Abstract
Purpose
A phase I study was conducted to determine the maximum-tolerated dose (MTD), dose-limiting toxicities (DLT), and pharmacokinetics of fenretinide delivered as an intravenous emulsion in relapsed/refractory hematologic malignancies.
Experimental Design
Fenretinide (80 – 1810 mg/m2/day) was administered by continuous infusion on Days 1 – 5, in 21-day cycles, using an accelerated titration design.
Results
Twenty-nine patients, treated with a median of three prior regimens (range, 1 to 7), were enrolled and received test drug. 97 courses were completed. An MTD was reached at 1280 mg/m2/day x 5 days. Course 1 DLTs included six patients with hypertriglyceridemia, four of which were asymptomatic; two patients experienced DLT thrombocytopenia (asymptomatic). Of eleven response-evaluable peripheral T-cell lymphomas, two had complete responses (CR, PFS 68+ months; unconfirmed CR, PFS 14+ months), two had unconfirmed partial responses (unconfirmed PR, PFS 5 months; unconfirmed PR, PFS 6 months), and five had stable disease (2 – 12 cycles). One mature B-cell lymphoma had an unconfirmed PR sustained for two cycles. Steady-state plasma levels were ~10 mcg/mL (mid-20’s μmol/L) at 640 mg/m2/day; ~14 mcg/mL (mid-30’s μmol/L) at 905 mg/m2/day; and ~22 mcg/mL (mid-50’s μmol/L) at 1280 mg/m2/day.
Conclusions
Intravenous fenretinide obtained significantly higher plasma levels than a previous capsule formulation, had acceptable toxicities, and evidenced anti-tumor activity in peripheral T-cell lymphomas. A recommended phase II dosing is 600 mg/m2 on Day 1, followed by 1200 mg/m2 on Days 2 – 5, every 21 days. A registration-enabling phase II study in relapsed/refractory PTCL (ClinicalTrials.gov identifier: NCT02495415) is ongoing.
Keywords: fenretinide, intravenous, lymphoma, phase I trial, pharmacokinetics
Introduction
The activity of synthetic retinoid, N-(4-hydroxyphenyl)retinamide (fenretinide, 4-HPR), has been widely studies in cell lines of multiple cancer types in vitro. Fenretinide is reported to induce cytotoxicity by multiple mechanisms, including p53- and caspase-independent apoptosis and/or non-apoptotic mechanisms independent of classic retinoid receptors (1, 2); reactive oxygen species (ROS) contributed to cytotoxicity in some leukemia and solid cancer cell lines (3); levels of potentially cytotoxic dihydroceramides (4) were increased in a dose- and time-dependent manner through concurrent stimulation of de novo synthesis and inhibition of dihydroceramide desaturase 1 (5–8). Significantly, fenretinide was cytotoxic to lymphoblastic leukemia cell lines in vitro (4, 9), but minimally toxic to fibroblasts, peripheral blood mononuclear cells, and marrow myeloid progenitors (6), suggesting a potential for use in hematologic malignancies. However, fenretinide is sparingly soluble in water, challenging clinical testing.
Previously, a capsule formulation was tested at 200 – 900 mg/day in multiple cancer types, obtaining 1 – 3 μmol/L plasma levels with minimal toxicity but limited evidence of activity (10, 11). Phase I and II high-dose capsule trials (500 – 4800 mg/m2/day) demonstrated minimal toxicities and some suggestions of activity, but still obtained plasma levels of only 7.5 – 12.5 μmol/L (12, 13).
We hypothesized that intravenous delivery would obtain higher plasma drug levels, increase tumor drug exposures, and possibly increase responses. The goals of the present study were to determine the maximum tolerated dose (MTD) of an oil-in-water fenretinide emulsion administered as a continuous intravenous infusion (c.i.v.) for five days, once every three weeks, describe toxicities, evaluate pharmacokinetics and, within the confines of a phase I trial, determine preliminary estimates of hematologic disease response.
Materials and Methods
Drug Sources and Formulation
N-(4-hydroxyphenyl)retinamide (fenretinide, 4-HPR; NSC 374551) formulated as a 20% soy oil-in-water emulsion was provided by the Rapid Access to Intervention Development (RAID) Program, Developmental Therapeutics Program (DTP), National Cancer Institute (NCI). Fenretinide, N-(4-methoxyphenyl)retinamide (4-MPR), and N-(4-ethoxyphenyl)retinamide (4-EPR) were obtained from the NCI/DTP Open Chemicals Repository, Bethesda, MD.
Patients
Patients were ≥ 18 years of age with documented, previously-treated leukemias, lymphomas, or multiple myeloma, with measurable or evaluable disease, excluding pre-existing central nervous system (CNS) disease, for whom standard therapies did not exist or were not effective, and who had absolute granulocytes ≥ 1500/μL, platelets ≥ 75,000/μL, creatinine 1.5 x upper limit of normal (ULN), bilirubin ≤ 1.5 x ULN, serum transaminases ≤ 2.5 x ULN, Eastern Cooperative Group (ECOG) performance status ≤ 2, and estimated survival of ≥ 3 months. The study, registered as ClinicalTrials.gov Identifier: NCT00104923, was approved by local investigational review boards in accordance with an assurance filed with and approved by the U.S. Department of Health and Human Services and the precepts of the Helsinki Declaration. ICH-GCP guidelines were followed. Written informed consents were obtained from each patient. Patient diagnoses were captured by assigned NCI Cancer Therapy Evaluation Program Clinical Data Update System (CDUS) codes supplemented by MedDRA code version 9.0 (Patients 1–10) or version 10.0 (Patients 11 – 29); for Patients #1 – 22, the diagnoses and responses of responding patients were confirmed by chart and scan review by an independent reviewer.
Clinical trial design and treatment
Fenretinide was given as a continuous intravenous (c.i.v.) infusion for five consecutive days (D1 through D5) on 21 day cycles. The starting dose was 80 mg/m2/day and escalation proceeded through a modified accelerated titration design (14) (Supplemental Table 1). Escalation was based on assessment of dose limiting toxicity (DLT) in the first cycle as scored according to NCI Common Terminology Criteria for Adverse Events (CTCAE) version 3.0, if toxicity was possibly, probably, or definitely attributed to fenretinide. For DLT assessment, patients had to receive ≥ 90% of the planned first course dose and be observed for ≥ 21 days from Day 1, or experience toxicity that met the definition of DLT at any time. Patients not evaluable for DLT assessment were replaced. Initially, one patient was treated per level and dosing advanced in every-other level increments; when two patients experienced ≥ grade 2, or one patient experienced a DLT, standard 3+3 rules applied. The MTD was the highest level with at least six patients treated at which < 33% of patients experienced DLT. Treatment was held for grade 3 or 4 toxicity until resolved to grade ≤ 1. After non-DLT toxicity resolution, treatment was restarted without dose reduction. For DLT, treatment was held until resolution and resumed at a one dose level reduction. Patients were removed from treatment if a scheduled cycle was delayed > 3 weeks due to toxicity or for recurrence of a same DLT. Patients with stable disease or better response were treated for six or more cycles or until evidence of disease progression. Once the original MTD was determined, the protocol was amended to exclude ‘minimally-symptomatic intralipid intolerance’ (defined below) as a DLT for dose escalation purposes, and escalation resumed using standard 3+3 rules to “backfill” levels to determine an MTD in ‘lipid tolerant’ patients. As an overly frequent occurrence of ‘intralipid intolerance’ could have impacted the practicality of treatment, the following stopping rule concerning ‘minimally-symptomatic intralipid intolerance’ was adopted but never invoked: for each dose level, 3 of the first 5 patients, or 4 of the first 8 patients, or 5 of the first 10 patients, or 6 of the first 12 patients. If the true probability of minimally-symptomatic intralipid intolerance was about 0.30, then there was a 34% chance that the flag would be exceeded; if the true probability was 0.50, then there was an 83% chance that the flag would be exceeded.
Toxicity management and definition of dose limiting toxicity
DLT was defined as (a) grade 4 thrombocytopenia, (b) grade 4 neutropenia lasting more than 7 days, or febrile neutropenia, (c) any toxicity resulting in treatment delays of > 3 weeks, or (d) any ≥ grade 3 non-hematological toxicity excluding: nyctalopia; ≥ grade 3 headache with history of migraines or grade 3 headache not due to pseudotumor cerebri treatable by medical management; grade 3 nausea, vomiting, or diarrhea controllable with medical management; grade 3 ALT or AST increase, or grade 3 or 4 alkaline phosphatase increase, that recovered to ≤ grade 2, or baseline, by Day 21; grade 3 fever or infection with ≤ grade 2 neutropenia; grade 4 fever or infection associated with a central venous catheter or other known cause; CNS toxicity attributable to disease that developed after enrollment. All patients who received any drug are included in toxicity summaries.
Definition of minimally-symptomatic intralipid intolerance
Due to decreased plasma clearance of the soy oil vehicle in some patients (i.e., hypertriglyceridemia), but in order to not limit dosing in the majority of patients who did not experience hypertriglyceridemia, ‘minimally-symptomatic intralipid intolerance’ was defined as asymptomatic grade 3 or 4 hypertriglyceridemia that returned to baseline within 96 hours after stopping infusion, with ≤ grade 1 lipase elevation that returned to baseline within 72 hours, and absent signs/symptoms of pancreatitis. Triglycerides were monitored every 12 hours during the first course. Patients with hypertriglyceridemia had their infusion stopped and were re-treated at a 25% dose reduction if resolved within 48 hours during the same course, or at a 50% dose reduction in the subsequent course; triglycerides were monitored for one further course after a dose reduction.
Evaluation of Response
Response was evaluated every two cycles. Complete response (CR) was defined as the disappearance of target and non-target lesions, no new lesions, and normalization of marrow, lasting > 4 weeks; Complete response, unconfirmed (CRu) was defined as a response otherwise qualifying as a CR but lacking marrow response testing; Partial response (PR) was defined by disease specific criteria and no unequivocal progression of non-target lesions, lasting > 4 weeks; Partial response, unconfirmed (PRu) was defined as a response otherwise qualifying as a PR but lacking marrow response testing (15–17). Progressive disease (PD) was defined as ≥ 25% increase in a perpendicular dimension of target lesions, new lesions, > 25% increase of leukemia in blood or marrow, or 25% increase in myeloma paraprotein within 8 weeks of entry. Stable disease (SD) was any condition not meeting other criteria. Patients completing one course were evaluable for disease response. Most responses were reviewed by an independent reviewer.
Pharmacokinetics
Blood samples were drawn pre-infusion, at hours +6, +12, +18, +24, +36, +48, +72, +96 and +120 during infusion, and at +2 and +48 hours after the end of infusion. Plasma levels of fenretinide, and inactive major metabolite, N-(4-methoxyphenyl)retinamide (4-MPR), were assayed by a validated high-performance liquid chromatography methodology (18).
Statistical analysis
Standard descriptive statistics were used to summarize results. Progression-free survival (PFS) was calculated from start of treatment to documentation of disease progression or death, if death occurred prior to progression, survival was calculated as time from start of treatment until death; alive patients were censored at the date of last contact. Kaplan-Meier plots summarized progression-free and overall survival (OS); standard errors were estimated using Greenwood’s formula; p-values were based on the log-rank test. Analysis of covariance was used to evaluate AUC (based on natural logarithm of plasma levels) with dose and lipid intolerance.
Results
Participant and disease characteristics, dose escalation and DLT’s
Twenty-nine patients (median 59 years, range: 23–79) were enrolled and received drug, most had ECOG status of 0 or 1 (Table 1, Supplemental Table 2). 101 courses were started of which 97 were completed, with a median of two courses per patient (range: <1 to 25); eleven patients completed 2 or 3 courses, seven completed ≥ four courses (Supplemental Table 2). During accelerated escalation, one patient each was treated at Dose Levels 1, 3, 5, 7, and 9, without DLT; only two experienced grade 1 toxicity at least possibly attributed to fenretinide (Supplemental Table 3). At Dose Level 10 (1810 mg/m2/day), two of three patients experienced DLT; therefore Dose Level 9 (1280 mg/m2/day) was expanded. Two of the next four patients experienced DLT, so Dose Level 8 (905 mg/m2/day) was expanded. Two of the next five patients experienced DLT, so Dose Level 7 (640 mg/m2) was expanded; five patients tolerated 640 mg/m2/day without DLT which was tentatively labeled the MTD (Supplemental Table 3). However, review revealed that four of six DLTs were ‘minimally-symptomatic intralipid intolerance’ that resolved once the infusion was stopped; two such patients went on to receive additional courses. To not limit dosing for the majority of patients who were lipid tolerant, the protocol was amended to consider ‘lipid-intolerant’ patients as a separate cohort; dose levels were re-opened, backfilled, and an MTD for ‘lipid tolerant’ patients was established at 1280 mg/m2/day (Supplemental Table 3). In an expansion cohort, a ‘ramped’ dosing schedule was tested (Day 1 dosing of 600 mg/m2 and Days 2 – 5 dosing of 1200 mg/m2/day), to determine if allowing for a reactive increase in serum lipase capacity during the first 24 hours could reduce intralipid intolerance. Of the four patients accrued prior to the exhaustion of drug supply, none experienced hypertriglyceridemia.
Table 1.
Patient Characteristics
| Characteristic | Patients (N = 29) No. (%) |
|---|---|
| Age, years | |
| Median | 59 |
| Range | 23–80 |
| Gender | |
| Male | 20 (69%) |
| Female | 9 (31%) |
| ECOG Performance Status | |
| 0 | 4 (14%) |
| 1 | 23 (79%) |
| 2 | 2 (7%) |
| Diagnosis | |
| Acute Leukemia | 3 (10%) |
| Mature B-Cell Lymphoma | 11 (38%) |
| T-Cell Lymphoma | 14 (48%) |
| Malignant Myeloma | 1 (3%) |
| Prior treatment | |
| Chemotherapy | 29 (100%) |
| Radiation Therapy | 8 (28%) |
| Marrow Transplant | 6 (21%) |
| No. prior chemotherapy regimens | |
| Median | 3 |
| 1 | 2 (7%) |
| 2 | 8 (28%) |
| 3–4 | 11 (38%) |
| 5–7 | 8 (28%) |
Adverse Events
Intravenous fenretinide was generally tolerated with minimal-modest toxicities and adverse events (AEs). Across all courses, grade 3 or 4 hematologic toxicity (Table 2) was seen in twenty patients without clear dose-response relationships, likely reflecting varied prior therapies. Thirteen patients experienced hematologic grade 3 or 4 toxicities at ≥ 905 mg/m2/day: grade 3 or 4 asymptomatic thrombocytopenia was seen in eight patients, none of whom required platelet transfusion, all of whom recovered to baseline by Day 28, and thrombocytopenia did not necessarily recur in subsequent cycles in the same patient; grade 3 or 4 neutropenia was seen in five patients who recovered to baseline without complications of infection and which did not necessarily recur in subsequent cycles; grade 3 anemia was seen in three patients. Other toxicities included metabolic abnormalities likely related to the load of the soy oil vehicle. These included nine patients with grade 3 and 4 hypertriglyceridemia - only one of which was symptomatic (elevated lipase and rapidly resolved grade 2 pancreatitis) which occurred at 1810 mg/m2/day prior to the institution of triglyceride monitoring.
Table 2.
Grade 3 and 4 AEs in three or more patients at least possibly related to study drug, all cycles
| AE | 80–640 mg/m2/day (n=8) | 905 mg/m2/day (n=6) | 1280 mg/m2/day (n=8) | 1810 mg/m2/day (n=3) | Expanded Cohort (n=4) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Grade 3 | Grade 4 | Grade 3 | Grade 4 | Grade 3 | Grade 4 | Grade 3 | Grade 4 | Grade 3 | Grade 4 | |
| Hypertriglyceridemia | 1 | 0 | 0 | 4 | 0 | 2 | 0 | 2 | 0 | 0 |
| Non-Hematologic, any | 2 | 0 | 1 | 4 | 3 | 4 | 0 | 3 | 1 | 3 |
| Hemoglobin reduced | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 |
| Leukocytes reduced | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Lymphocytes reduced | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 |
| Neutrophils reduced | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 |
| Platelets reduced | 0 | 0 | 0 | 0 | 3 | 1 | 0 | 1 | 0 | 3 |
| Hematologic, any | 0 | 0 | 1 | 1 | 3 | 3 | 0 | 1 | 1 | 3 |
Pharmacokinetic analysis
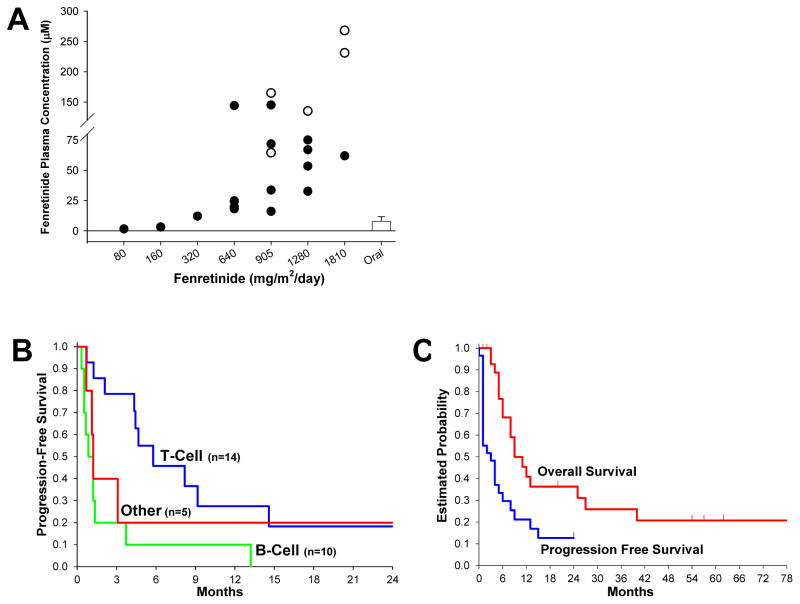
End-of-infusion plasma samples were available for twenty patients (Figure 1A). Analysis showed a dose-to-plasma level relationship with mean steady-state drug levels of ~10 mcg/mL (mid-20’s μmol/L) at 640 mg/m2/day; ~14 mcg/mL (mid-30’s μmol/L) at 905 mg/m2/day; and ~22 mcg/mL (mid-50’s μmol/L) at 1280 mg/m2/day. The AUC’s for fenretinide and its 4-MPR metabolite over the first 48 hours of the infusion were available for twenty-two patients (Supplemental Table 4); the presence of drug emulsion was visually noted in the PK blood samples from some lipid-intolerant patients.
Figure 1.
(A) End-infusion fenretinide plasma levels for dosing cohorts (+120 hours). Closed circles represent patients completing infusion course. Open circles represent last plasma sample of patients with infusions stopped due to hypertriglyceridemia. Bar represents reported achievable plasma levels using oral capsule dosed on seven-day schedule. (B) Progression Free Survival (PFS) of evaluable patients by tumor type; peripheral T-Cell (n=14), median 5.7 months (95% CI, 2.1 to 14.6); mature B-Cell lymphoma or leukemia (n=10), median 0.8 months (95% CI, 0.5 to 1.3) months; Other hematologic malignancy (n=5), median 1.2 months (95% CI, 0.6 to not available); difference in PFS between T-cell and B-cell, P < 0.011 (log-rank test). (C) Overall Survival (OS), median (95% CI): 9.3 (5.5, 25.4) months, and Progression Free Survival (PFS), median (95% CI): 3.0 (1.2, 5.7) months, for all patients (n = 29) over all dose levels.
Antitumor activity
A variety of hematologic cancer diagnoses were enrolled (Supplemental Table 2). Five of twenty-six response-evaluable patients demonstrated objective responses and seven had stable disease for 2 to 12 courses. Nine of eleven response-evaluable peripheral T-cell lymphomas had object response or stable disease (Table 3 and Supplemental Table 2); one of eleven B-cell malignancies achieved an unconfirmed PR sustained for two cycles at 320 mg/m2/day; one hairy cell leukemia and one B-cell lymphoma treated at 1280 mg/m2/day had stable disease for two and three cycles, respectively.
Table 3.
Summary of antitumor responses
| Best response | T-Cell NHL n, (%)a |
B-Cell NHL n, (%) |
leukemia / myeloma/other n, (%)a |
|---|---|---|---|
| CR/CRu | 2 (17%) | 0 | 0 |
| PRu | 2 (17%) | 1 (9%) | 0 |
| SD | 5 (42%) | 2 (18%) | 0 |
| PD | 2 (18%) | 8 (73%) | 4 (100%) |
| Inevaluableb | 2 | 0 | 1 |
Of evaluable patients
T-Cell: one patient withdrew after Course 1, one patient lost to follow-up; one ALL leukemia patient proceeded to marrow transplant after three courses.
Of response-evaluable peripheral T-cell lymphoma patients, four patients dosed at ≥ 905 mg/m2/day had objective responses and five had stable disease for 2 to 12 courses (Supplemental Table 2). Of the four responders, two were complete responders; one, with systemic cutaneous T-cell lymphoma (Sézary syndrome), treated at 1280 mg/m2/day (initial dosing) for 25 courses, achieved complete remission confirmed by CT PET and skin biopsy with a progression-free survival (PFS) of 68+ months; an angioimmunoblastic T-cell lymphoma patient treated at 905 mg/m2/day was an unconfirmed complete response (CRu) after four courses (marrow examination declined) and withdrew from study therapy - follow-up indicated PFS of ≥ 14 months and patient was confirmed alive at +118 months. One patient each with cutaneous and angioimmunoblastic lymphoma had unconfirmed partial responses (PRu), each receiving six cycles, with PFS of 5 and 6 months, respectively. These patients demonstrated ‘best response’ aggregate reductions in lymph node size of 94% and 78%, respectively; both had been previously treated with combination chemotherapy regimens and agents such as denileukin, anti-IL-1antibodies, depsipeptide, and bexaratone. Among the peripheral T-cells patients, one CR, one PRu, and one SD patient had failed previous HDACi therapy. PFS by disease type, and PFS and OS for all patients, are shown in Figure 1B and C.
Discussion
Fenretinide emulsion obtained plasma drug levels that were 5 to 7x higher than a previous capsule formulation (13) and demonstrated modest toxicities, including in patients of advanced age. Reversible asymptomatic hypertriglyceridemia related to the soy oil vehicle accounted for four of eight DLTs across all levels. Hypertriglyceridemia generally manifested in the first 48 hours of the first course, was detected by monitoring of non-fasting serum triglycerides, and was manageable in most patients by dose reduction. No specific pattern of predisposing factors was discernable from the limited number of such patients. Within the limitations of a phase I study, fenretinide emulsion demonstrated activity in eleven evaluable peripheral T-cell lymphomas from 905 – 1810 mg/m2/day, with a CR+PR+SD response rate of 82%, which is notable in the highly-pretreated patients enrolled. Only one of ten NHL B-cell malignancies responded but half of such patients were treated at less than the MTD level, or at non-tolerated dose levels, which limited interpretation. Too few patients were enrolled in other disease categories for assessment.
A recommended phase II schedule of 600 mg/m2/day on Day 1 and 1200 mg/m2/day on Days 2 – 5, c.i.v, every 21 days, is suggested to allow potentially lipid-intolerant patients to ‘metabolically induce’ serum lipase capacity for 24 hours prior to advancing to potentially therapeutic dosing. Pharmacokinetic analysis showed a dose-to-plasma level relationship with an expected mean steady-state fenretinide level in the 50’s μmol/L range at suggested phase II dosing. The data demonstrate that intravenous delivery solved the previous problem of limited fenretinide plasma levels; this may have contributed to the encouraging responses observed. A registration-enabling, phase II study of fenretinide emulsion in relapsed/refractory peripheral T-cell lymphomas using the recommended dosing schedule above is ongoing (ClinicalTrials.gov identifier: NCT02495415).
Future studies in hematologic cancers may confirm the association between systemic fenretinide exposure and clinical outcome and/or compare survival and quality of life endpoints. The modest toxicities of the present formulation also suggest that trials testing fenretinide emulsion in combination with other agents could be explored, such as the current phase I trial testing fenretinide emulsion in combination with the ceramide-modulating agent, safingol (ClinicalTrials.gov identifier: NCT01553071).
Supplementary Material
Translational Relevance.
Fenretinide is a cytotoxic retinoid possessed of potentially novel mechanisms of antitumor action, including increase of tumor cell dihydroceramide levels, but sparingly soluble in water has limited its clinical advancement. To overcome this limitation, we conducted a phase I trial of a novel oil-in-water intravenous emulsion formulation in hematologic malignancies. The emulsion was well-tolerated and obtained multi-fold higher plasma levels than a previously reported capsule formulation. Promising sustained complete and partial responses were observed in heavily pretreated patients with T-cell lymphomas.
Acknowledgments
Financial Support: This study was supported in part by NIH grants: U01 CA062505 and UM1 CA186717 (City of Hope, Duarte, CA); P30 CA014089, (USC/Norris Comprehensive Cancer Center, Los Angeles, CA); NCI DTP Rapid Access to Intervention Development (RAID) Grant, Cycle 2, and Cancer Research and Prevention Institute of Texas (CPRIT) Grant RP10072 (C. Patrick Reynolds).
The authors thank all of the patients and families that enrolled in this trial, Stella Khoo and Diana Calcanas-Perez of the Data Coordinating Center at City of Hope, the research staff at the Developmental Therapeutics Clinic Center for Cancer Research, NCI, NIH, and all participating treating physicians, with special thanks to Drs. Shanker Gupta and B. Rao Vishnuvajjala of the Developmental Therapeutics Program, NCI, NIH.
References
- 1.Clifford JL, Menter DG, Wang M, Lotan R, Lippman SM. Retinoid receptor-dependent and -independent effects of N-(4-hydroxyphenyl)retinamide in F9 embryonal carcinoma cells. Cancer Res. 1999;59:14–8. [PubMed] [Google Scholar]
- 2.Ponzoni M, Bocca P, Chiesa V, Decensi A, Pistoia V, Raffaghello L, et al. Differential effects of N-(4-hydroxyphenyl)retinamide and retinoic acid on neuroblastoma cells: apoptosis versus differentiation. Cancer Res. 1995;55:853–61. [PubMed] [Google Scholar]
- 3.Oridate N, Suzuki S, Higuchi M, Mitchell MF, Hong WK, Lotan R. Involvement of reactive oxygen species in N-(4-hydroxyphenyl)retinamide-induced apoptosis in cervical carcinoma cells. J Natl Cancer Inst. 1997;89:1191–8. doi: 10.1093/jnci/89.16.1191. [DOI] [PubMed] [Google Scholar]
- 4.Holliday MW, Cox SB, Kang MH, Maurer BJ. C22:0- and C24:0-dihydroceramides Confer Mixed Cytotoxicity in T-Cell Acute Lymphoblastic Leukemia Cell Lines. PLOS One. 2013;8:e74768. doi: 10.1371/journal.pone.0074768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maurer BJ, Metelitsa LS, Seeger RC, Cabot MC, Reynolds CP. Increase of ceramide and induction of mixed apoptosis/necrosis by N-(4-hydroxyphenyl)-retinamide in neuroblastoma cell lines. J Natl Cancer Inst. 1999;91:1138–46. doi: 10.1093/jnci/91.13.1138. [DOI] [PubMed] [Google Scholar]
- 6.Maurer BJ, Melton L, Billups C, Cabot MC, Reynolds CP. Synergistic cytotoxicity in solid tumor cell lines between N-(4-hydroxyphenyl)retinamide and modulators of ceramide metabolism. J Natl Cancer Inst. 2000;92:1897–909. doi: 10.1093/jnci/92.23.1897. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Maurer BJ, Reynolds CP, Cabot MC. N-(4-hydroxyphenyl)retinamide elevates ceramide in neuroblastoma cell lines by coordinate activation of serine palmitoyltransferase and ceramide synthase. Cancer Res. 2001;61:5102–5. [PubMed] [Google Scholar]
- 8.Rahmaniyan M, Curley RW, Jr, Obeid LM, Hannun YA, Kraveka JM. Identification of dihydroceramide desaturase as a direct in vitro target for fenretinide. J Biol Chem. 2011;286:24754–64. doi: 10.1074/jbc.M111.250779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Donnell PH, Guo WX, Reynolds CP, Maurer BJ. N-(4-hydroxyphenyl)retinamide increases ceramide and is cytotoxic to acute lymphoblastic leukemia cell lines, but not to non-malignant lymphocytes. Leukemia. 2002;16:902–10. doi: 10.1038/sj.leu.2402485. [DOI] [PubMed] [Google Scholar]
- 10.Costa A, Malone W, Perloff M, Buranelli F, Campa T, Dossena G, et al. Tolerability of the synthetic retinoid Fenretinide (HPR) Eur J Cancer Clin Oncol. 1989;25:805–8. doi: 10.1016/0277-5379(89)90124-7. [DOI] [PubMed] [Google Scholar]
- 11.Veronesi U, De Palo G, Marubini E, Costa A, Formelli F, Mariani L, et al. Randomized trial of fenretinide to prevent second breast malignancy in women with early breast cancer. J Natl Cancer Inst. 1999;91:1847–56. doi: 10.1093/jnci/91.21.1847. [DOI] [PubMed] [Google Scholar]
- 12.Jasti BR. Phase I clinical trial of Fenretinide (NSC374551) in advanced solid tumors. Proceedings of the American Society and Clinical Oncology; 2001; p. 122a. [Google Scholar]
- 13.Villablanca JG, London WB, Naranjo A, McGrady P, Ames MM, Reid JM, et al. Phase II study of oral capsular 4-hydroxyphenylretinamide (4-HPR/fenretinide) in pediatric patients with refractory or recurrent neuroblastoma: a report from the Children’s Oncology Group. Clin Cancer Res. 2011;17:6858–66. doi: 10.1158/1078-0432.CCR-11-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon R, Freidlin B, Rubinstein L, Arbuck SG, Collins J, Christian MC. Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst. 1997;89:1138–47. doi: 10.1093/jnci/89.15.1138. [DOI] [PubMed] [Google Scholar]
- 15.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 16.Blade J, Samson D, Reece D, Apperley J, Bjorkstrand B, Gahrton G, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102:1115–23. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 17.Kantarjian HM, Keating MJ, Estey EH, O’Brien S, Pierce S, Beran M, et al. Treatment of advanced stages of Philadelphia chromosome-positive chronic myelogenous leukemia with interferon-alpha and low-dose cytarabine. J Clin Oncol. 1992;10:772–8. doi: 10.1200/JCO.1992.10.5.772. [DOI] [PubMed] [Google Scholar]
- 18.Maurer BJ, Kang MH, Villablanca JG, Janeba J, Groshen S, Matthay KK, et al. Phase I trial of fenretinide delivered orally in a novel organized lipid complex in patients with relapsed/refractory neuroblastoma: a report from the New Approaches to Neuroblastoma Therapy (NANT) consortium. Pediatr Blood Cancer. 2013;60:1801–8. doi: 10.1002/pbc.24643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.