Abstract
Although manganese (Mn) is critical for proper function of various metabolic enzymes and cofactors, excess Mn in the brain causes neurotoxicity. While the exact transport mechanism of Mn has not been fully understood, several importers and exporters for Mn have been identified over the past decade. In addition to Mn-specific transporters, it has been demonstrated that iron transporters can mediate Mn transport in the brain and peripheral tissues. However, while the expression of iron transporters is regulated by body iron stores, whether or not disorders of iron metabolism modify Mn homeostasis has not been systematically discussed. The present review will provide an update on the role of altered iron status in the transport and toxicity of Mn.
Keywords: Anemia, divalent metal transporter 1, ferroportin, hepcidin, zinc
1. Introduction
Manganese (Mn) is an essential transition metal, which serves a vital role as a cofactor for various metabolic and antioxidant enzymes 1, 2. In addition, the metal is also involved in the physiological regulation of blood sugar, bone growth, blood clotting, and the immune system 3. Dietary sources of Mn include nuts, legumes, seafood and tea. Mn deficiency, although rare, is characterized by weight loss, poor bone formation, and reduced fertility 4. However, more common cases of human pathologies associated with Mn result from excessive exposure to this metal, rather than from Mn deficiency 5, 6, raising a huge concern in public health.
Historically, chronic Mn encephalopathy was first recognized among workers engaged in grinding of Mn ores. These workers displayed symptoms of motor and cognitive deficiencies, tremors, gait disturbances and hallucinations 7. Over the last few decades, welding, mining, and smelting have been recognized as high-risk occupations for developing Mn toxicity 8. The Bureau of Labor Statistics estimated about 400,000 people to be working in welding-related occupations in the U.S in 2015 9. Other subsets of population which can be exposed to high levels of Mn include infants fed formulas based on cow or soy milk 3, and patients receiving prolonged exposure to total parenteral nutrition 10. The use of methylcyclopentadienyl Mn tricarbonyl (MMT) as an antiknock agent increases Mn exposure in people living in urban, dense traffic zones 11, 12. Of note, consumption of contaminated water from domestic wells has also been found to be a source of Mn toxicity 13, 14. Due to the incomplete development of the blood-brain barrier (BBB), neonates are at a higher risk of Mn toxicity compared with adult rats 15–18.
While Mn has many oxidation states, Mn2+ and Mn3+ are the two common species found in human body 19. Since Mn2+ is chemically more stable than Mn3+ in the body, Mn is mainly incorporated into metalloenzymes in the form of Mn2+ 20, 21. However, Mn2+ can be oxidized to Mn3+ by ceruloplasmin 22 and transported by transferrin in the circulation 23. Importantly, the redox conversion between Mn2+ and Mn3+ provides a ‘double-sword’ effect on cellular homeostasis. For example, Mn serves as a cofactor for Mn superoxide dismutase (MnSOD) that catalyzes superoxide (O2•−) to hydrogen peroxide (H2O2) through the Mn2+/Mn3+ cycle and thereby detoxifies free radicals in the mitochondria to prevent oxidative stress. On the other hand, the Mn2+/Mn3+ cycle can trigger dopamine auto-oxidation, which is one of proposed mechanisms for Mn-induced neurotoxicity 24. Together, the Mn redox cycle contributes to both nutritional metabolism and toxic effects on biological function.
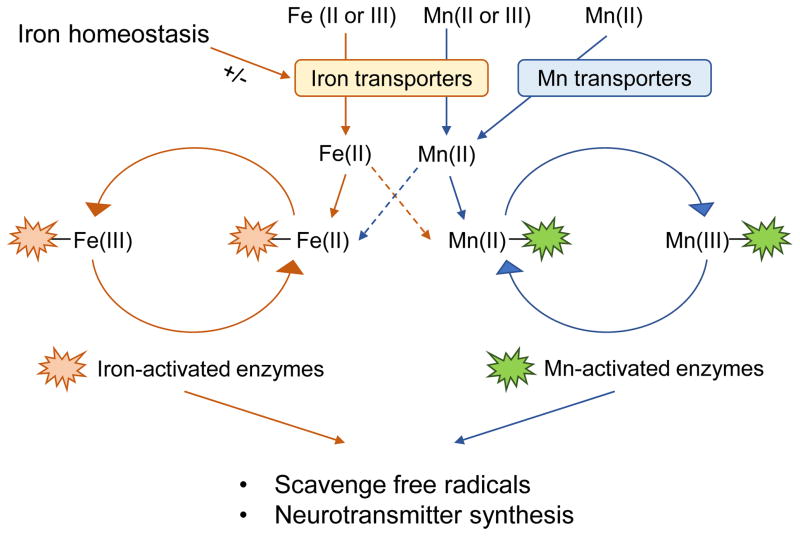
Iron (Fe) is another essential metal that provides the redox activity. In particular, iron is adjacent to Mn in the periodic table and shares similar chemical properties with Mn. As a result, iron can interact with Mn in several different physiological processes. First, Mn transport is in part mediated by iron transporters. Since the expression of iron transporters is affected by several conditions, such as iron deficient anemia and iron overload hemochromatosis, altered body iron status modifies Mn transport and consequently Mn-associated neurotoxicity 25–33, as discussed below. In addition, both metals are cofactors for a number of metalloenzymes, which play critical roles in antioxidant defense and neurochemistry in the brain. Due to structural and chemical similarities, it is possible that one metal, if in excess, could substitute the other and thereby modulate enzyme activities. For example, while the structure of the metal binding site of FeSOD is similar to that of MnSOD 34, excess iron could replace Mn in MnSOD. However, the redox potential of iron is incompatible with the function of MnSOD, and thereby the replacement of Mn with iron could deactivate the enzyme 36. Although some studies using in vitro systems have supported such possibility 35, 36, it is yet to be investigated whether imbalance of iron-Mn homeostasis will perturb the redox environment in mammals. Moreover, the interplay of Mn and iron can occur in the neurotransmission system, such as the dopaminergic pathway. For example, both metals support the function of tyrosine hydroxylase (TH) 37, 38, the rate-limiting enzyme for dopamine synthesis. This can allow one metal to compensate for the other in case of deficiency to correct impaired enzyme function. Together, these biochemical similarities between Mn and iron suggest the possible molecular interaction of the two metals in neurological function (Figure 1). Thus, the present review will focus on updates on iron-Mn interaction in the context of transport/toxicokinetics and toxicodynamics of metals. Readers are encouraged to consult more comprehensive reviews for detailed information on the molecular mechanisms of Mn homeostasis and Mn-induced neurotoxicity 21, 39, 40.
Figure 1. Proposed mechanisms of Mn-iron interaction.
The structural and chemical similarities between Mn and iron allow them to interact with each other in biological systems. Both Mn and iron can be transported as divalent forms by several divalent metal transporters (e.g. DMT1 and FPN) or as trivalent forms by the Tf/TfR system. It has been known that iron status can alter the expression of these transporters, thereby modifying Mn levels in the body. In addition, both Mn and iron serve as cofactors for several metalloproteins that play critical roles in antioxidant defense and neurological function. Since many of these enzymes have binding affinities for both metals, it is possible that they can substitute each other under certain conditions (dotted arrows), thereby alter the activity of these enzymes.
2. Absorption, Distribution and Disposal of Mn
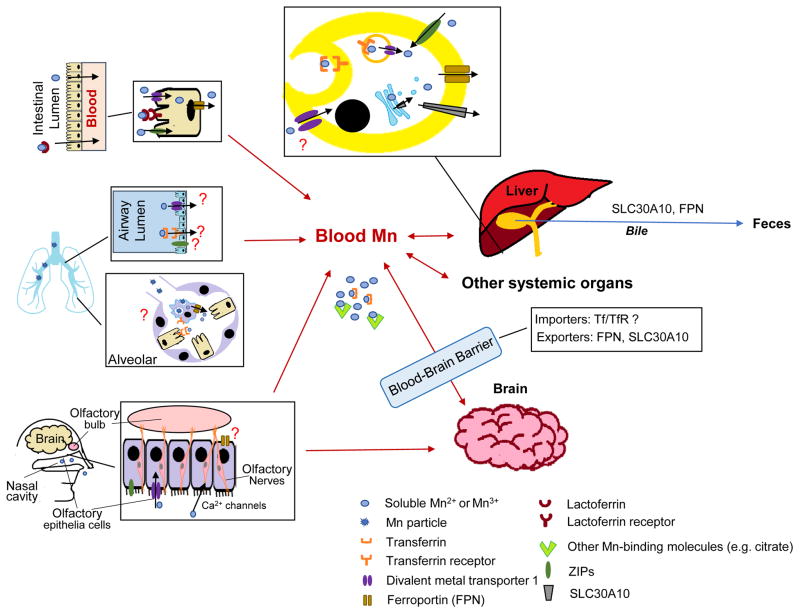
As an essential nutrient, Mn is absorbed via different routes and distributes into tissues (Figure 2). These transport processes are critical to maintain Mn homeostasis. While several Mn-specific exporters and regulatory proteins that play an important role in Mn homeostasis have recently been identified, it has long been recognized that Mn is also transported by iron transporters, since Mn possess similar chemical properties to iron. Moreover, several zinc transporters, especially ZIP (Zrt- and Irt-like proteins) family, mediate intracellular Mn uptake. The role of these transporters in Mn transport is reviewed in this section.
Figure 2. Absorption, distribution and disposal of Mn.
Mn is absorbed via intestinal, pulmonary and olfactory transport. At the intestine lumen, free Mn or lactoferrin-bound Mn can be taken into the enterocytes via divalent metal transporter 1 (DMT1), ZIP8 and lactoferrin receptor. The free Mn inside the enterocytes is released into blood for systemic circulation. Airborne Mn, especially Mn particles, is absorbed through the lung by inhalation. While both DMT1 and transferrin receptor (TfR) are expressed at the epithelial cells and transferrin (Tf) is found in bronchoalveolar fluid, it is unclear whether they are directly involved in pulmonary Mn transport. Ferroprotein (FPN) is expressed at the alveolar macrophages, where it could contribute to dissolution of Mn-containing particles and thereby absorption of soluble Mn. DMT1 is involved in the olfactory uptake of Mn into blood and brain. The nasal route also expresses several metal transporters, including FPN and ZIPs, but their roles in olfactory Mn transport have not been evaluated. Mn can be directly taken up into the brain by calcium channels expressed at the terminal of olfactory nerves. After absorption, Mn distributes into the tissues by several importers. Intracellular Mn is released into blood or excreted out of the body by metal exporters, such as FPN and SLC30A10. The liver is the major organ for Mn disposal, and Mn is mainly excreted by the bile into the feces. However, the exact mechanism of biliary Mn secretion is unknown. The mechanism of Mn uptake/export in the brain is incompletely understood and results are controversial.
2.1. Absorption
2.1.1. Intestinal absorption
Mn-containing food provides the major source of Mn intake in humans. The bioavailability of ingested Mn is about 3–5% in humans 41. Mn is absorbed from the intestine by either active transport or facilitated diffusion 42. While there is no specific Mn transporter identified in the gut, accumulating evidence has indicated that several iron transporters are involved in Mn absorption. The divalent metal transporter 1 (DMT1) plays an important role in intestinal uptake of Mn. DMT1, located in the apical membrane, mediates the uptake of multiple divalent metals into the cell, including iron (Fe), Mn and copper (Cu) 43. The homozygous Belgrade (b/b) rat carries a mutation in DMT1 protein that results in < 1% functional DMT1 44 and thus has provided a useful tool to investigate the role of DMT1 in Mn transport in vivo. Functional studies using the duodenum from b/b rats revealed a ~70% reduction in Mn transport activity 45, 46. Consistently, basal Mn levels are lower in the liver and blood of b/b rats compared with control rats 45, 47. However, Shawki et al. recently reported that specific-knockout of intestinal DMT1 does not affect the absorption or tissue distribution (including spleen, heart, kidney and liver) of Mn in mice 48. These conflicting results could be attributed to different experimental conditions of Mn transport: closed duodenal loops in situ or intestinal brush-border membrane vesicles from b/b rats were used along with relatively high concentrations of Mn 45, 46, whereas 54Mn radiotracer was administered by intragastric gavage to intestine-specific DMT1 knockout mice 48. Thus, it is possible that intestinal DMT1 significantly participates in Mn absorption when there is a substantial increase in the amount of Mn relative to iron in the gut (i.e. Mn overexposure or iron deficiency) 48.
The lactoferrin receptor is known to mediate the intestinal uptake of iron as well as Mn in multiple species, including humans, rhesus monkeys, mice and rabbits 49–51. A large portion of Mn in the milk is carried by lactoferrin 52, which can bind to lactoferrin receptors in the intestine and be absorbed into the bloodstream 53. The lactoferrin-mediated pathway is mainly responsible for intestinal Mn absorption during the lactation period.
Ferroportin (FPN; SLC40A1) is the only known metal exporter involved in the intestinal absorption of iron 54–56. After taken up by enterocytes via DMT1 and other intestinal importers, iron is released into blood by FPN for circulation. There is growing evidence that FPN transports Mn 57–60. Although the affinity of FPN to Mn is lower by three orders of magnitude than that to iron 61, deficiency of FPN in mice results in decreased intestinal uptake of Mn 57, 58, suggesting an important role of FPN in systemic absorption of Mn from diet.
While ZIP14 and ZIP8 were initially discovered as zinc (Zn) transporters 62, 63, in vitro studies demonstrated that they also participate in intracellular Mn uptake 64, 65. Since the transcript levels of ZIP14 are approximately 10 times higher than those of ZIP8 in the intestine 65 and since ZIP14 protein is also highly expressed at the basolateral membrane of the proximal intestine 66, ZIP14 is more likely involved in intestinal uptake of Mn than ZIP8. The proposed transport mechanism of ZIP14 is via endosome-mediated exocytosis 66. However, the role of ZIP14 in intestinal Mn uptake is questioned by a recent finding that human patients with ZIP14 mutations show elevated Mn levels in blood and brain 67. In contrast, human patients with loss of function mutations in ZIP8 have reduced Mn in blood along with severe neurological diseases related to Mn deficiency 68, 69. Combined, these results indicate that ZIP8, not ZIP14, plays a critical role in intestinal Mn absorption. Interestingly, the renal excretion of Mn is increased in individuals with ZIP8 mutation 68. An in vitro study has suggested that ZIP8 could play a significant role in renal reabsorption of Mn 70, and thereby ZIP8 mutation could impair Mn reabsorption in the kidney and contribute to low blood Mn. Pharmacokinetic studies for Mn using ZIP8/14 knockout mice will help to clarify the in vivo role of ZIPs in intestinal absorption as well as excretion of Mn.
2.1.2. Pulmonary absorption
Pulmonary epithelia provide a direct pathway for airborne Mn transport into the systemic circulation. However, the molecular mechanism of pulmonary transport is still obscure. Although DMT1 is expressed in the airway epithelia 71, it has been suggested that DMT1 is not a major transporter for Mn uptake across the lung: pulmonary absorption of 54Mn after intratracheal instillation is not different between DMT1-mutant b/b and control +/b rats 72. Also, iron deficiency enhances 54Mn uptake after intratracheal instillation, but the expression of DMT1 is not significantly altered 73.
While DMT1 transports divalent metals (e.g. Fe2+ and Mn2+), the transferrin-transferrin receptor (Tf-TfR) system is involved in the uptake of trivalent metals (e.g. Fe3+ and Mn3+) 74–76. Mn3+ is bound to transferrin (Tf) to form a Tf-Mn3+ complex, which is internalized into the cell via the transferrin receptor (TfR) and dissociates in the acidic endosomes. Mn3+ is reduced to Mn2+ and released into the cytosol via DMT1 expressed on the membrane of endosome75. Specifically, TfR is expressed in the type II alveolar epithelial cells, alveolar macrophages, bronchial epithelium and bronchus-associated lymphoid tissues 72. Heilig et al. demonstrated that increased expression of TfR protein in the lung upon iron deficiency is correlated with enhanced intratracheal absorption of 54Mn2+ 72, 73. However, iron deficiency does not change Tf concentrations in the lung and bronchoalveolar lavage fluid, and the majority of lung 54Mn2+ is not bound to Tf 72, suggesting that pulmonary absorption of Mn is Tf-independent. Since the Tf-TfR complex transports Mn3+, and not Mn2+, future studies using different species of Mn (i.e. Mn2+ and Mn3+) will help to identify the definitive role of the Tf-TfR system in pulmonary Mn uptake.
The same study demonstrated that L-type Ca2+ channels and TRPM7, a member of the transient receptor potential melastatin subfamily, are involved in Mn uptake alveolar epithelial cells in vitro. However, the role of these channels in pulmonary Mn transport has not been determined in vivo. Although FPN plays a role in intestinal Mn absorption, whether it is also involved in pulmonary Mn uptake is unknown. Interestingly, ZIP8 is abundantly expressed in the lung 65, 77, 78, but the correlation between ZIP8 or ZIP14 expression and pulmonary Mn absorption has not been explored. Further studies are needed to evaluate the impact of other metal transporters on pulmonary absorption of Mn.
2.1.3. Olfactory absorption
The olfactory transport pathway provides an efficient route of absorption of airborne Mn into blood due to the absence of hepatic first-pass elimination, thus enhancing Mn bioavailability compared with intestinal absorption of Mn 79, 80. Furthermore, the nasal-brain pathway allows for direct contact with the brain by circumventing the BBB, increasing access of Mn to the brain, the primary site of metal’s toxicity 80–82. Thus, airborne Mn exposure has long been recognized as a huge concern for Mn neurotoxicity in environmental and occupational health 83, particularly in workers employed in mining and Mn ore processing 84 and agricultural workers exposed to Mn-containing pesticide 85.
While olfactory absorption of Mn is rapid and efficient, the transport mechanisms remain largely unexplored. Only a few metal transporters have been found to be involved in olfactory Mn uptake. Earlier, Pautler et al. demonstrated that olfactory Mn transport can be inhibited by a calcium channel blocker and a microtubule disturbing compound, suggesting that Mn can enter the olfactory neurons via calcium channels, followed by a microtube-dependent transport system 86. DMT1 is also involved in olfactory transport of Mn, based on the finding that b/b rats showed decreased 54Mn uptake into blood after intranasal instillation of 54MnCl2 87. While some studies indicated that other metal transporters, including FPN, ZIP14 and ZIP8, are expressed in the olfactory epithelium or olfactory bulb 79, 88, it remains to be tested whether these transporters contribute to olfactory uptake of Mn in vivo.
2.2. Distribution
2.2.1. Tissue uptake
The Tf-TfR system transports both Fe and Mn into the cell, as reviewed elsewhere 40, 89. Mice with decreased circulating Tf display reduced 54Mn uptake as well as reduced steady-state levels of Mn in the liver 90, 91, indicating that the Tf-TfR system is important for tissue distribution of Mn. Tf-mediated transport of Mn requires normal function of DMT1, as evidenced by the finding that b/b rats have impaired uptake of Tf-bound Mn, but not free Mn in serum 45. However, the contribution of DMT1 to Mn distribution appears to be limited to the uptake process, and steady-state levels of Mn in the tissue could be more dependent on the export process. For example, b/b rats has lower or unchanged basal Mn levels in the liver compared with control rats 45, 47, whereas the distribution of intravenously injected 54Mn into the liver is greater in b/b rats 45. Since the liver transfers Mn into the bile for excretion 92, it is possible that Mn excretion is enhanced in b/b rats. Collectively, the Tf-TfR1 system and DMT1 work together to contribute, at least partially, to systemic Mn distribution.
Whether the Tf-TfR1 system mediates Mn uptake into the brain is not completely understood. Mice deficient in Tf showed no change in brain Mn levels after intravenous or subcutaneous 54Mn administration compared with wild-type mice 90, 93. Likewise, conflicting results exist about the role of DMT1 in Mn uptake into the brain. DMT1 is expressed in ependymal cells, neurons, astrocytes and vascular endothelial cells throughout the brain 94, 95. Crossgrove et al. demonstrated that dysfunction of DMT1 does not affect the transport of free 54Mn2+ in serum or Tf-54Mn into the brain, as assessed by both in vivo and in situ brain perfusion experiments 96, which is different from 59Fe transport 97. Han et al. revealed that b/b rats have normal levels of Mn in the brain 47. These studies suggest that DMT1 is not a primary transporter responsible for Mn uptake into the brain. However, it should be noted that gene knockout in the whole body could trigger compensatory response that activates the expression and/or function of other transporters or regulators, thereby masking the effects of single gene on brain Mn homeostasis. Although mice with hippocampal specific-knockout of DMT1 showed reduced iron levels in the hippocampus, Mn levels were not determined 98. Future studies utilizing tissue-specific gene knockout mice will increase our understanding of the role of these transporters in brain metal homeostasis.
Families of zinc transporters, especially ZIP14 and ZIP8, have been implicated in Mn uptake into cells. The expression levels of ZIP14 and ZIP8 are positively correlated with Mn uptake in vitro 99, 100. However, the tissue distribution patterns of ZIP14 and ZIP8 are different. ZIP14 is mostly expressed in the liver, pancreas, heart and duodenum, while ZIP8 is more abundant in the lung, testis and kidney 101. Therefore, it is likely that the role of ZIP14 and ZIP8 in Mn uptake is tissue-specific. However, the exact mechanism of ZIPs in Mn distribution and metabolism using in vivo experiments is yet to be characterized.
In the brain both active and facilitated transport mechanisms exist, including transport of a Mn-citrate complex by an organic anion transporter or a monocarboxylate transporter, Ca2+ channels (voltage-gated, store-operated, glutamatergic ionotropic), and choline transporters, as reviewed in elsewhere 21.
2.2.2. Mn export
Although an ex vivo study from Yokel et al. suggested that Mn efflux from the brain could be non-carrier-mediated 102, there has been increased understanding about the molecular mechanism of Mn export during the past decade. Several recent clinical studies have reported that human subjects with Solute Carrier Family 30 Member 10 (SLC30A10; ZnT10) deficiency/mutation display higher Mn accumulation in the liver 67 and blood 103, 104, as well as 10 times higher Mn in the basal ganglia, which is associated with dystonia, a typical phenotype of Mn neurotoxicity 105–107. SLC30A10 was thought to be a zinc exporter, but its amino acid structure is different from other zinc transporters in this family 108, and it was later suggested that SLC30A10 may not be involved in zinc transport 109. In contrast, the expression of SLC30A10 is inversely correlated with intracellular Mn accumulation in several in vitro systems, such as Hela cells, chicken DT40 cells and SH-SY5Y cells 100, 110, 111. SLC30A10 is localized the cell surface as well as intracellular compartments of secretory pathways 103. Both transmembrane and C-terminal domains of cell membrane-associated SLC30A10 are structurally critical for Mn export activity 112. Mutated SLC30A10 is unable to traffic to the cell surface and thereby decreases Mn efflux activity 110. Another study demonstrated that SLC30A10 forms heterodimers with several ZnTs in TfR-positive endosomes 113. However, whether the heterodimer is also critical for Mn transport and whether this mechanism also exists in vivo is not well understood. Although SLC30A10 is expressed in the intestine, whether it is involved in intestinal Mn absorption, like FPN, is yet to be investigated 114.
The secretory pathway Ca2+-ATPase isoform 1 (SPCA1) is another transporter involved in the Mn secretory pathway out of the cell. SPCA1 is a Ca2+-ATPase that can pump the cytoplasmic Mn2+ into the Golgi apparatus for secretion 115. Enhanced function of SPCA1 increases 54Mn levels in the Golgi apparatus and contributes to Mn detoxification 116, whereas deletion of SPCA1 reduces cell viability upon Mn exposure 117. In addition, ATP13A2, a P-type ATPase 118, reduces intracellular Mn accumulation in HEK293 and N2a cells and protects cells against Mn-induced toxicity 119. The contribution of these transporters to Mn homeostasis in vivo remains to be evaluated.
FPN mediates the export of Mn as well as iron. Induction of FPN expression in SH-SY5Y, HEK293T cells and oocytes increases Mn efflux and reduces Mn accumulation 57, 59, 60. Contradictory findings have been also reported. Mitchell et al. did not observe enhanced 54Mn efflux in oocytes overexpressing FPN 61. In animal studies, Seo et al. reported decreased Mn levels in several organs in flatiron mice that carry a mutation in FPN, but they did not differentiate the absorption and distribution processes of Mn into peripheral tissues 58. The same group also reported that 54Mn in blood is unchanged in the flatiron mice after intravenous injection of 54Mn, suggesting that FPN may not be responsible for systemic Mn clearance 57. It is possible that mutation in FPN and/or subsequent changes in iron status 58, as seen in the flatiron mice, could up-regulate other Mn exporters (e.g. SLC30A10) to control the excretion of Mn. FPN could also be involved in Mn export from the brain. The levels of Mn are elevated in the olfactory bulb and brain from FPN-deficient mice, especially in male mice, despite lower Mn levels in the peripheral tissues 58. In parallel, increased expression of FPN is correlated with reduced Mn accumulation in the brain 31. However, no studies have directly determined Mn export out of the brain, and pharmacokinetic studies after intracerebral injection of 54Mn using mice with FPN deficiency or overexpression will directly address the role of FPN in brain Mn export.
2.3. Disposal
The liver plays a major role in Mn excretion, and impairment of liver function results in excessive retention of Mn 120, 121. Mn is excreted from the liver through the bile into the feces 92. It has been suggested that Mn2+ cations may be secreted from blood into bile to form complexes with bile acids 122. Biliary excretion of Mn could be transporter-dependent 123, 124. Interestingly, FPN is expressed at the sinusoidal borders of the hepatocytes 125. However, there is no direct evidence that FPN is involved in biliary Mn excretion. Of note, SLC30A10 is highly expressed in the liver and the epithelium of bile ducts, and the expression of defective SLC30A10 leads to high levels of systemic Mn 103, suggesting that SLC30A10 could play a critical role in systemic excretion of Mn through bile. Tuschl et al. reported that mutations in ZIP14 increase blood Mn without affecting liver Mn, suggesting that ZIP14 is involved in Mn transport into the liver, followed by biliary excretion of Mn by SLC30A10 67.
3. Effect of iron status on Mn homeostasis
Factors that change the expression of metal transporters involved in Mn transport perturb Mn homeostasis. Specifically, imbalance in body iron status alters the expression of several key iron transporters that also mediate Mn transport. Both animal and human studies showed a ‘U-shape’ correlation between iron levels and Mn status in the body, likely due to the complex mechanisms in both causes and consequences of dysregulation of iron metabolism (i.e. genetic, nutritional and environmental), as discussed below.
3.1. Systemic Mn homeostasis in iron deficiency
Iron deficiency is the most prevalent nutritional disorder, affecting more than 2 billion people worldwide 126. The effects of iron deficiency on Mn transport have been well-documented. The transcripts of many iron transporters, including DMT1, FPN and TfR1, contain the iron-responsive element (IRE) 127. The iron-responsive proteins (IRP) bind to the IRE sequence and regulate transporter expression at the levels of transcription and post-transcription 128. Through this mechanism, the expression of iron transporters changes in response to body iron status. For example, dietary iron deficiency up-regulates the expression DMT1 in the intestine 43, 129, 130, resulting in increased basal levels of Mn in various tissues, including brain, heart, kidney, testis, femoral muscle and tibia 131, 132. Elevated blood Mn levels have been observed in human subjects with iron deficiency in children, infants and women 133–135. In addition, Mena et al. reported that anemic patients have a 2.5-fold greater intestinal 54Mn absorption compared with normal individuals 136, likely due to increased expression levels of duodenal iron transporters 137. In parallel, blood ferritin concentrations are inversely correlated with 54Mn absorption, suggesting that body iron stores could negatively influence intestinal Mn absorption 138, 139.
Many animal studies have characterized the effect of non-heme iron on Mn homeostasis, but little is known about the role of heme iron in Mn metabolism, although heme iron is the major form of iron source in non-vegetarian diet. Davis et al. examined the effect of different forms of iron (i.e. heme and non-heme) on serum Mn levels in human subjects provided with typical western diet and found that serum Mn levels are affected by the intake of non-heme iron, but not heme iron 140. More studies are warranted to better understand the exact role of heme iron in Mn transport using relevant animal models.
3.2. Brain homeostasis upon iron deficiency
Iron deficiency is associated with increased accumulation of Mn in the brain in a region-specific manner; elevated Mn levels observed with iron deficiency are more pronounced in the caudate putamen and globus pallidus 26. Iron deficiency also increases extracellular Mn concentrations in the striatum in rodents 141. Relatively less information is known about the Mn level in the brain of iron deficient/anemic patients. In a preliminary clinical study with a small sample size, it was reported that iron deficiency is correlated with increased Mn deposition in the basal ganglia 142. In contrast, Mn levels in the globus pallidus are minimally affected in anemic humans 143, which is different from animal studies 26 and other cases of Mn intoxication 144, 145. The reasons for these different results remain unclear.
The mechanism by which iron deficiency enhances Mn accumulation in the brain is not clearly understood. Although mixed results exist, it is believed that DMT1 and the Tf-TfR system may play a role in Mn retention upon iron deficiency. In vitro studies have shown that deferoxamine (DFO), an iron chelator, up-regulates the expression of DMT1 in several types of cells, including SH-SY5Y cells 25 and rat primary astrocytes 146, but this is not correlated with TfR expression 146. Similarly, Siddappa et al. demonstrated that the expression of DMT1 is up-regulated in the hippocampus and cerebral cortex of iron-deficient rats during the perinatal period 147. However, post-weaning iron deficiency by diet does not alter DMT1 levels in the brain 148. These results suggest that the regulation of brain DMT1 in response to iron stores could be more sensitive during early development. In addition to DMT1, the expression of the protein, and not the mRNA, of TfR1 is elevated in the brain of rats exposed to iron-deficient diet during either prenatal or postnatal period 147–149. These findings are different from the in vitro results that showed no change in Tf expression upon removal of iron 146. This discrepancy could result from a difference in the Tf-TfR1 system between in vitro and in vivo conditions. For example, the function of the Tf-TfR1 system is affected by several membrane-associated and/or soluble factors (such as hepcidin as discussed below) in vivo, but this mechanism could be lacking in cell culture conditions. Together, these studies suggest that enhanced Mn accumulation in vivo upon iron deficiency could be attributed to altered expression of transporters in an iron-responsive manner.
Thompson et al. demonstrated that dietary iron deficiency increases the expression of DMT1 in the rat olfactory epithelium, resulting in significantly enhanced blood Mn after a single dose of intranasal instillation of 54Mn isotope 87. However, iron deficiency does not alter 54Mn levels in the olfactory bulb and basal ganglia after 2 weeks post-instillation 87. Although both uptake and clearance affect brain Mn homeostasis, scarce information is known about the clearance from the brain. Chua et al. reported that iron deficiency does not affect blood Mn clearance after intravenous injection 131, but efflux kinetics of Mn from the brain was not investigated. Direct administration of Mn (e.g. intracerebral injection of 54Mn) will address this question by separating the absorption and elimination process of Mn transport under different body iron status. Moreover, Mn deposition in the brain is heterogeneous after intravenous MnCl2 injection upon iron deficiency; Mn distribution is increased in the brain stem, striatum and cortex, but decreased in the hippocampus 150. Whether or not the heterogeneous pattern of Mn distribution is correlated with the expression of transporters has not been evaluated. The influence of iron deficiency on Mn-specific exporters (e.g. SLC30A10 and SPCA1) has not yet been examined.
3.3. Effect of iron overload on Mn homeostasis
3.3.1. Iron overload and systemic Mn transport
Dietary iron overload
Compared with iron deficiency, the effects of iron overload on the expression of iron/Mn transporters are less understood. Iron overload suppresses the expression of DMT1 in enterocytes by an iron-responsive manner, which has been consistently reported by different researchers 151, 152. However, the effects of iron loading on DMT1 expression in other types of cells or tissues are mixed. Dietary iron overload increases hepatic DMT1 expression as well as DMT1 expression in rat primary astrocytes, resulting in increased Mn uptake 146, 152. However, Hansen et al. demonstrated that a high-iron diet decreases hepatic and duodenal Mn levels in pigs, along with decreased mRNA expression of DMT1, but the protein levels of DMT1 or FPN are not significantly altered 153. More mechanistic experiments are required to understand the effect of dietary iron overload on systemic Mn accumulation.
Genetic iron overload
Mutations in iron regulatory genes lead to genetic iron overload diseases. In particular, the HFE (High Fe or hyperferremia) protein is essential for proper control of iron transport 154–157, and mutations in the HFE gene are the major cause of hereditary hemochromatosis (HH), a most common genetic iron overload disorder among the North American Caucasian population 158–160. The C282Y mutation of the HFE gene is primarily involved in systemic iron loading, such as liver and heart 154, which predisposes to liver cirrhosis and cardiomyopathy. Importantly, the H63D variant of the HFE gene (22.9% gene variants worldwide) 161 receives increased attention due to a greater susceptibility to elevated iron and the development of neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis 154, 162.
It is generally accepted that systemic iron overload in HFE dysfunction is due to the altered expression of iron transporters, although there are some controversies. Several studies reported up-regulation of DMT1 and FPN protein expression in the intestine upon hemochromatosis 137. Elevated activity of intestinal FPN and DMT1 in HFE mutations is believed to be caused by the suppression of hepcidin, the master iron regulatory peptide produced in the liver and released into blood; hepcidin induces the degradation of FPN and DMT1 via a ubiquitination-dependent pathway 163, 164, while loss of HFE function results in hepcidin deficiency 165–167, which thereby up-regulates these iron transporters in several tissues.
The effects of HFE dysfunction on systemic Mn transport are mixed. On the one hand, Kim et al. found that blood levels of 54Mn are increased via gavage but unchanged after intravenous injection of 54MnCl2 79. On the other hand, the steady-state levels of Mn are decreased in both HFE-knockout mice and humans with HFE variants 168. Similarly, Jouihan et al. found that the mitochondrial Mn levels in the liver are lower in HFE-deficient mice 169. Together, these results suggest that HFE dysfunction could increase both absorption and elimination of Mn, and that the effect of HFE on elimination could outweigh that on absorption. It is unknown whether iron overload or HFE function modifies SLC30A10 and other Mn-specific transporters.
3.3.2. Iron overload and brain Mn transport
Dietary iron overload
Although it is conceivable that iron loading would decrease brain Mn transport due to the competition for the metal transporters, multiple studies reported different results 131, 150. Similar to iron deficiency, iron supplementation increased Mn deposition in the brain stem, striatum and cortex after intravenous MnCl2 injection, whereas hippocampal Mn accumulation was decreased 150. Iron loading throughout both pre-weaning and post-weaning periods also increases Mn accumulation in the brain via drinking water 131. In contrast, Fitsanakis et al. found no difference in brain Mn accumulation between iron-deficient and iron-loaded rats after intravenous injection of Mn 170. A recent study also indicated no change in striatal Mn accumulation after dietary iron treatment 31. Collectively, these results suggest that dietary iron loading may influence Mn transport in different brain regions by non-competitive mechanisms in time- and dose-dependent manners.
Genetic iron overload
In the brain, HFE mutation is associated with increased DMT1, but reduced Tf expression 171, and this implies that HFE dysfunction could alter Mn accumulation in the brain. Kim et al. reported that a single intranasal instillation of 54Mn increases 54Mn levels in the brain, but not in blood, with the expression of DMT1 and FPN unchanged in the olfactory bulb of Hfe-deficient mice 79. Interestingly, after repeated intranasal instillations of MnCl2, Hfe-knockout mice do not alter Mn levels in most brain regions except the cerebellum, in which Mn levels are decreased 33. These studies suggest that loss of HFE function could increase intranasal uptake of Mn into the brain as well as the clearance of the metal from the brain. A recent study by Ye et al. 31 demonstrated that mice carrying HFE H67D mutation, a mouse model with elevated brain iron 171, display decreased Mn levels in blood, liver and brain, especially in the striatum, after intranasal instillations of Mn for 3 days. The attenuated striatal Mn accumulation in H67D mutation is associated with increased transcript levels of FPN. Although the protein levels of FPN should be determined, this study underscores the effect of HFE-related hemochromatosis on regional expression of metal transporters and clearance of Mn from the brain after olfactory exposure 31. With respect to oral absorption of Mn, Alsulimani et al. reported that Mn accumulation is not different in the liver, but decreased in the brain in HFE-deficient mice after subchronic exposure to Mn via drinking water 172. Together, these studies suggest an important role of HFE function and iron overload in Mn homeostasis in the brain.
4. Effect of iron homeostasis on Mn-associated neurotoxicity
Excess Mn in the brain is neurotoxic and results in a number of neurobehavioral impairments known as manganism, including memory deficits, decreased motor skills and psychotic behavior resembling Parkinson’s disease 173. High levels of Mn are found in the striatum, globus pallidus and substantia nigra 174–177. While the exact mechanism of Mn-induced neurotoxicity is unclear, multiple mechanisms have been proposed, including oxidative stress, dysregulation of neurological signaling and apoptosis 178, 179. Several recent review papers have extensively discussed mechanisms of Mn-induced neurotoxicity in detail 21, 179–185.
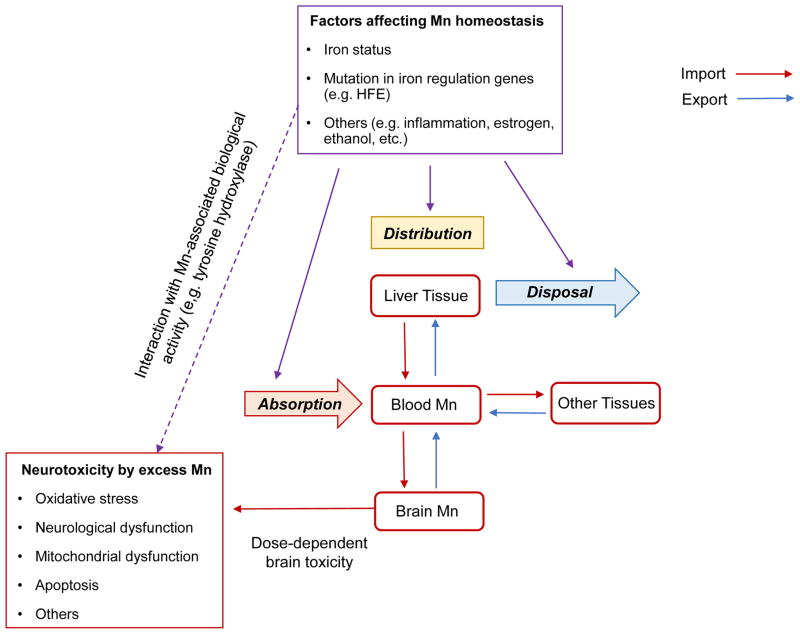
Since Mn transport is closely associated with body iron stores, it is likely that altered iron status would influence Mn homeostasis and Mn-associated biological function. Moreover, excess iron is known to cause neurological damage similar to Mn intoxication, which could exacerbate Mn-induced neurotoxicity. Specifically, both iron and Mn can accelerate dopamine auto-oxidation to form cytotoxic quinones and produce ROS 24, 186, 187. In addition, Mn induces hyperphosphorylation of tau protein 188, and iron promotes the aggregation of hyperphosphorylated tau protein189. It is thus plausible that high levels of both metals could synergistically lead to the accumulation of neurofibrillary tangles, a hall mark of Alzheimer’s diseases. Combined, altered iron status could modify Mn-induced neurotoxicity and result in abnormal behavioral consequences. In this section, we will review the impact of iron homeostasis on neurotoxic effects associated with Mn exposure.
4.1. Iron deficiency and Mn-induced toxicity
It is deduced that iron deficiency exacerbates neurological dysfunction caused by Mn exposure due to enhanced Mn accumulation in the brain (Figure 3). Alternatively, iron also serves as a cofactor for several antioxidant enzymes, such as catalase 190, and iron deficiency could reduce catalase activity and thereby decrease the protective capacity against Mn-induced oxidative stress. Co-incubation of DFO, an iron chelator, worsens the toxicity in rat pheochromocytoma cells, including cell apoptosis and mitochondrial dysfunction 191. In addition, DFO incubation additively increases markers for endoplasmic reticulum stress in human neuroblastoma cells 25. Consistent results have been reported by Seo et al. that dietary iron deficiency potentiates Mn-induced apoptosis in the olfactory bulb in rats after intranasal Mn instillation 25.
Figure 3. Impact of iron status on Mn-associated neurotoxicity.
Changes in iron homeostasis or gene mutations in iron transporters and regulatory proteins can alter the expression of metal transporters that mediate the import and export of Mn, which thereby influences the absorption, distribution and disposal of Mn. Consequently, Mn homeostasis in the brain could be perturbed, and the neurotoxicity associated with Mn accumulation could be altered.
Iron deficiency modifies Mn-induced neurochemical alterations. Mn via drinking water decreases extracellular concentrations of norepinephrine in the caudate putamen, which is enhanced by iron deficiency 26. There is a synergistic effect of oral Mn exposure and iron deficiency on increased gene expression, but not protein expression, of the γ-aminobutyric acid (GABA) transporter 141. Nevertheless, iron deficiency does not exacerbate the inhibition of striatal synaptosome uptake of GABA by Mn 192. Furthermore, Amos-Kroohs et al. 27 found that iron deficiency exacerbates Mn-induced psychiatric behavior, including anxiety, acoustic startle response and sociability, after oral administration of Mn. In addition, a combination of iron deficiency and Mn exposure increases the hippocampal levels of serotonin and norepinephrine, whereas Mn alone only moderately increases or does not affect the levels of these two monoamines 27. However, such synergistic effects of iron deficiency and Mn exposure are not observed on the levels of dopamine and its metabolites in the same study 27. Interestingly, it is possible that iron deficiency could counteract the effect of Mn intoxication on key proteins responsible for dopamine turnover. For example, iron is a cofactor for TH, and iron deficiency is associated with impaired function of TH 37. Mn can also support TH activity 38, and acute oral exposure of Mn increases the activity of TH 193. Mn supplementation could compensate for impaired TH in iron deficiency, as evidenced by a finding that olfactory Mn exposure corrects impaired dopamine release and motor dysfunction caused by iron deficiency 194.
4.2. Iron overload and Mn-induced toxicity
Compared with iron deficiency, the information about the effects of iron overload on Mn-induced neurotoxicity is scarce. On the one hand, since both excess iron and Mn could induce oxidative stress 195–200, they could enhance metal-associated toxicity. On the other hand, iron overload facilitates Mn disposal 31 such that Mn-related toxicity could be mitigated. Lines of evidence have suggested that co-exposure to Mn and iron appears to counteract oxidative stress each other. Sziraki et al. demonstrated that a co-treatment of MnCl2 with iron attenuates dopamine depletion, lipid peroxidation and neuronal degeneration induced by iron in the brain 28–30. One potential mechanism is that Mn may act as an anti-oxidant against iron-induced oxidative stress since Mn is an indispensable cofactor for MnSOD, a crucial antioxidant exclusively expressed in the mitochondria 29. Recent studies also demonstrated that olfactory or gavage exposure to Mn induces impulsivity, recognition memory impairment and motor dysfunction, and these abnormal behaviors are decreased in genetic iron loading caused by HFE dysfunction 32, 33, 172. The protective effect of genetic iron overload against Mn-induced behavioral toxicity is associated with attenuated oxidative stress, enhanced antioxidant capacity and restored function of monoaminergic proteins 31–33. On the other hand, Alsulimani et al. found that Hfe deficiency exacerbates impaired spatial memory capacity induced by Mn exposure via drinking water 172. Combined, these studies indicate that iron loading alters the physiological function of Mn in the brain, and further modifies Mn-induced neurological dysfunction. However, the effect of iron loading could vary depending on several factors, including routes of Mn exposure, exposure window, severity of iron loading, physiological factors (e.g. age, sex) and types of behavior examined.
5. Conclusions
Mn plays an important role in maintaining proper organ function by serving as a cofactor of several critical enzymes. However, Mn in excess causes a variety of neurotoxic effects. Therefore, the body develops multiple regulatory systems for Mn transport, which provide adaptive physiological responses and homeostasis. Besides Mn-specific transporters, iron transporters have been shown to mediate both import and export of Mn. Changes in iron status result in altered expression of iron transporters, which consequently modifies Mn transport and associated neurotoxicity. While Zn transporters also contribute to Mn transport and altered Zn levels affect Mn accumulation in vitro and vivo 201–203, it remains to be evaluated whether changes in Zn homeostasis impact Mn-associated neurotoxicity and behavioral deficits. Although molecular mechanisms of Mn transport and neurotoxicity are not completely understood, a better understanding about the interaction effects of these essential metals would provide an important insight into the role of nutrient metals in Mn deficiency and intoxication.
Several factors have been identified that can alter iron metabolism and transport by modulating the production of hepcidin; these include inflammation, erythropoiesis, alcohol and estrogen 204–207. Thus, it is possible that these factors may also affect Mn homeostasis and regulate Mn-induced neurotoxicity. For example, a pro-inflammatory cytokine IL-6 and erythropoietin alter the expression of iron transporters (TfR, DMT1 and FPN), and it has been shown that inflammation and erythropoiesis increase cellular Mn uptake in vitro 100, 208. Interestingly, it is reported that alcohol exposure worsens Mn-associated neurobehavioral problems 209. Further studies are required to determine if this is due to increased Mn uptake into the brain upon alcohol consumption and/or alcohol’s neurotoxic effects. In addition, Claro et al. reported that the transcript and protein levels of SLC30A10 are significantly up-regulated in subjects who receive vitamin D treatment 114, but Mn levels from these subjects are yet to be quantified. Both in vitro and in vivo transport studies along with exposure-response relationships will improve our understanding of Mn toxicity associated with these factors.
Supplementary Material
Acknowledgments
This work was supported in part by the NIH K99/R00 ES017781 (J.K.).
Abbreviations
- BBB
blood-brain barrier
- DFO
deferoxamine
- DMT1
divalent metal transporter 1
- FPN
ferroportin
- GABA
γ-aminobutyric acid
- HFE
high Fe or hyperferremia
- HH
hereditary hemochromatosis
- IRE
iron-responsive element
- IRP
iron-responsive protein
- ROS
reactive oxygen species
- SLC30A10
Solute Carrier Family 30 Member 10
- SPCA1
secretory pathway Ca2+-ATPase isoform 1
- SOD
superoxide dismutase
- Tf
transferrin
- TfR
transferrin receptor
- TH
tyrosine hydroxylase
- ZIP
Zrt- and Irt-like protein
Footnotes
The authors have no conflicting financial interests.
References
- 1.Takeda A. Manganese action in brain function. Brain Res Brain Res Rev. 2003;41:79–87. doi: 10.1016/s0165-0173(02)00234-5. [DOI] [PubMed] [Google Scholar]
- 2.Horning KJ, Caito SW, Tipps KG, Bowman AB, Aschner M. Manganese Is Essential for Neuronal Health. Annual review of nutrition. 2015 doi: 10.1146/annurev-nutr-071714-034419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aschner JL, Aschner M. Nutritional aspects of manganese homeostasis. Mol Aspects Med. 2005;26:353–362. doi: 10.1016/j.mam.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erikson KM, Thompson K, Aschner J, Aschner M. Manganese neurotoxicity: a focus on the neonate. Pharmacol Ther. 2007;113:369–377. doi: 10.1016/j.pharmthera.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kern CH, Stanwood GD, Smith DR. Preweaning manganese exposure causes hyperactivity, disinhibition and spatial learning and memory deficits associated with altered dopamine receptor and transporter levels. Synapse. 2010;64:363–378. doi: 10.1002/syn.20736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zoni S, Lucchini RG. Manganese exposure: cognitive, motor and behavioral effects on children: a review of recent findings. Curr Opin Pediatr. 2013;25:255–260. doi: 10.1097/MOP.0b013e32835e906b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mena I, Marin O, Fuenzalida S, Cotzias GC. Chronic manganese poisoning. Clinical picture and manganese turnover. Neurology. 1967;17:128–136. doi: 10.1212/wnl.17.2.128. [DOI] [PubMed] [Google Scholar]
- 8.Gerber GB, Leonard A, Hantson P. Carcinogenicity mutagenicity and teratogenicity of manganese compounds. Crit Rev Oncol Hematol. 2002;42:25–34. doi: 10.1016/s1040-8428(01)00178-0. [DOI] [PubMed] [Google Scholar]
- 9.Occupational Employment and Wages. 2015 May; https://www.bls.gov/oes/current/oes514121.htm#(1)
- 10.Santos D, Batoreu C, Mateus L, Marreilha Dos Santos AP, Aschner M. Manganese in human parenteral nutrition: considerations for toxicity and biomonitoring. Neurotoxicology. 2014;43:36–45. doi: 10.1016/j.neuro.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joselow MM, Tobias E, Koehler R, Coleman S, Bogden J, Gause D. Manganese pollution in the city environment and its relationship to traffic density. Am J Public Health. 1978;68:557–560. doi: 10.2105/ajph.68.6.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gulson B, Mizon K, Taylor A, Korsch M, Stauber J, Davis JM, Louie H, Wu M, Swan H. Changes in manganese and lead in the environment and young children associated with the introduction of methylcyclopentadienyl manganese tricarbonyl in gasoline--preliminary results. Environ Res. 2006;100:100–114. doi: 10.1016/j.envres.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Wasserman GA, Liu X, Parvez F, Ahsan H, Levy D, Factor-Litvak P, Kline J, van Geen A, Slavkovich V, LoIacono NJ, Cheng Z, Zheng Y, Graziano JH. Water manganese exposure, children's intellectual function in Araihazar Bangladesh. Environ Health Perspect. 2006;114:124–129. doi: 10.1289/ehp.8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouchard MF, Sauve S, Barbeau B, Legrand M, Brodeur ME, Bouffard T, Limoges E, Bellinger DC, Mergler D. Intellectual impairment in school-age children exposed to manganese from drinking water. Environ Health Perspect. 2011;119:138–143. doi: 10.1289/ehp.1002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shukla GS, Singh S, Chandra SV. The interaction between manganese and ethanol in rats. Acta Pharmacol Toxicol (Copenh) 1978;43:354–362. doi: 10.1111/j.1600-0773.1978.tb02278.x. [DOI] [PubMed] [Google Scholar]
- 16.Kontur PJ, Fechter LD. Brain regional manganese levels and monoamine metabolism in manganese-treated neonatal rats. Neurotoxicol Teratol. 1988;10:295–303. doi: 10.1016/0892-0362(88)90031-1. [DOI] [PubMed] [Google Scholar]
- 17.Pappas BA, Zhang D, Davidson CM, Crowder T, Park GA, Fortin T. Perinatal manganese exposure: behavioral, neurochemical and histopathological effects in the rat. Neurotoxicol Teratol. 1997;19:17–25. doi: 10.1016/s0892-0362(96)00185-7. [DOI] [PubMed] [Google Scholar]
- 18.Shukla GS, Dubey MP, Chandra SV. Managanese-induced biochemical changes in growing versus adult rats. Arch Environ Contam Toxicol. 1980;9:383–391. doi: 10.1007/BF01055290. [DOI] [PubMed] [Google Scholar]
- 19.Scheuhammer AM, Cherian MG. Binding of manganese in human and rat plasma. Biochimica et biophysica acta. 1985;840:163–169. doi: 10.1016/0304-4165(85)90115-1. [DOI] [PubMed] [Google Scholar]
- 20.Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function and diseases. Antioxidants & redox signaling. 2011;15:1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horning KJ, Caito SW, Tipps KG, Bowman AB, Aschner M. Manganese Is Essential for Neuronal Health. Annu Rev Nutr. 2015;35:71–108. doi: 10.1146/annurev-nutr-071714-034419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jursa T, Smith DR. Ceruloplasmin alters the tissue disposition, neurotoxicity of manganese, but not its loading onto transferrin. Toxicol Sci. 2009;107:182–193. doi: 10.1093/toxsci/kfn231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davidsson L, Lonnerdal B, Sandstrom B, Kunz C, Keen CL. Identification of transferrin as the major plasma carrier protein for manganese introduced orally or intravenously or after in vitro addition in the rat. The Journal of nutrition. 1989;119:1461–1464. doi: 10.1093/jn/119.10.1461. [DOI] [PubMed] [Google Scholar]
- 24.Shen XM, Dryhurst G. Iron- and manganese-catalyzed autoxidation of dopamine in the presence of L-cysteine: possible insights into iron- and manganese-mediated dopaminergic neurotoxicity. Chem Res Toxicol. 1998;11:824–837. doi: 10.1021/tx980036t. [DOI] [PubMed] [Google Scholar]
- 25.Seo YA, Li Y, Wessling-Resnick M. Iron depletion increases manganese uptake and potentiates apoptosis through ER stress. Neurotoxicology. 2013;38:67–73. doi: 10.1016/j.neuro.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson JG, Fordahl SC, Cooney PT, Weaver TL, Colyer CL, Erikson KM. Extracellular norepinephrine, norepinephrine receptor and transporter protein and mRNA levels are differentially altered in the developing rat brain due to dietary iron deficiency and manganese exposure. Brain research. 2009;1281:1–14. doi: 10.1016/j.brainres.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amos-Kroohs RM, Davenport LL, Gutierrez A, Hufgard JR, Vorhees CV, Williams MT. Developmental manganese exposure in combination with developmental stress and iron deficiency: Effects on behavior and monoamines. Neurotoxicol Teratol. 2016;56:55–67. doi: 10.1016/j.ntt.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sziraki I, Mohanakumar KP, Rauhala P, Kim HG, Yeh KJ, Chiueh CC. Manganese: a transition metal protects nigrostriatal neurons from oxidative stress in the iron-induced animal model of parkinsonism. Neuroscience. 1998;85:1101–1111. doi: 10.1016/s0306-4522(97)00660-x. [DOI] [PubMed] [Google Scholar]
- 29.Sziraki I, Rauhala P, Koh KK, van Bergen P, Chiueh CC. Implications for atypical antioxidative properties of manganese in iron-induced brain lipid peroxidation and copper-dependent low density lipoprotein conjugation. Neurotoxicology. 1999;20:455–466. [PubMed] [Google Scholar]
- 30.Sziraki I, Rauhala P, Chiueh CC. Novel protective effect of manganese against ferrous citrate-induced lipid peroxidation and nigrostriatal neurodegeneration in vivo. Brain research. 1995;698:285–287. doi: 10.1016/0006-8993(95)01056-2. [DOI] [PubMed] [Google Scholar]
- 31.Ye Q, Kim J. Mutation in HFE gene decreases manganese accumulation and oxidative stress in the brain after olfactory manganese exposure. Metallomics : integrated biometal science. 2016;8:618–627. doi: 10.1039/c6mt00080k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye Q, Kim J. Loss of hfe function reverses impaired recognition memory caused by olfactory manganese exposure in mice. Toxicological research. 2015;31:17–23. doi: 10.5487/TR.2015.31.1.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye Q, Kim J. Effect of olfactory manganese exposure on anxiety-related behavior in a mouse model of iron overload hemochromatosis. Environmental toxicology and pharmacology. 2015;40:333–341. doi: 10.1016/j.etap.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borgstahl GE, Parge HE, Hickey MJ, Beyer WF, Jr, Hallewell RA, Tainer JA. The structure of human mitochondrial manganese superoxide dismutase reveals a novel tetrameric interface of two 4-helix bundles. Cell. 1992;71:107–118. doi: 10.1016/0092-8674(92)90270-m. [DOI] [PubMed] [Google Scholar]
- 35.Mizuno K, Whittaker MM, Bachinger HP, Whittaker JW. Calorimetric studies on the tight binding metal interactions of Escherichia coli manganese superoxide dismutase. The Journal of biological chemistry. 2004;279:27339–27344. doi: 10.1074/jbc.M400813200. [DOI] [PubMed] [Google Scholar]
- 36.Kang Y, He YX, Zhao MX, Li WF. Structures of native and Fe-substituted SOD2 from Saccharomyces cerevisiae. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011;67:1173–1178. doi: 10.1107/S1744309111029186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beard JL, Unger EL, Bianco LE, Paul T, Rundle SE, Jones BC. Early postnatal iron repletion overcomes lasting effects of gestational iron deficiency in rats. The Journal of nutrition. 2007;137:1176–1182. doi: 10.1093/jn/137.5.1176. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto K, Kobayashi N, Yoshitama K, Teramoto S, Komamine A. Isolation and purification of tyrosine hydroxylase from callus cultures of Portulaca grandiflora. Plant Cell Physiol. 2001;42:969–975. doi: 10.1093/pcp/pce125. [DOI] [PubMed] [Google Scholar]
- 39.Tuschl K, Mills PB, Clayton PT. Manganese and the brain. Int Rev Neurobiol. 2013;110:277–312. doi: 10.1016/B978-0-12-410502-7.00013-2. [DOI] [PubMed] [Google Scholar]
- 40.Chen P, Chakraborty S, Mukhopadhyay S, Lee E, Paoliello MM, Bowman AB, Aschner M. Manganese homeostasis in the nervous system. Journal of neurochemistry. 2015;134:601–610. doi: 10.1111/jnc.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Finley JW, Johnson PE, Johnson LK. Sex affects manganese absorption and retention by humans from a diet adequate in manganese. The American journal of clinical nutrition. 1994;60:949–955. doi: 10.1093/ajcn/60.6.949. [DOI] [PubMed] [Google Scholar]
- 42.Bai SP, Lu L, Luo XG, Liu B. Kinetics of manganese absorption in ligated small intestinal segments of broilers. Poult Sci. 2008;87:2596–2604. doi: 10.3382/ps.2008-00117. [DOI] [PubMed] [Google Scholar]
- 43.Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 44.Fleming MD, Romano MA, Su MA, Garrick LM, Garrick MD, Andrews NC. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:1148–1153. doi: 10.1073/pnas.95.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chua AC, Morgan EH. Manganese metabolism is impaired in the Belgrade laboratory rat. Journal of comparative physiology. B, Biochemical, systemic, and environmental physiology. 1997;167:361–369. doi: 10.1007/s003600050085. [DOI] [PubMed] [Google Scholar]
- 46.Knopfel M, Zhao L, Garrick MD. Transport of divalent transition-metal ions is lost in small-intestinal tissue of b/b Belgrade rats. Biochemistry. 2005;44:3454–3465. doi: 10.1021/bi048768+. [DOI] [PubMed] [Google Scholar]
- 47.Han M, Chang J, Kim J. Loss of divalent metal transporter 1 function promotes brain copper accumulation and increases impulsivity. Journal of neurochemistry. 2016;138:918–928. doi: 10.1111/jnc.13717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shawki A, Anthony SR, Nose Y, Engevik MA, Niespodzany EJ, Barrientos T, Ohrvik H, Worrell RT, Thiele DJ, Mackenzie B. Intestinal DMT1 is critical for iron absorption in the mouse but is not required for the absorption of copper or manganese. American journal of physiology. Gastrointestinal and liver physiology. 2015;309:G635–647. doi: 10.1152/ajpgi.00160.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davidson LA, Lonnerdal B. Specific binding of lactoferrin to brush-border membrane: ontogeny and effect of glycan chain. The American journal of physiology. 1988;254:G580–585. doi: 10.1152/ajpgi.1988.254.4.G580. [DOI] [PubMed] [Google Scholar]
- 50.Hu WL, Mazurier J, Sawatzki G, Montreuil J, Spik G. Lactotransferrin receptor of mouse small-intestinal brush border. Binding characteristics of membrane-bound and triton X-100-solubilized forms. The Biochemical journal. 1988;249:435–441. doi: 10.1042/bj2490435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mazurier J, Montreuil J, Spik G. Visualization of lactotransferrin brush-border receptors by ligand-blotting. Biochimica et biophysica acta. 1985;821:453–460. doi: 10.1016/0005-2736(85)90050-1. [DOI] [PubMed] [Google Scholar]
- 52.Lonnerdal B, Keen CL, Hurley LS. Manganese binding proteins in human and cow's milk. The American journal of clinical nutrition. 1985;41:550–559. doi: 10.1093/ajcn/41.3.550. [DOI] [PubMed] [Google Scholar]
- 53.Davidson LA, Lonnerdal B. Fe-saturation and proteolysis of human lactoferrin: effect on brush-border receptor-mediated uptake of Fe and Mn. The American journal of physiology. 1989;257:G930–934. doi: 10.1152/ajpgi.1989.257.6.G930. [DOI] [PubMed] [Google Scholar]
- 54.Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, Paw BH, Drejer A, Barut B, Zapata A, Law TC, Brugnara C, Lux SE, Pinkus GS, Pinkus JL, Kingsley PD, Palis J, Fleming MD, Andrews NC, Zon LI. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403:776–781. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]
- 55.Ganz T. Cellular iron: ferroportin is the only way out. Cell metabolism. 2005;1:155–157. doi: 10.1016/j.cmet.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 56.McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, Miret S, Bomford A, Peters TJ, Farzaneh F, Hediger MA, Hentze MW, Simpson RJ. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell. 2000;5:299–309. doi: 10.1016/s1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- 57.Seo YA, Wessling-Resnick M. Ferroportin deficiency impairs manganese metabolism in flatiron mice. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015;29:2726–2733. doi: 10.1096/fj.14-262592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seo YA, Elkhader JA, Wessling-Resnick M. Distribution of manganese and other biometals in flatiron mice. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine. 2016;29:147–155. doi: 10.1007/s10534-015-9904-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yin Z, Jiang H, Lee ES, Ni M, Erikson KM, Milatovic D, Bowman AB, Aschner M. Ferroportin is a manganese-responsive protein that decreases manganese cytotoxicity and accumulation. Journal of neurochemistry. 2010;112:1190–1198. doi: 10.1111/j.1471-4159.2009.06534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Madejczyk MS, Ballatori N. The iron transporter ferroportin can also function as a manganese exporter. Biochimica et biophysica acta. 2012;1818:651–657. doi: 10.1016/j.bbamem.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mitchell CJ, Shawki A, Ganz T, Nemeth E, Mackenzie B. Functional properties of human ferroportin, a cellular iron exporter reactive also with cobalt and zinc. American journal of physiology. Cell physiology. 2014;306:C450–459. doi: 10.1152/ajpcell.00348.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Begum NA, Kobayashi M, Moriwaki Y, Matsumoto M, Toyoshima K, Seya T. Mycobacterium bovis BCG cell wall, lipopolysaccharide induce a novel gene, BIGM103, encoding a 7-TM protein: identification of a new protein family having Zn-transporter and Zn-metalloprotease signatures. Genomics. 2002;80:630–645. doi: 10.1006/geno.2002.7000. [DOI] [PubMed] [Google Scholar]
- 63.Taylor KM, Morgan HE, Johnson A, Nicholson RI. Structure-function analysis of a novel member of the LIV-1 subfamily of zinc transporters, ZIP14. FEBS letters. 2005;579:427–432. doi: 10.1016/j.febslet.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 64.He L, Girijashanker K, Dalton TP, Reed J, Li H, Soleimani M, Nebert DW. ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: characterization of transporter properties. Molecular pharmacology. 2006;70:171–180. doi: 10.1124/mol.106.024521. [DOI] [PubMed] [Google Scholar]
- 65.Girijashanker K, He L, Soleimani M, Reed JM, Li H, Liu Z, Wang B, Dalton TP, Nebert DW. Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: similarities to the ZIP8 transporter. Molecular pharmacology. 2008;73:1413–1423. doi: 10.1124/mol.107.043588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guthrie GJ, Aydemir TB, Troche C, Martin AB, Chang SM, Cousins RJ. Influence of ZIP14 (slc39A14) on intestinal zinc processing and barrier function. American journal of physiology. Gastrointestinal and liver physiology. 2015;308:G171–178. doi: 10.1152/ajpgi.00021.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tuschl K, Meyer E, Valdivia LE, Zhao N, Dadswell C, Abdul-Sada A, Hung CY, Simpson MA, Chong WK, Jacques TS, Woltjer RL, Eaton S, Gregory A, Sanford L, Kara E, Houlden H, Cuno SM, Prokisch H, Valletta L, Tiranti V, Younis R, Maher ER, Spencer J, Straatman-Iwanowska A, Gissen P, Selim LA, Pintos-Morell G, Coroleu-Lletget W, Mohammad SS, Yoganathan S, Dale RC, Thomas M, Rihel J, Bodamer OA, Enns CA, Hayflick SJ, Clayton PT, Mills PB, Kurian MA, Wilson SW. Mutations in SLC39A14 disrupt manganese homeostasis and cause childhood-onset parkinsonism-dystonia. Nature communications. 2016;7:11601. doi: 10.1038/ncomms11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boycott KM, Beaulieu CL, Kernohan KD, Gebril OH, Mhanni A, Chudley AE, Redl D, Qin W, Hampson S, Kury S, Tetreault M, Puffenberger EG, Scott JN, Bezieau S, Reis A, Uebe S, Schumacher J, Hegele RA, McLeod DR, Galvez-Peralta M, Majewski J, Ramaekers VT, Care4Rare Canada C, Nebert DW, Innes AM, Parboosingh JS, Abou Jamra R. Autosomal-Recessive Intellectual Disability with Cerebellar Atrophy Syndrome Caused by Mutation of the Manganese and Zinc Transporter Gene SLC39A8. American journal of human genetics. 2015;97:886–893. doi: 10.1016/j.ajhg.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park JH, Hogrebe M, Gruneberg M, DuChesne I, von der Heiden AL, Reunert J, Schlingmann KP, Boycott KM, Beaulieu CL, Mhanni AA, Innes AM, Hortnagel K, Biskup S, Gleixner EM, Kurlemann G, Fiedler B, Omran H, Rutsch F, Wada Y, Tsiakas K, Santer R, Nebert DW, Rust S, Marquardt T. SLC39A8 Deficiency: A Disorder of Manganese Transport and Glycosylation. American journal of human genetics. 2015;97:894–903. doi: 10.1016/j.ajhg.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fujishiro H, Yano Y, Takada Y, Tanihara M, Himeno S. Roles of ZIP8, ZIP14, and DMT1 in transport of cadmium and manganese in mouse kidney proximal tubule cells. Metallomics : integrated biometal science. 2012;4:700–708. doi: 10.1039/c2mt20024d. [DOI] [PubMed] [Google Scholar]
- 71.Ghio AJ, Wang X, Silbajoris R, Garrick MD, Piantadosi CA, Yang F. DMT1 expression is increased in the lungs of hypotransferrinemic mice. American journal of physiology. Lung cellular and molecular physiology. 2003;284:L938–944. doi: 10.1152/ajplung.00225.2002. [DOI] [PubMed] [Google Scholar]
- 72.Heilig EA, Thompson KJ, Molina RM, Ivanov AR, Brain JD, Wessling-Resnick M. Manganese and iron transport across pulmonary epithelium. American journal of physiology. Lung cellular and molecular physiology. 2006;290:L1247–1259. doi: 10.1152/ajplung.00450.2005. [DOI] [PubMed] [Google Scholar]
- 73.Heilig E, Molina R, Donaghey T, Brain JD, Wessling-Resnick M. Pharmacokinetics of pulmonary manganese absorption: evidence for increased susceptibility to manganese loading in iron-deficient rats. American journal of physiology. Lung cellular and molecular physiology. 2005;288:L887–893. doi: 10.1152/ajplung.00382.2004. [DOI] [PubMed] [Google Scholar]
- 74.Garrick LM, Dolan KG, Romano MA, Garrick MD. Non-transferrin-bound iron uptake in Belgrade and normal rat erythroid cells. Journal of cellular physiology. 1999;178:349–358. doi: 10.1002/(SICI)1097-4652(199903)178:3<349::AID-JCP9>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 75.Gunter TE, Gerstner B, Gunter KK, Malecki J, Gelein R, Valentine WM, Aschner M, Yule DI. Manganese transport via the transferrin mechanism. Neurotoxicology. 2013;34:118–127. doi: 10.1016/j.neuro.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aschner M, Gannon M. Manganese (Mn) transport across the rat blood-brain barrier: saturable and transferrin-dependent transport mechanisms. Brain research bulletin. 1994;33:345–349. doi: 10.1016/0361-9230(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 77.Dalton TP, He L, Wang B, Miller ML, Jin L, Stringer KF, Chang X, Baxter CS, Nebert DW. Identification of mouse SLC39A8 as the transporter responsible for cadmium-induced toxicity in the testis. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:3401–3406. doi: 10.1073/pnas.0406085102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang B, Schneider SN, Dragin N, Girijashanker K, Dalton TP, He L, Miller ML, Stringer KF, Soleimani M, Richardson DD, Nebert DW. Enhanced cadmium-induced testicular necrosis and renal proximal tubule damage caused by gene-dose increase in a Slc39a8-transgenic mouse line. American journal of physiology. Cell physiology. 2007;292:C1523–1535. doi: 10.1152/ajpcell.00409.2006. [DOI] [PubMed] [Google Scholar]
- 79.Kim J, Buckett PD, Wessling-Resnick M. Absorption of manganese and iron in a mouse model of hemochromatosis. PloS one. 2013;8:e64944. doi: 10.1371/journal.pone.0064944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brenneman KA, Wong BA, Buccellato MA, Costa ER, Gross EA, Dorman DC. Direct olfactory transport of inhaled manganese ((54)MnCl(2)) to the rat brain: toxicokinetic investigations in a unilateral nasal occlusion model. Toxicol Appl Pharmacol. 2000;169:238–248. doi: 10.1006/taap.2000.9073. [DOI] [PubMed] [Google Scholar]
- 81.Dorman DC, Brenneman KA, McElveen AM, Lynch SE, Roberts KC, Wong BA. Olfactory transport: a direct route of delivery of inhaled manganese phosphate to the rat brain. J Toxicol Environ Health A. 2002;65:1493–1511. doi: 10.1080/00984100290071630. [DOI] [PubMed] [Google Scholar]
- 82.Tjalve H, Henriksson J, Tallkvist J, Larsson BS, Lindquist NG. Uptake of manganese and cadmium from the nasal mucosa into the central nervous system via olfactory pathways in rats. Pharmacol Toxicol. 1996;79:347–356. doi: 10.1111/j.1600-0773.1996.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 83.Avila DS, Puntel RL, Aschner M. Manganese in health and disease. Metal ions in life sciences. 2013;13:199–227. doi: 10.1007/978-94-007-7500-8_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park RM, Schulte PA, Bowman JD, Walker JT, Bondy SC, Yost MG, Touchstone JA, Dosemeci M. Potential occupational risks for neurodegenerative diseases. American journal of industrial medicine. 2005;48:63–77. doi: 10.1002/ajim.20178. [DOI] [PubMed] [Google Scholar]
- 85.Lucchini RG, Martin CJ, Doney BC. From manganism to manganese-induced parkinsonism: a conceptual model based on the evolution of exposure. Neuromolecular medicine. 2009;11:311–321. doi: 10.1007/s12017-009-8108-8. [DOI] [PubMed] [Google Scholar]
- 86.Pautler RG, Koretsky AP. Tracing odor-induced activation in the olfactory bulbs of mice using manganese-enhanced magnetic resonance imaging. NeuroImage. 2002;16:441–448. doi: 10.1006/nimg.2002.1075. [DOI] [PubMed] [Google Scholar]
- 87.Thompson K, Molina RM, Donaghey T, Schwob JE, Brain JD, Wessling-Resnick M. Olfactory uptake of manganese requires DMT1 and is enhanced by anemia. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2007;21:223–230. doi: 10.1096/fj.06-6710com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ma C, Schneider SN, Miller M, Nebert DW, Lind C, Roda SM, Afton SE, Caruso JA, Genter MB. Manganese accumulation in the mouse ear following systemic exposure. Journal of biochemical and molecular toxicology. 2008;22:305–310. doi: 10.1002/jbt.20241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Irie S, Tavassoli M. Transferrin-mediated cellular iron uptake. The American journal of the medical sciences. 1987;293:103–111. doi: 10.1097/00000441-198702000-00007. [DOI] [PubMed] [Google Scholar]
- 90.Dickinson TK, Devenyi AG, Connor JR. Distribution of injected iron 59 and manganese 54 in hypotransferrinemic mice. The Journal of laboratory and clinical medicine. 1996;128:270–278. doi: 10.1016/s0022-2143(96)90028-1. [DOI] [PubMed] [Google Scholar]
- 91.Herrera C, Pettiglio MA, Bartnikas TB. Investigating the role of transferrin in the distribution of iron, manganese, copper, and zinc. J Biol Inorg Chem. 2014;19:869–877. doi: 10.1007/s00775-014-1118-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Papavasiliou PS, Miller ST, Cotzias GC. Role of liver in regulating distribution and excretion of manganese. The American journal of physiology. 1966;211:211–216. doi: 10.1152/ajplegacy.1966.211.1.211. [DOI] [PubMed] [Google Scholar]
- 93.Malecki EA, Devenyi AG, Beard JL, Connor JR. Transferrin response in normal and iron-deficient mice heterozygotic for hypotransferrinemia; effects on iron and manganese accumulation. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine. 1998;11:265–276. doi: 10.1023/a:1009280922387. [DOI] [PubMed] [Google Scholar]
- 94.Burdo JR, Menzies SL, Simpson IA, Garrick LM, Garrick MD, Dolan KG, Haile DJ, Beard JL, Connor JR. Distribution of divalent metal transporter 1 and metal transport protein 1 in the normal and Belgrade rat. Journal of neuroscience research. 2001;66:1198–1207. doi: 10.1002/jnr.1256. [DOI] [PubMed] [Google Scholar]
- 95.Pelizzoni I, Zacchetti D, Smith CP, Grohovaz F, Codazzi F. Expression of divalent metal transporter 1 in primary hippocampal neurons: reconsidering its role in non-transferrin-bound iron influx. Journal of neurochemistry. 2012;120:269–278. doi: 10.1111/j.1471-4159.2011.07578.x. [DOI] [PubMed] [Google Scholar]
- 96.Crossgrove JS, Yokel RA. Manganese distribution across the blood-brain barrier III. The divalent metal transporter-1 is not the major mechanism mediating brain manganese uptake. Neurotoxicology. 2004;25:451–460. doi: 10.1016/j.neuro.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 97.Moos T, Skjoerringe T, Gosk S, Morgan EH. Brain capillary endothelial cells mediate iron transport into the brain by segregating iron from transferrin without the involvement of divalent metal transporter 1. Journal of neurochemistry. 2006;98:1946–1958. doi: 10.1111/j.1471-4159.2006.04023.x. [DOI] [PubMed] [Google Scholar]
- 98.Carlson ES, Tkac I, Magid R, O'Connor MB, Andrews NC, Schallert T, Gunshin H, Georgieff MK, Petryk A. Iron is essential for neuron development and memory function in mouse hippocampus. The Journal of nutrition. 2009;139:672–679. doi: 10.3945/jn.108.096354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fujishiro H, Ohashi T, Takuma M, Himeno S. Suppression of ZIP8 expression is a common feature of cadmium-resistant and manganese-resistant RBL-2H3 cells. Metallomics : integrated biometal science. 2013;5:437–444. doi: 10.1039/c3mt00003f. [DOI] [PubMed] [Google Scholar]
- 100.Fujishiro H, Yoshida M, Nakano Y, Himeno S. Interleukin-6 enhances manganese accumulation in SH-SY5Y cells: implications of the up-regulation of ZIP14 and the down-regulation of ZnT10. Metallomics : integrated biometal science. 2014;6:944–949. doi: 10.1039/c3mt00362k. [DOI] [PubMed] [Google Scholar]
- 101.Jenkitkasemwong S, Wang CY, Mackenzie B, Knutson MD. Physiologic implications of metal-ion transport by ZIP14 and ZIP8. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine. 2012;25:643–655. doi: 10.1007/s10534-012-9526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yokel RA, Crossgrove JS, Bukaveckas BL. Manganese distribution across the blood-brain barrier. II. Manganese efflux from the brain does not appear to be carrier mediated. Neurotoxicology. 2003;24:15–22. doi: 10.1016/s0161-813x(02)00090-6. [DOI] [PubMed] [Google Scholar]
- 103.Quadri M, Federico A, Zhao T, Breedveld GJ, Battisti C, Delnooz C, Severijnen LA, Di Toro Mammarella L, Mignarri A, Monti L, Sanna A, Lu P, Punzo F, Cossu G, Willemsen R, Rasi F, Oostra BA, van de Warrenburg BP, Bonifati V. Mutations in SLC30A10 cause parkinsonism, dystonia with hypermanganesemia, polycythemia and chronic liver disease. American journal of human genetics. 2012;90:467–477. doi: 10.1016/j.ajhg.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tuschl K, Clayton PT, Gospe SM, Jr, Gulab S, Ibrahim S, Singhi P, Aulakh R, Ribeiro RT, Barsottini OG, Zaki MS, Del Rosario ML, Dyack S, Price V, Rideout A, Gordon K, Wevers RA, Chong WK, Mills PB. Syndrome of hepatic cirrhosis, dystonia, polycythemia, hypermanganesemia caused by mutations in SLC30A10, a manganese transporter in man. American journal of human genetics. 2012;90:457–466. doi: 10.1016/j.ajhg.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lechpammer M, Clegg MS, Muzar Z, Huebner PA, Jin LW, Gospe SM., Jr Pathology of inherited manganese transporter deficiency. Annals of neurology. 2014;75:608–612. doi: 10.1002/ana.24131. [DOI] [PubMed] [Google Scholar]
- 106.Mukhtiar K, Ibrahim S, Tuschl K, Mills P. Hypermanganesemia with Dystonia, Polycythemia and Cirrhosis (HMDPC) due to mutation in the SLC30A10 gene. Brain & development. 2016;38:862–865. doi: 10.1016/j.braindev.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 107.Quadri M, Kamate M, Sharma S, Olgiati S, Graafland J, Breedveld GJ, Kori I, Hattiholi V, Jain P, Aneja S, Kumar A, Gulati P, Goel M, Talukdar B, Bonifati V. Manganese transport disorder: novel SLC30A10 mutations and early phenotypes. Movement disorders : official journal of the Movement Disorder Society. 2015;30:996–1001. doi: 10.1002/mds.26202. [DOI] [PubMed] [Google Scholar]
- 108.Seve M, Chimienti F, Devergnas S, Favier A. In silico identification and expression of SLC30 family genes: an expressed sequence tag data mining strategy for the characterization of zinc transporters' tissue expression. BMC Genomics. 2004;5:32. doi: 10.1186/1471-2164-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen P, Bowman AB, Mukhopadhyay S, Aschner M. SLC30A10: A novel manganese transporter. Worm. 2015;4:e1042648. doi: 10.1080/21624054.2015.1042648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Leyva-Illades D, Chen P, Zogzas CE, Hutchens S, Mercado JM, Swaim CD, Morrisett RA, Bowman AB, Aschner M, Mukhopadhyay S. SLC30A10 is a cell surface-localized manganese efflux transporter, and parkinsonism-causing mutations block its intracellular trafficking and efflux activity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:14079–14095. doi: 10.1523/JNEUROSCI.2329-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nishito Y, Tsuji N, Fujishiro H, Takeda TA, Yamazaki T, Teranishi F, Okazaki F, Matsunaga A, Tuschl K, Rao R, Kono S, Miyajima H, Narita H, Himeno S, Kambe T. Direct Comparison of Manganese Detoxification/Efflux Proteins and Molecular Characterization of ZnT10 Protein as a Manganese Transporter. The Journal of biological chemistry. 2016;291:14773–14787. doi: 10.1074/jbc.M116.728014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zogzas CE, Aschner M, Mukhopadhyay S. Structural Elements in the Transmembrane and Cytoplasmic Domains of the Metal Transporter SLC30A10 Are Required for Its Manganese Efflux Activity. The Journal of biological chemistry. 2016;291:15940–15957. doi: 10.1074/jbc.M116.726935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhao Y, Feresin RG, Falcon-Perez JM, Salazar G. Differential Targeting of SLC30A10/ZnT10 Heterodimers to Endolysosomal Compartments Modulates EGF-Induced MEK/ERK1/2 Activity. Traffic. 2016;17:267–288. doi: 10.1111/tra.12371. [DOI] [PubMed] [Google Scholar]
- 114.Claro da Silva T, Hiller C, Gai Z, Kullak-Ublick GA. Vitamin D3 transactivates the zinc and manganese transporter SLC30A10 via the Vitamin D receptor. The Journal of steroid biochemistry and molecular biology. 2016;163:77–87. doi: 10.1016/j.jsbmb.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 115.Durr G, Strayle J, Plemper R, Elbs S, Klee SK, Catty P, Wolf DH, Rudolph HK. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+, Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Molecular biology of the cell. 1998;9:1149–1162. doi: 10.1091/mbc.9.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mukhopadhyay S, Linstedt AD. Identification of a gain-of-function mutation in a Golgi P-type ATPase that enhances Mn2+ efflux and protects against toxicity. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:858–863. doi: 10.1073/pnas.1013642108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Leitch S, Feng M, Muend S, Braiterman LT, Hubbard AL, Rao R. Vesicular distribution of Secretory Pathway Ca(2)+-ATPase isoform 1 and a role in manganese detoxification in liver-derived polarized cells. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine. 2011;24:159–170. doi: 10.1007/s10534-010-9384-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ramonet D, Podhajska A, Stafa K, Sonnay S, Trancikova A, Tsika E, Pletnikova O, Troncoso JC, Glauser L, Moore DJ. PARK9-associated ATP13A2 localizes to intracellular acidic vesicles and regulates cation homeostasis and neuronal integrity. Human molecular genetics. 2012;21:1725–1743. doi: 10.1093/hmg/ddr606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tan J, Zhang T, Jiang L, Chi J, Hu D, Pan Q, Wang D, Zhang Z. Regulation of intracellular manganese homeostasis by Kufor-Rakeb syndrome-associated ATP13A2 protein. The Journal of biological chemistry. 2011;286:29654–29662. doi: 10.1074/jbc.M111.233874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zeron HM, Rodriguez MR, Montes S, Castaneda CR. Blood manganese levels in patients with hepatic encephalopathy. Journal of trace elements in medicine and biology : organ of the Society for Minerals and Trace Elements (GMS) 2011;25:225–229. doi: 10.1016/j.jtemb.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 121.Hauser RA, Zesiewicz TA, Rosemurgy AS, Martinez C, Olanow CW. Manganese intoxication and chronic liver failure. Annals of neurology. 1994;36:871–875. doi: 10.1002/ana.410360611. [DOI] [PubMed] [Google Scholar]
- 122.Tichy M, Cikrt M. Manganese transfer into the bile in rats. Arch Toxikol. 1972;29:51–58. doi: 10.1007/BF00316514. [DOI] [PubMed] [Google Scholar]
- 123.Klaassen CD. Biliary excretion of manganese in rats, rabbits, and dogs. Toxicology and applied pharmacology. 1974;29:458–468. doi: 10.1016/0041-008x(74)90117-3. [DOI] [PubMed] [Google Scholar]
- 124.Madejczyk MS, Boyer JL, Ballatori N. Hepatic uptake, biliary excretion of manganese in the little skate, Leucoraja erinacea. Comparative biochemistry and physiology Toxicology & pharmacology : CBP. 2009;149:566–571. doi: 10.1016/j.cbpc.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. The Journal of biological chemistry. 2000;275:19906–19912. doi: 10.1074/jbc.M000713200. [DOI] [PubMed] [Google Scholar]
- 126.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System 1993–2005. Public Health Nutr. 2009;12:444–454. doi: 10.1017/S1368980008002401. [DOI] [PubMed] [Google Scholar]
- 127.Anderson CP, Shen M, Eisenstein RS, Leibold EA. Mammalian iron metabolism and its control by iron regulatory proteins. Biochimica et biophysica acta. 2012;1823:1468–1483. doi: 10.1016/j.bbamcr.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hubert N, Hentze MW. Previously uncharacterized isoforms of divalent metal transporter (DMT)-1: implications for regulation and cellular function. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:12345–12350. doi: 10.1073/pnas.192423399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Canonne-Hergaux F, Gruenheid S, Ponka P, Gros P. Cellular and subcellular localization of the Nramp2 iron transporter in the intestinal brush border and regulation by dietary iron. Blood. 1999;93:4406–4417. [PubMed] [Google Scholar]
- 130.Lymboussaki A, Pignatti E, Montosi G, Garuti C, Haile DJ, Pietrangelo A. The role of the iron responsive element in the control of ferroportin1/IREG1/MTP1 gene expression. J Hepatol. 2003;39:710–715. doi: 10.1016/s0168-8278(03)00408-2. [DOI] [PubMed] [Google Scholar]
- 131.Chua AC, Morgan EH. Effects of iron deficiency and iron overload on manganese uptake and deposition in the brain and other organs of the rat. Biol Trace Elem Res. 1996;55:39–54. doi: 10.1007/BF02784167. [DOI] [PubMed] [Google Scholar]
- 132.Yokoi K, Kimura M, Itokawa Y. Effect of dietary iron deficiency on mineral levels in tissues of rats. Biol Trace Elem Res. 1991;29:257–265. doi: 10.1007/BF03032682. [DOI] [PubMed] [Google Scholar]
- 133.Park S, Sim CS, Lee H, Kim Y. Blood manganese concentration is elevated in infants with iron deficiency. Biological trace element research. 2013;155:184–189. doi: 10.1007/s12011-013-9782-9. [DOI] [PubMed] [Google Scholar]
- 134.Rahman MA, Rahman B, Ahmed N. High blood manganese in iron-deficient children in Karachi. Public Health Nutr. 2013;16:1677–1683. doi: 10.1017/S1368980013000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kim Y, Lee BK. Iron deficiency increases blood manganese level in the Korean general population according to KNHANES 2008. Neurotoxicology. 2011;32:247–254. doi: 10.1016/j.neuro.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 136.Mena I, Horiuchi K, Burke K, Cotzias GC. Chronic manganese poisoning. Individual susceptibility and absorption of iron. Neurology. 1969;19:1000–1006. doi: 10.1212/wnl.19.10.1000. [DOI] [PubMed] [Google Scholar]
- 137.Zoller H, Koch RO, Theurl I, Obrist P, Pietrangelo A, Montosi G, Haile DJ, Vogel W, Weiss G. Expression of the duodenal iron transporters divalent-metal transporter 1 and ferroportin 1 in iron deficiency and iron overload. Gastroenterology. 2001;120:1412–1419. doi: 10.1053/gast.2001.24033. [DOI] [PubMed] [Google Scholar]
- 138.Meltzer HM, Brantsaeter AL, Borch-Iohnsen B, Ellingsen DG, Alexander J, Thomassen Y, Stigum H, Ydersbond TA. Low iron stores are related to higher blood concentrations of manganese, cobalt, cadmium in non-smoking, Norwegian women in the HUNT 2 study. Environ Res. 2010;110:497–504. doi: 10.1016/j.envres.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 139.Finley JW. Manganese absorption and retention by young women is associated with serum ferritin concentration. Am J Clin Nutr. 1999;70:37–43. doi: 10.1093/ajcn/70.1.37. [DOI] [PubMed] [Google Scholar]
- 140.Davis CD, Malecki EA, Greger JL. Interactions among dietary manganese, heme iron, and nonheme iron in women. Am J Clin Nutr. 1992;56:926–932. doi: 10.1093/ajcn/56.5.926. [DOI] [PubMed] [Google Scholar]
- 141.Anderson JG, Fordahl SC, Cooney PT, Weaver TL, Colyer CL, Erikson KM. Manganese exposure alters extracellular GABA, GABA receptor and transporter protein and mRNA levels in the developing rat brain. Neurotoxicology. 2008;29:1044–1053. doi: 10.1016/j.neuro.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Herrero Hernandez E, Valentini MC, Discalzi G. T1-weighted hyperintensity in basal ganglia at brain magnetic resonance imaging: are different pathologies sharing a common mechanism? Neurotoxicology. 2002;23:669–674. doi: 10.1016/S0161-813X(02)00016-5. [DOI] [PubMed] [Google Scholar]
- 143.Kim Y, Park JK, Choi Y, Yoo CI, Lee CR, Lee H, Lee JH, Kim SR, Jeong TH, Yoon CS, Park JH. Blood manganese concentration is elevated in iron deficiency anemia patients, whereas globus pallidus signal intensity is minimally affected. Neurotoxicology. 2005;26:107–111. doi: 10.1016/j.neuro.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 144.Kim Y, Kim KS, Yang JS, Park IJ, Kim E, Jin Y, Kwon KR, Chang KH, Kim JW, Park SH, Lim HS, Cheong HK, Shin YC, Park J, Moon Y. Increase in signal intensities on T1-weighted magnetic resonance images in asymptomatic manganese-exposed workers. Neurotoxicology. 1999;20:901–907. [PubMed] [Google Scholar]
- 145.Park NH, Park JK, Choi Y, Yoo CI, Lee CR, Lee H, Kim HK, Kim SR, Jeong TH, Park J, Yoon CS, Kim Y. Whole blood manganese correlates with high signal intensities on T1-weighted MRI in patients with liver cirrhosis. Neurotoxicology. 2003;24:909–915. doi: 10.1016/S0161-813X(03)00111-6. [DOI] [PubMed] [Google Scholar]
- 146.Erikson KM, Aschner M. Increased manganese uptake by primary astrocyte cultures with altered iron status is mediated primarily by divalent metal transporter. Neurotoxicology. 2006;27:125–130. doi: 10.1016/j.neuro.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 147.Siddappa AJ, Rao RB, Wobken JD, Casperson K, Leibold EA, Connor JR, Georgieff MK. Iron deficiency alters iron regulatory protein and iron transport protein expression in the perinatal rat brain. Pediatric research. 2003;53:800–807. doi: 10.1203/01.PDR.0000058922.67035.D5. [DOI] [PubMed] [Google Scholar]
- 148.Ke Y, Chang YZ, Duan XL, Du JR, Zhu L, Wang K, Yang XD, Ho KP, Qian ZM. Age-dependent and iron-independent expression of two mRNA isoforms of divalent metal transporter 1 in rat brain. Neurobiology of aging. 2005;26:739–748. doi: 10.1016/j.neurobiolaging.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 149.Moos T, Oates PS, Morgan EH. Expression of the neuronal transferrin receptor is age dependent and susceptible to iron deficiency. J Comp Neurol. 1998;398:420–430. [PubMed] [Google Scholar]
- 150.Fitsanakis VA, Zhang N, Avison MJ, Erikson KM, Gore JC, Aschner M. Changes in dietary iron exacerbate regional brain manganese accumulation as determined by magnetic resonance imaging. Toxicol Sci. 2011;120:146–153. doi: 10.1093/toxsci/kfq376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tandy S, Williams M, Leggett A, Lopez-Jimenez M, Dedes M, Ramesh B, Srai SK, Sharp P. Nramp2 expression is associated with pH-dependent iron uptake across the apical membrane of human intestinal Caco-2 cells. J Biol Chem. 2000;275:1023–1029. doi: 10.1074/jbc.275.2.1023. [DOI] [PubMed] [Google Scholar]
- 152.Trinder D, Oates PS, Thomas C, Sadleir J, Morgan EH. Localisation of divalent metal transporter 1 (DMT1) to the microvillus membrane of rat duodenal enterocytes in iron deficiency, but to hepatocytes in iron overload. Gut. 2000;46:270–276. doi: 10.1136/gut.46.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hansen SL, Trakooljul N, Liu HC, Moeser AJ, Spears JW. Iron transporters are differentially regulated by dietary iron, and modifications are associated with changes in manganese metabolism in young pigs. J Nutr. 2009;139:1474–1479. doi: 10.3945/jn.109.105866. [DOI] [PubMed] [Google Scholar]
- 154.Nandar W, Connor JR. HFE gene variants affect iron in the brain. J Nutr. 2011;141:729S–739S. doi: 10.3945/jn.110.130351. [DOI] [PubMed] [Google Scholar]
- 155.Zhou XY, Tomatsu S, Fleming RE, Parkkila S, Waheed A, Jiang J, Fei Y, Brunt EM, Ruddy DA, Prass CE, Schatzman RC, O'Neill R, Britton RS, Bacon BR, Sly WS. HFE gene knockout produces mouse model of hereditary hemochromatosis. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:2492–2497. doi: 10.1073/pnas.95.5.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fleming RE, Sly WS. Mechanisms of iron accumulation in hereditary hemochromatosis. Annu Rev Physiol. 2002;64:663–680. doi: 10.1146/annurev.physiol.64.081501.155838. [DOI] [PubMed] [Google Scholar]
- 157.Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A, Dormishian F, Domingo R, Jr, Ellis MC, Fullan A, Hinton LM, Jones NL, Kimmel BE, Kronmal GS, Lauer P, Lee VK, Loeb DB, Mapa FA, McClelland E, Meyer NC, Mintier GA, Moeller N, Moore T, Morikang E, Prass CE, Quintana L, Starnes SM, Schatzman RC, Brunke KJ, Drayna DT, Risch NJ, Bacon BR, Wolff RK. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nature genetics. 1996;13:399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 158.Bradley LA, Johnson DD, Palomaki GE, Haddow JE, Robertson NH, Ferrie RM. Hereditary haemochromatosis mutation frequencies in the general population. Journal of medical screening. 1998;5:34–36. doi: 10.1136/jms.5.1.34. [DOI] [PubMed] [Google Scholar]
- 159.Pietrangelo A. Hereditary hemochromatosis--a new look at an old disease. N Engl J Med. 2004;350:2383–2397. doi: 10.1056/NEJMra031573. [DOI] [PubMed] [Google Scholar]
- 160.Merryweather-Clarke AT, Pointon JJ, Jouanolle AM, Rochette J, Robson KJ. Geography of HFE C282Y and H63D mutations. Genet Test. 2000;4:183–198. doi: 10.1089/10906570050114902. [DOI] [PubMed] [Google Scholar]
- 161.Hanson EH, Imperatore G, Burke W. HFE gene and hereditary hemochromatosis: a HuGE review. Human Genome Epidemiology. American journal of epidemiology. 2001;154:193–206. doi: 10.1093/aje/154.3.193. [DOI] [PubMed] [Google Scholar]
- 162.Nielsen JE, Jensen LN, Krabbe K. Hereditary haemochromatosis: a case of iron accumulation in the basal ganglia associated with a parkinsonian syndrome. Journal of neurology, neurosurgery, and psychiatry. 1995;59:318–321. doi: 10.1136/jnnp.59.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 164.Brasse-Lagnel C, Karim Z, Letteron P, Bekri S, Bado A, Beaumont C. Intestinal DMT1 cotransporter is down-regulated by hepcidin via proteasome internalization and degradation. Gastroenterology. 2011;140:1261–1271. e1261. doi: 10.1053/j.gastro.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 165.Nicolas G, Bennoun M, Devaux I, Beaumont C, Grandchamp B, Kahn A, Vaulont S. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:8780–8785. doi: 10.1073/pnas.151179498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fleming RE, Sly WS. Hepcidin: a putative iron-regulatory hormone relevant to hereditary hemochromatosis and the anemia of chronic disease. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:8160–8162. doi: 10.1073/pnas.161296298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ahmad KA, Ahmann JR, Migas MC, Waheed A, Britton RS, Bacon BR, Sly WS, Fleming RE. Decreased liver hepcidin expression in the Hfe knockout mouse. Blood cells, molecules & diseases. 2002;29:361–366. doi: 10.1006/bcmd.2002.0575. [DOI] [PubMed] [Google Scholar]
- 168.Claus Henn B, Kim J, Wessling-Resnick M, Tellez-Rojo MM, Jayawardene I, Ettinger AS, Hernandez-Avila M, Schwartz J, Christiani DC, Hu H, Wright RO. Associations of iron metabolism genes with blood manganese levels: a population-based study with validation data from animal models. Environ Health. 2011;10:97. doi: 10.1186/1476-069X-10-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jouihan HA, Cobine PA, Cooksey RC, Hoagland EA, Boudina S, Abel ED, Winge DR, McClain DA. Iron-mediated inhibition of mitochondrial manganese uptake mediates mitochondrial dysfunction in a mouse model of hemochromatosis. Molecular medicine. 2008;14:98–108. doi: 10.2119/2007-00114.Jouihan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fitsanakis VA, Zhang N, Anderson JG, Erikson KM, Avison MJ, Gore JC, Aschner M. Measuring brain manganese and iron accumulation in rats following 14 weeks of low-dose manganese treatment using atomic absorption spectroscopy and magnetic resonance imaging. Toxicol Sci. 2008;103:116–124. doi: 10.1093/toxsci/kfn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nandar W, Neely EB, Unger E, Connor JR. A mutation in the HFE gene is associated with altered brain iron profiles and increased oxidative stress in mice. Biochimica et biophysica acta. 2013;1832:729–741. doi: 10.1016/j.bbadis.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 172.Alsulimani HH, Ye Q, Kim J. Effect of Hfe Deficiency on Memory Capacity and Motor Coordination after Manganese Exposure by Drinking Water in Mice. Toxicol Res. 2015;31:347–354. doi: 10.5487/TR.2015.31.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cotzias GC. Manganese in health and disease. Physiol Rev. 1958;38:503–532. doi: 10.1152/physrev.1958.38.3.503. [DOI] [PubMed] [Google Scholar]
- 174.Dietz MC, Ihrig A, Wrazidlo W, Bader M, Jansen O, Triebig G. Results of magnetic resonance imaging in long-term manganese dioxide-exposed workers. Environ Res. 2001;85:37–40. doi: 10.1006/enrs.2000.4068. [DOI] [PubMed] [Google Scholar]
- 175.Josephs KA, Ahlskog JE, Klos KJ, Kumar N, Fealey RD, Trenerry MR, Cowl CT. Neurologic manifestations in welders with pallidal MRI T1 hyperintensity. Neurology. 2005;64:2033–2039. doi: 10.1212/01.WNL.0000167411.93483.A1. [DOI] [PubMed] [Google Scholar]
- 176.Klos KJ, Chandler M, Kumar N, Ahlskog JE, Josephs KA. Neuropsychological profiles of manganese neurotoxicity. Eur J Neurol. 2006;13:1139–1141. doi: 10.1111/j.1468-1331.2006.01407.x. [DOI] [PubMed] [Google Scholar]
- 177.Uchino A, Noguchi T, Nomiyama K, Takase Y, Nakazono T, Nojiri J, Kudo S. Manganese accumulation in the brain: MR imaging. Neuroradiology. 2007;49:715–720. doi: 10.1007/s00234-007-0243-z. [DOI] [PubMed] [Google Scholar]
- 178.Dobson AW, Erikson KM, Aschner M. Manganese neurotoxicity. Annals of the New York Academy of Sciences. 2004;1012:115–128. doi: 10.1196/annals.1306.009. [DOI] [PubMed] [Google Scholar]
- 179.HaMai D, Bondy SC. Oxidative basis of manganese neurotoxicity. Annals of the New York Academy of Sciences. 2004;1012:129–141. doi: 10.1196/annals.1306.010. [DOI] [PubMed] [Google Scholar]
- 180.Peres TV, Schettinger MR, Chen P, Carvalho F, Avila DS, Bowman AB, Aschner M. Manganese-induced neurotoxicity: a review of its behavioral consequences and neuroprotective strategies. BMC Pharmacol Toxicol. 2016;17:57. doi: 10.1186/s40360-016-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tarale P, Chakrabarti T, Sivanesan S, Naoghare P, Bafana A, Krishnamurthi K. Potential Role of Epigenetic Mechanism in Manganese Induced Neurotoxicity. Biomed Res Int. 2016;2016:2548792. doi: 10.1155/2016/2548792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bouabid S, Tinakoua A, Lakhdar-Ghazal N, Benazzouz A. Manganese Neurotoxicity: behavioral disorders associated with dysfunctions in the basal ganglia and neurochemical transmission. Journal of neurochemistry. 2015 doi: 10.1111/jnc.13442. [DOI] [PubMed] [Google Scholar]
- 183.Sidoryk-Wegrzynowicz M. Impairment of glutamine/glutamate-gamma-aminobutyric acid cycle in manganese toxicity in the central nervous system. Folia Neuropathol. 2014;52:377–382. doi: 10.5114/fn.2014.47838. [DOI] [PubMed] [Google Scholar]
- 184.Chen P, Parmalee N, Aschner M. Genetic factors and manganese-induced neurotoxicity. Frontiers in genetics. 2014;5:265. doi: 10.3389/fgene.2014.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Austin RN, Freeman JL, Guilarte TR. Neurochemistry of lead and manganese. Metallomics : integrated biometal science. 2016;8:561–562. doi: 10.1039/c6mt90017h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Brenneman KA, Cattley RC, Ali SF, Dorman DC. Manganese-induced developmental neurotoxicity in the CD rat: is oxidative damage a mechanism of action? Neurotoxicology. 1999;20:477–487. [PubMed] [Google Scholar]
- 187.Sloot WN, Korf J, Koster JF, De Wit LE, Gramsbergen JB. Manganese-induced hydroxyl radical formation in rat striatum is not attenuated by dopamine depletion or iron chelation in vivo. Exp Neurol. 1996;138:236–245. doi: 10.1006/exnr.1996.0062. [DOI] [PubMed] [Google Scholar]
- 188.Cai T, Che H, Yao T, Chen Y, Huang C, Zhang W, Du K, Zhang J, Cao Y, Chen J, Luo W. Manganese induces tau hyperphosphorylation through the activation of ERK MAPK pathway in PC12 cells. Toxicol Sci. 2011;119:169–177. doi: 10.1093/toxsci/kfq308. [DOI] [PubMed] [Google Scholar]
- 189.Yamamoto A, Shin RW, Hasegawa K, Naiki H, Sato H, Yoshimasu F, Kitamoto T. Iron (III) induces aggregation of hyperphosphorylated tau and its reduction to iron (II) reverses the aggregation: implications in the formation of neurofibrillary tangles of Alzheimer's disease. Journal of neurochemistry. 2002;82:1137–1147. doi: 10.1046/j.1471-4159.2002.t01-1-01061.x. [DOI] [PubMed] [Google Scholar]
- 190.Yoo JH, Maeng HY, Sun YK, Kim YA, Park DW, Park TS, Lee ST, Choi JR. Oxidative status in iron-deficiency anemia. J Clin Lab Anal. 2009;23:319–323. doi: 10.1002/jcla.20335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Roth JA, Feng L, Dolan KG, Lis A, Garrick MD. Effect of the iron chelator desferrioxamine on manganese-induced toxicity of rat pheochromocytoma (PC12) cells. Journal of neuroscience research. 2002;68:76–83. doi: 10.1002/jnr.10207. [DOI] [PubMed] [Google Scholar]
- 192.Anderson JG, Cooney PT, Erikson KM. Brain manganese accumulation is inversely related to gamma-amino butyric acid uptake in male and female rats. Toxicol Sci. 2007;95:188–195. doi: 10.1093/toxsci/kfl130. [DOI] [PubMed] [Google Scholar]
- 193.Bonilla E. L-tyrosine hydroxylase activity in the rat brain after chronic oral administration of manganese chloride. Neurobehav Toxicol. 1980;2:37–41. [PubMed] [Google Scholar]
- 194.Kim J, Li Y, Buckett PD, Bohlke M, Thompson KJ, Takahashi M, Maher TJ, Wessling-Resnick M. Iron-responsive olfactory uptake of manganese improves motor function deficits associated with iron deficiency. PloS one. 2012;7:e33533. doi: 10.1371/journal.pone.0033533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Erikson KM, Dobson AW, Dorman DC, Aschner M. Manganese exposure and induced oxidative stress in the rat brain. The Science of the total environment. 2004;334–335:409–416. doi: 10.1016/j.scitotenv.2004.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Fernsebner K, Zorn J, Kanawati B, Walker A, Michalke B. Manganese leads to an increase in markers of oxidative stress as well as to a shift in the ratio of Fe(II)/(III) in rat brain tissue. Metallomics : integrated biometal science. 2014;6:921–931. doi: 10.1039/c4mt00022f. [DOI] [PubMed] [Google Scholar]
- 197.Piloni NE, Perazzo JC, Fernandez V, Videla LA, Puntarulo S. Sub-chronic iron overload triggers oxidative stress development in rat brain: implications for cell protection. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine. 2016;29:119–130. doi: 10.1007/s10534-015-9902-4. [DOI] [PubMed] [Google Scholar]
- 198.Chtourou Y, Fetoui H, Gdoura R. Protective effects of naringenin on iron-overload-induced cerebral cortex neurotoxicity correlated with oxidative stress. Biological trace element research. 2014;158:376–383. doi: 10.1007/s12011-014-9948-0. [DOI] [PubMed] [Google Scholar]
- 199.Piloni NE, Fermandez V, Videla LA, Puntarulo S. Acute iron overload and oxidative stress in brain. Toxicology. 2013;314:174–182. doi: 10.1016/j.tox.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 200.Maaroufi K, Save E, Poucet B, Sakly M, Abdelmelek H, Had-Aissouni L. Oxidative stress and prevention of the adaptive response to chronic iron overload in the brain of young adult rats exposed to a 150 kilohertz electromagnetic field. Neuroscience. 2011;186:39–47. doi: 10.1016/j.neuroscience.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 201.Fujimura T, Terachi T, Funaba M, Matsui T. Reduction of liver manganese concentration in response to the ingestion of excess zinc: identification using metallomic analyses. Metallomics. 2012;4:847–850. doi: 10.1039/c2mt20100c. [DOI] [PubMed] [Google Scholar]
- 202.Aschner M. Manganese homeostasis in the CNS. Environ Res. 1999;80:105–109. doi: 10.1006/enrs.1998.3918. [DOI] [PubMed] [Google Scholar]
- 203.Wedler FC, Ley BW. Cu(II) and Zn(II) ions alter the dynamics and distribution of Mn(II) in cultured chick glial cells. Neurochem Res. 1990;15:1221–1228. doi: 10.1007/BF01208583. [DOI] [PubMed] [Google Scholar]
- 204.Harrison-Findik DD, Schafer D, Klein E, Timchenko NA, Kulaksiz H, Clemens D, Fein E, Andriopoulos B, Pantopoulos K, Gollan J. Alcohol metabolism-mediated oxidative stress down-regulates hepcidin transcription and leads to increased duodenal iron transporter expression. The Journal of biological chemistry. 2006;281:22974–22982. doi: 10.1074/jbc.M602098200. [DOI] [PubMed] [Google Scholar]
- 205.Hou Y, Zhang S, Wang L, Li J, Qu G, He J, Rong H, Ji H, Liu S. Estrogen regulates iron homeostasis through governing hepatic hepcidin expression via an estrogen response element. Gene. 2012;511:398–403. doi: 10.1016/j.gene.2012.09.060. [DOI] [PubMed] [Google Scholar]
- 206.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. The Journal of clinical investigation. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006;108:3730–3735. doi: 10.1182/blood-2006-06-028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ziecik A. Influence of erythropoiesis stimulation on 54Mn distribution in rats. Acta Physiol Pol. 1976;27:287–292. [PubMed] [Google Scholar]
- 209.Ellingsen DG, Kusraeva Z, Bast-Pettersen R, Zibarev E, Chashchin M, Thomassen Y, Chashchin V. The interaction between manganese exposure and alcohol on neurobehavioral outcomes in welders. Neurotoxicol Teratol. 2014;41:8–15. doi: 10.1016/j.ntt.2013.11.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.