline, miRNA
Introduction
Prenatal alcohol exposure can disrupt development of the fetus via multiple mechanisms. Alcohol may alter cell proliferation, migration, differentiation, synaptogenesis, and myelination, depending on the developmental timing and other exposure parameters (Camarillo and Miranda, 2008; Gil-Mohapel et al., 2010; Kane et al., 2012; O'Leary-Moore et al., 2011; Santillano et al., 2005; Tingling et al., 2013; Wilhelm and Guizzetti, 2016). But alcohol may also lead to long-lasting changes in cell function, altering gene expression (Downing et al. 2012; Downing et al., 2011) and compromising synaptic plasticity (Gil-Mohapel et al., 2010; Medina, 2011), all of which contribute to long-lasting pathology in neural structure and function. Such disruptions lead to behavioral and cognitive alterations (Hamilton et al., 2010; Mattson et al., 2011), effects that have serious consequences on the quality of life of individuals prenatally exposed to alcohol and their families.
Protection against alcohol’s teratogenic effects is critical, as prevention efforts have not effectively reduced alcohol consumption among many pregnant women (Centers for Disease Control and Prevention, 2015; May et al., 2008). In fact, recent estimates suggest that the global prevalence of fetal alcohol spectrum disorders (FASD), the range of effects caused by prenatal alcohol exposure on the offspring, nears 177 per 1000 live births; estimates range from 7–319 per 1000 in South Africa, from 34–61 per 1000 in Italy, and from 24–43 per 1000 in the United States (Roozen et al., 2016). Given that 10% of women report some alcohol consumption during pregnancy in the U.S. (Center for Behavioral Health Statistics and Quality, 2015) and that almost half of pregnancies are unplanned (Finer and Zolna, 2011), FASD will continue to be a problem. Elucidation of factors that can reduce the severity of FASD may lead to effective intervention strategies.
Alcohol’s teratogenic effects may be modified by a number of factors, including nutritional variables (Abel, 1995; Young et al., 2014). We have shown that administration of choline, an essential nutrient, can reduce the severity of alcohol’s teratogenic effects, including physical (Thomas et al., 2009), neuropathological (Monk et al., 2012; Otero et al., 2012), and behavioral outcomes (Monk et al., 2012; Ryan et al., 2008; Thomas et al., 2004a; Thomas et al., 2010; Thomas et al., 2000; Thomas and Tran, 2012). Specifically, we have shown that prenatal choline supplementation during prenatal alcohol exposure mitigates ethanol-related reductions in birth and brain weights, delays in incisor emergence, and delays in reflex development, including impairments in hindlimb coordination (Thomas et al., 2009). Choline also mitigates delays in the development of spontaneous alternation and long-lasting deficits on working memory induced by prenatal alcohol exposure (Thomas et al., 2010), both of which depend on the functional integrity of the hippocampus. But importantly, choline can reduce the severity of fetal alcohol effects, even when administered postnatally and after the alcohol exposure has occurred. When administered during the early postnatal period, choline targets ethanol’s effects on behaviors associated predominately with hippocampal function, reducing the severity of open field overactivity (Monk et al., 2012; Thomas et al., 2004a), and deficits in reversal learning (Thomas et al., 2004a), working memory (Thomas et al., 2000), spatial learning (Ryan et al., 2008; Thomas et al., 2010), and trace classical conditioning (Thomas and Tran, 2012; Wagner and Hunt, 2006). In contrast, when administered during this period of development, choline does not reduce ethanol’s effects on behaviors that depend on the functional integrity of the cerebellum (Thomas et al., 2004b; Thomas and Tran, 2012; Wagner and Hunt, 2006), although one study reported that choline supplementation from postnatal day (PD) 1–20 reduces the severity of motor deficits associated with acute ethanol on PD 5 (Bearer et al., 2015). These data suggest that when administered after PD 10, choline targets the hippocampus.
However, it is not clear how choline achieves these behavioral effects. Choline acts, in part, as a precursor to the neurotransmitter acetylcholine (Zeisel, 2013; Zeisel and Niculescu, 2006) and we have found that choline supplementation attenuates alcohol-related changes in hippocampal cholinergic receptors (Monk et al., 2012). Choline also acts as a methyl donor and can, therefore, influence gene expression (Niculescu and Zeisel, 2002; Zeisel, 2006). In fact, we have found that choline attenuates alcohol-related changes in hippocampal DNA methylation (Otero et al., 2012). Choline’s actions as an epigenetic factor mean that it may influence many target pathways that influence development. In this study, we tested the hypothesis that choline reduces the effects of developmental alcohol exposure, in part, by attenuating the effects of alcohol on a class of small non-protein-coding RNAs termed microRNAs (miRNAs).
MiRNAs regulate cellular function and maturation state by repressing the translation of networks of protein-coding genes (for review, see (Miranda, 2014)). We previously showed that early developmental exposure to alcohol resulted in alterations in miRNAs (Sathyan et al., 2007). Moreover, these alterations could explain ethanol’s teratogenic effects on neural and craniofacial development (Pappalardo-Carter et al., 2013; Sathyan et al., 2007; Tsai et al., 2014). Pertinent to the presumptive mechanisms of action of choline, we also previously showed that ethanol-sensitive miRNAs were regulated by epigenetic mechanisms (Pappalardo-Carter et al., 2013) and that the effects of ethanol on miRNAs could be prevented and reversed by ligands that acted at nicotinic acetylcholine receptors (Balaraman et al., 2012; Tsai et al., 2014). These data suggested to us that choline supplementation may be a means to reverse the effects of developmental alcohol exposure on miRNA pathways. Therefore, the present study examined whether the nutrient choline would modify ethanol’s effects on miRNAs. To examine this, we used a 3rd trimester model of ethanol exposure, which produces hippocampal pathology. Subjects received choline both during and after ethanol exposure to maximize effects.
Materials and Methods
Subjects were offspring from the Center for Behavioral Teratology mating colony at San Diego State University. The mating colony is housed in a temperature and humidity-controlled environment, and food and water was available ad lib. Male and female Sprague-Dawley rats were housed overnight and the presence of a seminal plug at the bottom of the cage indicated mating. Pregnant dams were then singly housed. The day after birth, litters were culled to 8, with 4 males and 4 females, whenever possible.
On PD 4, pups were randomly assigned to groups in a 2 (ethanol, sham) × 2 (choline, saline) × 2 (male, female) design. No more than one sex pair per litter was assigned to a treatment group to minimize litter effects. A total of 48 subjects (6/group) were generated. Ethanol was administered via oral intubation. Consistent with our previous studies of 3rd trimester alcohol exposure (Monk et al., 2012; Ryan et al., 2008;Thomas and Tran, 2012; Schneider and Thomas, 2016), subjects received 2.625 g/kg ethanol in a nutritionally balanced milk formula (11.9% v/v) twice per day, 2 hours apart, for a daily dose of 5.25 g/kg/day from PD 4–9, to mimic exposure during the third trimester-equivalent period of human development (Workman et al., 2013). Ethanol-exposed subjects were also given 2 additional intubations of milk formula with no alcohol (2 hours apart) to minimize growth differences. Sham controls received intubations, but no formula during the 4 daily intubations. Intubations took just a few minutes and subjects remained with the dam in between intubations. From PD 4–21, subjects were injected subcutaneously (s.c.) with choline chloride (100 mg/kg/day) or saline, as accomplished in previous studies showing benefits of choline chloride at this dose during postnatal development (Monk et al., 2012; Otero et al., 2012; Ryan et al., 2008; Thomas et al., 2007).
On PD 6, 20 µL of blood was collected via a tail clip, 1.5 hrs after the second ethanol treatment to determine peak blood alcohol concentration (BACs; mg/dL). Blood samples were centrifuged and plasma samples were analyzed using the Analox Alcohol Analyzer (Model AM1, Analox Instruments; Lunenburg, MA). On PD 7, each subject’s paws were tattooed with non-toxic Indian ink for individual identification.
On PD 22, juvenile subjects were sacrificed, brains were rapidly removed, the hippocampus dissected out, and tissue was flash frozen using methylbutane cooled to −20°C using dry ice. Tissue was sent to Texas A&M University (RCM laboratory) for miRNA analyses. RNA was isolated from the hippocampus and miRNA expression was assessed with TaqMan Low-Density Arrays (TLDA; Taqman Human microRNA A and B cards v.3.0; Applied Biosystems, Foster City, CA). All procedures were approved by the Institutional Animal Use and Care Committee at SDSU.
Preparation of total RNA
Total RNA was extracted from the hippocampus using the mirVana miRNA kit (Ambion, Austin, TX) according to the manufacturer’s instructions. RNA yield and purity were evaluated with a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies/Thermo Scientific). A260/A280 ratios between 1.96 and 2.04 were obtained for all samples of isolated RNA, confirming their purity. For each treatment group, equal amounts of RNA from two males or two females were combined into one experimental sample, so that each experimental sample represented two biological samples of the same sex. A total of 24 experimental samples were assessed for miRNA expression patterns, with 3 male and 3 female samples represented in each treatment group.
miRNA quantification
Expression of mature miRNAs was determined using TaqMan Human microRNA A and B cards v.3.0 (Applied Biosystems, Foster City, CA). Card A and Card B together assessed 768 elements including 750 mature miRNAs and other small RNAs including U6 small nucleolar RNA (snRNA) that was used as a normalization control. As per manufacturer's instructions, 50 ng of RNA was reverse transcribed using the TaqMan MicroRNA RT kit, followed by polymerase chain reaction (PCR) using Multiplex RT target-specific stem-loop primers (Applied Biosystems, Foster City, CA). Complementary DNA products were diluted, mixed with TaqMan master mix, and loaded onto miRNA TaqMan Low-Density Arrays for amplification on an Applied Biosystems 7900HT fast Real-time PCR system (ABI/Life Technologies, Grand Island, NY).
Data Analysis
TaqMan Human MicroRNA Panel Low-Density Arrays card A and card B miRNAs were analyzed separately to assess the stability of the normalization U6 snRNA control transcript. The normalization control U6 snRNA was expressed in all samples. There were no statistically significant differences in U6 snRNA expression as a function of group (Wilk’s lambda, F(6,30)=0.215, p<0.97) or sex (F(2,15)=1.29, p<0.305), nor was there a significant interaction between these two factors (F(6,30)=1.4, p<0.25). Therefore, all miRNA expression was calculated as ΔCT relative to their respective panel 1 or panel 2 U6 snRNA transcript CT values. Data were analyzed with GeneSifter® Analysis Edition (GSAE, Geospiza/Perkin Elmer, Seattle, WA), or by SPSS (ver. 24, IBM, Armonk, New York). Data were subjected to the Wilk’s Lambda Multivariate Analysis of Variance (MANOVA) test, followed by Analysis of Variance (ANOVA) with Benjamini and Hochberg false discovery rate (FDR) correction for multiple comparisons (α=0.05), and the Scheffe post-hoc pairwise comparison test to control for multiple comparisons. The miRNAs that were found to be affected when correcting for FDR, as well as miRNAs that exceeded an unadjusted p<0.05 criterion for main effect of treatment were further analyzed with a 3-way ANOVA with ethanol, choline and sex as factors. Cluster analysis with Euclidean distance and average linkage, with rows centered on miRNAs was conducted on miRNAs that exceeded an unadjusted p<0.05 criterion by ANOVA, to assess the relatedness among the various groups. Candidate miRNAs derived from ANOVA (FDR-corrected and unadjusted p<0.05) were subject to pathway overrepresentation analysis, using DIANA tools, MirPath V3 pathway analysis suite, and the MicroT-CDS v5 prediction algorithm (Vlachos et al., 2015), using a modified Fisher’s Exact test with FDR-corrected criterion of p<0.05 and a MicroT-CDS threshold score of 0.8. Pathway definitions were as defined in the Kyoto Encylopedia of Genes and Genomes release v78.0 (KEGG, (Kanehisa et al., 2016)).
Results
Body Weights
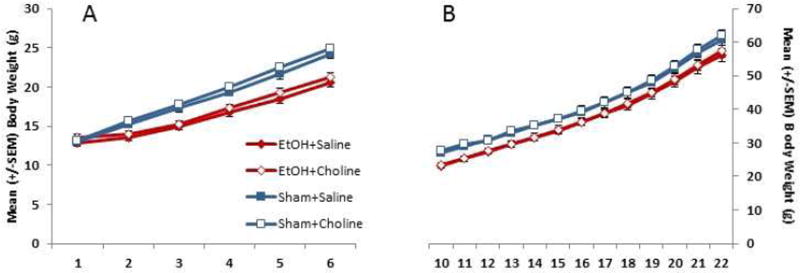
Body weight data from PD 4–9 are shown in Figure 1A. There were no differences in body weight among groups on PD 4, but on subsequent days, ethanol-exposed subjects lagged in growth, producing a significant interaction of day × ethanol [F(5,200) = 49.9, p<.001] and a main effect of ethanol [F(1,40), = 25.5, p<.001]. All groups gained weight over days [F(5,200) = 1459.7, p<.001] and males weighed more than females [F(1,40) = 5.0, p<.05]. During choline treatment (PD 10–22; Figure 1B), ethanol-exposed subjects continued to weigh less than controls [F(1,40) = 12.1, p<.01]. Choline did not significantly affect body growth, nor did sex interact with ethanol or choline on body weight.
Figure 1.
Ethanol-exposed subjects lagged in growth following the first day of ethanol exposure. However, choline did not alter this growth pattern.
Blood Alcohol Concentrations
No significant sex or treatment differences were detected in the ethanol-exposed subjects’ BAC levels (all p’s >.05; Ethanol + Saline group (mean ± SEM): 376.5 ± 15.3 mg/dL; Ethanol ± Choline group: 365.4 ± 15.3 mg/dL).
Alcohol and Choline Effects on miRNA
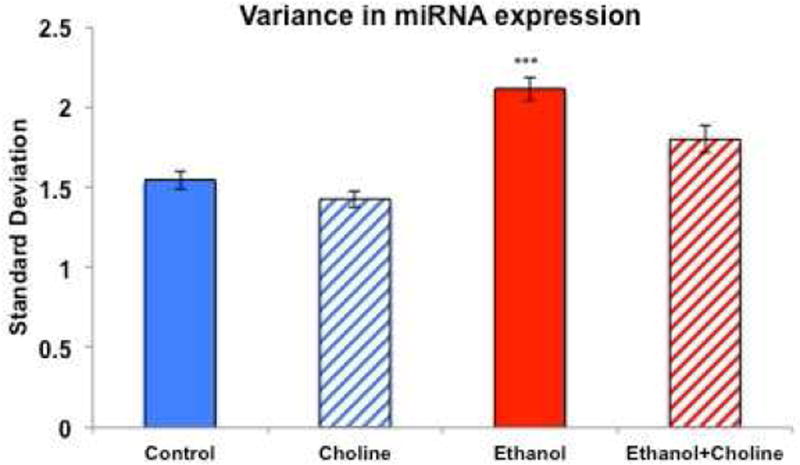
To determine whether early alcohol exposure dysregulates global miRNA expression, we assessed the Standard Deviation (SD) for each expressed miRNA. Because the Levene's Test of Equality of Error Variances was statistically significant [F(7,2592) = 22.426, p<1.18 e−28], indicating heterogeneity of variance across exposure groups, we used non-parametric tests to assess the effects of experimental treatment on SD separately for both males and females [Kruskal-Wallis Independent-Samples Test, p<0.05 for both males and females]. Post-hoc, planned pair-wise comparisons (Mann-Whitney U) showed that third trimester ethanol exposure resulted in a significant increase in the SD in both males and females (all p’s< 0.003). Choline treatment significantly reduced ethanol-related increases in SD in both males and females (all p’s<0.034). Thus, ethanol exposure significantly increased variance in miRNA expression and this effect of ethanol was significantly reduced with choline treatment in both sexes (Figure 2).
Figure 2.
Ethanol exposure during the third trimester equivalent significantly increased variance in miRNA expression, and this effect was attenuated with choline supplementation.
*** significantly different from all other groups
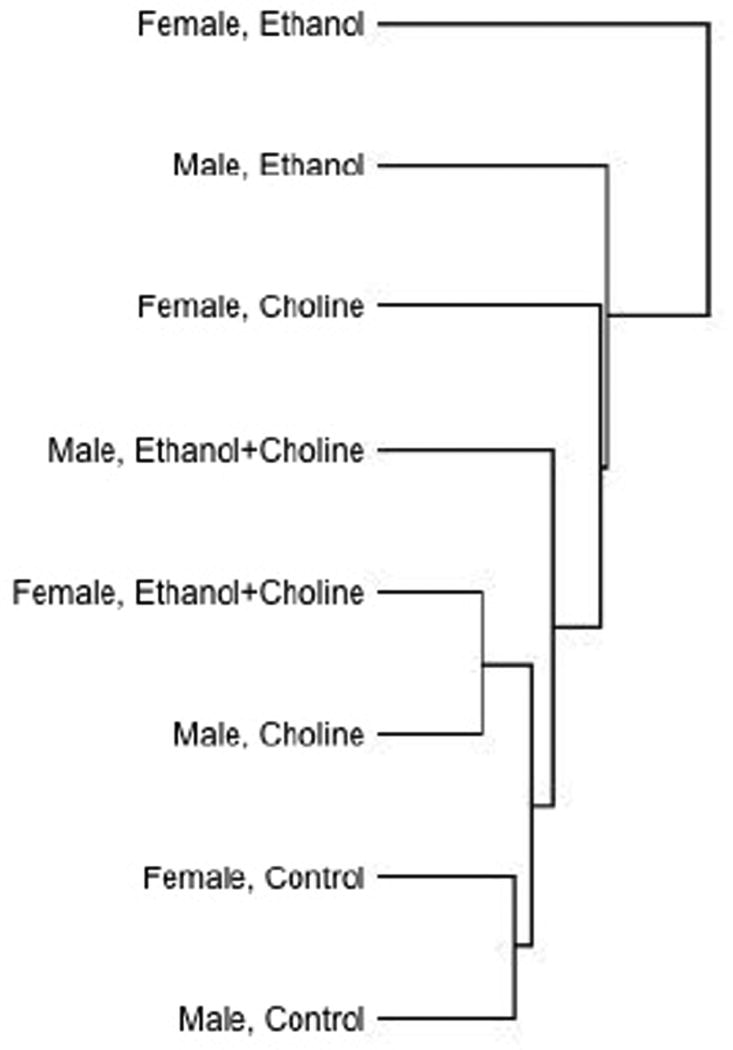
In an overall two-way ANOVA with sex and exposure group as factors, miRNAs that exceeded a raw p-value criterion of p<0.1 were subject to cluster analysis with Euclidean distance and average linkage, with rows centered on miRNAs. Cluster analysis showed that control and ethanol-exposed animals were most different from each other, with Sham + Choline and Ethanol + Choline groups in between (see Figure 3). As with variance analysis, these data suggest that early alcohol exposure disrupts juvenile hippocampal miRNA expression patterns and that choline exposure has a general normalization effect on miRNAs in the ethanol-exposed hippocampus.
Figure 3.
Cluster analysis with Euclidean distance and average linkage, with rows centered on miRNAs for miRNAs that exceeded, by ANOVA, a raw p<0.1 for the interaction between sex and treatment group. Cluster analysis showed that control and ethanol-exposed animals were most different from each other, with Sham (Control) + Choline and Ethanol + Choline treatment groups in between.
For further analyses, we selected miRNAs that were expressed in every sample, and to assess the effects of choline on ethanol’s effects specifically, we selected those expressed miRNAs that exhibited an average of 1 CT (2-fold) difference between control and ethanol-exposed groups, i.e., those miRNAs that may be vulnerable to developmental alcohol exposure. Of the 760 assessed miRNAs, 97 miRNAs (55 from card A and 42 from card B, 13% in total) fit this criterion and were subject to a MANOVA followed by ANOVAs, with sex and group as independent variables. Multivariate analysis showed that there was a significant global sex by group interaction (Wilk’s Lambda, F(48,4)=8.65, p<0.028). Post-hoc ANOVAs showed that 5 miRNAs, miR-130b, miR-326, miR-374-5p, miR-878-3p, and miR-327 exceeded an unadjusted criterion of p<0.05 for the main effects of group. In general, with the exception of miR-878-3p, ethanol increased these miRNA levels and choline attenuated these effects. However, one miRNA, miR-200c exceeded a B&H FDR criterion of p<0.05 for the main effect of group. Analysis for the effect of sex identified 3 miRNAs, miR-326, miR-200c, and miR-22 as exceeding the unadjusted criterion of p<0.05, though none exceeded the B&H FDR adjusted criterion of p<0.05. Finally, analysis of the interaction between sex and treatment showed that four miRNAs, miR-326, miR-200c, miR-15a, and miR-494 exceeded the unadjusted criterion of p<0.05, though again, none exceeded the B&H FDR adjusted criterion of p<0.05 (see Table 1).
Table 1.
miRNA altered as a function of treatment group and sex (n.s.=not significant).
| miRNA | Treatment | Sex | Sex × Treatment |
|---|---|---|---|
| miR-200c | 0.0004067* | 0.009 | 0.02 |
| miR-130b | 0.01 | n.s. | n.s. |
| miR-326 | 0.015 | 0.006 | 0.019 |
| miR-374-5p | 0.024 | n.s. | n.s. |
| miR-878-3p | 0.033 | n.s. | n.s. |
| miR-327 | 0.037 | n.s. | n.s. |
| miR-22 | n.s. | 0.018 | n.s. |
| miR-15a | n.s. | n.s. | 0.026 |
exceeds B&H FDR Criterion
Ethanol-choline interactions and miR-200c
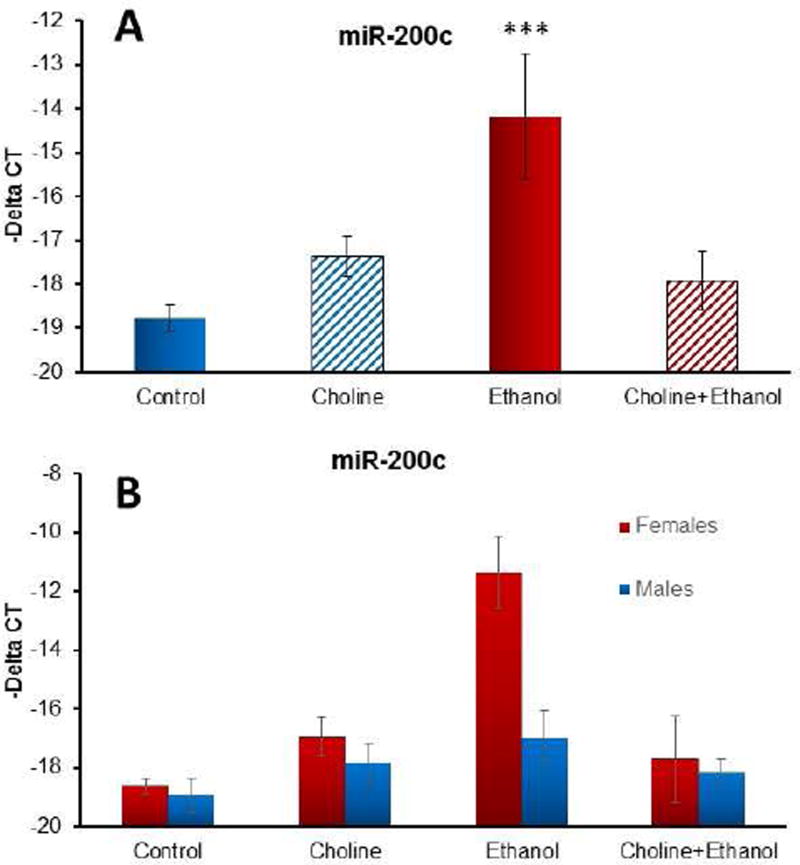
Post-hoc analyses showed that ethanol by itself significantly increased miR-200c and that choline treatment mitigated ethanol’s effects, producing a significant interaction of choline × ethanol [F(1,16) = 17.6, p<.001] as well as a main effect of ethanol [F(1,16) = 11.0, p<.01]. Importantly, choline treatment mitigated ethanol’s effects, so that the miR-200c levels within the Ethanol + Choline group were not significantly different from that of controls (p<0.866), but were significantly lower compared to the Ethanol + Saline group (p<0.005) (see Figure 4A). Although the sex × group interaction did not exceed the B&H FDR adjusted criterion, this effect was more robust in females compared to males, producing a significant interaction of choline × ethanol × sex [F(1,16) = 5.6, p<.05] and main effect of sex [F(1,16) = 5.6, p<.05]. Choline by itself did not alter miR-200c (p>.4); however, choline did reduce alcohol-related increases among the females, producing a significant ethanol × choline interaction [F(1,8) = 15.8, p<.005] (Scheffe’s post-hoc test, p<0.005) (see Figure 4B). A similar pattern was seen with males, although the interaction of ethanol and choline did not reach significance (p = .14). Finally, the effect of ethanol and choline on miR-200c was specific. Family members, miR-200a and miR-200b on rat chromosome 5, were not significantly altered by treatment (p<0.737 and p<0.283, respectively), and miR-141, which lies in close proximity to miR-200c on rat chromosome 4, was also not significantly altered by treatment (p<0.895). MiR-200 family member, miR-429, was expressed in only 20% of assessed samples. These data suggest that ethanol and choline specifically target miR-200c.
Figure 4.
Ethanol exposure during the third trimester equivalent significantly increased expression of miR-200c, an effect that was mitigated with choline supplementation (A). This effect was driven largely by effects among females (B).
*** significantly different from all other groups
Ethanol-choline interactions and other miRNAs
A pattern similar to that of miR-200c was seen with miR-326 expression, with ethanol increasing expression and choline mitigating this effect, producing an interaction of ethanol × choline [F(1,16) = 5.3, p<.05], as well as a main effect of choline [F(1,16) = 4.9, p<.05](see Figure 5). Although the sex × group interaction failed to reach significance using the B&H FDR criterion, as did the interaction of ethanol × choline × sex in the follow-up analyses (p<.08), this effect was driven by the females, producing interactions of ethanol × sex [F(1,16) = 4.7, p<.05], choline × sex [F(1,16) = 5.1, p<.05], and a main effect of sex [F(1,16) = 9.9, p<.01]. Follow-up analyses confirmed that miR-326 expression was significantly elevated in ethanol-exposed females, but not males, and that choline supplementation significantly mitigated this effect.
Figure 5.
Although effects in other miRNAs did not reach significance given the conservative B&H FDR adjusted criterion, with the exception of miR 878-3p, similar patterns of ethanol-induced increases in miRNA expression and choline attenuation of this effect were seen across other miRNAs.
Ethanol also increased miR-374-5p expression above control levels and choline supplementation attenuated ethanol’s effects to control levels, although only the overall choline effect was significant [F(1,16) = 7.0, p<.05], an effect driven by reduced expression in ethanol-exposed subjects treated with choline (Figure 5). Likewise, ethanol also significantly increased expression of miR-130a [F(1,16) = 4.3, p<.05] and choline reduced expression [F(1,16) = 8.7, p<.01], although the interaction did not reach statistical significance (p=.10). However, there was heterogeneity in variance in miR-130b-3p expression [Levene’s test: F(7,16) = 3.5, p<.05] and non-parametric analyses failed to reach statistical significance (p=.07). Finally, there was a significant interaction of ethanol × choline [F(1,16) = 8.2, p<.05] in miR878-3p, as both ethanol only or choline only significantly increased expression above control levels, whereas expression among subjects exposed to the combination of ethanol and choline did not differ significantly from any other group, including controls (Figure 5).
Pathway Overrepresentation analysis
miR-200c (ANOVA FDR-adjusted p<0.05) alone was compared to a combination of miRNAs, miR-130b-3p, miR-326, and miR-374a-5p, that exceeded the unadjusted criterion of p<0.05 for pathway overrepresentation analysis. MiR-878-3p and miR-327 are designated as a rodent-specific miRNA (miRBase release 21), not annotated as present in the human genome GRCh38/hg38 assembly, and were therefore eliminated from further analysis, since they would not be expected to predict function in the human. This analysis showed that MAP kinase and FoxO signaling pathways were predicted to be common targets. However, miR-200c was also predicted to target non-overlapping cellular functions including neurotrophin and ErbB2 signaling and axon guidance pathways, compared to the combination of miR-130b-3p, miR-326, and miR-374a-5p (Table 2).
Table 2.
Predicted miRNA-targeted signaling pathways.
| miR-200c | miR-130b-3p miR-326 miR-374a-5p |
|
|---|---|---|
| KEEG Pathway | p-value | p-value |
| Glycosphingolipid biosynthesis - lacto and neolacto series | 2.81E-06 | n.s. |
| Biotin metabolism | 4.02E-05 | n.s. |
| ErbB signaling pathway | 0.000129347 | n.s. |
| Neurotrophin signaling pathway | 0.000281105 | n.s. |
| Glycosaminoglycan biosynthesis - heparan sulfate / heparin | 0.000914285 | n.s. |
| Glycosaminoglycan biosynthesis - chondroitin sulfate / dermatan sulfate | 0.006683496 | n.s. |
| Thyroid hormone signaling pathway | 0.017464465 | n.s. |
| Axon guidance | 0.031128048 | n.s. |
| Lysine degradation | 0.034593547 | n.s. |
| Gap junction | n.s. | 0.04972998 |
| Morphine addiction | n.s. | 0.04972998 |
| Rap1 signaling pathway | n.s. | 0.039875166 |
| Ras signaling pathway | n.s. | 0.036203142 |
| Hippo signaling pathway | n.s. | 0.036203142 |
| Renin-angiotensin system | n.s. | 0.025151429 |
| MAPK signaling pathway | 0.011135814 | 0.0161786 |
| Fatty acid metabolism | n.s. | 0.012709576 |
| Endocytosis | n.s. | 0.012709576 |
| GABAergic synapse | n.s. | 0.002414972 |
| FoxO signaling pathway | 0.049946489 | 0.000125049 |
| Biosynthesis of unsaturated fatty acids | n.s. | 2.36E-09 |
| TGF-beta signaling pathway | n.s. | 1.81E-10 |
| Prion diseases | n.s. | 2.09E-16 |
Discussion
The present study illustrates that choline may modify some of ethanol’s adverse consequences on development by attenuating effects on miRNAs. Developmental ethanol exposure increased miRNA variance and increased levels of several specific miRNAs. Choline supplementation mitigated general ethanol-related increases in variance, as well as specific ethanol-related increases in miR-200c. In contrast, choline supplementation, by itself, did not significantly influence these outcomes among controls. The effect of developmental ethanol exposure on increased inter-subject variation in miRNA content in the juvenile hippocampus is particularly significant, since it suggests that such exposure has a pervasive and persistent destabilizing effect on homeostatic mechanisms that control miRNA expression, contributing to a global destabilization in miRNA expression. Such global destabilization of miRNAs may also contribute to commensurate destabilization of gene expression and the preferential down regulation of gene expression, as has been observed in a recent meta-analysis of several prenatal alcohol exposure studies (Rogic et al., 2016). Importantly, choline supplementation reduced the increase in miRNA expression variance suggesting that this nutritional supplement may be a useful tool for long-term normalization of miRNA regulatory networks.
This is the first report, to our knowledge, specifically tying miR-200c to the brain effects of developmental alcohol exposure. We observed that third-trimester-equivalent ethanol exposure resulted in a significant and specific induction in hippocampal miR-200c and that choline attenuated this effect. Ethanol has recently been shown to induce liver miR-200c in an adult rat model of alcohol-induced steatosis (Chen et al., 2014), and we previously found that in utero 3-trimester-equivalent exposure to ethanol in a sheep model resulted in significantly increased levels of a related family member, miR-200a, in the plasma of the newborn lamb (Balaraman et al., 2014). Although other miR-200 family members (miR-200a/b and miR-429) were not altered in the current study, these data nevertheless show that miR-200c may represent a target of ethanol across cell-types and developmental periods.
The miR-200 family of miRNAs is highly conserved through vertebrate evolution, and during development, they can control neurogenesis and gliogenesis by fine-tuning levels of target protein expression rather than by inducing large changes in gene expression (reviewed in (Trumbach and Prakash, 2015)). In fact, miR-200 regulates neuronal differentiation in the developing olfactory bulb, an effect that is mediated by inhibition of the zinc finger protein Zeb2 (Beclin et al, 2016). Previous research has shown that miR-200c expression is increased in the adult rodent spinal cord following injury (Yu et al., 2014), in the adult cerebral cortex following ischemic preconditioning (Lee et al., 2010), and following cerebrovascular stroke (Stary et al., 2015). These data suggest that miR-200c may be generally elevated during episodes of brain stress, and further suggest that developmental ethanol exposure may function as a persistent source of brain stress. The implications of elevated miR-200c are unclear however. Although data from cell culture models show that miR-200c overexpression can improve survival in microglia (Yu et al., 2014) and neuroblastoma cells following oxygen glucose deprivation (Lee et al., 2010), studies in whole animal models show that elevated levels of brain miR-200c contribute to the severity of brain damage (Stary et al., 2015). It remains to be determined whether the persistent increase in miR-200c in the juvenile hippocampus represents ongoing damage due to developmental ethanol exposure, adaptive neuroprotection, or persistence of aberrant developmental neurogenic programs.
Nevertheless, the ability of developmental choline supplementation to attenuate the ethanol induction of miR-200c suggests that choline supplementation is mechanistically antagonistic to the effects of prenatal ethanol exposure. In fact, choline supplementation can protect against a number of central nervous system (CNS) insults e.g. (Holmes et al., 2002; Kolb et al., 2000; Ricceri et al., 2011) whether administered prenatally or postnatally. To date, little is known of the effects of choline supplementation on miRNA expression within the brain. Certainly, dietary choline deficiency-induced liver injury alters miRNA expression in plasma (Clarke et al., 2014; Tryndak, 2012) and liver (Tryndak et al., 2016), and choline supplementation can protect against cardiac hypertrophy following an ischemic event, normalizing aberrant increases in miR-133 (Zhao, et al., 2013). But effects of choline supplementation on miRNAs in the brain have not been investigated. Interestingly, prenatal choline supplementation reduces apoptosis and increases cell division and neurogenesis in the fetal hippocampus (Albright et al., 1999; Craciunescu et al., 2003; Niculescu et al., 2006). Moreover, perinatal choline supplementation enhances hippocampal neurogenesis throughout the lifespan, resulting in increased levels of newly proliferated cells (Glenn et al., 2007; Glenn et al., 2008). This effect may be mediated by long-lasting choline-induced increases in neurotrophic support, including neurotrophin-3 and brain-derived neurotrophic factor (BDNF) (Glenn et al., 2007; Glenn et al., 2008). Prenatal choline supplementation may also enhance phosphorylation of MAP kinase and CREB following hippocampal stimulation (Mellott et al., 2004). If choline protects against early apoptosis or a persistent pathological hippocampal environment associated with developmental alcohol exposure, it may explain the attenuation in miR-200c, which is known to influence neurogenesis and neurotrophic support (see Table 2). Given that tissue was collected 10 days after ethanol exposure, choline’s effects could represent modifications of ethanol’s acute and/or long-lasting actions that are reflected as modifications in miR-200c.
As a methyl donor, choline also acts as an epigenetic factor and may also act directly on miRNA expression. We have reported that alcohol exposure during the 3rd trimester equivalent leads to global DNA hypermethylation in both the hippocampus and the prefrontal cortex, an effect attenuated by early postnatal choline supplementation (Otero et al., 2012). This suggests that choline is altering ethanol’s effects on gene expression. In fact, Sarkar’s group reported that prenatal choline supplementation can normalize ethanol’s effects on gene methylation and mRNA expression of POMC genes, as well as β-EP peptide production, in the hypothalamus (Bekdash et al., 2013). Specifically, choline both increased and decreased, respectively, levels of activating and repressing methylation marks. Choline also altered the expression of various histone-modifying enzymes, and normalized DNA methylation in areas where POMC neurons are found. Altogether, such effects may ultimately reduce stress hyperresponsivity associated with prenatal alcohol exposure (Bekdash et al., 2013). The present study provides further evidence that choline acts, at least in part, by altering gene expression.
But the effects of choline on brain structure and function following an alcohol insult are still not well understood. First, we have shown that postnatal choline supplementation up to PD 30 mitigates ethanol’s effects on muscarinic cholinergic receptors in the hippocampus (Monk et al., 2012). Although choline did not mitigate ethanol-related reductions in M1 receptors, it did mitigate ethanol-related increases in M2/4 receptors back down to control levels, leading to M1:M2/4 ratios similar to that of controls. High ratios of M1:M2/4 receptors correspond to increases in cAMP, CREB, and BDNF, all of which have well-documented roles in memory and neuronal plasticity (Alonso et al., 2002a; Alonso et al., 2002b; Chen et al., 2010; Countryman et al., 2005). These data suggest that choline mitigates long-lasting ethanol-related changes in cholinergic functioning. Interestingly, in the present study, we did not find significant effects in miR-132, a miRNA that is associated with cholinergic activity (Zhu et al., 2016). Nevertheless, given that cholinergic inputs into the hippocampus promote plasticity (Rasmusson, 2000), it is possible that it contributes to the long-lasting beneficial effects choline has on the alcohol-exposed brain.
In addition, choline may also alter lipid metabolism (Thomas et al., 2016). In fact, pre-exposure with choline protects against ethanol-related disruptions in lipid rafts (Tang et al., 2014). So in addition to changes in gene expression and cholinergic functioning, choline can lead to long-lasting changes in cell membranes and lipid homeostasis that will ultimately affect cell survival and signaling. Given that miR-200c may influence glycosphingolipid synthesis, this represents another potential intersection by which choline may modify ethanol’s effects on brain development and function.
There are several limitations of this study. One limitation of the present study is that miRNAs were examined only during one developmental timepoint. It is possible that ethanol alters developmental timing of events, which may consequently affect miRNA expression. Although choline did not alter body growth, choline may mitigate ethanol-related changes in developmental timing, which are reflected in miRNA expression. In addition, we examined miRNA changes shortly after cessation of choline treatment, but it is not known how long changes in miRNAs persist following choline treatment. Only follow-up analyses at different ages can address these questions. Finally, it should be noted that we used an analytical approach that focused specifically on potential ethanol-regulated miRNAs to identify those ethanol-sensitive miRNAs that were protected by choline exposure. It is also possible that with greater power, one might find choline attenuates additional alcohol-related effects of miRNAs. In addition, it is possible that choline may affect other pathways, including miRNA targets that are independent of ethanol’s effects, but still act to protect against ethanol-associated neuropathology and functional deficits. Nevertheless, the specificity of effects of combined ethanol and choline provide strong evidence that choline does selectively alter some of ethanol’s effects, particularly with miR-200c.
In sum, choline likely exerts multiple actions to protect the ethanol-exposed brain. Given that choline targets the hippocampus during this period of early postnatal development (Monk et al., 2012; Otero et al., 2012; Wagner and Hunt, 2006; Thomas et al., 2004a; Thomas et al., 2004b; Thomas and Tran, 2012), it would also be important to determine whether ethanol-related changes in miRNAs in the cerebellum or other CNS regions are unaffected by choline supplementation to provide further evidence that such changes relate to behavioral outcome. Nevertheless, the present study illustrates that choline modification of ethanol’s effects on miRNAs provide another avenue through which choline may protect against ethanol’s teratogenic effects.
HIGHLIGHTS.
Developmental alcohol exposure dysregulates hippocampal miRNA expression
Developmental alcohol exposure specifically increases several miRNAs
Choline supplementation normalized ethanol-related increases in miRNA variance
Choline specifically attenuated ethanol-related increases in hippocampal miR-200c
Choline may reduce fetal alcohol spectrum disorders by altering miRNAs
Acknowledgments
This work was supported by the NIAAA (AA12446 & AA013440).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel EL. An update on incidence of FAS: FAS is not an equal opportunity birth defect. Neurotoxicology and Teratology. 1995;17(4):437–443. doi: 10.1016/0892-0362(95)00005-c. [DOI] [PubMed] [Google Scholar]
- Albright CD, Friedrich CB, Brown EC, Mar MH, Zeisel SH. Maternal dietary choline availability alters mitosis, apoptosis and the localization of TOAD-64 protein in the developing fetal rat septum. Brain Research Developmental Brain Research. 1999;115(2):123–129. doi: 10.1016/s0165-3806(99)00057-7. [DOI] [PubMed] [Google Scholar]
- Alonso M, Vianna MR, Depino AM, Mello e Souza T, Pereira P, Szapiro G, Viola H, Pitossi F, Izquierdo I, Medina JH. BDNF-triggered events in the rat hippocampus are required for both short- and long-term memory formation. Hippocampus. 2002a;12(4):551–560. doi: 10.1002/hipo.10035. [DOI] [PubMed] [Google Scholar]
- Alonso M, Vianna MR, Izquierdo I, Medina JH. Signaling mechanisms mediating BDNF modulation of memory formation in vivo in the hippocampus. Cellular and Molecular Neurobiology. 2002b;22(5–6):663–674. doi: 10.1023/A:1021848706159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaraman S, Lunde ER, Sawant O, Cudd TA, Washburn SE, Miranda RC. Maternal and neonatal plasma microRNA biomarkers for fetal alcohol exposure in an ovine model. Alcoholism: Clinical and Experimental Research. 2014;38(5):1390–1400. doi: 10.1111/acer.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaraman S, Winzer-Serhan UH, Miranda RC. Opposing actions of ethanol and nicotine on microRNAs are mediated by nicotinic acetylcholine receptors in fetal cerebral cortical-derived neural progenitor cells. Alcoholism: Clinical and Experimental Research. 2012;36(10):1669–1677. doi: 10.1111/j.1530-0277.2012.01793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearer CF, Wellmann KA, Tang N, He M, Mooney SM. Choline ameliorates deficits in balance caused by acute neonatal ethanol exposure. Cerebellum. 2015;14(4):413–420. doi: 10.1007/s12311-015-0691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beclin C, Follert P, Stappers E, Barral S, Nathalie C, de Chevigny A, Magnone V, Lebrigand K, Bisselss U, Huylebroeck D, Bosio A, Barbry P, Seuntjens E, Cremer H. miR-200 family controls late steps of postnatal forebrain neurogenesis via Zeb2 inhibition. Scientific Reports. 2016;6:25729. doi: 10.1038/srep35729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekdash RA, Zhang C, Sarkar DK. Gestational choline supplementation normalized fetal alcohol-induced alterations in histone modifications, DNA methylation, and proopiomelanocortin (POMC) gene expression in beta-endorphin-producing POMC neurons of the hypothalamus. Alcoholism: Clinical and Experimental Research. 2013;37(7):1133–1142. doi: 10.1111/acer.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarillo C, Miranda RC. Ethanol exposure during neurogenesis induces persistent effects on neural maturation: evidence from an ex vivo model of fetal cerebral cortical neuroepithelial progenitor maturation. Gene Expression. 2008;14(3):159–171. [PMC free article] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality. Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health (HHS Publication No. SMA 15-4927, NSDUH Series H-50) 2015 Retrieved from http://www.samhsa.gov/ data/
- Centers for Disease Control and Prevention. Fetal Alcohol Spectrum Disorders (FASD) 2015. [Google Scholar]
- Chen LY, Rex CS, Pham DT, Lynch G, Gall CM. BDNF signaling during learning is regionally differentiated within hippocampus. Journal of Neuroscience. 2010;30(45):15097–15101. doi: 10.1523/JNEUROSCI.3549-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YP, Jin X, Kong M, Li YM. Pattern of microRNA expression associated with different stages of alcoholic liver disease in rat models. Molecular Medicine Reports. 2014;10(3):1195–1204. doi: 10.3892/mmr.2014.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Countryman RA, Orlowski JD, Brightwell JJ, Oskowitz AZ, Colombo PJ. CREB phosphorylation and c-Fos expression in the hippocampus of rats during acquisition and recall of a socially transmitted food preference. Hippocampus. 2005;15(1):56–67. doi: 10.1002/hipo.20030. [DOI] [PubMed] [Google Scholar]
- Craciunescu CN, Albright CD, Mar MH, Song J, Zeisel SH. Choline availability during embryonic development alters progenitor cell mitosis in developing mouse hippocampus. Journal of Nutrition. 2003;133(11):3614–3618. doi: 10.1093/jn/133.11.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing C, Flink S, Florez-McClure ML, Johnson TE, Tabakoff B, Kechris KJ. Gene expression changes in C57BL/6J and DBA/2J mice following prenatal alcohol exposure. Alcoholism: Clinical and Experimental Research. 2012;36(9):1519–1529. doi: 10.1111/j.1530-0277.2012.01757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing C, Johnson TE, Larson C, Leakey TI, Siegfried RN, Rafferty TM, Cooney CA. Subtle decreases in DNA methylation and gene expression at the mouse Igf2 locus following prenatal alcohol exposure: Effects of a methyl-supplemented diet. Alcohol. 2011;45(1):65–71. doi: 10.1016/j.alcohol.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finer LB, Zolna MR. Unintended pregnancy in the United States: Incidence and disparities, 2006. Contraception. 2011;84(5):478–485. doi: 10.1016/j.contraception.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Mohapel J, Boehme F, Kainer L, Christie BR. Hippocampal cell loss and neurogenesis after fetal alcohol exposure: insights from different rodent models. Brain research reviews. 2010;64(2):283–303. doi: 10.1016/j.brainresrev.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Glenn MJ, Gibson EM, Kirby ED, Mellott TJ, Blusztajn JK, Williams CL. Prenatal choline availability modulates hippocampal neurogenesis and neurogenic responses to enriching experiences in adult female rats. The European Journal of Neuroscience. 2007;25(8):2473–2482. doi: 10.1111/j.1460-9568.2007.05505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn MJ, Kirby ED, Gibson EM, Wong-Goodrich SJ, Mellott TJ, Blusztajn JK, Williams CL. Age-related declines in exploratory behavior and markers of hippocampal plasticity are attenuated by prenatal choline supplementation in rats. Brain Research. 2008;1237:110–123. doi: 10.1016/j.brainres.2008.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DA, Akers KG, Rice JP, Johnson TE, Candelaria-Cook FT, Maes LI, Rosenberg M, Valenzuela CF, Savage DD. Prenatal exposure to moderate levels of ethanol alters social behavior in adult rats: Relationship to structural plasticity and immediate early gene expression in frontal cortex. Behavioural brain research. 2010;207(2):290–304. doi: 10.1016/j.bbr.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes GL, Yang Y, Liu Z, Cermak JM, Sarkisian MR, Stafstrom CE, Neill JC, Blusztajn JK. Seizure-induced memory impairment is reduced by choline supplementation before or after status epilepticus. Epilepsy Research. 2002;48(1–2):3–13. doi: 10.1016/s0920-1211(01)00321-7. [DOI] [PubMed] [Google Scholar]
- Kane CJ, Phelan KD, Drew PD. Neuroimmune mechanisms in fetal alcohol spectrum disorder. Developmental Neurobiology. 2012;72(10):1302–1316. doi: 10.1002/dneu.22035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Research. 2016;44(D1):D457–D462. doi: 10.1093/nar/gkv1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Gibb R, Gorny G. Cortical plasticity and the development of behavior after early frontal cortical injury. Developmental Neuropsychology. 2000;18(3):423–444. doi: 10.1207/S1532694208Kolb. [DOI] [PubMed] [Google Scholar]
- Lee ST, Chu K, Jung KH, Yoon HJ, Jeon D, Kang KM, Park KH, Bae EK, Kim M, Lee SK, Roh JK. MicroRNAs induced during ischemic preconditioning. Stroke. 2010;41(8):1646–1651. doi: 10.1161/STROKEAHA.110.579649. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais AS, Hendricks LS, Snell CL, Tabachnick BG, Stellavato C, Buckley DG, Brooke LE, Viljoen DL. Maternal risk factors for fetal alcohol syndrome and partial fetal alcohol syndrome in South Africa: A third study. Alcoholism: Clinical and Experimental Research. 2008;32(5):738–753. doi: 10.1111/j.1530-0277.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Crocker N, Nguyen TT. Fetal alcohol spectrum disorders: Neuropsychological and behavioral features. Neuropsychology Review. 2011;21:81–101. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina AE. Fetal alcohol spectrum disorders and abnormal neuronal plasticity. The Neuroscientist. 2011;17(3):274–287. doi: 10.1177/1073858410383336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellott TJ, Williams CL, Meck WH, Blusztajn JK. Prenatal choline supplementation advances hippocampal development and enhances MAPK and CREB activation. FASEB Journal. 2004;18(3):545–547. doi: 10.1096/fj.03-0877fje. [DOI] [PubMed] [Google Scholar]
- Miranda RC. MicroRNAs and Ethanol Toxicity. International Review of Neurobiology. 2014;115:245–284. doi: 10.1016/B978-0-12-801311-3.00007-X. [DOI] [PubMed] [Google Scholar]
- Monk BR, Leslie FM, Thomas JD. The effects of perinatal choline supplementation on hippocampal cholinergic development in rats exposed to alcohol during the brain growth spurt. Hippocampus. 2012;22(8):1750–1757. doi: 10.1002/hipo.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niculescu MD, Craciunescu CN, Zeisel SH. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J. 2006;20(1):43–49. doi: 10.1096/fj.05-4707com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niculescu MD, Zeisel SH. Diet, methyl donors and DNA methylation: Interactions between dietary folate, methionine and choline. The Journal of Nutrition. 2002;132(8 Suppl):2333S–2335S. doi: 10.1093/jn/132.8.2333S. [DOI] [PubMed] [Google Scholar]
- O'Leary-Moore SK, Parnell SE, Lipinski RJ, Sulik KK. Magnetic resonance-based imaging in animal models of feteal alcohol spectrum disorder. Neuropsychology Review. 2011;21(2):167–185. doi: 10.1007/s11065-011-9164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero NK, Thomas JD, Saski CA, Xia X, Kelly SJ. Choline supplementation and DNA methylation in the hippocampus and prefrontal cortex of rats exposed to alcohol during development. Alcoholism: Clinical and Experimental Research. 2012;36(10):1701–1709. doi: 10.1111/j.1530-0277.2012.01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappalardo-Carter DL, Balaraman S, Sathyan P, Carter ES, Chen WJ, Miranda RC. Suppression and epigenetic regulation of MiR-9 contributes to ethanol teratology: evidence from zebrafish and murine fetal neural stem cell models. Alcoholism: Clinical and Experimental Research. 2013;37(10):1657–1667. doi: 10.1111/acer.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson DD. The role of acetylcholine in cortical synaptic plasticity. Behavioural Brain Research. 2000;115(2):205–218. doi: 10.1016/s0166-4328(00)00259-x. [DOI] [PubMed] [Google Scholar]
- Ricceri L, De Filippis B, Fuso A, Laviola G. Cholinergic hypofunction in MeCP2-308 mice: beneficial neurobehavioural effects of neonatal choline supplementation. Behavioural Brain Research. 2011;221(2):623–629. doi: 10.1016/j.bbr.2011.03.051. [DOI] [PubMed] [Google Scholar]
- Rogic S, Wong A, Pavlidis P. Meta-Analysis of gene expression patterns in animal models of prenatal alcohol exposure suggests role for protein synthesis inhibition and chromatin remodeling. Alcoholism: Clinical and Experimental Research. 2016;40(4):717–727. doi: 10.1111/acer.13007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozen S, Peters GJ, Kok G, Townend D, Nijhuis J, Curfs L. Worldwide prevalence of fetal alcohol spectrum disorders: A systematic literature review including meta-analysis. Alcoholism: Clinical and Experimental Research. 2016;40(1):18–32. doi: 10.1111/acer.12939. [DOI] [PubMed] [Google Scholar]
- Ryan SH, Williams JK, Thomas JD. Choline supplementation attenuates learning deficits associated with neonatal alcohol exposure in the rat: effects of varying the timing of choline administration. Brain Research. 2008;1237:91–100. doi: 10.1016/j.brainres.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santillano DR, Kumar LS, Prock TL, Camarillo C, Tingling JD, Miranda RC. Ethanol induces cell-cycle activity and reduces stem cell diversity to alter both regenerative capacity and differentiation potential of cerebral cortical neuroepithelial precursors. BMC Neuroscience. 2005;6:59. doi: 10.1186/1471-2202-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyan P, Golden HB, Miranda RC. Competing interactions between micro-RNAs determine neural progenitor survival and proliferation after ethanol exposure: evidence from an ex vivo model of the fetal cerebral cortical neuroepithelium. Journal of Neuroscience. 2007;27(32):8546–8557. doi: 10.1523/JNEUROSCI.1269-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider RD, Thomas JD. Adolescent choline supplementation attenuates working memory deficits in rats exposed to alcohol during the third trimester equivalent. Alcoholism: Clinical and Experimental Research. 2016;40(4):897–905. doi: 10.1111/acer.13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stary CM, Xu L, Sun X, Ouyang YB, White RE, Leong J, Li J, Xiong X, Giffard RG. MicroRNA-200c contributes to injury from transient focal cerebral ischemia by targeting Reelin. Stroke. 2015;46(2):551–556. doi: 10.1161/STROKEAHA.114.007041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N, Bamford P, Jones J, He M, Kane MA, Mooney SM, Bearer CF. Choline partially prevents the impact of ethanol on the lipid raft dependent functions of l1 cell adhesion molecule. Alcoholism: Clinical and Experimental Research. 2014;38(11):2722–2730. doi: 10.1111/acer.12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, Biane JS, O'Bryan KA, O'Neill TM, Dominguez HD. Choline supplementation following third-trimester-equivalent acohol expoure attenuates behavioral alterations in rats. Behavioral Neuroscience. 2007;121(1):120–130. doi: 10.1037/0735-7044.121.1.120. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Abou EJ, Dominguez HD. Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Neurotoxicology and Teratology. 2009;31(5):303–311. doi: 10.1016/j.ntt.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, Garrison M, O'Neill TM. Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicology and Teratology. 2004a;26(1):35–45. doi: 10.1016/j.ntt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Idrus NM, Monk BR, Dominguez HD. Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in rats. Birth Defects Research. 2010;88(10):827–837. doi: 10.1002/bdra.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, Jeanguenin L, Herr DR, Walls SM, Idrus NM, Harris GL. Choline supplementation attenuates increased ceramide levels associated with developmental alcohol exposure. Alcoholism: Clinical and Experimental Research. 2016;40(Supple1):157A. [Google Scholar]
- Thomas JD, La Fiette MH, Quinn VR, Riley EP. Neonatal choline supplementation ameliorates the effects of prenatal alcohol exposure on a discrimination learning task in rats. Neurotoxicology and Teratology. 2000;22(5):703–711. doi: 10.1016/s0892-0362(00)00097-0. [DOI] [PubMed] [Google Scholar]
- Thomas JD, O'Neill TM, Dominguez HD. Perinatal choline supplementation does not mitigate motor coordination deficits associated with neonatal alcohol exposure in rats. Neurotoxicology and Teratology. 2004b;26(2):223–229. doi: 10.1016/j.ntt.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Tran TD. Choline supplementation mitigates trace, but not delay, eyeblink conditioning deficits in rats exposed to alcohol during development. Hippocampus. 2012;22(3):619–630. doi: 10.1002/hipo.20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingling JD, Bake S, Holgate R, Rawlings J, Nagsuk PP, Chandrasekharan J, Schneider SL, Miranda RC. CD24 expression identifies teratogen-sensitive fetal neural stem cell subpopulations: evidence from developmental ethanol exposure and orthotopic cell transfer models. PLoS One. 2013;8(7):e69560. doi: 10.1371/journal.pone.0069560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumbach D, Prakash N. The conserved miR-8/miR-200 microRNA family and their role in invertebrate and vertebrate neurogenesis. Cell and Tissue Research. 2015;359(1):161–177. doi: 10.1007/s00441-014-1911-z. [DOI] [PubMed] [Google Scholar]
- Tsai PC, Bake S, Balaraman S, Rawlings J, Holgate RR, Dubois D, Miranda RC. MiR-153 targets the nuclear factor-1 family and protects against teratogenic effects of ethanol exposure in fetal neural stem cells. Biology Open. 2014;3(8):741–758. doi: 10.1242/bio.20147765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachos IS, Zagganas K, Paraskevopoulou MD, Georgakilas G, Karagkouni D, Vergoulis T, Dalamagas T, Hatzigeorgiou AG. DIANA-miRPath v3.0: deciphering microRNA function with experimental support. Nucleic Acids Research. 2015;43:W460–W466. doi: 10.1093/nar/gkv403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AF, Hunt PS. Impaired trace fear conditioning following neonatal ethanol: reversal by choline. Behavioral Neuroscience. 2006;120(2):482–487. doi: 10.1037/0735-7044.120.2.482. [DOI] [PubMed] [Google Scholar]
- Wilhelm CJ, Guizzetti M. Fetal alchol spectrum disorders: An overview from the glia perspective. Frontieers in Integrative Neuroscience. 2016;9:1–16. doi: 10.3389/fnint.2015.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman AD, Charvet CJ, Clancy B, Darlington RB, Finlay BL. Modeling transformations of neurodevelopmental sequences across mammalian species. Journal of Neuroscience. 2013;33(17):7368–7383. doi: 10.1523/JNEUROSCI.5746-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JK, Giesbrecht HE, Eskin MN, Aliani M, Suh M. Nutrition implications for fetal alcohol spectrum disorder. Advances in Nutrition. 2014;5(6):675–692. doi: 10.3945/an.113.004846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu DS, Lv G, Mei XF, Cao Y, Wang YF, Wang YS, Bi YL. MiR-200c regulates ROS-induced apoptosis in murine BV-2 cells by targeting FAP-1. Spinal cord. 2014;53:182–189. doi: 10.1038/sc.2014.185. [DOI] [PubMed] [Google Scholar]
- Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annual Review of Nutrition. 2006;26:229–250. doi: 10.1146/annurev.nutr.26.061505.111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH. Nutrition in pregnancy: the argument for including a source of choline. International Journal of Women's Health. 2013;5:193–199. doi: 10.2147/IJWH.S36610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH, Niculescu MD. Perinatal choline influences brain structure and function. Nutrition Reviews. 2006;64(4):197–203. doi: 10.1111/j.1753-4887.2006.tb00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]