Abstract
Background
Translational studies suggest that excess perioperative release of catecholamines and prostaglandins may facilitate metastasis and reduce disease-free survival. This trial tested the combined perioperative blockade of these pathways in breast cancer patients.
Methods
In a randomized placebo-controlled biomarker trial, 38 early-stage breast cancer patients received 11 days of perioperative treatment with a beta-adrenergic antagonist (propranolol) and a cyclooxygenase-2 (COX-2) inhibitor (etodolac), beginning five days before surgery. Excised tumors and sequential blood samples were assessed for pro-metastatic biomarkers.
Results
Drugs were well tolerated with adverse event rates comparable to placebo. Transcriptome profiling of the primary tumor tested a priori hypotheses and indicated that drug treatment significantly (i) decreased epithelial-to-mesenchymal transition, (ii) reduced activity of pro-metastatic/proinflammatory transcription factors (GATA-1, GATA-2, early-growth-response-3/EGR3, signal transducer and activator of transcription-3/STAT-3), and (iii) decreased tumor-infiltrating monocytes while increasing tumor-infiltrating B cells. Drug treatment also significantly abrogated presurgical increases in serum interleukin-6 (IL-6) and C-reactive protein levels, abrogated perioperative declines in stimulated interleukin-12 and interferon-gamma production, abrogated postoperative mobilization of CD16− “classical” monocytes, and enhanced expression of CD11a on circulating natural killer cells.
Conclusions
Perioperative inhibition of COX-2 and β-adrenergic signaling provides a safe and effective strategy for inhibiting multiple cellular and molecular pathways related to metastasis and disease recurrence in early-stage breast cancer.
Keywords: Breast cancer, COX-2 inhibition, β-adrenergic blockade
Introduction
The removal of a primary tumor, and the abolition of its potential immunosuppressive and metastasis-promoting effects (1), presents a window of opportunity to eliminate or control any remaining minimal residual disease. Unfortunately, the perioperative period and the excision of a primary tumor also trigger a variety of physiological processes that may potentially accelerate the progression of pre-existing micrometastases and promote the initiation of new metastases (2,3). As such, the perioperative period plays a critical role in determining long-term cancer outcomes, disproportionally to its short duration (4). Importantly, pre-clinical animal models of cancer suggest that pharmacological modification of perioperative physiology could be exploited to reduce the burden of residual disease (4).
Specifically, animal studies using syngeneic or human xenograft models of cancer have implicated peri-surgical high levels of catecholamines and prostaglandins in mediating many of the pro-metastatic effects of surgery and perioperative stress (2,4,5). Catecholamines and prostaglandins are released by tumor cells, stromal cells within the tumor microenvironment, and by host physiological systems as a result of physiological and psychological stress responses to coping with cancer, tissue damage, pain, and a variety of surgical impacts (6). These signaling pathways can act directly on tumor cells to enhance their proliferation, motility, invasive capacity, resistance to anoikis, secretion of angiogenic factors (5,7–9), and epithelial-to-mesenchymal transition (EMT) (7,10). Catecholamines and prostaglandins can also indirectly promote metastasis by suppressing cell-mediated immunity (2), increasing pro-metastatic cytokines (e.g., interleukin-8; IL-8) (11), and inducing inflammation, which is a hallmark of cancer progression (12).
During the last decade, we and others have found that pharmacologic inhibition of β-adrenoceptors and/or prostaglandin synthesis can reduce the pro-metastatic and immune-suppressive effects of stress and surgery (9,13–18). In these preclinical studies, the simultaneous administration of a β-blocker (propranolol) and a COX-2 inhibitor (etodolac) in combination (rather than each drug alone) has generally proven most effective, and sometimes constitutes the only effective approach (19–21). The synergistic effect of combined treatment protocols may stem from the fact that catecholamines and prostaglandins are both elevated perioperatively and each can increase metastatic propensity through converging pathways (i.e., activation of the cAMP-Protein Kinase A signaling system). Simultaneous inhibition of COX-2 and β-adrenergic signaling has reduced postoperative metastasis (and in some cases improved overall survival) in multiple pre-clinical tumor models including breast, colon, lung, melanoma, and leukemia (19,20,22–24). Consistent with these pre-clinical studies, several pharmaco-epidemiological studies have also documented reductions in breast cancer progression or recurrence in patients who happened to be taking β-blockers at or before initial diagnosis (25,26). Long-term use of COX-inhibiting NSAIDS was also associated with reduced risk of colorectal cancer (27). To assess the potential biological impact of combined perioperative COX-2 and β-adrenergic inhibition in human breast cancer, we herein conducted a randomized placebo-controlled biomarker trial employing etodolac and propranolol. Primary outcome analyses tested whether this combined drug treatment would reduce proinflammatory and prometastatic transcriptome profiles in the malignant tissue.
Three specific transcriptome signatures were targeted a priori based on previous research implicating them in COX-2 and/or β-adrenergic influences on breast cancer progression and metastasis. (i) The primary tumor’s EMT profile was assessed as mesenchymal polarization has been shown to promote intravasation and extravasation of epithelial tumor cells (28), and because both COX-2 activation and β-adrenergic signaling can promote EMT (COX-2 by inducing matrix metalloproteinase-1 (MMP-1) and MMP-2 (10) and inhibiting Smad signaling (29), and β-adrenergic signaling by upregulating SNAIL and TWIST transcription factors (7)). (ii) Transcriptome signatures of tumor-infiltrating leukocyte subpopulations were assessed based on data linking monocyte/macrophage infiltration to breast cancer metastasis and B lymphocyte infiltration to reduced progression. (iii) Pro-inflammatory and pro-metastatic transcription control pathways previously implicated in breast cancer progression were also assessed (nuclear factor-kappaB [NF-κB]/cRel, activator-protein-1 [AP-1], GATA family, STAT family, NRF-2, EGR family transcription factors and the glucocorticoid receptor; GR). Secondary outcome analyses addressed peripheral immune parameters, including serum and ex-vivo stimulated Th1 and inflammatory cytokines (IL-12, IFN-γ), NK cell activation markers, and circulating leukocyte populations (with a particular focus on monocytes due to their involvement in metastasis). This is the first clinical trial to test the efficacy of a combined perioperative treatment with a β-blocker and a COX-2 inhibitor in breast cancer patients.
Methods
Patients and inclusion/exclusion criteria
Thirty-eight women (age 33–70) diagnosed with stage I–III breast cancer were enrolled from three medical centers in Israel. Exclusion criteria included (i) any contraindication for the drugs, such as diabetes, asthma, cardiovascular disease, or low blood pressure, (ii) chronic use of any β-blocker or COX inhibitor, and, (iii) chronic autoimmune disease. The study protocol (ClinicalTrials.gov Identifier: NCT00502684) was approved by IRBs at each study site, and written informed consent was obtained from patients before performing any study-related procedures.
Study design and drug treatment
This multicenter double-blind placebo-controlled randomized biomarker trial employed two equal-sized arms of drug- and placebo-treatment (Fig. 1 A and B). Patient randomization was stratified by age within each medical center (below or above 50). Drug/placebo was administered for 11 consecutive days, starting five days before resection of the primary tumor (Fig. 1 B). Oral BID etodolac (400 mg) was administered throughout the treatment period. Propranolol was administered orally using extended release formulations: 20 mg BID during the five days preceding surgery; 80 mg on the morning of surgery and on the evening and morning following surgery; and 20 mg BID thereafter during five postoperative days. Identical schedules were used for placebo and medication.
Fig. 1A.
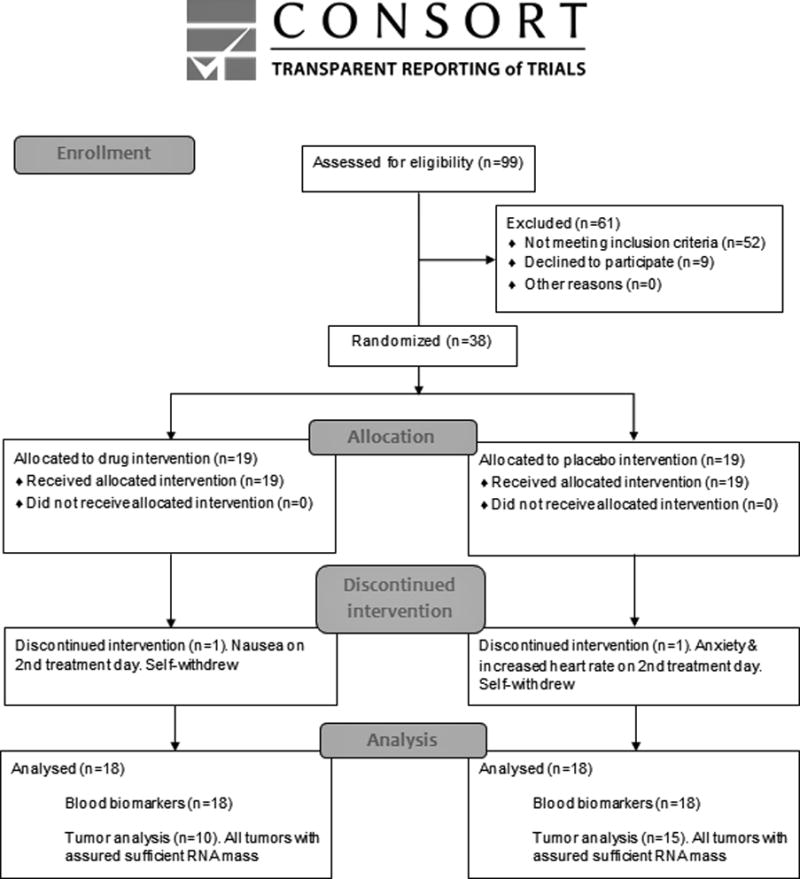
CONSORT diagram of clinical trial enrollment and treatment.
Fig. 1B. Schematic presentation of the design and time schedule of the study.

A double-blind placebo-controlled biomarker trial was conducted in early stage breast cancer patients, treating patients with placebo or with propranolol and etodolac for 11 consecutive days, starting 5 days before surgery. Propranolol doses were increased on the day of surgery. Of the 38 patients recruited, one from each group self-withdraw before surgery. Blood samples were collected before drug initiation (T1), on the morning before surgery (T2), on the morning after surgery (T3), and several days after cessation of drug treatment (T4). Tumor tissue was collected during surgery.
Endpoints and assessments
Excised tumor tissue was fixed in 4% formaldehyde and stored as a formalin-fixed-paraffin-embedded (FFPE) block. Five 5μm sections were used for gene expression profiling as described below. Four blood samples were obtained between 7–11 AM. The first was taken before medication initiation (T1); the second and third were taken on the mornings before and after surgery (T2 and T3, respectively), at least 1 hr after the morning medication dose; and the fourth was taken at least 2 days after treatment cessation (T4; median of 16 days post-medication) (Fig. 1B).
Gene expression profiling and bioinformatic analysis
Detailed methods and references for gene expression profiling and bioinformatic analysis are presented in the Supplementary Methods. Briefly, RNA was extracted from five 5μm FFPE sections of breast tumors, tested for sufficient mass, and subjected to genome-wide transcriptional profiling using Illumina Human HT-12 v4 Expression BeadChips (Illumina Inc., San Diego, California) with quantile normalization (30). Linear model analyses of log2-transformed expression values quantified the difference in average expression between groups (drug treatment vs. placebo) after controlling for tumor stage. A priori hypotheses regarding EMT polarization and tumor-associated leukocyte transcriptomes were tested using Transcript Origin Analyses to relate all genes showing ≥ 1.25-fold differential expression in this study to previously published reference transcriptome profiles derived from mesenchymal- vs. epithelial-polarized breast cancer cells (GSE13915) or isolated leukocyte subsets (GSE1133). A priori hypotheses regarding activity of breast cancer-relevant transcription control pathways were tested using TELiS bioinformatic analysis of transcription factor binding motifs (TFBMs) in the promoters of all genes showing ≥ 1.25-fold differential expression, using TRANSFAC position-specific weight matrices for inflammation-related pathways (NF-κB/cRel, AP-1), GATA family factors GATA1-GATA3, cytokine response factors STAT1 and STAT3, the oxidative stress response factor NRF-2, the neuroendocrine response factor GR, and EGR family transcription factors EGR1-EGR4/NGFIC, as previously described. Statistical testing of bioinformatics results was based on standard errors derived from bootstrap resampling of linear model residual vectors over all genes assayed (which accounts for any potential correlation across genes).
Blood collection, ELISA, flow-cytometry, and induced cytokine production
The Supplementary Methods detail the standard procedures used to assess serum IL-6, CRP, IL-10, and cortisol; ex-vivo lipopolysaccharide- (LPS) & phytohaemagglutinin- (PHA) stimulated production of interferon-gamma (IFN-γ) and IL-12; and flow cytometric analyses of NK cell activation markers and leukocyte subset prevalence.
Statistical Analysis
All analyses were two-sided and conducted based on a priori hypotheses. Our primary hypothesis was that drug treatment would reduce three progression-related transcriptome profiles in malignant tissue (EMT, tumor-associated monocyte/macrophage transcriptomes, and proinflammatory/prometastatic transcription factors). Our secondary hypothesis was that drug treatment would shift circulating immune parameters towards lower inflammatory and higher anti-metastatic immunity as indicated by serum and ex-vivo stimulated cytokine levels, NK cell activation markers, and circulating “classical” (CD14++CD16−) monocytes.
For tumor transcriptome analyses, the statistical significance of bioinformatic result ratios (Drugs/Placebo) was tested by Student’s t-test. For blood-measures analyses, a planned contrast was used to compare the impact of drug treatment (average of T2 and T3) to untreated levels (average of T1 and T4) (i.e., Drugs [(T2+T3) − (T1+T4)] – Placebo [(T2+T3) − (T1+T4)]), and post-hoc comparisons were performed to assess group differences at specific time points. For serum cytokine levels and gene expression assessments, data were log transformed to stabilize variance. Blood sample data during treatment were expressed as a % of the average value at no-treatment time points (i.e., average of T1 and T4).
Results
Demographics, adverse events, and drug compliance
The two groups did not differ on any demographic or cancer-related characteristic assessed (Table 1 and 2). Two patients reported physical discomfort within the first 2 days of treatment (before hospitalization): one placebo-treated patient reported anxiety and showed increased heart rate and blood pressure; a second drug-treated patient reported nausea. Both self-withdrew without further medical examination, and no additional samples were collected from these patients. The other 36 women reported no adverse events and consumed at least 95% of their medication/placebo doses.
Table 1.
Baseline Patient Demographic and Clinical Characteristics (36 patients providing blood samples)
| Control Group n=18 | Treatment Group n=18 | p | |||
|---|---|---|---|---|---|
| Age Mean (MIN, MAX) | 55.2 (33, 70) | 55.3 (41, 70) | .97 | ||
| BMI Mean (MIN, MAX) | 25.7 (20.3, 32.0) | 26.3 (19.4, 36.5) | .73 | ||
| Weight Mean (MIN, MAX) | 68.1 (52, 86) | 69.5 (50, 103) | .75 | ||
| Smoking – Present | NO YES (<5 cigarette per day) YES (>5 cigarette per day) NA |
15 1 1 1 |
NO YES (<5 cigarette per day) YES (>5 cigarette per day) NA |
9 2 6 1 |
.06 |
| T Staging | Tis T1 T2 T3 NA |
2 9 5 0 2 |
Tis T1 T2 T3 NA |
0 13 3 0 2 |
.33 |
| Histological Grade (HG) | HG1 HG2 HG2/3 HG3 DCIS/LCIS |
5 6 1 2 4 |
HG1 HG2 HG2/3 HG3 DCIS/LCIS |
3 10 1 1 3 |
.74 |
| Surgical Resection | Lumpectomy Mastectomy Other a,b,c |
13 2 3 |
Lumpectomy Mastectomy Other d |
15 2 1 |
.56 |
| Metastatic Spread | No NA |
18 0 |
No NA Axillary metastasis |
16 1 1 |
.34 |
| ER Status | Negative Positive |
2 16 |
Negative Positive |
1 17 |
.83 |
| PR Status | Negative Positive |
6 12 |
Negative Positive |
5 13 |
.93 |
| HER2/neu status | Negative NA Positive |
9 4 5 |
Negative NA Positive |
8 3 7 |
.76 |
| Tumor Max. Diameter | 1.6 cm | 1 cm | .11 | ||
| Carcinoma | Invasive Non-invasive NA |
13 3 2 |
Invasive Non-invasive NA |
15 1 2 |
.56 |
Mastectomy +Immediate reconstruction with silicone,
Lumpectomy (double- Lt&Rt),
Lumpectomy (+Intraoperative radiation),
Mastectomy with axillary sentinel lymph node excision
NA - not available;
DCIS – Ductal Carcinoma In Situ;
LCIS – Lobular Carcinoma In Situ
Table 2.
Baseline Patient Demographic and Clinical Characteristics for Transcriptome Profiling*
| Control Group n=15 | Treatment Group n=10 | p | |||
|---|---|---|---|---|---|
| Age Mean (MIN, MAX) | 57 (43,70) | 57.3 (46, 70) | .93 | ||
| BMI Mean (MIN, MAX) | 25.1 (20.3, 32.0) | 26.4 (19.4, 34.5) | .48 | ||
| Weight Mean (MIN, MAX) | 66.1 (52, 86) | 67.7 (50, 94) | .76 | ||
| Smoking – Present | NO YES (<5 cigarette per day) YES (>5 cigarette per day) NA |
13 1 0 1 |
NO YES (<5 cigarette per day) YES (>5 cigarette per day) NA |
5 2 2 1 |
.08 |
| T Staging | Tis T1 T2 T3 |
2 8 4 1 |
Tis T1 T2 T3 |
0 9 1 0 |
.40 |
| Histological Grade(HG) | HG1 HG2 HG2/3 HG3 DCIS/LCIS |
5 6 1 1 2 |
HG1 HG2 HG2/3 HG3 DCIS/LCIS |
2 6 0 1 1 |
.79 |
| Surgical Resection | Lumpectomy Mastectomy Other a,b,c |
11 1 3 |
Lumpectomy Mastectomy Other |
9 1 0 |
.31 |
| Metastatic Spread | No | 15 | No | 10 | 1 |
| ER Status | Negative Positive |
0 15 |
Negative Positive |
1 9 |
.45 |
| PR Status | Negative Positive |
3 12 |
Negative Positive |
3 7 |
.84 |
| HER2/neu status | Negative NA Positive |
8 3 4 |
Negative NA Positive |
6 0 4 |
.30 |
| Tumor Max. Diameter | 1.8 cm | 1.2 cm | .32 | ||
| Carcinoma | Invasive Non-invasive NA |
12 2 1 |
Invasive Non-invasive NA |
8 1 1 |
.93 |
Twenty-five tumors yielded RNA quality assured for sufficient mas
Mastectomy + Immediate reconstruction with silicone,
Lumpectomy (double- Lt&Rt),
Lumpectomy (+Intraoperative radiation)
NA - not available;
DCIS – Ductal Carcinoma In Situ;
LCIS – Lobular Carcinoma In Situ
Tumor gene expression
Genome-wide transcriptional profiling of tumor tissues identified 163 genes showing >1.25 fold up-regulation in tumors from drug-treated patients vs. placebo-treated controls, and 141 genes were equivalently down-regulated. A priori-specified bioinformatic analyses, using previously published mesenchymal and epithelial breast cancer cell transcriptomes as reference points, showed that drug treatment reduced the extent of mesenchymal polarization (diagnosticity z-score: mean = −0.43 ± SE 0.09, p <.0001) but had no significant effect on epithelial-characteristic gene expression (+0.13 ± 0.11, p = .106; Fig. 2A). Similar a priori-specified tumor transcriptome analyses using isolated leukocyte subpopulations as reference points (Fig. 2B) indicated that drug treatment reduced expression of CD14+ monocyte-related transcripts (−0.42 ± 0.15, p=.0036) and increased expression of genes characteristic of CD19+ B cells (+0.45 ± 0.17, p=.0033). We also tested a priori hypotheses regarding specific transcription control pathways that are linked to pro-metastatic processes of inflammation, tissue invasion, and EMT. Promoter-based bioinformatic analyses (Fig. 2C) indicated down-regulated activity of GATA-1 (log2 fold difference in promoter binding site prevalence: mean = −0.48 ± 0.10, p<.0001), GATA-2 (−0.40 ± 10, p=.0001), STAT3 (−1.61 ± 0.66, p=.0154), EGR-3 (−0.70 ± 0.35, p=.048) and GRE (−0.85 ± 0.42, p=.043) in tumors from drug-treated patients.
Fig. 2. Effect of drug treatment on primary tumor transcriptome indicators of EMT, tumor-associated leukocytes, and pro-metastatic transcription factors.
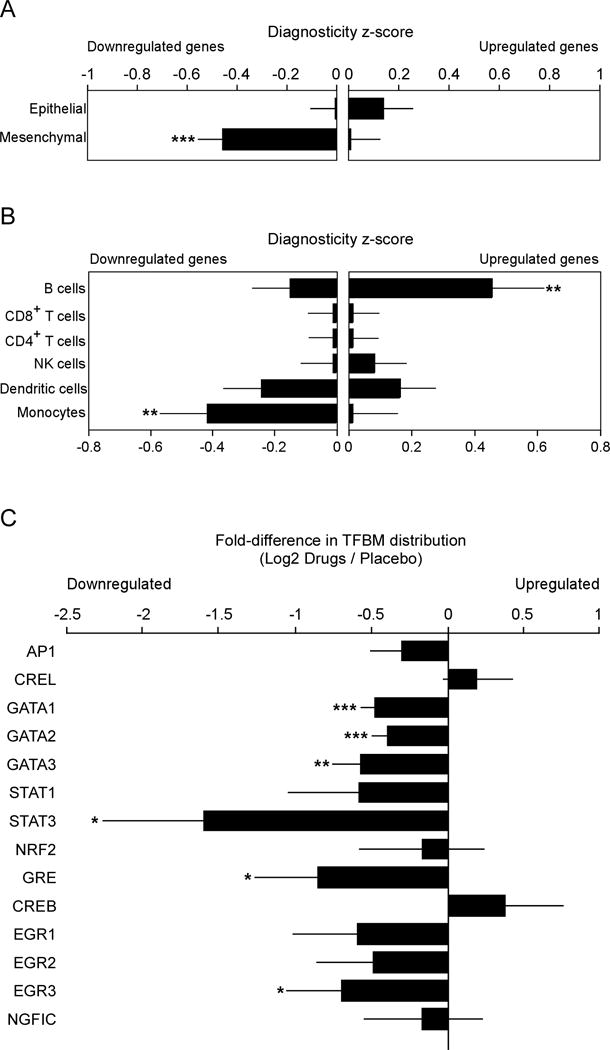
Twenty-five tumors yielded RNA of sufficient quality for transcriptome profiling (10 drug-treated and 15 placebo). (A) Effects of drug treatment on primary tumor EMT gene expression were quantified by Transcript Origin Analysis (58) of 163 genes showing > 1.25-fold up-regulation and 141 genes showing equivalent down-regulation in tumors from drug-treated patients vs. controls, using reference transcriptome profiles derived from mesenchymal- vs. epithelial-polarized breast cancer cells (59). (B) Transcript Origin Analysis also assessed the effects of drug treatment on expression of genes derived from monocytes, dendritic cells, CD4+ and CD8+ T cells, B cells and NK cells, using reference data derived from isolated samples of each cell type (22). (C) Effect of drug treatment on transcription control pathways as indicated by bioinformatics analysis of transcription factor-binding motifs in promoters of differentially expressed genes. Data is presented as mean ± SEM. Group differences are indicated by * (p < 0.05), ** (p < 0.01), or *** (p < 0.001).
Serum levels of soluble factors
As expected, given that psychological and physiological stress responses intensify in the lead-up to surgery (31), serum levels of IL-6 increased by 24% ± 12.1% from T1 to T2 in the placebo-treated group and CRP levels similarly increased by 41.5% ± 20.5% (Fig. 3A–B). However, this pattern was significantly reversed in the drug-treated group (11.3% ±5.5% decline for IL-6, p=.0009; 10% ± 10.7% decline for CRP, p=.034). On the morning after surgery (T3), both placebo- and drug-treated groups showed increases in IL-6 and CRP above pre-surgical levels (IL-6: +573% ± 97% and +442% ± 70% for placebo- and drug-treated groups, respectively; CRP: +828% ± 285% and +635% ± 281%; all p <.001). A planned contrast of drug- vs. placebo-treated groups during treatment (average of T2 and T3) vs. off treatment (average of T1 and T4) showed a significant reduction in IL-6 for the drug-treated group (p =.011), and a marginally significant reduction in CRP. Drug treatment did not significantly affect serum cortisol or IL-10 concentrations at any time point (Fig. 3C–D).
Fig. 3. Effect of drug treatment on circulating levels of IL-6, CRP, IL-10 and cortisol levels (n=18 per group).
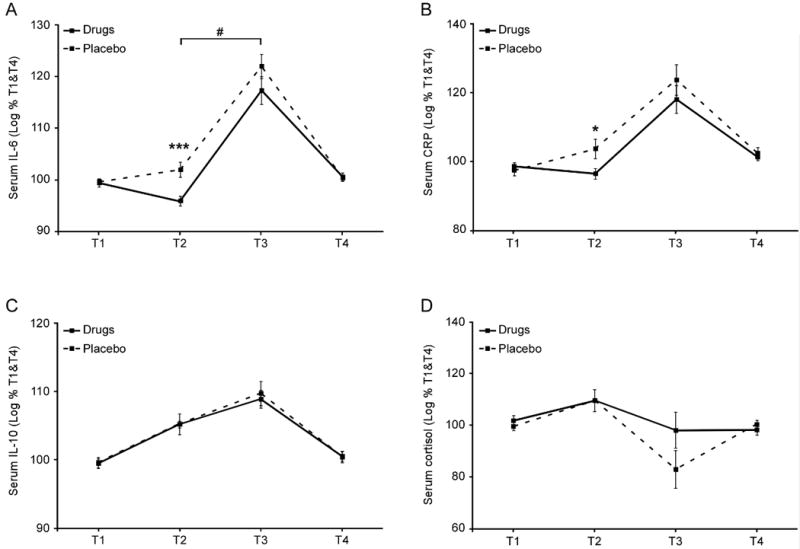
Serum levels of IL-6 (A), C-reactive protein (B), cortisol (C), and IL-10 (D) were assessed by commercial enzyme-linked immunosorbent assay (high-sensitivity ELISA kits for IL-6 and IL-10). Data represent mean ± SEM. Group differences at a specific time point are indicated by * (p<.05), *** (p<.001). A significant contrast between drug and placebo conditions during treatment (T2+T3) [vs off treatment (T1+T4)] is indicated by #.
Immune indices in blood samples
In the placebo-treated group, ex-vivo LPS- and PHA-stimulated production of IL-12 and IFN-γ decreased progressively from T1 to T3 (IL-12: −38% ± 10%; IFN-γ: −30.2% ± 9.1%; both p<.0001), as previously reported (31). Drug treatment blocked this decrease, resulting in higher levels of these cytokines at T2 and T3 (i.e., for the planned contrast described above, IL-12: +50.8% ± 15.2%, p=.028; IFN-γ: +31% ± 8.6%, p=.024) (Fig. 4A–B).
Fig. 4. Effect of drug treatment on ex vivo stimulated production of IL-12 and IFN-γ, on numbers of circulating CD16− monocytes, and on CD11a (LFA-1) expression levels on NK cells (n=18 per group).
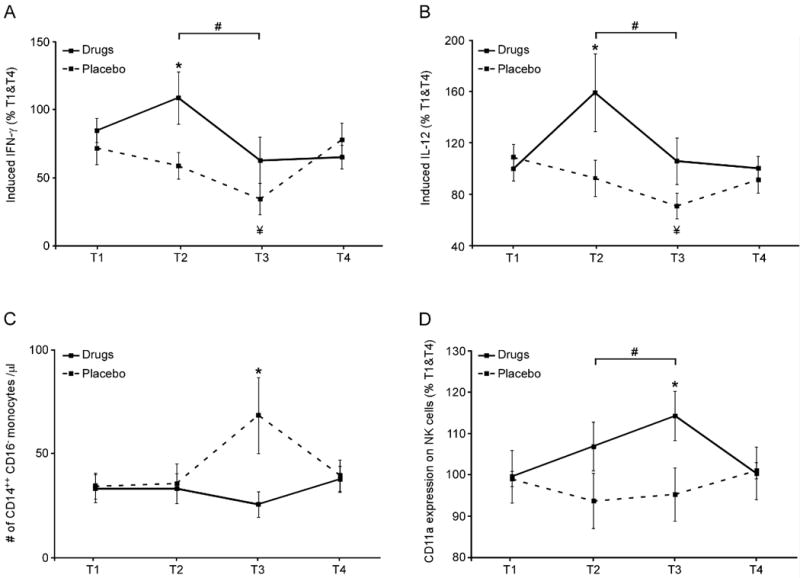
Venipuncture blood samples were assayed for: (A and B) Induced cytokine levels following 21-hrs LPS & PHA-stimulation, assessed in culture supernatant by enzyme-linked immunosorbent assay (ELISA); (C) circulating frequency of CD14++CD16− “classical” monocytes, and (D) expression levels of the activation marker CD11a on NK cells (CD3−CD56+CD16+ lymphocytes), assessed by flow cytometry. Data represent mean ± SEM. Group differences at a specific time point are indicated by * (p < 0.05). A significant contrast between drug and placebo treatments at T2+T3 [vs off treatment (T1+T4)] is indicated by #. A significant decrease from T1 to T3 within the placebo group is indicated by ¥.
Drug treatment also blocked an influx of CD14++CD16− classical monocytes into circulation on the morning after surgery (T3; difference = 85% ± 15%, p=.032) (Fig. 4C), and increased expression of the activation marker CD11a on NK cells (CD3−CD56+CD16+) during treatment (average of T2 and T3) vs. off treatment (average of T1 and T4; difference = +16% ± 6.3%, p=.024) (Fig. 4D).
Discussion
These data show that perioperative administration of the β-adrenergic antagonist propranolol and the COX-2 inhibitor etodolac induces multiple favorable impacts on (i) primary tumor gene expression profiles (bioinformatic indications of reduced EMT; reduced activity of GATA-1, GATA-2, EGR3, GRE, and STAT3 transcription factors; and reduced tumor-associated monocytes and increased tumor-associated B cells) and on (ii) circulating immune parameters (serum and ex vivo-induced cytokine levels, reduced classical monocyte influx, and increased NK cell activation markers). Each of these outcomes has previously been linked to reduced tumor progression in pre-clinical animal models and/or human clinical studies. This study was designed solely as a randomized controlled test of perioperative propranolol and etodolac effects on biomarker outcomes, and involved no long-term assessment of clinical outcomes. However, the favorable safety profile and favorable impacts on tumor transcriptome profiles and immune parameters provide a rationale for future clinical trials employing more robust sample sizes and long-term follow-up to assess impacts of perioperative COX-2 and β-adrenergic inhibition on clinical outcomes in early-stage breast cancer.
Tumor molecular characteristics
Drug treatment reduced tumor molecular biomarkers of EMT. This finding corresponds well with animal studies indicating that COX-2 inhibitors and β-adrenergic antagonists can inhibit EMT in human tumor xenografts (5,32). In metastatic breast cancer, a mesenchymal phenotype is more prevalent in circulating tumor cells than in the primary tumor (33), suggesting the clinical significance of EMT for metastasis. Moreover, the EMT profile of the primary tumor predicts long-term cancer outcomes, including overall survival (34), in several cancer types.
Drug treatment also decreased intra-tumoral molecular indicators of several pro-metastatic transcription control pathways, including GATA-1, GATA-2, and EGR3. GATA-1 exerts anti-apoptotic activity (35) and promotes EMT by down-regulating E-cadherin (5), and GATA-2 can inhibit the tumor suppressor gene, phosphatase and tensin homolog (PTEN) (36). Both factors promote breast cancer development and progression (37). EGR3 is induced by estrogen signaling (38), and has been linked to lymph node status, metastatic spread, and poor prognosis (39). Drug treatment also reduced indicated activity of the glucocorticoid receptor (GR), which can enhance tumor cell survival by inducing Bcl-xL (40), inducing anti-apoptotic signaling (41,42), suppressing p53 activity (43), and inducing chemoresistance (8).
Inflammatory indicators in the tumor and circulation
Previous studies have reported that psychological and surgery-related sympathetic nervous system stress responses can elevate levels of proinflammatory ligands, including IL-6 and CRP (44). In the current study, both IL-6 and CRP levels increased before surgery in the placebo group (from T1 to T2), but drug treatment reversed this effect and reduced levels of both inflammatory indicators at T2. IL-6 and CRP are associated with tumor progression and poor prognosis in multiple solid tumor types, including breast, lung, and prostate, and hematopoietic malignancies (37,45). IL-6 activates the janus-kinase-STAT signaling pathway, which is well known to promote tumor cell proliferation, survival, and invasion, as well as immunosuppression and inflammation. STAT3 and STAT5 are strongly associated with cancer progression (37). Here, both plasma IL-6 levels and indicators of tumor STAT3 activity were reduced by the drug treatment. Drug treatment also effectively blocked a marked postoperative (T3) mobilization of “classical” pro-inflammatory CD14++CD16− monocytes. Thus, combined perioperative β-blockade and COX-2 inhibition may inhibit stress-induced inflammatory and metastatic processes through multiple cellular and molecular pathways.
Immune status in the tumor and circulation
Transcriptome profiling of the primary tumor also indicated potential effects of the combined perioperative drug treatment in increasing tumor-infiltrating B cells and decreasing tumor-associated monocyte/macrophages. Tumor-infiltrating B cells comprise up to 60% of the tumor-associated lymphocytes (46,47), and predict increased survival rates in breast cancer (47,48). Monocyte recruitment by tumors was shown in several animal models to be enhanced by β-adrenergic signaling, and to promote cancer progression (5). In human cancers, tumor-infiltrating monocytes, which often transform into M2-macrophages, are correlated with decreased survival in many solid tumors including breast, thyroid, and bladder cancers (49,50). Thus, the profile of immune cell alterations reflected in whole-tumor transcriptome profiling indicates favorable effects of this drug regimen on local immune cell mediators of disease progression.
Consistent with previous reports in animal models, (31) stress and surgery also decreased LPS- & PHA-induced production of IFN-γ and IL-12 by circulating leukocytes. However, this suppression was abrogated by drug treatment. These Th1 cytokines are prominent activators of anti-tumor CTL and NK cells (2). The drug treatment also increased expression of CD11a (lymphocyte function-associated antigen-1; LFA-1) on circulating NK cells. This membrane glycoprotein is a marker of NK cell activation and interacts with intercellular adhesion molecule-1 (ICAM-1) and other tumor ligands to promote tumor lysis by NK cells (51). In a pre-clinical model, where a spontaneously metastasizing orthotopic primary tumor was excised, surgery reduced CD11a expression on NK-cells and our perioperative drug treatment (propranolol and etodolac) blocked that effect and improved long-term survival rates (20).
Perioperative significance of the treatment and safety concerns
Combined administration of propranolol and etodolac had favorable impacts on multiple biomarkers assessed before, during, and following surgery. Given that both catecholamines and prostaglandins are abundant throughout the perioperative period, and that micrometastases and residual disease may exist before and following surgery, these data suggest that treatment throughout the entire perioperative period is optimal.
The safety of this drug regimen is discussed in greater detail in the Supplementary Methods. Briefly, for patients without contraindications, the safety profiles of propranolol and etodolac are well established, especially for the short duration employed here (52,53). Concerns and variable findings have been reported regarding the perioperative use of β1-selective antagonists (54), but no evidence indicates a risk associated with use of non-selective β-adrenergic antagonists such as propranolol. Tissue healing was shown in animal studies not to be adversely affected by either drug or by their combined use (55). In the current study and in a previous study in cancer patients (56), we observed no serious or moderate adverse events associated with this treatment regimen. This favorable safety profile is important in evaluating the overall cost-benefit ratio for the present drug regimen, especially as both chronic and perioperative use of COX inhibitors or β-adrenergic blockers have been associated with improved cancer outcomes (4,7,57). Thus, any hypothetical long-term risk associated with the combined use of propranolol and etodolac appears empirically unlikely, and should be weighed against the positive outcomes reported by translational, epidemiological, and clinical studies, as well as by the favorable profile of molecular and cellular biomarkers observed in this trial.
Limitations
This study was designed solely as a randomized controlled proof-of-concept test of perioperative propranolol and etodolac effects on metastasis-related biomarkers in early-stage breast cancer. This study was powered only to detect those biomarker outcomes (based on effect sizes previously observed in pre-clinical studies) and it provides no information about long-term clinical outcomes (e.g., effects on relapse-free or overall survival). The generality of these results also needs to be examined in future studies beyond the present single-nation context, and possibly examining alternative treatment durations and regimens (including the use of each agent alone in addition to combined use) and selective targeting of high-risk disease settings (e.g., ER-/PR-/her2- breast cancer) and other cancer types. It is also important to note that the biomarkers examined in this trial were selected a priori as outcomes (based on previous clinical and preclinical research) and are not intended to provide a prognostic biomarker for clinical disease progression.
Summary
Data from this first clinical trial of perioperative treatment with a COX-2 inhibitor and a β-adrenergic antagonist in early stage breast cancer finds a favorable safety profile and favorable impact on multiple tumor and circulating biomarkers associated with cancer progression and metastasis. These findings provide a biological rationale for future clinical trials to assess the impact of this easily implemented, safe, and inexpensive treatment regimen on long-term clinical outcomes (e.g., overall and recurrence-free survival).
Supplementary Material
Translational relevance.
The clinical trial reported here supports the safety and efficacy of pharmacologically inhibiting β-adrenergic and COX-2 pathways during the perioperative period in early-stage breast cancer. Preclinical studies have shown that simultaneous blockade of these two pathways improves long-term survival rates in several models of primary tumor excision. Moreover, this treatment is inexpensive and clinically feasible for patients without contraindications for the medications used (~50% of patients). Given the positive biomarker indications reported in the present proof-of-concept trial, future studies assessing clinical impact on disease progress/recurrence and overall survival are justified. This treatment may also be beneficial in a variety of cancer types and does not contraindicate other cancer therapies. As such, brief perioperative inhibition of β-adrenergic and COX-2 signaling may provide a novel strategy for improving long-term cancer outcomes.
Acknowledgments
We thank Ella Rosenne, Hagar Lavon, and Eli Elbaz for their devoted technical work supporting this project, and for Dr. Patricia Ganz for her critical evaluation of the manuscript.
Funding: This work was supported by the National Cancer Institute Network on Biobehavioral Pathways in Cancer (grant number 13XS084) to SB-E; the Israel Science Foundation (grant 1406/10) to SB-E; and the National Institutes of Health/National Institute on Aging (grant P30 AG017265) to SC.
Footnotes
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org
AUTHOR CONTRIBUTIONS
Conception and design: Shamgar Ben-Eliyahu, Steve Cole, Lee Shaashua, Oded Zmora
Collection and assembly of data: All authors
Data analysis and interpretation: Shamgar Ben-Eliyahu, Steve Cole, Maytal Shabat-Simon, Lee Shaashua, Rita Haldar
Manuscript writing: Shamgar Ben-Eliyahu, Steve Cole, Lee Shaashua, Maytal Shabat-Simon
Final approval of manuscript: All authors
References
- 1.Motz GT, Coukos G. Deciphering and reversing tumor immune suppression. Immunity. 2013;39(1):61–73. doi: 10.1016/j.immuni.2013.07.005. S1074-7613(13)00289-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neeman E, Ben-Eliyahu S. Surgery and stress promote cancer metastasis: new outlooks on perioperative mediating mechanisms and immune involvement. Brain, behavior, and immunity. 2013;30(Suppl):S32–40. doi: 10.1016/j.bbi.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shakhar G, Ben-Eliyahu S. Potential prophylactic measures against postoperative immunosuppression: could they reduce recurrence rates in oncological patients? Annals of surgical oncology. 2003;10(8):972–92. doi: 10.1245/aso.2003.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Horowitz M, Neeman E, Sharon E, Ben-Eliyahu S. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat Rev Clin Oncol. 2015;12(4):213–26. doi: 10.1038/nrclinonc.2014.224. nrclinonc.2014.224 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armaiz-Pena GN, Gonzalez-Villasana V, Nagaraja AS, Rodriguez-Aguayo C, Sadaoui NC, Stone RL, et al. Adrenergic regulation of monocyte chemotactic protein 1 leads to enhanced macrophage recruitment and ovarian carcinoma growth. Oncotarget. 2015;6(6):4266–73. doi: 10.18632/oncotarget.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neeman E, Zmora O, Ben-Eliyahu S. A new approach to reducing postsurgical cancer recurrence: perioperative targeting of catecholamines and prostaglandins. Clin Cancer Res. 2012;18(18):4895–902. doi: 10.1158/1078-0432.CCR-12-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole SW, Sood AK. Molecular pathways: beta-adrenergic signaling in cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(5):1201–6. doi: 10.1158/1078-0432.CCR-11-0641. 1078-0432.CCR-11-0641 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55(1):115–22. doi: 10.1136/gut.2004.047100. doi gut.2004.047100 [pii] 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nature medicine. 2006;12(8):939–44. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 10.Hugo HJ, Saunders C, Ramsay RG, Thompson EW. New Insights on COX-2 in Chronic Inflammation Driving Breast Cancer Growth and Metastasis. J Mammary Gland Biol Neoplasia. 2015;20(3–4):109–19. doi: 10.1007/s10911-015-9333-4. 10.1007/s10911-015-9333-4 [pii] [DOI] [PubMed] [Google Scholar]
- 11.Singh B, Berry JA, Vincent LE, Lucci A. Involvement of IL-8 in COX-2-mediated bone metastases from breast cancer. J Surg Res. 2006;134(1):44–51. doi: 10.1016/j.jss.2006.03.018. doi doi S0022-4804(06)00127-2 [pii] 10.1016/j.jss.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Cordon-Cardo C. At the crossroad of tumorigenesis: drivers and hitchhikers. Hum Pathol. 1999;30(9):1001–3. doi: 10.1016/s0046-8177(99)90215-0. [DOI] [PubMed] [Google Scholar]
- 13.Cole SW, Arevalo JM, Takahashi R, Sloan EK, Lutgendorf SK, Sood AK, et al. Computational identification of gene-social environment interaction at the human IL6 locus. Proc Natl Acad Sci U S A. 2010;107(12):5681–6. doi: 10.1073/pnas.0911515107. 0911515107 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188(1):21–8. doi: 10.4049/jimmunol.1101029. doi 188/1/21 [pii] 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yakar I, Melamed R, Shakhar G, Shakhar K, Rosenne E, Abudarham N, et al. Prostaglandin e(2) suppresses NK activity in vivo and promotes postoperative tumor metastasis in rats. Annals of surgical oncology. 2003;10(4):469–79. doi: 10.1245/aso.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Shakhar G, Ben-Eliyahu S. In vivo beta-adrenergic stimulation suppresses natural killer activity and compromises resistance to tumor metastasis in rats. J Immunol. 1998;160(7):3251–8. [PubMed] [Google Scholar]
- 17.Ben-Eliyahu S, Shakhar G, Page GG, Stefanski V, Shakhar K. Suppression of NK cell activity and of resistance to metastasis by stress: a role for adrenal catecholamines and beta-adrenoceptors. Neuroimmunomodulation. 2000;8(3):154–64. doi: 10.1159/000054276. doi 54276 [pii] 54276. [DOI] [PubMed] [Google Scholar]
- 18.Nowell Le CP, Kim-Fuchs CJ, Botteri C, Hiller E, Ismail JGH, et al. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nature communications. 2016;7:10634. doi: 10.1038/ncomms10634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benish M, Bartal I, Goldfarb Y, Levi B, Avraham R, Raz A, et al. Perioperative use of beta-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Annals of surgical oncology. 2008;15(7):2042–52. doi: 10.1245/s10434-008-9890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glasner A, Avraham R, Rosenne E, Benish M, Zmora O, Shemer S, et al. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J Immunol. 2010;184(5):2449–57. doi: 10.4049/jimmunol.0903301. [DOI] [PubMed] [Google Scholar]
- 21.Sorski L, Melamed R, Matzner P, Lavon H, Shaashua L, Rosenne E, et al. Reducing liver metastases of colon cancer in the context of extensive and minor surgeries through β-adrenoceptors blockade and COX2 inhibition. Brain, behavior, and immunity. 2016 doi: 10.1016/j.bbi.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melamed R, Rosenne E, Shakhar K, Schwartz Y, Abudarham N, Ben-Eliyahu S. Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a beta-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain, behavior, and immunity. 2005;19(2):114–26. doi: 10.1016/j.bbi.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Inbar S, Neeman E, Avraham R, Benish M, Rosenne E, Ben-Eliyahu S. Do stress responses promote leukemia progression? An animal study suggesting a role for epinephrine and prostaglandin-E2 through reduced NK activity. PLoS One. 2011;6(4):e19246. doi: 10.1371/journal.pone.0019246. PONE-D-10-06187 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaashua RS-F, EK S, Ben-Eliyahu S. Surgical excision of a primary tumor enhances spontaneous metastasis of breast cancer through COX-2 and β-adrenergic pathways. Cold Spring Harbor Laboratory; NY, USA: 2015. [Google Scholar]
- 25.Powe DG, Voss MJ, Zanker KS, Habashy HO, Green AR, Ellis IO, et al. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;1(7):628–38. doi: 10.18632/oncotarget.197. doi 101009 [pii] 10.18632/oncotarget.101009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population- based study. J Clin Oncol. 2011;29(19):2635–44. doi: 10.1200/JCO.2010.33.5422. JCO.2010.33.5422 [pii] [DOI] [PubMed] [Google Scholar]
- 27.Friis S, Riis AH, Erichsen R, Baron JA, Sorensen HT. Low-Dose Aspirin or Nonsteroidal Anti-inflammatory Drug Use and Colorectal Cancer Risk: A Population-Based, Case-Control Study. Ann Intern Med. 2015;163(5):347–55. doi: 10.7326/M15-0039. [DOI] [PubMed] [Google Scholar]
- 28.Kang Y, Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118(3):277–9. doi: 10.1016/j.cell.2004.07.011. S0092867404007020 [pii] [DOI] [PubMed] [Google Scholar]
- 29.Neil JR, Johnson KM, Nemenoff RA, Schiemann WP. Cox-2 inactivates Smad signaling and enhances EMT stimulated by TGF-beta through a PGE2-dependent mechanisms. Carcinogenesis. 2008;29(11):2227–35. doi: 10.1093/carcin/bgn202. bgn202 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19(2):185–93. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 31.Greenfeld K, Avraham R, Benish M, Goldfarb Y, Rosenne E, Shapira Y, et al. Immune suppression while awaiting surgery and following it: dissociations between plasma cytokine levels, their induced production, and NK cell cytotoxicity. Brain, behavior, and immunity. 2007;21(4):503–13. doi: 10.1016/j.bbi.2006.12.006. doi S0889-1591(07)00002-5 [pii] 10.1016/j.bbi.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 32.Adhim Z, Matsuoka T, Bito T, Shigemura K, Lee KM, Kawabata M, et al. In vitro and in vivo inhibitory effect of three Cox-2 inhibitors and epithelial-to-mesenchymal transition in human bladder cancer cell lines. Br J Cancer. 2011;105(3):393–402. doi: 10.1038/bjc.2011.262. bjc2011262 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339(6119):580–4. doi: 10.1126/science.1228522. 339/6119/580 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bringuier PP, Umbas R, Schaafsma HE, Karthaus HF, Debruyne FM, Schalken JA. Decreased E-cadherin immunoreactivity correlates with poor survival in patients with bladder tumors. Cancer Res. 1993;53(14):3241–5. [PubMed] [Google Scholar]
- 35.Boidot R, Vegran F, Jacob D, Chevrier S, Cadouot M, Feron O, et al. The transcription factor GATA-1 is overexpressed in breast carcinomas and contributes to survivin upregulation via a promoter polymorphism. Oncogene. 2010;29(17):2577–84. doi: 10.1038/onc.2009.525. onc2009525 [pii] [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, He X, Ngeow J, Eng C. GATA2 negatively regulates PTEN by preventing nuclear translocation of androgen receptor and by androgen-independent suppression of PTEN transcription in breast cancer. Hum Mol Genet. 2012;21(3):569–76. doi: 10.1093/hmg/ddr491. ddr491 [pii] [DOI] [PubMed] [Google Scholar]
- 37.Yu H, Lee H, Herrmann A, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nature reviews Cancer. 2014;14(11):736–46. doi: 10.1038/nrc3818. nrc3818 [pii] [DOI] [PubMed] [Google Scholar]
- 38.Inoue A, Omoto Y, Yamaguchi Y, Kiyama R, Hayashi SI. Transcription factor EGR3 is involved in the estrogen-signaling pathway in breast cancer cells. J Mol Endocrinol. 2004;32(3):649–61. doi: 10.1677/jme.0.0320649. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki T, Inoue A, Miki Y, Moriya T, Akahira J, Ishida T, et al. Early growth responsive gene 3 in human breast carcinoma: a regulator of estrogen-meditated invasion and a potent prognostic factor. Endocr Relat Cancer. 2007;14(2):279–92. doi: 10.1677/ERC-06-0005. doi 14/2/279 [pii] 10.1677/ERC-06-0005. [DOI] [PubMed] [Google Scholar]
- 40.Schorr K, Furth PA. Induction of bcl-xL expression in mammary epithelial cells is glucocorticoid-dependent but not signal transducer and activator of transcription 5-dependent. Cancer Res. 2000;60(21):5950–3. [PubMed] [Google Scholar]
- 41.Moran TJ, Gray S, Mikosz CA, Conzen SD. The glucocorticoid receptor mediates a survival signal in human mammary epithelial cells. Cancer Res. 2000;60(4):867–72. [PubMed] [Google Scholar]
- 42.Mikosz CA, Brickley DR, Sharkey MS, Moran TW, Conzen SD. Glucocorticoid receptor-mediated protection from apoptosis is associated with induction of the serine/threonine survival kinase gene, sgk-1. J Biol Chem. 2001;276(20):16649–54. doi: 10.1074/jbc.M010842200. M010842200 [pii] [DOI] [PubMed] [Google Scholar]
- 43.Sengupta S, Wasylyk B. Ligand-dependent interaction of the glucocorticoid receptor with p53 enhances their degradation by Hdm2. Genes Dev. 2001;15(18):2367–80. doi: 10.1101/gad.202201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain, behavior, and immunity. 2007;21(7):901–12. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 45.Asegaonkar SB, Asegaonkar BN, Takalkar UV, Advani S, Thorat AP. C-Reactive Protein and Breast Cancer: New Insights from Old Molecule. International journal of breast cancer. 2015;2015:145647. doi: 10.1155/2015/145647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nelson BH. CD20+ B cells: the other tumor-infiltrating lymphocytes. J Immunol. 2010;185(9):4977–82. doi: 10.4049/jimmunol.1001323. 185/9/4977 [pii] [DOI] [PubMed] [Google Scholar]
- 47.Coronella-Wood JA, Hersh EM. Naturally occurring B-cell responses to breast cancer. Cancer Immunol Immunother. 2003;52(12):715–38. doi: 10.1007/s00262-003-0409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmidt M, Bohm D, von Torne C, Steiner E, Puhl A, Pilch H, et al. The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res. 2008;68(13):5405–13. doi: 10.1158/0008-5472.CAN-07-5206. 68/13/5405 [pii] [DOI] [PubMed] [Google Scholar]
- 49.Gustafson MP, Lin Y, Bleeker JS, Warad D, Tollefson MK, Crispen PL, et al. Intratumoral CD14+ Cells and Circulating CD14+HLA−DRlo/neg Monocytes Correlate with Decreased Survival in Patients with Clear Cell Renal Cell Carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21(18):4224–33. doi: 10.1158/1078-0432.CCR-15-0260. 1078-0432.CCR-15-0260 [pii] [DOI] [PubMed] [Google Scholar]
- 50.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nature reviews Cancer. 2009;9(4):239–52. doi: 10.1038/nrc2618. nrc2618 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsumoto G, Omi Y, Lee U, Nishimura T, Shindo J, Penninger JM. Adhesion mediated by LFA-1 is required for efficient IL-12-induced NK and NKT cell cytotoxicity. Eur J Immunol. 2000;30(12):3723–31. doi: 10.1002/1521-4141(200012)30:12<3723::AID-IMMU3723>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 52.Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77–137. doi: 10.1016/j.jacc.2014.07.944. S0735-1097(14)05536-3 [pii] [DOI] [PubMed] [Google Scholar]
- 53.Wattchow DA, De Fontgalland D, Bampton PA, Leach PL, McLaughlin K, Costa M. Clinical trial: the impact of cyclooxygenase inhibitors on gastrointestinal recovery after major surgery - a randomized double blind controlled trial of celecoxib or diclofenac vs. placebo. Aliment Pharmacol Ther. 2009;30(10):987–98. doi: 10.1111/j.1365-2036.2009.04126.x. APT4126 [pii] [DOI] [PubMed] [Google Scholar]
- 54.Group PS. Devereaux PJ, Yang H, Yusuf S, Guyatt G, Leslie K, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371(9627):1839–47. doi: 10.1016/S0140-6736(08)60601-7. [DOI] [PubMed] [Google Scholar]
- 55.Hazut O, Shaashua L, Benish M, Levi B, Sorski L, Benjamin B, et al. The effect of beta-adrenergic blockade and COX-2 inhibition on healing of colon, muscle, and skin in rats undergoing colonic anastomosis. Int J Clin Pharmacol Ther. 2011;49(9):545–54. doi: 10.5414/CP201550. doi 8913 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zmora O, Shaashua L, Gutman M, Ben-Eliyahu S. The perioperative use of a beta-adrenergic blocker and a COX-2 inhibitor in colorectal cancer patients for the prevention of cancer recurrence: A preliminary study assessing feasibility and safety. Brighton, UK: BBI; 2016. [Google Scholar]
- 57.Ricon I, Hiller JG, Ben-Eliyahu S. The Combined Blockade of beta-Adrenoceptor and COX-2 During the Perioperative Period to Improve Long-term Cancer Outcomes. International anesthesiology clinics. 2016;54(4):72–91. doi: 10.1097/AIA.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 58.Cole SW, Hawkley LC, Arevalo JM, Cacioppo JT. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc Natl Acad Sci U S A. 2011;108(7):3080–5. doi: 10.1073/pnas.1014218108. 1014218108 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choi YL, Bocanegra M, Kwon MJ, Shin YK, Nam SJ, Yang JH, et al. LYN is a mediator of epithelial-mesenchymal transition and a target of dasatinib in breast cancer. Cancer Res. 2010;70(6):2296–306. doi: 10.1158/0008-5472.CAN-09-3141. 0008-5472.CAN-09-3141 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


