Abstract
Importance
Active surveillance is an important option to reduce prostate cancer overtreatment, but it remains underutilized in many countries. Models from the United States show that greater use of active surveillance is important for prostate cancer screening to be cost-effective.
Objectives
To perform an up-to-date, nationwide, population-based study on use of active surveillance for localized prostate cancer in Sweden.
Design
Cross-sectional study in the National Prostate Cancer Register (NPCR) of Sweden from 2009 through 2014.
Setting
The NPCR has data on 98% of prostate cancers diagnosed in Sweden and has comprehensive linkages to other nationwide databases.
Participants
Overall, 32 518 men with a median age of 67 years were diagnosed with favorable-risk prostate cancer, including 4 693, 15 403 and 17 115 men with very-low-risk (subset of the low-risk group) (clinical stage, T1c; Gleason score, ≤6; prostate-specific antigen [PSA], <10 ng/ml; PSA density <0.15ng/mL/cm3; and <8-mm total cancer length in ≤4 positive biopsy cores), low-risk (including all men in the very-low-risk group) (T1–T2; Gleason score, ≤6; and PSA, <10ng/mL), and intermediate-risk disease (T1–T2 with Gleason score, 7 and/or PSA, 10–20ng/mL).
Exposures
Diagnosis with favorable-risk prostate cancer
Major Outcome
Utilization of active surveillance
Results
The use of active surveillance increased in men of all ages from 57% (380 of 665) to 91% (939 of 1027) for very-low-risk prostate cancer and from 40% (1159 of 2895) to 74% (1951 of 2644) for low-risk prostate cancer, with the strongest increase occurring from 2011 onward. Among men aged 50 to 59 years, 88% (211 of 240) with very-low-risk and 68% (351 of 518) with low-risk disease chose active surveillance in 2014. Use of active surveillance for intermediate-risk disease remained lower, 19% (561 of 3030) in 2014.
Conclusions and Relevance
Active surveillance has become the dominant management for low-risk prostate cancer among men in Sweden, with the highest rates yet reported and almost complete uptake for very-low-risk cancer. These data should serve as a benchmark to compare the use of active surveillance for favorable-risk disease around the world.
Keywords: prostate cancer, active surveillance, watchful waiting, treatment, low-risk
Brief Report
Low-risk prostate cancer has a prolonged natural history and can be safely managed with active surveillance and selective, deferred use of curative treatment. Despite this, recent US studies have reported that most low-risk prostate cancer is managed with immediate curative treatment,1 often reducing quality of life. Moreover, a recent modeling study showed that prostate cancer screening is generally not cost-effective based on current US treatment patterns but would be cost-effective if low-risk prostate cancer were managed conservatively.2 Although Sweden does not have a national screening program, more than 50% of Swedish men aged 55 to 69 years have been exposed to prostate-specific antigen (PSA) screening.3,4
Our group previously reported increasing use of active surveillance in Sweden through 2011.5 The goal of the present study is to perform an updated analysis of nationwide use of active surveillance using comprehensive, population-based Swedish data through 2014.
The National Prostate Cancer Register (NPCR) includes 98% of all prostate cancer cases in Sweden.6,7 Designated staff at each health care unit extract data on cancer features and treatment from the medical records to an online registration in NPCR and have distinguished “watchful waiting” from “active surveillance” since 2007. In addition, the Longitudinal Integration Database for Health Insurance and Labor Market Studies provided socioeconomic data, and the Patient Register provided information on comorbidities.7
The Umeå University Research Ethics Board approved the study, waiving patient written informed consent.
Descriptive statistics and the Mann-Kendall test for trend based on the Kendall rank correlation were used to examine active surveillance utilization from 2009 through 2014 by age and clinical risk category, classified as very-low-risk (clinical stage, T1c; Gleason score, ≤6; PSA, <10 ng/mL; PSA density <0.15 ng/mL/cm3; <8-mm total cancer length in ≤4 positive biopsy cores8), low-risk (T1–T2; Gleason score, ≤6; and PSA, <10 ng/mL), and intermediate-risk disease (T1–T2 with Gleason score, 7 and/or PSA, 10–20 ng/mL). Subset analysis was performed for intermediate-risk cancer with Gleason, 6 and PSA, 10–20 ng/mL, and Gleason, 7 (3 + 4) and PSA less than 10 ng/mL, and using alternate risk classifications (CAPRA scores [Cancer and the Prostate Risk Assessment], Epstein criteria, and PRIAS criteria [Prostate Cancer Research International: Active Surveillance]). Multivariable logistic regression was performed within clinical risk categories to determine predictors of active surveillance, including variables not used in the risk categorization. All P values were 2 sided and P < .05 was considered statistically significant.
Between 2009 and 2014, 32 518 men, median age of 67 years, were diagnosed with favorable-risk prostate cancer (Table). Use of active surveillance and watchful waiting increased from 64% to 93% for very-low-risk disease, 50% to 79% for low-risk disease, and decreased from 26% to 25% for intermediate-risk cancer from 2009 to 2014. Use of active surveillance increased from 57% to 91% for very-low-risk cancer and from 40% to 74% for low-risk cancer (P < .001 for all comparisons), whereas use of watchful waiting decreased over time (Figure). The greatest increase in active surveillance for very-low-risk and low-risk cancer occurred after 2011. Classifying men using CAPRA scores, we found that active surveillance increased from 17% to 62% in men with CAPRA 0, 50% to 85% in CAPRA 1, 33% to 55% in CAPRA 2, and 22% to 29% in CAPRA 3. In men with CAPRA scores higher than 3, use of active surveillance was relatively stable (eFigure 1 in the Supplement).
Table.
Demographic and Clinical Characteristics in the National Prostate Cancer Register of Sweden of Men Diagnosed With Favorable-Risk Prostate Cancer, 2009–2014
| Characteristic | Participants, No. (%) | |||
|---|---|---|---|---|
|
| ||||
| Very Low Riska (n = 4693) |
Low Riskb (n = 15 403) |
Intermediate- Riskc (n = 17 115) |
All Men (N = 32 518) |
|
|
| ||||
| Calendar year | ||||
| 2009 | 665 (14) | 2895 (19) | 3002 (18) | 5897 (18) |
| 2010 | 741 (16) | 2683 (17) | 2837 (17) | 5520 (17) |
| 2011 | 727 (15) | 2582 (17) | 2882 (17) | 5464 (17) |
| 2012 | 718 (15) | 2214 (14) | 2532 (15) | 4746 (15) |
| 2013 | 815 (17) | 2385 (15) | 2832 (17) | 5217 (16) |
| 2014 | 1027 (22) | 2644 (17) | 3030 (18) | 5674 (17) |
| Age at diagnosis, y | ||||
| <50 | 67 (1) | 282 (2) | 161 (1) | 443 (1) |
| 50–59 | 1048 (22) | 3136 (20) | 2122 (12) | 5258 (16) |
| 60–69 | 2810 (60) | 8330 (54) | 8191 (48) | 16 521 (51) |
| ≥70 | 768 (16) | 3655 (24) | 6641 (39) | 10 296 (32) |
| Charlson comorbidity index | ||||
| 0 | 4096 (87) | 12 895 (84) | 13 560 (79) | 26 455 (81) |
| 1 | 356 (8) | 1426 (9) | 1964 (11) | 3390 (10) |
| ≥2 | 241 (5) | 1082 (7) | 1591 (9) | 2673 (8) |
| Type of hospital | ||||
| University | 660 (14) | 2126 (14) | 2311 (14) | 4437 (14) |
| Nonuniversity | 4033 (86) | 13 277 (86) | 14 804 (86) | 28 081 (86) |
| Marital statusd | ||||
| Married | 2015 (71) | 7216 (70) | 7714 (69) | 14 930 (69) |
| Divorced, widower, or never married | 835 (29) | 3155 (30) | 3535 (31) | 6690 (31) |
| Educational leveld,e | ||||
| Low | 676 (24) | 2847 (27) | 3692 (33) | 6539 (30) |
| Middle | 1151 (40) | 4330 (42) | 4483 (40) | 8813 (41) |
| High | 1016 (36) | 3157 (30) | 3016 (27) | 6173 (29) |
| Missing data | 8 | 40 | 62 (1) | 102 |
| PSA level at diagnosis, median (IQR), ng/mL | 4.6 (3.7–5.9) | 5.3 (4.0–7.0) | 8.2 (5.4–12.0) | 6.4 (4.5–9.3) |
| PSA density at diagnosis, ng/mL/cm3 | ||||
| <0.15 | 4693 (100) | 8155 (53) | 4674 (27) | 12 829 (39) |
| ≥0.15 | 0 | 6358 (41) | 11 419 (67) | 17 777 (55) |
| Missing data | 0 | 890 (6) | 1022 (6) | 1912 (6) |
| Clinical stage | ||||
| T1 | 4693 (100) | 12 362 (80) | 10 751 (63) | 23 113 (71) |
| T2 | 0 | 3041 (20) | 6364 (37) | 9405 (29) |
| Gleason score | ||||
| <7 | 4693 (100) | 15 403 (100) | 3219 (19) | 18 622 (57) |
| 7 | 0 | 0 | 13 896 (81) | 13 896 (43) |
| Positive cores, No. | ||||
| 1 | 2597 (55) | 5553 (36) | 2753 (16) | 8306 (26) |
| 2–3 | 1908 (41) | 5652 (37) | 5794 (34) | 11 446 (35) |
| 4–6 | 188 (4) | 2609 (17) | 5534 (32) | 8143 (25) |
| ≥7 | 0 | 541 (4) | 2312 (14) | 2853 (9) |
| Missing data | 0 | 1048 (7) | 722 (4) | 1770 (5) |
| Cancer extent in biopsies, mm | ||||
| <8 | 4693 (100) | 10 121 (66) | 6308 (37) | 16 429 (51) |
| 8–16 | 0 | 2083 (14) | 3677 (21) | 5760 (18) |
| ≥16 | 0 | 1495 (10) | 5717 (33) | 7212 (22) |
| Missing data | 0 | 1704 (11) | 1413 (8) | 3117 (10) |
| Primary treatment | ||||
| Active surveillance | 3436 (73) | 8389 (54) | 2868 (17) | 11 257 (35) |
| Watchful waiting | 211 (4) | 1200 (8) | 1468 (9) | 2668 (8) |
| ADT | 13 (0) | 172 (1) | 1466 (9) | 1638 (5) |
| Curative treatment | 1033 (22) | 5642 (37) | 11 313 (66) | 16 955 (52) |
CCI = Charlson comorbidity index, PSA = Prostate-specific antigen, IQR = Interquartile range, ADT = Androgen deprivation therapy
Very-low-risk disease defined as not N1/M1; clinical T1c; PSA, less than 10 ng/mL; Gleason 6 or lower; PSA density, less than 0.15 ng/mL/cm3; 4 positive cores or fewer; and less than 8 mm of cancer in biopsies.8
Low-risk disease defined as not N1/M1; clinical T1–T2; PSA, less than 10 ng/mL, Gleason 6 or lower. Low-risk category includes also all men in very-low- risk group.
Intermediate-risk disease defined as Gleason grading group 3: not N1/M1; clinical T1–T2; PSA, 10–20 ng/mL; or Gleason 7 (3 + 4 or 4 + 3).
Only available for men diagnosed between 2009 and 2012.
Educational levels in years: low, compulsory school (<10 y); middle, upper secondary school (10–12 y); high, college, or university (>12 y).
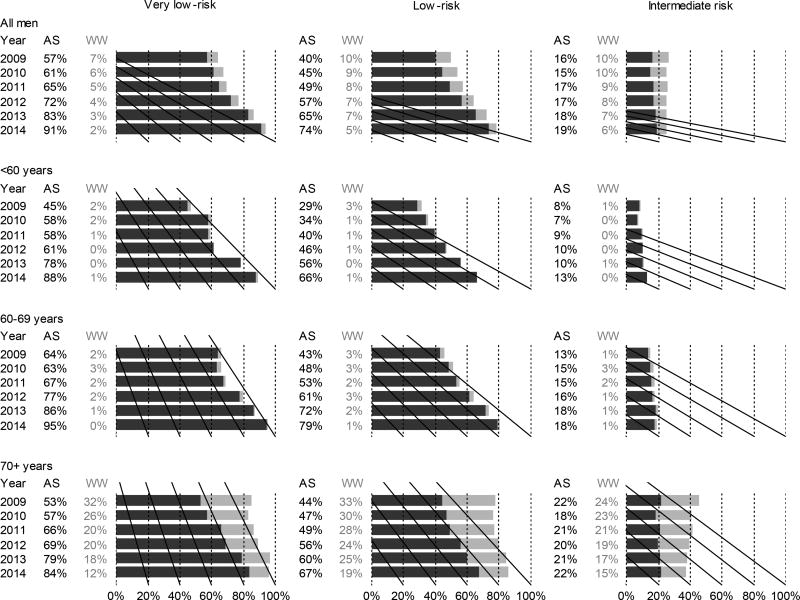
Figure.
Use of active surveillance (AS) and watchful waiting (WW) in Sweden from 2009–2014. Results are shown by age group and clinical risk category: very low-risk (clinical stage T1c, Gleason score ≤6, PSA <10 ng/ml, PSA density <0.15ng/ml/cm3, <8mm total cancer length in ≤4 positive biopsy cores)[8], low-risk (T1–T2, Gleason score ≤6 and PSA <10ng/ml), and intermediate-risk (T1–T2 with Gleason score 7 and/or PSA 10–20ng/ml).
Active surveillance increased over time in all age groups (<60, 60–69, and ≥70 years) (Figure). In men with very-low-risk cancer, active surveillance increased from 44% to 88% in those aged 50 to 59, 64% to 95% in those aged 60 to 69 years, and from 53% to 84% in those aged 70 years or older. In men with low-risk disease, active surveillance also increased, from 30% to 68% among those aged 50 to 59 years, from 43% to 79% in those aged 60 to 69 years, and from 44% to 67% in those aged 70 years or older. Even among men younger than 50 years, active surveillance increased, to 89% and 43% among men with very-low-risk and low-risk disease by 2014. Using the Epstein and PRIAS criteria for active surveillance yielded almost identical results, 93% and 90%, respectively, compared with 91% by the NPCR very-low-risk definition (eFigure 2 in the Supplement).
Active surveillance use among men in the intermediate-risk category remained low during the entire study period. However, use of active surveillance increased from 31% in 2009 to 53% in 2014 in the intermediate-risk subset with Gleason, 6 and PSA, 10–20 ng/mL. Use of active surveillance remained low in those with Gleason, 7 (3 + 4) and PSA, less than 10 ng/mL (17% in 2014) (eFigure 3 in the Supplement).
Within each clinical risk category, later year of diagnosis, diagnosis at a university hospital, older age, and status of not currently married were associated with the use of active surveillance, while comorbidity and educational level were not associated.
This population-based study shows the highest uptake of active surveillance ever reported: by 2014, 91% of Swedish men diagnosed with very-low-risk prostate cancer and 74% with low-risk disease initiated active surveillance. This use of active surveillance is substantially higher than that reported in the United States.1,9,10 Potential facilitating factors for the rapid uptake of active surveillance in Sweden include 2007 national guidelines recommending active surveillance for men with low-risk prostate cancer and a life expectancy of 10 to 20 years. In 2014, the 20-year upper limit for life expectancy was abandoned, and active surveillance was recommended for all men with very-low-risk prostate cancer. Importantly, NPCR provides real-time feedback to units on their adherence to these national guidelines and also in annual reports publically available online.11,12. The creation in 2010 of 6 regional cancer networks, supporting cancer services from primary to tertiary care, likely also increased adherence to the guidelines. The Swedish health care system is dominated by equal-access, tax-funded care without financial incentives for clinicians to recommend curative treatment. Furthermore, there may also be cultural differences in attitudes toward conservative management between Sweden and other countries such as the United States with lower use of these strategies. However, use of conservative management in the United States also rapidly increased from 2010 through 2013, suggesting more widespread acceptance worldwide.
A limitation of our study is that the interpretation of the definition of active surveillance among some clinicians became less stringent for men 70 years or older, indicated by the fact that more than twice as many men younger than 70 years on active surveillance underwent a repeat biopsy compared with men 70 years or older (eTable in the Supplement).
Another limitation is that our study does not include follow-up data on active surveillance outcomes. However, the objective was to study initial treatment selection by contemporary cases, and our group previously demonstrated that the majority of Swedish men on active surveillance had not converted to curative treatment at 5 years.13
A strength of our study is that, unlike data from US registries that do not differentiate active surveillance from watchful waiting or low-risk from very-low-risk prostate cancer,1,9 the Swedish NPCR provides population-based data on all of these features, and in addition, provides information on potential confounders including socioeconomic status and comorbidity from other nationwide databases. To our knowledge, there are no other reports from Europe on nationwide active surveillance use except our groups earlier Swedish data.5
In summary, active surveillance can preserve the benefits of screening while minimizing harm caused by overtreatment of indolent cancer.2 The Göteborg randomized prostate cancer screening trial14 showed the highest mortality reduction ever reported (44%) in a population in which almost half the screen-detected cancers were managed with active surveillance.14 Our results show that treatment patterns can be changed on a national level within a few years. Active surveillance is now the dominant management strategy for low-risk prostate cancer across Sweden, and there is an almost complete uptake of active surveillance for very-low-risk cancer. These findings should encourage clinicians, healthcare authorities, and cancer network administrators in other countries to reduce the harms of screening by minimizing overtreatment of low-risk prostate cancer.
Supplementary Material
Acknowledgments
This project was made possible by the continuous work of the National Prostate Cancer Register of Sweden (NPCR) steering group: Pär Stattin (chairman), Anders Widmark, Camilla Thellenberg, Ove Andrén, Eva Johansson Ann-Sofi Fransson, Magnus Törnblom, Stefan Carlsson, Marie Hjälm Eriksson, David Robinson, Mats Andén, Johan Stranne, Jonas Hugosson, Ingela Franck Lissbrant, Maria Nyberg, Göran Ahlgren, René Blom, Calle Waller, Kenth Leven, Per Fransson, Fredrik Sandin, Karin Hellström. Yasin Folkvaljon had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis
Funding
This work was supported by The Swedish Research Council 825-2012-5047 (Funded PCBaSe including infrastructure, project management, data management, cross linkages, steering group meetings etc since 2009); The Swedish Cancer Society 14 0570 (provided funding for statistical analysis for this study); Västerbotten County Council; the Swedish Cancer Society (2012/475) (salary to OB); the Louis Feil Charitable Lead Trust to SL (research support to SL); the Laura and Isaac Perlmutter Cancer Center at NYU Langone Medical Center (research support to SL) (P30CA016087); and the National Institutes of Health to SL (Award Number K07CA178258). The content is solely the responsibility of the authors and does not represent the official views of the NIH.
Footnotes
Related presentations: An abstract based on these data will be presented at the American Urological Association meeting.
Contributor Information
Stacy Loeb, New York University and Manhattan Veterans Affairs Medical Center, New York, NY, USA.
Yasin Folkvaljon, Regional Cancer Centre Uppsala Örebro, Uppsala University Hospital, Uppsala, Sweden.
Caitlin Curnyn, New York University and Manhattan Veterans Affairs Medical Center, New York, NY, USA.
David Robinson, Department of Urology, Ryhov County Hospital, Jönköping, Sweden, Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå.
Ola Bratt, Department of Urology, CamPARI Clinic, Addenbrooke’s Hospital, Cambridge, UK and Department of Translational Sciences, Lund University, Sweden.
Pär Stattin, Department of Surgical Sciences, Uppsala University, Sweden and Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå.
References
- 1.Cooperberg MR, Carroll PR. Trends in Management for Patients With Localized Prostate Cancer, 1990–2013. JAMA. 2015 Jul 7;314(1):80–82. doi: 10.1001/jama.2015.6036. [DOI] [PubMed] [Google Scholar]
- 2.Roth JA, Gulati R, Gore JL, Cooperberg MR, Etzioni R. Economic Analysis of Prostate-Specific Antigen Screening and Selective Treatment Strategies. JAMA oncology. 2016 Mar 24; doi: 10.1001/jamaoncol.2015.6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jonsson H, Holmstrom B, Duffy SW, Stattin P. Uptake of prostate-specific antigen testing for early prostate cancer detection in Sweden. Int J Cancer. 2011 Oct 15;129(8):1881–1888. doi: 10.1002/ijc.25846. [DOI] [PubMed] [Google Scholar]
- 4.Nordstrom T, Aly M, Clements MS, Weibull CE, Adolfsson J, Gronberg H. Prostate-specific antigen (PSA) testing is prevalent and increasing in Stockholm County, Sweden, Despite no recommendations for PSA screening: results from a population-based study, 2003–2011. European urology. 2013 Mar;63(3):419–425. doi: 10.1016/j.eururo.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Loeb S, Berglund A, Stattin P. Population Based Study of Use and Determinants of Active Surveillance and Watchful Waiting for Low and Intermediate Risk Prostate Cancer. The Journal of urology. 2013 May 30; doi: 10.1016/j.juro.2013.05.054. [DOI] [PubMed] [Google Scholar]
- 6.Tomic K, Berglund A, Robinson D, et al. Capture rate and representativity of The National Prostate Cancer Register of Sweden. Acta Oncol. 2015 Feb;54(2):158–163. doi: 10.3109/0284186X.2014.939299. [DOI] [PubMed] [Google Scholar]
- 7.Van Hemelrijck M, Wigertz A, Sandin F, et al. Cohort Profile: The National Prostate Cancer Register of Sweden and Prostate Cancer data Base Sweden 2.0. Int J Epidemiol. 2012 May 4; doi: 10.1093/ije/dys068. [DOI] [PubMed] [Google Scholar]
- 8.Bratt O, Folkvaljon Y, Loeb S, Klotz L, Egevad L, Stattin P. Upper limit of cancer extent on biopsy defining very low-risk prostate cancer. BJU Int. 2015 Aug;116(2):213–219. doi: 10.1111/bju.12874. [DOI] [PubMed] [Google Scholar]
- 9.Womble PR, Montie JE, Ye Z, et al. Contemporary Use of Initial Active Surveillance Among Men in Michigan with Low-risk Prostate Cancer. Eur Urol. 2015 Jan;67(1):44–50. doi: 10.1016/j.eururo.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 10.Ingimarsson JP, Celaya MO, Laviolette M, Rees JR, Hyams ES. Trends in initial management of prostate cancer in New Hampshire. Cancer Causes Control. 2015 Jun;26(6):923–929. doi: 10.1007/s10552-015-0574-8. [DOI] [PubMed] [Google Scholar]
- 11. [Accessed June 1, 2016]; www.npcr.se.
- 12.Stattin P, Sandin F, Sandback T, et al. Dashboard report on performance on select quality indicators to cancer care providers. Scandinavian journal of urology. 2016;50(1):21–28. doi: 10.3109/21681805.2015.1063083. [DOI] [PubMed] [Google Scholar]
- 13.Loeb S, Folkvaljon Y, Makarov DV, Bratt O, Bill-Axelson A, Stattin P. Five-year Nationwide Follow-up Study of Active Surveillance for Prostate Cancer. Eur Urol. 2014 Jun 30; doi: 10.1016/j.eururo.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hugosson J, Carlsson S, Aus G, et al. Mortality results from the Goteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010;11(8):725–732. doi: 10.1016/S1470-2045(10)70146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.