Abstract
J Clin Hypertens(Greenwich). 2010;12:578–587. © 2010 Wiley Periodicals, Inc.
The authors evaluated the relationship of hypertensive target organ damage to masked hypertension assessed by ambulatory blood pressure (BP) and home blood pressure (HBP) monitoring in 129 participants without taking antihypertensive medication. Masked hypertension was defined as office BP ≤140/90 mm Hg and 24‐hour ambulatory BP ≥130/80 mm Hg. The masked hypertensive participants defined by 24‐hour ambulatory BP (n=13) had a higher serum glucose level (126 vs 96 mg/dL, P=.001) and urinary albumin‐creatinine ratio (38.0 vs 7.5 mg/gCr, P<.001) than the normotensive participants (n=74); however, these relationships were not observed when the authors defined groups using HBP (≥135/85 mm Hg). Masked hypertension by both 24‐hour ambulatory BP and HBP had a higher urinary albumin‐creatinine ratio than normotension by both 24‐hour ambulatory BP and HBP (62.1 vs 7.4 mg/gCr, P=.001), and than masked hypertension by HBP alone (9.3 mg/gCr, P=.009). Masked hypertension defined by 24‐hour ambulatory BP is associated with an increased serum glucose level and urinary albumin‐creatinine ratio, but these relationships are not observed in masked hypertension defined by HBP.
Masked hypertension (MHT), 1 a state in which ambulatory blood pressure (BP) is high despite a normal office blood pressure (BP) of <140/90 mm Hg, is reported to be seen in about 10%–20% of patients in general populations. 2 MHT is associated with hypertensive target organ damage 3 , 4 and cardiovascular events. 5 , 6
Several factors have been reported to contribute to MHT (ie, smoking, alcohol drinking, physical activity, and stress). 2 In addition, it has been reported that the prevalence of MHT is high in outpatients with diabetes, 7 , 8 , 9 and that MHT is related to increases in sympathetic nerve activity and insulin resistance. 10 However, these previous findings were based on samples of treated hypertensives, clinic outpatients with comorbid diseases, or healthy volunteers within limited age ranges, and thus the conditions related to MHT in the general population remain unclear.
Moreover, the out‐of‐office BP is more frequently measured using self‐measured home BP (HBP) monitoring than ambulatory BP monitoring. HBP is reported to have a higher predictive value of cardiovascular events than clinic BP. 11 , 12 In the studies of clinic outpatients, MHT defined by HBP monitoring has also been associated with a higher risk of increased carotid intima‐media thickness (c‐IMT) 13 and cardiovascular events. 14 Stergiou and coworkers 15 reported that HBP was interchangeable with ambulatory BP for diagnosing MHT in some patients, but the 2 modalities disagreed in others. Only limited data exist on the relationship between markers of hypertensive target organ damage and MHT diagnosed by ambulatory BP or HBP; Sega and coworkers 4 reported that patients with MHT diagnosed by both ambulatory BP and HBP monitoring had a greater left ventricular mass index (LVMI) than normotensive patients.
The main purposes of the present study were: (1) to compare the prevalence of MHT defined by ambulatory BP and HBP monitoring in a sample from the general population; (2) to compare the characteristics of the participants with MHT defined by ambulatory BP and HBP monitoring; and (3) to evaluate the differences in the relationships of hypertensive target organ damage to MHT defined by ambulatory BPand HBP.
Methods
Participants
This cross‐sectional study was conducted in 1998, in the rural community of Kinugawa, Tochigi, in the Miyori district of Japan. Details of the participants in the present study have been reported previously. 16 Briefly, a nurse sent a letter to all residents of Kinugawa aged 20 years or older (N=541 residents), inviting them to participate in this study at the time of their annual health examination, and a total of 181 adults (33% of the residents) responded and participated.
Office BP, 24‐Hour Ambulatory BP and HBP Monitoring
Office BP was measured using a cuff oscillometric device (UA‐631; A&D Company, Ltd, Tokyo, Japan). 17 Two consecutive readings were taken by a physician, during a clinic visit, at 1‐minute intervals after 5 minutes of rest in a sitting position.
Noninvasive ambulatory BP monitoring was carried out on a weekday, with an automatic device (TM‐2425; A&D Company, Ltd, Tokyo, Japan) that recorded BP by the oscillometric method and heart rate every 30 minutes for 24 hours. Sleep BP was defined as the average BP over the period from bedtime to rising, and awake BP was defined as the average BP over the rest of the day. According to the guidelines of the American Heart Association, 18 we defined the BP cut‐off levels as follows: clinic BP ≥140/90 mm Hg, 24‐hour average BP (ABP) ≥130/80 mm Hg, awake ABP ≥135/85 mm Hg, sleep BP ≥120/70 mm Hg, and HBP ≥135/85 mm Hg.
All patients were instructed to measure HBP for 3 days using a validated oscillometric device (UA‐631; A&D Company, Ltd) 17 on the same upper arm. Patients were asked to perform 2 consecutive self‐measurements of BP after 5 minutes of rest in the sitting position twice daily, once in the morning between 5 and 10 am and once in the evening between 5 and 10 pm. The self‐measured HBP was the average of all 6 pairs of readings collected over 3 days.
Echocardiography
M‐mode echocardiography was performed with 2‐dimensional monitoring. Left ventricular chamber recording was obtained at the tip of the mitral valve. The interventricular septal thickness and posterior wall thickness were measured at end‐diastole. The left ventricular internal dimensions were measured at end‐diastole, in accordance with the recommendations of the American Society of Echocardiography. The LVMI was calculated with the equation described by Devereux and Reichek 19 and indexed by body surface area. The relative wall thickness (RWT) was calculated as 2 × posterior wall thickness / left ventricular internal dimensions.
Carotid Ultrasonography
Imaging of the right and left extracranial carotid arteries was performed using a 7.5‐MHz transducer with the participant in a supine position with hyperextension of the neck. Measurement of the c‐IMT of the far wall at the end of diastole was performed in B‐mode.
Blood and Urinary Sample Tests
Impaired fasting glucose (IFG) was defined as a fasting glucose level of 6.2–6.9 mmol/L (111–125 mg/dL) and/or hemoglobin A1c of 5.9%–6.1%. Diabetes mellitus (DM) was defined as a fasting glucose level ≥7.0 mmol/L (126 mg/dL), hemoglobin A1c≥6.2% or the use of hypoglycemic agents. Hyperlipidemia was defined as a total cholesterol level ≥6.2 mmol/L (≥240 mg/dL) or the use of lipid‐lowering agents. Body mass index (BMI) was calculated as weight (kilograms)/height (meters). 2
Fasting blood samples were drawn on the morning after ambulatory BP monitoring. The serum levels of insulin were measured using an enzyme immunoassay (Eiken Chemical Co., Tokyo, Japan). As an index of insulin resistance, homeostasis model assessment (HOMA‐R) 20 was calculated as follows: insulin (μU/mL) × fasting blood glucose (mg/dL)/405. The plasma samples were stored at −80°C in a refrigerator until the measurements were made. All blood samples were measured in the same laboratory (SRL Inc., Tokyo, Japan). To minimize the confounding influence of daily physical activity, and to facilitate sample collection, the participants were asked to collect urine from 7 pm to 7 am (ie, over a period of 12 hours) during ambulatory BP monitoring. Urinary microalbumin, creatinine, sodium, chloride, potassium, and urinary salt excretion per 24 hours were calculated afterward. The urinary albumin per creatinine excretion ratio (UAR) was also calculated afterward.
Statistical Analysis
Among the 181 study participants, both office BP measurement and ambulatory BP monitoring were successfully conducted in 172 participants. After excluding 43 people who were taking antihypertensive medication, eligible data were available for 129 participants. Among them, data of HBP monitoring was available in 120 participants.
A global test of differences in means among normotensives, white coat hypertensives, masked hypertensives, and sustained hypertensives was evaluated using analysis of variance, and the differences between each pair of hypertensive groups were evaluated using Tukey’s honestly significant differences test. For categorical measures, the global differences in percentages among hypertensive groups were tested using the chi‐squared test. These data are shown as the mean ± standard deviation or as percentages. Because associations were observed between numerous potential confounders and the parameters of hypertensive target organ damage and/or serum glucose levels, analysis of covariance was used to evaluate the overall differences in the means of serum glucose level, log‐transformed UAR, LVMI, RWT, and c‐IMT after adjustment. We used 2 models for the adjustment: model 1 (age, gender, BMI, current smoking, and presence of hyperlipidemia), and model 2 (the preceding covariates plus presence of IFG or DM, office systolic BP [SBP] and office diastolic BP [DBP]). The differences in means among the hypertensive groups were analyzed using the Bonferroni procedure to control the risk of a type I error. The data of analysis of covariance are shown as the adjusted mean ± standard error. Values of P<.05 were considered statistically significant. The SPSS software package, version 16.0 (SPSS Inc., Chicago, IL) was used for the analysis.
Results
Participants
The mean age was 59.9±10.9 years, and 47.3% of the participants were men. The mean BMI was 24.0±3.1 kg/m2. The average of office SBP/DBP, 24‐hour ABP, awake ABP, sleep ABP, and HBP were 132±18/82±10 mm Hg, 121±14/74±8 mm Hg, 126±15/77±9 mm Hg, 110±14/67±9 mm Hg, and 124±22/77±11 mm Hg, respectively. Of the participants, 33.1% were current smokers, 15.5% had IFG or DM, and 38.8% had hyperlipidemia.
Characteristics of Participants With Masked Hypertension Defined by 24‐Hour ABP
There were 74 participants (57.4%) with true normotension (office BP <140/90 mm Hg and 24‐hour ABP <130/80 mm Hg), 11 participants (8.5%) with white coat hypertension (WCHT; office BP ≥140/90 mm Hg and 24‐hour ABP <130/80 mm Hg), 13 participants (10.0%) with MHT (office BP <140/90 mm Hg and 24‐hour ABP ≥130/80 mm Hg), and 31 participants (24.0%) with sustained hypertension (office BP ≥140/90 mm Hg and 24‐hour ABP ≥130/80 mm Hg) defined by office BP and 24‐hour ABP. The characteristics of the participants are shown in Table I. Age was significantly higher in the participants with WCHT than those with normotension.
Table I.
Comparison of BP Subgroups Defined by Office BP and 24‐hour ABP (N=129)
| Office BP 24‐Hour BP | NT (n=74) <140/90 mm Hg <130/80 mm Hg | WCHT (n=11) ≥140/90 mm Hg <130/80 mm Hg | MHT (n=13) <140/90 mm Hg ≥130/80 mm Hg | SHT (n=31) ≥140/90 mm Hg ≥130/80 mm Hg | P Value |
|---|---|---|---|---|---|
| Age, y | 58.6±11.0 | 67.5±10.8 | 57.8±8.5 | 61.2±10.9 | .061 |
| Female, % | 59.5 | 54.5 | 53.8 | 35.5 | .17 |
| Body mass index, kg/m2 | 23.3±2.7 | 24.6±3.0 | 24.0±3.9 | 25.4±3.3c | .013 |
| Current smokers, % | 28.6 | 30.0 | 30.0 | 46.4 | .39 |
| Fasting glucose, mg/dL | 94±22 | 105±15 | 117±44b,g | 95±12 | .008 |
| Hemoglobin A1c, % | 5.4±0.7 | 5.7±1.0 | 6.0±1.6 | 5.4±0.4 | .13 |
| IFG or diabetes, % | 13.5 | 27.3 | 23.1 | 12.9 | .55 |
| Total cholesterol, mg/dL | 195±35 | 203±31 | 167±36a,g | 198±35 | .033 |
| Hyperlipidemia, % | 36.5 | 45.5 | 23.1 | 48.4 | .40 |
| LVMI, g/m2 | 106±27 | 132±44 | 110±29 | 133±39b | .001 |
| Relative wall thickness | 0.40±0.10 | 0.49±0.13a | 0.43±0.08 | 0.49±0.11c | <.001 |
| Carotid IMT, mm | 0.6±0.1 | 0.8±0.1 | 0.7±0.2 | 0.8±0.2b | <.001 |
| Geometric mean UAR, mg/gCr | 7.5 (4.5–12.6) | 12.8 (5.2–31.2) | 27.8c (5.4–143.7) | 15.3b (4.7–50.0) | <.001 |
| Creatinine, mg/dL | 0.88±0.14 | 0.88±0.15 | 0.88±0.20 | 0.88±0.14 | .999 |
| Urinary salt excretion, g/d | 9.3±5.4 | 10.4±3.1 | 9.9±8.5 | 10.4±4.6 | .77 |
| Office SBP, mm Hg | 121±11 | 149±14c | 126±7f,i | 153±15c | <.001 |
| Office DBP, mm Hg | 77±7 | 90±7c | 80±7e,i | 93±9c | <.001 |
| 24‐hour SBP, mm Hg | 112±8 | 120±7a | 136±7c,f | 137±11c,f | <.001 |
| 24‐hour DBP, mm Hg | 69±6 | 74±4 | 79±6c | 82±8c | <.001 |
| Awake SBP, mm Hg | 117±9 | 124±8 | 142±5c,f | 143±13c,f | <.001 |
| Awake DBP, mm Hg | 72±6 | 77±4 | 82±6c | 86±8c,f | <.001 |
| Sleep SBP, mm Hg | 102±9 | 111±7a | 121±15c | 125±11c,f | <.001 |
| Sleep DBP, mm Hg | 63±6 | 70±4b | 70±8b | 75±8c | <.001 |
| Home SBP, mm Hg | 114±12 | 135±25b | 123±12g | 141±29c | <.001 |
| Home DBP, mm Hg | 73±8 | 83±13a | 76±9 | 83±14c | <.001 |
Data are shown as mean ± standard deviation (SD) or percentage. Data of urinary albumin per creatinine excretion ratio (UAR) are shown as geometric mean (±1 SD range). Overall P values were calculated using analysis of variance, and the differences in means among the hypertensive groups were calculated using Tukey’s honestly significant difference test. Abbreviations: ABP, average blood pressure; BP, blood pressure; DBP, diastolic blood pressure; IFG, impaired fasting glucose; IMT, intima‐media thickness; LVMI, left ventricular mass index; MHT, masked hypertension; NT, normotension; SBP, systolic blood pressure; SHT, sustained hypertension; UAR, urinary albumin per creatinine ratio; WCHT, white coat hypertension;. a P<.05, b P<.01, c P<.001 vs NT group. d P<.05, e P<.01, f P<.001 vs WCHT group, g P<.05, h P<.01, i P<.001 vs SHT group. Values of P<.05 were considered statistically significant.
The participants with MHT defined by 24‐hour ABP had a significantly higher serum glucose level than those with normotension and those with sustained hypertension (Table I). These differences remained significant (P=.001) after adjustment for age, gender, BMI, current smoking, and presence of hyperlipidemia. Even when presence of IFG or DM, office SBP, and office DBP were also statistically controlled, these differences remained significant (Figure 1). Additionally, the participants with MHT had a significantly higher UAR than those with normotension (Table I). These differences also remained significant after adjustment for the potential confounding factors of age, gender, BMI, current smoking, presence of hyperlipidemia, presence of IFG or DM, office SBP, and office DBP (Figure 2).
Figure 1.
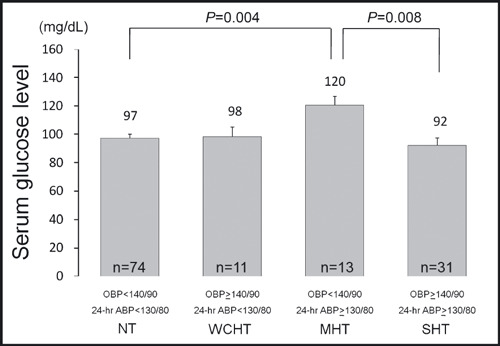
Serum glucose level in subjects with masked hypertension defined by 24‐hour average blood pressure (ABP). Data are shown as adjusted mean (±1 standard error). P values are based on an analysis of covariance that adjusts for age, gender, body mass index, current smoking, presence of hyperlipidemia, presence of impaired fasting glucose or diabetes, office systolic blood pressure, and office diastolic blood pressure. The Bonferroni procedure was used to test the differences in adjusted means among hypertension groups. Values of P<.05 were considered statistically significant. OBP and ABP values were measured in mm Hg MHT indicates masked hypertension; NT, normotension; OBP, office blood pressure; SHT, sustained hypertension; WCHT, white coat hypertension.
Figure 2.
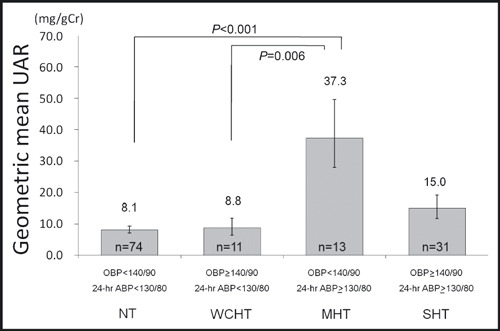
Urinary albumin per creatinine excretion ratio (UAR) in subjects with masked hypertension defined by 24‐hour average blood pressure (ABP). Data are shown as adjusted geometric mean (±1 standard error). P values are based on an analysis of covariance that adjusts for age, gender, body mass index, current smoking, presence of hyperlipidemia, presence of impaired fasting glucose or diabetes, office systolic blood pressure, and office diastolic blood pressure. The Bonferroni procedure was used to test the differences in adjusted geometric means among hypertension groups. Values of P<.05 were considered statistically significant. OBP and ABP values were measured in mm Hg. MHT indicates masked hypertension; NT, normotension; OBP, office blood pressure; SHT, sustained hypertension; WCHT, white coat hypertension.
The participants with sustained hypertension (SHT) defined by 24‐hour ABP had a significantly greater LVMI, RWT, and c‐IMT than those with normotension (Table I); the differences in LVMI and RWT remained after adjustment for potential confounding factors (mean ± standard error: LVMI, 137±9 g/m2 vs 107±5 g/m2, P=.048; RWT, 0.52±0.03 vs 0.38±0.02, P=.002) while the difference in c‐IMT was no longer statistically significant.
The participants with WCHT defined by 24‐hour ABP had a greater RWT than those with normotension (Table I), and the difference remained significant after adjustment for the confounding factors (0.50±0.03 vs 0.38±0.02, P=.030).
Masked Hypertension Defined by HBP
Among the participants in this study, 120 successfully performed HBP monitoring. The predictive values of HBP for diagnosing each type of hypertension identified by 24‐hour ABP are shown in Figure 3. Among the participants with MHT diagnosed by HBP, there were 30.0% of the participants with MHT diagnosed by 24‐hour ABP. Among the participants with WCHT diagnosed by HBP, there were 31.2% of the participants with WCHT diagnosed by 24‐hour ABP. The kappa statistic for the agreement of BP classification defined by HBP was 0.57 (P<.001) for that defined by 24‐hour ABP (0.63 [P<.001] for that defined by awake ABP). Seventy percent of the participants with MHT defined by HBP were classified as normotensive by 24‐hour ABP, and 68.8% of participants with WCHT defined by HBP were classified as sustained hypertensive by 24‐hour ABP. When both clinic BP and HBP were below the cut‐off levels, 89.9% of the participants had normotension defined by 24‐hour ABP. Similarly, when both clinic BP and HBP were above the cut‐off levels, 76.0% of the participants had sustained hypertension defined by 24‐hour ABP.
Figure 3.
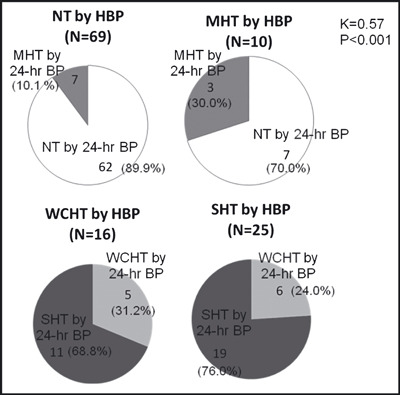
The relationships between hypertensive groups defined by home blood pressure (HBP) and those defined by 24‐hour average blood pressure (BP). Data are shown as the numbers (percentages) of subjects in the hypertensive groups defined by HBP, or the numbers (percentages) of subjects classified in the hypertensive groups by 24‐hour ambulatory BP. K indicates kappa statistic; MHT, masked hypertension; NT, normotension; SHT, sustained hypertension; WCHT, white coat hypertension.
The characteristics of participants with MHT and WCHT defined by HBP are shown in Table II. The participants with MHT defined by HBP did not have significant differences in serum glucose level, UAR, LVMI, RWT, or c‐IMT in comparison to those with normotension.
Table II.
Comparison of BP Subgroups Defined From Office BP and Home BP (N=120)
| Office BP Home BP | NT (n=69) <140/90 mm Hg <135/85 mm Hg | WCHT (n=16) ≥140/90 mm Hg <135/85 mm Hg | MHT (n=10) <140/90 mm Hg ≥135/85 mm Hg | SHT (n=25) ≥140/90 mm Hg ≥135/85 mm Hg | P Value |
|---|---|---|---|---|---|
| Age, y | 59.4±10.5 | 62.8±10.7 | 55.8±10.9 | 63.0±11.8 | .21 |
| Female, % | 58.0 | 43.8 | 70.0 | 40.0 | .25 |
| Body mass index, kg/m2 | 23.4±3.0 | 25.7±3.7a | 24.5±3.4 | 24.9±3.0 | .024 |
| Current smokers, % | 26.9 | 56.3 | 33.3 | 31.8 | .17 |
| Glucose, mg/dL | 97±24 | 95±10 | 104±38 | 99±16 | .79 |
| Hemoglobin A1c, % | 5.4±0.7 | 5.6±0.3 | 5.7±1.5 | 5.3±0.8 | .60 |
| IFG or diabetes, % | 14.5 | 18.8 | 20.0 | 12.0 | .91 |
| Total cholesterol, mg/dL | 192±35 | 183±27 | 189±33 | 208±35 | .09 |
| Hyperlipidemia, % | 33.3 | 37.5 | 50.0 | 52.0 | .36 |
| LVMI, g/m2 | 107±27 | 119±36 | 110±35g | 144±40c | <.001 |
| Relative wall thickness | 0.40±0.10 | 0.51±0.13b | 0.41±0.11 | 0.48±0.11a | .001 |
| Carotid IMT, mm | 0.6±0.1 | 0.7±0.2 | 0.7±0.3 | 0.8±0.2c | .001 |
| Geometric mean UAR, mg/gCr | 8.4 (3.8–18.4) | 9.3 (4.8–18.2) | 13.7 (6.2–30.2) | 20.2c,d (5.9–69.7) | <.001 |
| Creatinine, mg/dL | 0.89±0.14 | 0.88±0.15 | 0.78±0.15 | 0.88±0.14 | .17 |
| Urinary salt excretion, g/d | 9.8±6.1 | 11.4±4.7 | 8.7±4.1 | 9.7±3.9 | .63 |
| Office SBP, mm Hg | 121±11 | 145±9c | 129±6e,h | 157±15c,e | <.001 |
| Office DBP, mm Hg | 77±6 | 89±7c | 82±7h | 95±9c | <.001 |
| 24‐hour SBP, mm Hg | 114±10 | 129±10c | 124±14a,g | 136±14c | <.001 |
| 24‐hour DBP, mm Hg | 70±6 | 78±7c | 75±7g | 82±8c | <.001 |
| Awake SBP, mm Hg | 119±12 | 131±11b | 128±14g | 142±15c,d | <.001 |
| Awake DBP, mm Hg | 73±7 | 80±8b | 77±8g | 86±8c | <.001 |
| Sleep SBP, mm Hg | 102±9 | 122±12c | 116±19b | 121±12c | <.001 |
| Sleep DBP, mm Hg | 63±6 | 74±8c | 69±8 | 73±8c | <.001 |
| Home SBP, mm Hg | 113±11 | 116±15 | 129±8b,h | 155±23c,d | <.001 |
| Home DBP, mm Hg | 72±7 | 72±11 | 83±9b,e | 91±10c,f | <.001 |
Data are shown as mean ± standard deviation (SD) or percentage. Data of urinary albumin per creatinine excretion ratio (UAR) are shown as geometric mean (±1 SD range). Overall P values were calculated using analysis of variance. The differences in mean among the hypertension categories were calculated using Tukey’s honestly significant difference test. Abbreviations: BP, blood pressure; DBP, diastolic blood pressure; IFG, impaired fasting glucose; IMT, intima‐media thickness; LVMI, left ventricular mass index; MHT, masked hypertension; NT, normotension; SBP, systolic blood pressure; SHT, sustained hypertension; WCHT, white coat hypertension. a P<.05, b P<.01, c P<.001 vs NT group. d P<.05 e P<.01, f P<.001 vs WCHT group. g P<.05, h P<.001 vs SHT group. Probability .05 was considered statistically significant.
The participants with SHT defined by HBP had a greater LVMI, RWT, and c‐IMT than those with normotension (Table II). The differences in LVMI and RWT remained significant even after adjustment for age, gender, BMI, presence of hyperlipidemia, presence of IFG or DM, office SBP, and office DBP (LVMI, 147±9 vs 105±5 g/m2, P=.006; RWT, 0.52±0.03 vs 0.39±0.02, P=.013). The difference in c‐IMT was significant in the model adjusted for age, gender, BMI, presence of hyperlipidemia, and presence of IFG or DM (P=.001), but not when office SBP and office DBP were also statistically controlled. The participants with SHT defined by HBP had a greater UAR than those with normotension and those with WCHT (Table II). The differences in UAR remained significant in the model adjusted for age, gender, BMI, presence of hyperlipidemia, and presence of IFG or DM (P=.001 for normotension, P=.044 for WCHT), but not when office SBP and office DBP were also controlled.
The participants with WCHT defined by HBP had a greater RWT than those with normotension (Table II), and the difference remained significant after the adjustment (0.52±0.03 vs 0.38±0.02, P=.003).
Masked Hypertension Defined by HBP or 24‐Hour ABP
Among the participants who had an office BP of <140/90 mm Hg (n=89), there were 62 participants with normotension as assessed by both HBP and 24‐hour ABP (HBP <135/85 mm Hg and 24‐hour ABP <130/80 mm Hg), 7 participants with MHT defined by HBP alone (HBP ≥135/85 mm Hg and 24‐hour ABP <130/80 mm Hg), 7 participants with MHT defined by 24‐hour BP alone (24‐hour ABP ≥130/80 mm Hg and HBP <135/85 mm Hg), and 3 participants with MHT diagnosed by both HBP and 24‐hour ABP (Table III). The characteristics of the participants with normotension defined by both HBP and 24‐hour ABP, MHT defined by HBP alone, MHT defined by 24‐hour ABP alone, and MHT defined by both HBP and 24‐hour ABP are shown in Table III. The participants with MHT defined by both HBP and 24‐hour ABP had a higher UAR than those with normotension by both HBP and 24‐hour ABP and than those with MHT defined by HBP alone (Table III). These differences remained significant even after adjustment for age, gender, BMI, presence of hyperlipidemia, and presence of IFG or DM (Figure 4). Additionally, the participants with MHT by both HBP and 24‐hour ABP had a greater UAR than those with MHT defined by HBP alone (Table III). The difference in UAR was also significant after adjustment for covariates (Figure 4). The participants with MHT defined by 24‐hour ABP alone tended to have a higher UAR than those with MHT defined by HBP in the adjusted model (P=.07; Figure 4).
Table III.
Comparison of MHT Defined by Either of Home BP or 24‐Hour ABP (N=79)
| N HBP 24‐Hour ABP | NT by Both HBP and 24‐Hour ABP 62 <135/85 mm Hg <130/80 mm Hg | MHT by HBP Alone 7 ≥135/85 mm Hg <130/80 mm Hg | MHT by 24‐Hour ABP Alone 7 <135/85 mm Hg ≥130/80 mm Hg | MHT by Both HBP and 24‐Hour ABP 3 ≥135/85 mm Hg ≥130/80 mm Hg | P Value |
|---|---|---|---|---|---|
| Age, y | 59.5±10.8 | 58.1±12.0 | 58.6±7.4 | 50.3±6.0 | .53 |
| Female, % | 58.1 | 71.4 | 57.1 | 66.7 | .91 |
| Body mass index, kg/m2 | 23.3±2.8 | 24.2±2.9 | 24.4±4.4 | 25.3±4.9 | .51 |
| Current smokers, % | 26.7 | 28.6 | 28.6 | 50.0 | .91 |
| Glucose, mg/dL | 96±23 | 91±7 | 108±34 | 132±69 | .070 |
| Hemoglobin A1c, % | 5.4±0.8 | 5.4±0.3 | 5.5±0.6 | 6.5±2.8 | .21 |
| IFG or diabetes, % | 14.5 | 14.3 | 14.3 | 33.3 | .85 |
| Total cholesterol, mg/dL | 194±34 | 193±37 | 176±40 | 178±21 | .53 |
| Hyperlipidemia, % | 33.9 | 57.1 | 28.6 | 33.3 | .65 |
| Serum creatinine, mg/dL | 0.89±0.14 | 0.77±0.16 | 0.84±0.11 | 0.80±0.17 | .13 |
| LVMI, g/m2 | 107±27 | 115±36 | 108±24 | 98±36 | .82 |
| Relative wall thickness | 0.40±0.10 | 0.42±0.13 | 0.44±0.10 | 0.39±0.05 | .76 |
| Carotid IMT, mm | 0.6±0.1 | 0.7±0.2 | 0.7±0.1 | 0.9±0.3a | .056 |
| Geometric mean UAR, mg/gCr | 7.5 (4.4–12.9) | 9.2 (7.1–11.9) | 21.8b (4.0–119.0) | 35.0b,d (15.0–81.6) | <.001 |
| Urinary salt excretion, g/d | 9.5±5.7 | 10.2±3.6 | 12.5±9.3 | 5.0±3.2 | .32 |
| Office SBP, mm Hg | 121±11 | 130±6 | 127±7 | 128±8 | .06 |
| Office DBP, mm Hg | 76±6 | 82±7 | 82±4 | 81±10 | .011 |
| 24‐hour SBP, mm Hg | 112±8 | 116±4 | 133±6c,f | 143±6c,f | <.001 |
| 24‐hour DBP, mm Hg | 69±5 | 73±7 | 80±3c | 79±7b | <.001 |
| Awake SBP, mm Hg | 117±10 | 119±6 | 140±6c,f | 147±3c,f | <.001 |
| Awake DBP, mm Hg | 72±6 | 75±9 | 84±4c,d | 82±6a | <.001 |
| Sleep SBP, mm Hg | 101±8 | 110±9 | 117±11c | 131±29c,e | <.001 |
| Sleep DBP, mm Hg | 62±6 | 68±6a | 69±4b | 69±14 | <.001 |
| Home SBP, mm Hg | 112±11 | 130±7c | 120±13 | 128±12 | <.001 |
| Home DBP, mm Hg | 72±7 | 84±8c | 74±8 | 82±13 | <.001 |
Data are shown as mean ± standard deviation (SD) or percentage. Data of urinary albumin per creatinine excretion ratio (UAR) are shown as geometric mean (±1 SD range). Overall P values were calculated using analysis of variance. Intergroup differences were calculated using Tukey’s honestly significant difference test. Abbreviations: ABP, average blood pressure; BP, blood pressure; DBP, diastolic blood pressure; HBP, home blood pressure; IFG, impaired fasting glucose; IMT, intima‐media thickness; LVMI, left ventricular mass index; MHT, masked hypertension; NT, normotension; SBP, systolic blood pressure; WCHT, white coat hypertension. a P<.05, b P<.01, c P<.001 vs NT by both 24‐hour and home BP. d P<.05, e P<.01, f P<.001 vs MHT only by home BP.
Figure 4.
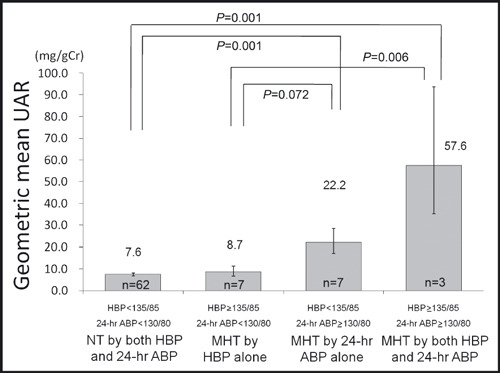
Urinary albumin per creatinine excretion ratio (UAR) in subjects with normotension (NT) or masked hypertension (MHT) defined by home blood pressure (HBP) and/or 24‐hour average blood pressure (ABP) (includes only those with office blood pressure <140/90 mm Hg). Data are shown as the adjusted geometric means (±1 standard error). P values are based on an analysis of covariance that adjusts for age, gender, body mass index, current smoking, presence of hyperlipidemia, and presence of impaired fasting glucose or diabetes. Intergroup differences in the adjusted geometric means were tested using Bonferroni test. Values of P<.05 were considered statistically significant. HBP and ABP values were measured in mm Hg.
Discussion
The main findings of this study are as follows: compared to normotensives, (1) MHT defined by ambulatory BP monitoring was associated with a higher serum glucose level and UAR; (2) participants with WCHT had increased LVMI and c‐IMT, but these differences were not statistically significant after controlling for confounding factors; (3) there were discrepancies in the diagnosis of MHT between ambulatory BP and HBP monitoring; and (4) MHT defined by HBP monitoring was not associated with increased serum glucose level or UAR.
MHT defined by ambulatory BP monitoring was associated with a high glucose level and UAR at a relatively younger age. Lurbe and coworkers 21 have reported that MHT in youth can be a precursor to the development of SHT, and was associated with obesity and increased LVMI. On the other hand, LVMI and c‐IMT in this general population were greater in participants with WCHT than in those with normotension. It has been reported that aging increases both ambulatory and clinic BP, but the rate of increase of office BP is greater than that of ambulatory BP. 22 Therefore, office BP is more likely to exceed ambulatory BP in elderly patients. 23 In the Pressione Arteriose Monitorate E Loro Associazioni (PAMELA) study, 4 LVMI in WCHT was increased similarly to that in MHT (the age of participants with WCHT was again older than that of the MHT group). In general populations, the differences among hypertensive groups defined by office BP and 24‐hour ABP (especially WCHT) in cardiovascular risk markers may be partially/substantially due to differences in age.
In the present study, MHT was associated with a high serum glucose level and UAR, even though the average age was lower in the MHT group than the other hypertensive groups. Glucose‐related sympathetic nerve overactivity 24 (increased daytime BP), and decreased sodium excretion capacity that is needed to compensate during nighttime (nocturnal hypertension) 25 have been proposed as possible mechanisms; however, there were no significant differences in urinary salt excretion or physical activity (measured by actigraphy) among the hypertensive groups in this study. Our data support previous reports that have demonstrated a relationship between MHT and increased glucose levels 3 or presence of diabetes, 8 although Kotsis and coworkers 26 reported in participants who were referred to hypertension centers that there were no significant differences in serum glucose level between participants with MHT and normotensives. However, in the Kotsis study, the study participants were all suspected of having hypertension, even those in the normotensive groups.
The finding of increased glucose level and UAR in those with MHT, compared to normotensives, persists when we use other measures of ambulatory BP (mean awake or mean sleep ABP) to define MHT (data not shown). On the other hand, MHT defined by HBP monitoring was not associated with increased glucose level or UAR. Although HBP monitoring is predictive of MHT defined by ambulatory BP, 15 it is usually measured in a sitting position at rest while ambulatory BP can be measured under a variety of conditions including during physical activity or while resting/sleeping in a supine position. Therefore, depending on exactly what it is one is trying to measure, HBP may not be an adequate substitute for 24‐hour ambulatory BP monitoring. We previously reported that nocturnal hypertension defined by ambulatory BP monitoring among participants with HBP <135/85 mm Hg was associated with increased left ventricular mass and c‐IMT. 27 However, MHT defined by HBP has also been reported to be associated with increased c‐IMT, 13 pulse wave velocity, 13 and risk of cardiovascular events 14 in clinic outpatients.
HBP monitoring with office BP measurements was useful for detecting normotensives and sustained hypertensives defined by 24‐hour ABP monitoring. However, the ability of HBP monitoring to identify those with MHT or WCHT defined by 24‐hour ABP was poor, and we may need to perform ambulatory BP monitoring for participants with such borderline BP levels.
Study Limitations
There were limitations in this study as follows: (1) This was a cross‐sectional study, and therefore cannot demonstrate that the relationships of increased glucose level and UAR with MHT are causal. (2) We were only able to enroll 33% of the residents in the study population, and the analysis of participants performing HBP analysis was based on 120 of the 181 study participants, leaving open the possibility of selection bias. (3) There was a relatively high prevalence of smoking in this population (33.1%), consistent with national rates in Japan at the time (52.8% for men and 13.4% for women) (http://www1.mhlw.go.jp/houdou/1111/h1111‐2_11.html#no1), in comparison with that in the United States of about 20% of adults 28 ; thus we are not sure whether this population can be generalized to others (eg, US population). (4) Finally, the comparison of masked hypertension defined by HBP vs 24‐hour BP was limited by very low numbers in each subgroup, and further research needs to be done in a larger population to confirm these relationships.
Conclusions
Masked hypertension defined by ambulatory BP monitoring was associated with increased glucose level and urinary albumin‐creatinine excretion ratio. Masked hypertension defined by HBP monitoring may not have the same ability to predict cardiovascular risk factors and hypertensive target organ damage as masked hypertension defined by ambulatory BP. Further research needs to be done to investigate the utility of home monitoring for diagnosing masked hypertension.
Disclosure: Dr Ishikawa received support from the Mitsubishi Tanabe Pharma Research Foundation.
Acknowledgments
Acknowledgment: Source of funding: This study was supported by a grant from the Division of Cardiovascular Medicine, Jichi Medical University, School of Medicine.
All authors declare that they participated in this study, and that they saw and approved the final version. They also declare that they have no conflict of interest in connection with this paper.
References
- 1. Pickering TG, Davidson K, Gerin W, et al. Masked hypertension. Hypertension. 2002;40:795–796. [DOI] [PubMed] [Google Scholar]
- 2. Pickering TG, Eguchi K, Kario K. Masked hypertension: a review. Hypertens Res. 2007;30:479–488. [DOI] [PubMed] [Google Scholar]
- 3. Liu JE, Roman MJ, Pini R, et al. Cardiac and arterial target organ damage in adults with elevated ambulatory and normal office blood pressure. Ann Intern Med. 1999;131:564–572. [DOI] [PubMed] [Google Scholar]
- 4. Sega R, Trocino G, Lanzarotti A, et al. Alterations of cardiac structure in patients with isolated office, ambulatory, or home hypertension: data from the general population (Pressione Arteriose Monitorate E Loro Associazioni [PAMELA] study). Circulation. 2001;104:1385–1392. [DOI] [PubMed] [Google Scholar]
- 5. Ohkubo T, Kikuya M, Metoki H, et al. Prognosis of “masked” hypertension and “white‐coat” hypertension detected by 24‐h ambulatory blood pressure monitoring: 10‐year follow‐up from the Ohasama study. J Am Coll Cardiol. 2005;46:508–515. [DOI] [PubMed] [Google Scholar]
- 6. Bjorklund K, Lind L, Zethelius B, et al. Isolated ambulatory hypertension predicts cardiovascular morbidity in elderly men. Circulation. 2003;107:1297–1302. [DOI] [PubMed] [Google Scholar]
- 7. Eguchi K, Ishikawa J, Hoshide S, et al. Masked hypertension in diabetes mellitus: a potential risk. J Clin Hypertens (Greenwich). 2007;9:601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ben‐Dov IZ, Ben‐Ishay D, Mekler J, et al. Increased prevalence of masked blood pressure elevations in treated diabetic subjects. Arch Intern Med. 2007;167:2139–2142. [DOI] [PubMed] [Google Scholar]
- 9. Leitao CB, Canani LH, Kramer CK, et al. Masked hypertension, urinary albumin excretion rate, and echocardiographic parameters in putatively normotensive type 2 diabetic patients. Diabetes Care. 2007;30:1255–1260. [DOI] [PubMed] [Google Scholar]
- 10. Grassi G, Seravalle G, Trevano FQ, et al. Neurogenic abnormalities in masked hypertension. Hypertension. 2007;50:537–542. [DOI] [PubMed] [Google Scholar]
- 11. Ohkubo T, Imai Y, Tsuji I, et al. Home blood pressure measurement has a stronger predictive power for mortality than does screening blood pressure measurement: a population‐based observation in Ohasama, Japan. J Hypertens. 1998;16:971–975. [DOI] [PubMed] [Google Scholar]
- 12. Sega R, Facchetti R, Bombelli M, et al. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow‐up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111:1777–1783. [DOI] [PubMed] [Google Scholar]
- 13. Matsui Y, Eguchi K, Ishikawa J, et al. Subclinical arterial damage in untreated masked hypertensive subjects detected by home blood pressure measurement. Am J Hypertens. 2007;20:385–391. [DOI] [PubMed] [Google Scholar]
- 14. Bobrie G, Chatellier G, Genes N, et al. Cardiovascular prognosis of “masked hypertension” detected by blood pressure self‐measurement in elderly treated hypertensive patients. JAMA. 2004;291:1342–1349. [DOI] [PubMed] [Google Scholar]
- 15. Stergiou GS, Salgami EV, Tzamouranis DG, et al. Masked hypertension assessed by ambulatory blood pressure versus home blood pressure monitoring: is it the same phenomenon? Am J Hypertens. 2005;18:772–778. [DOI] [PubMed] [Google Scholar]
- 16. Hoshide S, Kario K, Hoshide Y, et al. Associations between nondipping of nocturnal blood pressure decrease and cardiovascular target organ damage in strictly selected community‐dwelling normotensives. Am J Hypertens. 2003;16:434–438. [DOI] [PubMed] [Google Scholar]
- 17. Longo D, Bertolo O, Toffanin G, et al. Validation of the A&D UA‐631 (UA‐779 Life Source) device for self‐measurement of blood pressure and relationship between its performance and large artery compliance. Blood Press Monit. 2002;7:243–248. [DOI] [PubMed] [Google Scholar]
- 18. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716. [DOI] [PubMed] [Google Scholar]
- 19. Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–618. [DOI] [PubMed] [Google Scholar]
- 20. Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 21. Lurbe E, Torro I, Alvarez V, et al. Prevalence, persistence, and clinical significance of masked hypertension in youth. Hypertension. 2005;45:493–498. [DOI] [PubMed] [Google Scholar]
- 22. Pickering TG. The natural history of hypertension: prehypertension or masked hypertension? J Clin Hypertens (Greenwich). 2007;9:807–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Minutolo R, Borrelli S, Chiodini P, et al. Effects of age on hypertensive status in patients with chronic kidney disease. J Hypertens. 2007;25:2325–2333. [DOI] [PubMed] [Google Scholar]
- 24. Huggett RJ, Scott EM, Gilbey SG, et al. Impact of type 2 diabetes mellitus on sympathetic neural mechanisms in hypertension. Circulation. 2003;108:3097–3101. [DOI] [PubMed] [Google Scholar]
- 25. Sachdeva A, Weder AB. Nocturnal sodium excretion, blood pressure dipping, and sodium sensitivity. Hypertension. 2006;48:527–533. [DOI] [PubMed] [Google Scholar]
- 26. Kotsis V, Stabouli S, Toumanidis S, et al. Target organ damage in “white coat hypertension” and “masked hypertension”. Am J Hypertens. 2008;21:393–399. [DOI] [PubMed] [Google Scholar]
- 27. Hoshide S, Ishikawa J, Eguchi K, et al. Masked nocturnal hypertension and target organ damage in hypertensives with well‐controlled self‐measured home blood pressure. Hypertens Res. 2007;30:143–149. [DOI] [PubMed] [Google Scholar]
- 28. Centers for Disease Control and Prevention (CDC) . State‐specific prevalence and trends in adult cigarette smoking‐‐United States, 1998–2007. MMWR Morb Mortal Wkly Rep. 2009;58:221–226. [PubMed] [Google Scholar]


