Abstract
Women appear to be more vulnerable to the depressogenic effects of inflammation than men. Chronic stress, one of the most pertinent risk factors of depression and anxiety, is known to induce behavioral and affective-like deficits via neuroimmune alterations including activation of the brain’s immune cells, proinflammatory cytokine expression, and subsequent changes in neurotransmission and synaptic plasticity within stress-related neural circuitry. Despite well-established sexual dimorphisms in the stress response, immunity, and prevalence of stress-linked psychiatric illnesses, much of current research investigating the neuroimmune impact of stress remains exclusively focused on male subjects. We summarize and evaluate here the available data regarding sex differences in the neuro-immune consequences of stress, and some of the physiological factors contributing to these differences. Furthermore, we discuss the extent to which sex differences in stress-related neuroinflammation can account for the overall female bias in stress-linked psychiatric disorders including major depressive disorder and anxiety disorders. The currently available evidence from rodent studies does not unequivocally support the peripheral inflammatory changes seen in women following stress. Replication of many recent findings in stress-related neuroinflammation in female subjects is necessary in order to build a framework in which we can assess the extent to which sex differences in stress-related inflammation contribute to the overall female bias in stress-related affective disorders.
Keywords: Sex differences, neuroinflammation, stress, female
1. Introduction
Stressful experiences can precipitate depression and anxiety, and stress-induced changes in physiology include an immune component. Throughout the evolution of mammalian physiology, stress-inducing situations reliably required activation of the immune system, and vice versa. As such, the endocrine and the immune system have come to be intricately co-regulated (Miller & Raison 2016), generating a physiological concordance that subserves vital functions such as regulation of energy allocation, reproduction, learning, mood, and behavior (Maier & Watkins 1998). The “pathogen host defense” hypothesis of depression posits that in response to a variety of environmental threats and challenges, stress perception and immune activation have co-evolved to generate “sickness behavior,” a set of behaviors that traditionally have protected ancestral humans from pathogens but have come to manifest as depressed mood and behavior in modern humans whose environmental challenges have largely shifted away from predators and immediate survival to psychosocial demands (Miller & Raison 2016, Raison & Miller 2013). In support of this hypothesis, considerable evidence indicates that psychological stress induces immune activation via the same signaling pathways as physiological stress – via the process of “sterile” inflammation, and that stress-evoked inflammation may be linked to the pathophysiology of depression (Iwata et al 2013).
Several lines of evidence suggest that the female sex may be associated with increased susceptibility to mood deficits following both long- and short-term inflammation in the body. Although mixed, some evidence for sex differences is provided by studies examining the effects of interferon-α treatment on mood in patients with Hepatitis. In some cases, interferon-α treatment generated greater depressive symptoms in women compared to men (Koskinas et al 2002); however, no sex differences in depressive symptoms following interferon-α treatment have also been reported (Bonaccorso et al 2002). In contrast to these smaller individual studies, a recent meta-analysis by Udina et al (2012a) found the female sex to be predictive of major depressive episodes following antiviral treatment. In addition, a series of recent studies in healthy humans have suggested that women are behaviorally more vulnerable to the acute depressogenic effects of endotoxin-induced inflammation (Eisenberger et al 2009, Moieni et al 2015). Although particularly the latter experimental design cannot fully recapitulate the behavioral and neurobiological correlates of depression in women (DellaGioia & Hannestad 2010), it provides a proof-of-concept demonstrating the greater short-term influence of immune activation on women’s mood. It has been argued that given the negative impact of inflammation on reproduction, the greater sensitivity to inflammation in women may have conferred reproductive benefits by increasing sickness or depressive-like behavior, thus resolving and avoiding inflammation (Miller & Raison 2016). While this conceptual framework may help explain the modern female bias in stress disorders, whether females mount a more pronounced immune response following stressors, and therefore experience greater maladaptive consequences with regards to mood and behavior, has not been critically evaluated.
Recent progress in our understanding of stress-related alterations in immune function has prompted the consideration of psychotropic drugs with primary immunomodulatory actions – including non-steroidal anti-inflammatory drugs and other anti-cytokine agents – for the treatment of stress-related psychiatric and somatic illnesses, with several clinical trials demonstrating modest therapeutic benefits (Kohler et al 2014) and others showing no effect (Eyre et al 2015). However, conflicting evidence exists regarding the involvement of inflammatory dysregulation in males and females with depression. Although depressed women report a higher prevalence of somatic symptoms (Silverstein 1999) and are more vulnerable to the harmful effects of inflammation (Derry et al 2015), the link between stress-related psychiatric illnesses and low-grade inflammation is more consistently found in men than women (Liukkonen et al 2011, Ramsey et al 2016). In fact, C-reactive protein (CRP), one of the most consistently reported inflammatory biomarkers of mood disorders, was recently found to be associated with anxiety and comorbid anxiety and depression in men, but not women (Liukkonen et al 2011). Moreover, when large-scale data from the Netherlands Study of Depression and Anxiety (NESDA) were re-analyzed with sex as a variable, several immune markers previously reported to be associated with depression turned out to be male-specific (Ramsey et al 2016). These reports highlight the inherent etiological heterogeneity in mood disorders whereby inflammation occurs in only a subset of affected populations, and potentially demonstrate clinically relevant implications for the generalizability of anti-inflammatory treatments for mood disorders. While efforts to reduce the sex gap in stress research has yielded some significant mechanistic insights into the underlying biological mechanisms, some areas remain to be investigated. This review summarizes and evaluates the available data on sex differences in the neuroimmune consequences of stress, and some of the physiological factors contributing to them.
2. Sex differences in stress response
The female bias in mood disorders has traditionally been attributed to dysregulation of the hypothalamic-pituitary-adrenal (HPA) and hypothalamic-pituitary-gonadal (HPG) axes; however, the past few decades have led to significant discoveries in the neuroimmune consequences of stress including the identification of inflammasomes and signaling pathways mediating stress-related cytokine expression within the brain, a better delineation of immune cell subsets responsible for the neuroimmune effects of stress, and recognition that inflammatory signaling impacts synaptic plasticity and neurotransmission in both health and disease (Deak et al 2015). Considerable evidence demonstrates that males and females respond differently to stressors in terms of behavioral outcomes, activation of the HPA axis and the sympathetic nervous system, and, research into sex differences in inflammatory and immune processes have revealed nuanced effects of sex on different aspects of immunity such as wound healing, immunosuppression, host-defense mechanisms, and chronic, low-grade inflammation.
Stress is an undercurrent in most aspects of health, an effect mediated by the mutual regulation between endocrine and immune systems in both normal and pathological conditions. Stress can both enhance and suppress immune function depending on the type, duration, and frequency of stressors, combination of stress hormones released, and the particular aspect of immunity being examined (Dhabhar 2003). Acute stress occurring before or at the time of immune activation has been shown to enhance wound healing via increasing the mobilization and trafficking of immune cells. Longer exposure to stressors involving acute activation of the hypothalamic-pituitary-adrenal (HPA) axis and the glucocorticoid receptor (GR) can suppress inflammatory processes primarily through the anti-inflammatory transcriptional actions of the GR. Both the immune-enhancing and immunosuppressive actions of stress hormones have been framed as an adaptive strategy that either promotes inflammatory processes necessary for wound healing in situations where wound acquisition would have been likely, or one that conserves energy during critical situations by suppressing energy-consuming immune processes (Dhabhar 2003). Experiencing stressors of yet longer durations such as hours to weeks, which may constitute pathological degrees of stress exposure, can permanently alter the underlying biology that enables normal enhancement or suppression of the immune system by stress, and cause either chronic excessive inflammation or a state of immunosuppression, both of which are detrimental to the health and well-being of an organism. Mood disorders represent one such pathological state where excessive inflammation and dampened stress hormone signaling are concurrently present. Interestingly, impaired balance between stress and immune axis output, i.e. plasma levels of the anti- and pro-inflammatory mediators cortisol (Cort) and CRP, appears to be linked to distinct behavioral deficits in men and women. Namely, decreased Cort/CRP ratio, indicating increased inflammatory output relative to stress axis activity, was shown to be associated with increasing severity of depressive symptoms in women; whereas, increased Cort/CRP ratio, which suggests enhanced HPA axis output relative to inflammation, was associated with greater anxiety in men (Suarez et al 2015).
Numerous studies conducted both in humans and in animal models detail profound sex differences in the endocrine, behavioral, and neural aspects of the stress response, driven primarily by adrenal and gonadal influences. An important between-species difference emerges with regards to sex differences in HPA axis activity following a stressor. In addition to a more constitutively active HPA axis at baseline conditions, female rodents display a more robust and prolonged activation of the axis following acute stress (Handa et al 1994). In contrast, men display greater induction of ACTH and cortisol compared to women following the Trier Social Stress Test (TSST), a highly standardized laboratory acute stressor (Stephens et al 2016). Sex-dependent disparity in the prevalence of stress disorders is partially attributed to the impact of gonadal hormones on the neuroendocrine system throughout development (Panagiotakopoulos & Neigh 2014). In both rodents and humans, testosterone is negatively correlated with ACTH and corticosterone/cortisol (Stephens et al 2016); whereas, estrogen acts on both the hypothalamus and the adrenal gland to stimulate the output of the HPA axis (Panagiotakopoulos & Neigh 2014). Furthermore, the expression and regulation of GR, as well as interaction of sex steroids with GR, are sexually dimorphic (Bourke et al 2012, Bourke & Neigh 2011, Bourke et al 2013), and may lead to differential onset, magnitude, and resolution of endocrine responses in males and females during stressor exposure.
3. Sex differences in neuroinflammation and its consequences
Inflammatory processes in the brain influence many aspects of normal and pathological physiology in the brain such as alterations in synaptic (Pribiag & Stellwagen 2014) and neuronal plasticity (Kubera et al 2011), neurotransmission (Haroon et al 2016, Merali et al 1997), and related behavioral outcomes (Howerton et al 2014). Males and females display distinct vulnerabilities to different types of inflammatory dysregulation which suggests underlying biological differences. Neuroinflammatory diseases such as multiple sclerosis, Alzheimer’s disease, and chronic pain are more common among females (Loram et al 2012), and dysregulation of immunocyte function, steroid hormone signaling (Oertelt-Prigione 2012), and gut microbiome-driven changes in autoimmunity (Markle et al 2013) have been proposed as potential mediators. However, females are behaviorally and/or immunologically protected in some models of inflammatory diseases such as experimental autoimmune encephalomyelitis (Harpaz et al 2013), hypoxic-ischemic encephalopathy (Mirza et al 2015), and microembolic stroke (Nemeth et al 2014), and this protection is thought to be derived, at least partially, from the anti-inflammatory actions of estrogen and progesterone (Czlonkowska et al 2006). Both estrogen and progesterone suppress inflammation at physiological concentrations, and women suffering from autoimmune disorders experience a profound remission during pregnancy, throughout which the levels of estrogen and progesterone maintain significantly elevated concentrations. These underlying sex differences in neuroimmunity are likely to impact stress-evoked inflammation in the brain, and potentially contribute to the differential behavioral outcome of stress in males and females.
Furthermore, some evidence suggests that females may be more vulnerable to the central effects of peripheral inflammation. While both healthy men and women display increases in pro-inflammatory cytokines when administered a small dose of endotoxin (lipopolysaccharide, LPS), cytokine induction was associated with depressed mood and increased feelings of social disconnectedness in women only (Moieni et al 2015). Interestingly, however, no sex differences in anxiety and mood responses were found when lower LPS doses were administered or when only women using hormonal contraceptives are included in between-sex comparisons (Engler et al 2015, Lasselin et al 2016). Furthermore, the relationship between plasma increases in IL-6 and depressed mood was found to be mediated by an association between IL-6 and increased activity in dorsal anterior cingulate cortex, anterior insula – areas involved in social pain – in women who received LPS but not men (Eisenberger et al 2009). Similarly, intranasal endotoxin challenge led to depressive-like behavior, and increased expression of TNF-α and IL-6 in the hippocampus and brainstem of female, but not male, rats (Tonelli et al 2008). Taken together, these results suggest that systemic inflammation impairs mood and affective behavior of females to a greater extent than of males. It is conceivable that chronic stress, via inducing a low-grade, generalized inflammatory state in the body and brain, exerts similar sexually dimorphic effects as immune activation.
Sex differences in peripheral and central inflammatory and immune processes have been studied both in vivo and ex vivo. Peripheral immune cells, which contribute to neuroinflammation both indirectly via extravasation of peripherally released cytokines and directly by trafficking and infiltration, exhibit considerable sexual dimorphism (Klein 2012). A recent study by De Leon-Nava et al (2009) demonstrated similar levels of estrogen-α receptor in T- and B-lymphocytes from males and females, but a greater expression of progesterone receptor on lymphocytes from males, a difference that was abolished by gonadectomy. Immune cells within the brain can also contribute to sexually dimorphic neuroimmune processes via displaying differential activity, number, and regulation in males and females. As the primary immune effector cells in the CNS, microglia constitute the first-line response to injury and infection, and later on promote repair and resolution of inflammation. When unactivated, microglia survey the environment, and regulate neuronal excitability, synaptic architecture, neurogenesis, and programmed cell death (Lenz & McCarthy 2015). Microglia are known to display a dynamic sexual dimorphism in their number and morphology throughout development (Schwarz et al 2012). As the rat brain develops, the proportion of round or amoeboid-shaped microglia decreases, and microglia with thicker or long and ramified morphology become increasingly common in several brain regions regardless of sex. However, the rate of microglial colonization and morphological development differs between males and females such that males have more microglia early in post-natal development, which may confer heightened vulnerability to the negative consequences of immune insult during this time in males. Indeed, exposure to inflammatory insults during early development, especially in utero immune stressors, has been suggested to be linked to developmental disorders such as autism and schizophrenia particularly in males (Bale 2015). In contrast, female rats possess more microglia with activated morphology starting around early puberty and in adulthood (Schwarz et al 2012), which interestingly coincides with the onset of a significantly greater prevalence of mood disorders in women but these two temporally congruent events have not yet been mechanistically linked.
Consistent with the contribution of developmental processes to sex differences in neuroimmunity, glia from males and females have been shown to respond differentially to sex steroids during early development and in adulthood. Loram et al (2012) demonstrated that microglia and astrocytes isolated from neonatal male rats release more IL-1β when stimulated with LPS compared to glia from neonatal females. Furthermore, co-stimulation with estradiol suppressed LPS-induced IL-1β expression in neonatal male microglia but enhanced IL-1β expression in female microglia. Conversely, estradiol attenuated LPS-induced IL-1β expression in adult hippocampal microglia of ovariectomized female rats, but not intact male rats. These dynamic sex differences in both stress response and inflammation may influence stress-induced inflammatory processes in the brain, and thereby, the resulting changes in mood and behavior, as discussed in the next section.
4. Sex differences in the neuro-immune consequences of stress
One of the main outcomes of stress-induced inflammation is induction of “sickness behavior,” a set of highly conserved physiological and behavioral changes that promote the effective resolution of ongoing inflammation in the body and prevent further inflammation. During the initial phases of sickness, peripherally released inflammatory mediators such as cytokines and acute phase proteins initiate an inflammatory response within the brain itself via activating the brain’s resident immune cells (Dantzer 2006). Activation of hypothalamic nuclei involved in homeostatic regulation by immune or stress signals then initiate physiological and behavioral changes such as hyperthermia, anorexia, increased sleep, and decreased mood and social withdrawal. Although genetic variants that promote enhanced stress-induced inflammation have had enough evolutionary success to remain in the modern genetic pool, in the vastly different environmental needs of today, these same genetic variants may contribute to a predisposition towards mood disorders (Raison & Miller 2013).
Much of our knowledge regarding the relationship between stress, inflammation, and depressive behaviors is derived from chronic stress paradigms commonly used in rodent research. The neuroimmune consequences of chronic stress have been studied primarily within the context of 1) stress-induced cytokine expression in frontal-limbic structures, 2) alterations in the number and function of glial cells, including microglia, the brain’s resident immune cells, 3) inflammatory pathways that mediate the “priming” effect of stress to cause subsequent hyperinflammation upon later immune stimulation, and more recently 4) the infiltration and trafficking of peripheral immune cells to stress-responsive brain regions. Understanding the impact of neuroimmune consequences of stress has helped linkinflammatory processes to stress-driven alterations in synaptic plasticity and neurotransmission.
Converging evidence from animal and human studies demonstrate that males and females display differences in the immune response to an acute stressor or direct HPA axis activation. The concurrent immunosuppressive and hyper-inflammatory effects of stress may each manifest in the periphery as reduced wound healing capacity and in the central nervous system as excessive inflammation that is detrimental to neurotransmission, synaptic plasticity, and growth and metabolism in the brain – processes that are thought to underlie the effects of stress on mood and behavior. Stress perception, coping and management styles may be different in men and women, thus making studying the biological mechanisms of sex differences in stress-linked disorders more challenging. Therefore animal models offer a controlled setting to tease out the biological mechanisms of stress-linked inflammation. However, similar to the diverging findings on sexual dimorphism in the stress response across species, it is possible that humans and rodents display different trends in neuroimmune activation following stress. The following sub-sections review the existing literature on stress-induced immune activation generated by investigation of humans and assessment of rodent models.
4.1. Evidence from human literature
In the absence of easy access to immune measures in the brain following stress, most human studies have investigated the stress-induced response of peripheral immune cells. Although such peripheral immune measures are unlikely to completely recapitulate stress-induced neuroimmune alterations, converging evidence points to the usefulness of assessing stress-evoked peripheral inflammation in identifying susceptibility to stress-related neural activity. Functional imaging studies in particular have highlighted the validity of assessing the peripheral immune response as a proxy to studying stress-related processes in the brain. Among women who underwent a brief social stressor, individuals showing the greatest plasma cytokine response following stress also showed a greater activation of the amygdala, a key regulatory region of the HPA axis (Muscatell et al 2015). Similarly, larger increases in peripheral soluble receptor for tumor necrosis factor-α (sTNFαRII) following the TSST were found to be correlated with greater activity in the dorsal anterior cingulate cortex and anterior insula while experiencing social rejection (Slavich et al 2010). These brain regions have previously been associated with processing social pain and rejection, and their activation was unrelated to baseline levels of sTNFαRII. In addition, peripheral blood cells have been used to establish a link between genetic variability in inflammation-related genes and brain structure. An interaction between polymorphisms in the GR and interleukin-1 genes, and early adverse life events was found to be significantly associated with thinning of the subgenual cingulate cortex, a region linked to treatment-resistant depression and emotional arousal (Gupta et al 2016). The following sub-sections summarize both acute laboratory stress-induced alterations in leukocytes and chronic stress-related changes in wound healing and antibody titer development in men and women.
4.1.1. Stress-induced changes in human leukocyte population and function
Among a depressed group of patients, an increase in the number of white blood cells, also known as leukocytosis and a hallmark of depression, was found to occur to a greater extent in depressed men than depressed women (Maes et al 1992). While this pronounced leukocytosis in depressed men was primarily driven by increases in monocytes regardless of depression diagnosis, women overall displayed a greater percentage of lymphocytes than men, potentially suggesting differential roles of leukocyte subtypes in depression in men and women. Given the stringent regulation of immune cells by stress hormones it is therefore reasonable to hypothesize that stress leads to a greater activation and number of total leukocytes, as well as specific subsets such as monocytes, in males. The most recent meta-analyses examining the association between immune measures and stress in humans discovered 1) alterations in the number of blood cells including an overall leukocytosis, changes in the levels of cytotoxic lymphocytes, T-cells, CD4/CD8 ratios, and natural killer (NK) cell cytotoxicity, as well as 2) functional changes such as altered antibody titers to several different viruses, reduced lymphocyte proliferation, and increased lymphocyte adhesiveness (Segerstrom & Miller 2004, Zorrilla et al 2001). Many of these findings have been confirmed in both sexes; however, systematic analysis of sex differences in these immune measures are scarce. The few studies in which male and female responses to a laboratory stressor have been compared suggest that females may mount a more robust mobilization of immune cells. Acute mental stress consisting of the Stroop color-word interference and cold pressor test has been reported to increase the number of natural killer cells in the blood of women in both the follicular and luteal phases, whereas theses stressors decreased the number of natural killer cells in the blood of men (Pehlivanoglu et al 2012). The use of oral contraceptives by females has been associated with significantly higher stress-induced responses in number of total leukocytes, neutrophils and CD19+ B cells than the response of either females without the use of oral contraceptives or males, suggesting a strong influence of female sex steroids on immune cells responsivity (Maes et al 1999). Notably, the results from these acute stress studies are not consistent with the afore-mentioned hypothesis of greater immune responsivity to stress in males, but the cause of the discrepancy has not yet been delineated. Further studies examining specific subsets of immune cells in chronically stressed men and women are necessary to investigate potential sex-specific mechanisms mediating the link between mood disorders and immune cell alterations that occur in these conditions.
In addition to the sex-specific changes to their number, leukocytes from men and women also display differential function and activity. Post-menopausal women display greater induction of the pro-inflammatory cytokine IL-6 at 45 and 75 minutes following a brief mental stress paradigm compared to men of the same age (Endrighi et al 2016). Following acute stress, peripheral blood cells from men and women exhibit differential kinetics of pro-inflammatory cytokine production when stimulated with LPS ex vivo (Prather et al 2009). Leukocytes obtained from men immediately after cessation of the acute stress displayed a reduction in LPS-induced expression of IL-6 and TNF-α compared to cells obtained just before the stressor. This stress-mediated suppression of cytokine production was absent in women, an effect found to be driven primarily by responses from postmenopausal subjects. Men have also been shown to display greater immunosuppression by acute stress in studies that utilized dexamethasone, a synthetic glucocorticoid that is able to suppress antigen-stimulated cytokines. Following exposure to the TSST, men and women displayed similar increases in plasma cortisol (Rohleder et al 2001). However, glucocorticoid sensitivity – defined as the capacity of dexamethasone to suppress antigen-induced pro-inflammatory cytokines ex vivo – was increased in leukocytes from men, and unchanged or even slightly decreased in cells from women in the luteal phase of their estrous cycle. It is therefore possible that following an acute psychosocial stressor, women are exposed for a longer duration to, and therefore more susceptible to, the potentially harmful effects of pro-inflammatory processes. In a follow-up study by these authors, healthy men and women were exposed to up to one minute of nauseogenic body rotation stress, which increases plasma expression of the stress hormones ACTH, cortisol, and vasopressin, over four days (Rohleder et al 2006). Consistent with the sex-specific effects of TSST described above, men displayed increased glucocorticoid sensitivity on cytokine suppression on the first day. However, by day three of the stress paradigm, men started to show decreased glucocorticoid sensitivity, whereas, women continued to show no significant changes due to rotation stress. Taken together, these results suggest that acute and chronic stressors may differentially impact sex-specific immune responses, although the latter study’s small sample size is a noted limitation.
4.1.2. Stress-induced changes in human wound healing and antibody titer
Both acute and chronic stress have been linked to slower wound healing in samples consisting of men and women or women only; yet sex differences have not yet been systematically evaluated. Acute stressors as brief as the TSST or a slightly longer, but temporary, stressful episode such as examination stress, have been shown to delay skin barrier recovery (Robles 2007) and mucosal wound healing (Marucha et al 1998) respectively, in gender-mixed samples. Considering that men displayed significantly greater cortisol responses to the TSST compared to women in the first study, it is surprising that wound healing outcomes have not been stratified by sex in these experiments. Chronic stress experienced by healthy female caregivers has also been associated with a significant delay in wound healing and decreased cellular immunity (Kiecolt-Glaser et al 1995). Although caregiver stress has since been linked to greater inflammatory activity and glucocorticoid resistance in gender-mixed samples (Miller et al 2014), the wound healing paradigm has not been replicated in male subjects to allow investigation of sex differences. The only study to compare how chronic stress impacts male and female immunity suggests that despite the excessive inflammation potentially experienced by women following acute stress, when chronically stressed, women may experience greater suppression of immunity compared to men. Among first-year law students, a group characterized by unusually high experience of chronic stress, men displayed greater delayed-type hypersensitivity (DTH) response to a skin antigen challenge compared to women despite there being no differences between the sexes in the non-stressed population (Flynn et al 2009). Lower DTH response in chronically stressed women indicates decreased cellular immunity which is associated with impaired resistance to infection. In addition to wound healing, stress-induced immune changes have been investigated in the context of antibody titer development in response to vaccination. It is well-established that psychosocial stress is associated with decreased antibody titer to various viruses or vaccines in both men and women (Pedersen et al 2009). One study examining healthy men and women’s antibody response to influenza vaccination reported that gender was found not to predict antibody titer, but cumulative stress did predict antibody titer (Miller et al 2004). Collectively, the aforementioned studies offer some evidence that chronic stress leads to a greater suppression of cell-mediated immunity in women; however, replication of many landmark experiments in both sexes are necessary to fully assess sex differences in stress-related immune alterations.
4.2. Evidence from rodent literature
4.2.1. Stress-induced changes in rodent leukocyte populations and function
Several studies that have characterized rodent leukocytes at basal and stress conditions reveal sex differences both in gross immune measures such as stress-induced thymic involution, as well as, in specific subsets of leukocytes belonging to the innate and adaptive immune systems. On a gross scale, restraint stress ranging in chronicity from 1 to 14 days showed that stressed male mice displayed greater thymic involution (Dominguez-Gerpe & Rey-Mendez 1998) although between-sex statistics were not provided. Among studies that examined changes in total number of leukocytes, Stefanski and Gruner (2006) found that exposure to a two-hour-long social confrontation stressor led to a greater number of leukocytes in male rats compared to females, an effect primarily driven by a male sex-stress interaction leading to a dramatic increase in the granulocyte population. In contrast, another study that utilized a six-week restraint stressor reported that stressed female rats had a greater number of total leukocytes and antibody response compared to stressed males (Baldwin et al 1997) although the sex-stress interaction effect did not reach statistical significance. These two studies potentially highlight the contrasting immunological consequences of short-term and long-term stress exposure, a theme that is also present in sex differences in specific subsets of immune cells as detailed below.
Of the studies that examined distinct populations of immune cells following stress, a general trend of sex differences in the innate immune system emerges, whereas sex-specific changes to cells of the adaptive immune system are less consistent. The innate arm of the immune system includes granulocytes which are professional phagocytic cells and natural killer cells which display cytotoxicity toward infected host cells. These cells of the innate immune system are fast-acting agents that respond to damage and infection non-specifically, in contrast with the adaptive immune system which responds to specific pathogens. Under basal conditions, female Sprague-Dawley rats displayed greater splenic natural killer cell cytotoxicity and lymphokine-activated killer cell cytotoxicity compared to male rats (Pitychoutis et al 2009). Exposure to chronic mild stress (CMS) for 7 weeks decreased natural killer cell cytotoxicity in females, but increased it in males; however, a sex-stress interaction was not tested. Additionally, CMS reduced lymphokine-activated killer cell cytotoxicity in female rats only, which suggests potentially sex-specific immunosuppressive effects of CMS. Another study showed that among adult Long-Evans rats, females at baseline were shown to have fewer NK cells in the blood than males (Stefanski & Gruner 2006). Following a two-hour resident-intruder stressor, stressed male rats displayed a greater number of granulocytes and an increase in phagocytic activity, but no difference in lymphocyte proliferation compared to females exposed to stress. It is possible that acute stress temporarily enhances immunity by promoting circulation of fast-acting immune cells whereas chronic stress suppresses immunity by decreasing the cytotoxic activity of cells in the spleen, a reservoir of immune cells that can be deployed upon demand. The kinetics of stress-induced changes in leukocyte number appear to be similar in male and female rats (Neeman et al 2012). Namely, the number of leukocytes decreases during stress, followed by an increase upon cessation of the stress, thus illustrating stressor duration-dependent changes.
A series of studies by de Coupade and colleagues demonstrated a sex-specific response of neutrophils, the largest group of granulocytes, to a sub-chronic, non-habituating sound stress. Exposure to intermittent sound stress over 4 days suppressed reactive oxygen species (ROS) production by peripheral blood neutrophils of male, but not female, rats, an effect found to be mediated by adrenal hormones (Brown et al 2008). Furthermore, following this non-habituating sound stress paradigm, neutrophils from male rats display enhanced LPS-induced trafficking, but this alteration in neutrophils was not observed in female rats. In contrast, neutrophils isolated from female rats exhibited both greater β2-adrenergic receptor binding as well as increased non-directed migration upon β2 signaling (de Coupade et al 2004). These results are potentially of relevance to stress-induced neuroinflammation because another group (Wohleb et al 2011) recently demonstrated that β-adrenergic receptor antagonism reduced social defeat-induced trafficking of peripheral macrophages and normalized anxiety-like behavior in male mice.
4.2.2. Stress-induced changes in rodent wound healing
Rodent studies of wound healing highlight sex differences in both outcome and mechanisms. Hermes et al. 2005 reported that chronic isolation stress in male and female rats differentially altered the non-specific immune response induced by carrageenin, in which immune cells produce a granuloma as part of the healing process. Compared to same-sex group-housed rats, male, but not female, rats subjected to chronic isolation stress showed a decrease in carrageenin-induced exudate volume, suggesting decreased wound healing capacities (Hermes et al 2006). Furthermore, a single exposure to restraint two weeks prior to carrageenin facilitated healing in female rats as indicated by a reduction in exudate volume, and the number of lymphocytes per volume of exudate present on day 10 of the inflammatory response. However, male rats exposed to restraint displayed increased exudate volume and lymphocyte count, and unaltered healing score at this chronic stage of the inflammatory response. These results indicate that both chronic and acute stressors lead to a more robust non-specific inflammatory response in female rodents, a conclusion at odds with findings from chronic stress in humans which may reflect both differences in rodent and human reproductive axes (Becker et al 2005) and differences in rodent and human immune responses (Mestas & Hughes 2004).
4.2.3. Stress-induced neuroinflammation
Sex differences in stress-induced neuroinflammation may arise from sexual dimorphism at multiple levels including cellular, molecular, and endocrine regulation. The few existing reports on the effects of stress on microglia in the male and female brain have used different stressors and developmental timelines, and examined a variety of brain regions, thus making direct comparisons untenable (see Table 1). Bollinger et al (2016) reported that although unstressed adult male and female rats displayed similar numbers of total microglia in the medial prefrontal cortex (mPFC), the ratio of primed to ramified microglia was higher in unstressed females. Increased ratio of primed microglia would traditionally be interpreted as indicative of a heightened basal state of activation; however, it should be noted that microglial morphology alone does not necessary indicate their activation state (Beynon & Walker 2012). Furthermore, the significance of greater expression in female PFC of CX3CL1 and CX3CLR, which exert anti-inflammatory and neuroprotective influences in some settings (Morganti et al 2012), remains to be elucidated, and thus may challenge the notion that unstressed female rats display more microglial activation. Bollinger et al (2016) also found that acute (1-day) and chronic (10 days) restraint stress reduced the ratio of microglia with a “primed” morphology relative to its basal, ramified morphology in females but not males, indicating female sex-specific changes following stress. This result contrasts with the findings of Chocyk et al (2011) who employed a paradigm of rat maternal separation during postnatal days 1–14. This paradigm decreased the number of non-neuronal (glial) cells in the substantia nigra pars compacta and ventral tegmental area of male, but not female, rats (Chocyk et al 2011). Using prenatal stressors of same duration and chronicity but different nature, Diz-Chaves and colleagues demonstrated increased Iba-1+ (microglial marker) cells in female dentate gyrus and increased Iba-1+ and morphologically active cells in male CA1 region of the hippocampus (Diz-Chaves et al 2013, Diz-Chaves et al 2012). Finally, prenatal exposure to dexamethasone has been reported to cause sex-specific, long-lasting structural changes in prefrontal microglia of adult offspring (Caetano et al 2016). In this study, pregnant dams received an injection of dexamethasone on gestational days 18 and 19, and microglial morphology was assessed in adult offspring. Prenatal dexamethasone led to longer and more numerous microglial processes in males, whereas it was associated with fewer and shorter processes in females. This hyper-ramification found in stressed males was interpreted to be indicative of behavioral deficits. Collectively, these studies suggest that sex differences in stress-induced neuroinflammation may arise from sexual dimorphism at multiple levels including cellular, molecular, and endocrine regulation.
Table 1.
Summary of stress-induced neuroimmune alterations in females, and males if included in the same or similar study.
| Stressor | Stressor duration | Sex | Outcome | Region/Organ | Age at assessment | Species | Reference | |
|---|---|---|---|---|---|---|---|---|
| Stress-driven changes in baseline expression of inflammatory mediators | Maternal chronic variable stress | E1–E7 | M | ↑ inflammation-related genes | Placenta | E 12.5 | Mouse | Bronson et al. 2014* |
| F | → inflammation-related genes | |||||||
| Prenatal bright light | 45 min * 3 times/day during E12-PND0 | M | ↑ IL-1β, TNF-α | HC | PND 120 | Mouse | Diz-Chaves et al. 2013 | |
| Prenatal restraint | F | ↑ IL-6 | HC | PND 135 | Mouse | Diz-Chaves et al. 2012† | ||
| Maternal deprivation on PND 9 | 24 hr | M | ↑ synaptic expression of IL-1R1 | HC | PND 45 | Rat | Viviani et al. 2014* | |
| F | → synaptic expression of IL-1R1 | |||||||
| Maternal deprivation on PND 9 | 24 hr | M | → IL-1β, TNF-α, IL-6, CD11b, GFAP | HC, PFC | PND 103 | Rat | Burke et al. 2000 * | |
| F | → IL-1β, TNF-α, IL-6, CD11b, GFAP | |||||||
| Prenatal repeated restraint, prenatal | 45 min * 3 times/day during E14–21 | M | ↓ IL-1β immunoreacvity | Hilus of DG | PND 147 –161 | Rat | Mandyam et al. 2008 * | |
| F | → IL-1β immunoreacvity | |||||||
| Prolonged restraint stress | 6 hr/day for 28 days | F | ↓ IL-10 after 1–4 weeks of restraint, ↑ IL-4, IL-1β and ↓ TNF-α after 2 weeks of stress, ↑ IFN after 2 and 4 weeks of stress | Cortex, HC | Adult (> PND 50) | Mouse | Voorhees et al. 2010 | |
| Footshock | F | ↑ mRNA expression of IL-1 | HC | > PND 77 | Rat | Arakawa et al. 2014† | ||
| Stress-driven changes in acute stress-stimulated immune response | Variable stressor | 3 days | M | Stress history potentiated IL-1β following re-exposure to restraint (↑) | HC | In adulthood, 6 weeks after initial stress exposure | Mouse | Hudson et al. 2014* |
| F | Stress history did not potentiate IL-1β following re-exposure to restraint (→) | |||||||
| Stress-driven changes in antigen-stimulated immune response | Chronic adolescent stress | 60 min of restraint or 5 min of social defeat/day during PND 37–48 | M | Stress history potentiated LPS-induced IL-1β, TNF-α (↑) | HC | PND 80 (4.5 weeks after the end of stress) | Rat | Pyter et al. 2013* |
| F | Stress history did not potentiate LPS-induced IL-1β, TNF-α (→) | |||||||
| Stress-induced glial alterations | Prenatal bright light stress | 45 min * 3 times/day during E12-PND0 | M | ↑ percent of Iba-1 cells with reactive morphology | CA1 | PND 120 | Mouse | Diz-Chaves et al. 2013 |
| Prenatal restraint | 45 min * 3 times/day | F | ↑ percent of Iba-1-posive cells | DG | PND 120 | Mouse | Diz-Chaves et al. 2012† | |
| Maternal separation | 3 hr/day during PND 1 –14 | M | ↓ number of glia | SNpc, VTA | PND 15 | Rat | Chocyk et al. 2011* | |
| F | → number of glia | |||||||
| Acute and chronic restraint | 3 hr/day for 1 or 10 days | M | → ratio of primed to ramified microglia | mPFC | Adult (> PND 70) | Rat | Bollinger et al. 2016* | |
| F | ↓ ratio of primed to ramified microglia | |||||||
| Prenatal dexamethasone administration | 1 mg/kg on E18 and E19 | M | ↑ number and length of microglia processes | PFC | PND 90 | Rat | Caetano et al. 2016* | |
| F | ↓ number and length of microglia processes | |||||||
| Stress-driven trafficking of peripheral immune cells to the brain | Witnessing footshock of cagemate | 1 hr/day for 5 days | M | ↑ trafficking of bone marrow-derived cells into the brain | PVN | Adult | Mouse | Ataka et al. 2013 |
| Footshock | 1 hr/day for 1,2, or 5 days | F | ↑ trafficking of bone marrow-derived cells into the brain | Ventral HC | Adult (> PND 98) | Mouse | Brevet et al. 2010 |
both males and females assessed in the same study;
female rats were ovariectomized.
M = male; F = female. E = embryonic day; PND = postnatal day. HC = hippocampus; DG = dentate gyrus; PFC = prefrontal cortex; mPFC = medial prefrontal cortex, SNpc = substantia nigra pars compacta; VTA = ventral tegmental area; PVN = paraventricular nucleus. ↑ increase; ↓ decrease; → no change.
The sex-specific neuroimmune consequences of stress likely impact not only microglia, but also neurons and their properties. Viviani et al (2014) found that even a relatively mild version of the maternal deprivation paradigm, a single episode of separation for 24 hour on postnatal day 9, led to increased synaptic expression of the receptor for IL-1 in the hippocampus of male rats, but not female rats, assessed in adolescence (Viviani et al 2014). Furthermore, this result was accompanied by an increase in the protein-protein interaction between the IL-1 receptor and GluN2B, a subunit of NMDA receptor, in hippocampal synapses of male rats, pointing to immune-mediated effects of early life stress on synaptic plasticity. Chronic variable stress applied to pregnant dams during embryonic days E1–7 was found to increase the expression of a number of cytokines, chemokines, and their receptors in male, but not female, placenta. Concurrent treatment of pregnant dams with NSAID during E1–7 partially reverted the effect of stress on placental gene expression (Bronson & Bale 2014). Furthermore, when assessed in adulthood, prenatally stressed male offspring displayed aberrant dopaminergic signaling and locomotor hyperactivity.
These cellular and molecular changes likely reflect modulation of inflammatory processes by adrenal and gonadal hormones. Although ROS production in both male and female rodents has been documented following chronic stress (Lucca et al 2009, Pascuan et al 2015), to our knowledge, a systematic exploration of sex differences in stress-induced ROS production has not yet been performed. However, 8-week-old female mice have been demonstrated to have more resilience in response to the oxidative stress-inducing and behavioral effects of D-galactose, a reducing sugar that generates ROS, compared to both 24-week-old females and 8-week-old males (Hao et al 2014), which may suggest ovarian hormones as a potential basis for sex differences in stress-related ROS production. Using an experimental autoimmune encephalomyelitis (EAE) model in which female mice are generally more resistant, Harpaz et al (2013) found that exposure to chronic variable stress predisposed females to a more pro-inflammatory profile following EAE compared to similarly stressed males. Abolishment of the female-sex protection by chronic variable stress was found to be mediated by corticosterone signaling (Harpaz et al 2013). This result may be attributable to dysregulated glucocorticoid signaling and sensitivity. In addition to adrenal influences, ovarian hormones have been shown to modulate stress-induced increases in pro-inflammatory cytokines within the female rat brain (Arakawa et al 2014). Arakawa et al (2014) used a footshock paradigm in which 80 footshocks were delivered within two hours to naturally cycling female rats. This stressor led to a robust increase in the expression of IL-1β within the paraventricular nucleus at all stages of the estrous cycle except metestrus. Stress-induced cytokines were further elevated in ovariectomized females compared to sham-operated animals, an effect that was abolished by administration of estradiol and progesterone to ovariectomized females. Although this study by Arakawa et al (2014) suggests anti-inflammatory functions of ovarian hormones in stressed animals, it is not clear whether a reduction in neuroinflammation necessarily translates to better behavioral outcomes following stress. In a study employing a six-day chronic unpredictable stress (CUS) paradigm, LaPlant et al (2009) found that ovariectomized female mice were protected against the pro-depressive effects of CUS compared to intact females, an effect that was mediated by nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) signaling in the nucleus accumbens (LaPlant et al 2009). While NFκB is best recognized as a major pro-inflammatory signaling pathway that can lead to the release of cytokines and chemokines, apoptosis and cell death, it is also constitutively involved in synaptic plasticity and neural transmission. Thus, contrary to the commonly held belief that neuroinflammation is generally detrimental, it is feasible that a basal degree of inflammatory signaling may be protective in the context of chronic stress.
In many chronic stress paradigms, a history of stressor exposure primes the underlying physiology toward enhanced susceptibility to a re-encounter of similar stressors such that the impact of the chronic stress is manifest not necessarily under unstimulated or basal conditions but upon exposure to a subsequent acute stressor. Chronic unpredictable stress and inescapable shocks in rats have been demonstrated to prime the cytokine and microglial response in the brain to a peripheral immune challenge (Frank et al 2007). Several studies to date have revealed sex differences in the neuroinflammatory priming effect of chronic or repeated stress. Research from our laboratory has demonstrated that chronically stressed male and female rats display distinct neuroinflammatory profiles in the hippocampus (Pyter et al 2013). In this study, male and female rats underwent a chronic adolescent stress (CAS) paradigm, in which experimental rats are exposed to randomized episodes of restraint stress and social defeat by same-sex aggressors. Consistent with the findings of others (Munhoz et al 2006), when challenged with LPS intraperitoneally in adulthood, male CAS rats displayed exaggerated induction of the pro-inflammatory cytokines IL-1β and TNF-α. Interestingly, CAS females did not display a similar inflammatory priming by a history of chronic stress, suggesting sex differences in stress-related neuroimmune mechanisms. Similarly, Hudson et al (2014) found that prior exposure to stress led to sensitization of the cytokine response to an acute re-exposure stress in male, but not female, mice. In this study, CD-1 mice were exposed to a 3-day variable stressor consisting of restraint, forced swim, and wet bedding, followed by a re-exposure to a brief episode of restraint stress weeks removed from the initial stress experience. Male mice that underwent both the initial and re-exposure stress displayed exaggerated induction of hippocampal IL-1β compared to males exposed to either stress alone. While female mice displayed elevated IL-1β expression compared to their male counterparts both at baseline and following acute stress, no potentiating effect of re-exposure was evident.
In addition to the previously-described central mechanisms, the peripheral immune system also influences stress-induced neuroinflammation. Repeated social defeat stress has been shown to increase trafficking of “primed” monocytes from the spleen to neural circuitry relevant to modulating the stress response including the PFC, amygdala, and hippocampus (Wohleb et al 2013). While this particular social defeat paradigm has not been performed in female mice, similar demonstration of increased leukocyte trafficking has been reported in female mice that have been subjected to footshock stress (Brevet et al 2010). It should be noted that some of the anti-inflammatory properties of gonadal hormones could be speculated to protect from such harmful effects of defeat stress. For example, estrogen has been found to protect the blood–brain barrier from endotoxin-induced disruption and to suppress the subsequent lymphocyte trafficking (Maggioli et al 2016). Astrocytes, which are essential for the integrity of the blood-brain barrier, from males also respond more to LPS stimulation compared to those from females (Santos-Galindo et al 2011).
5. Conclusions and future directions
Although the literature is sparse, some synopses and conclusions can be drawn from the available studies. Evidence to date, at least from rodent literature, does not readily support the hypothesis that female susceptibility to mood disorders is fueled by sex-specific neuroimmune responses following stressor exposure (see Figure 1). Data from human studies suggest that women respond to acute stressors in a more pro-inflammatory fashion with increased mobilization of various immune cells and decreased glucocorticoid sensitivity. Furthermore, some evidence suggests that chronic stress may lead to exaggerated immunosuppression in women compared to men. These data are consistent with the behavioral susceptibility of women to inflammatory challenges, yet they do not explain the mechanisms by which inflammatory biomarkers are more consistently linked to depression in men compared to women. However, much of the evidence generated from rodent studies suggest that males may be more likely to display stress-induced inflammatory changes. It should be noted that important methodological issues such as developmental timeline of stressor exposure, usage of behavioral assays validated in males only, and technical aspects of accurately measuring inflammatory outcomes in naturally cycling females may be introducing a potential bias toward increased immune changes in stressed males and continued efforts towards maximizing experimental control in preclinical models will be essential.
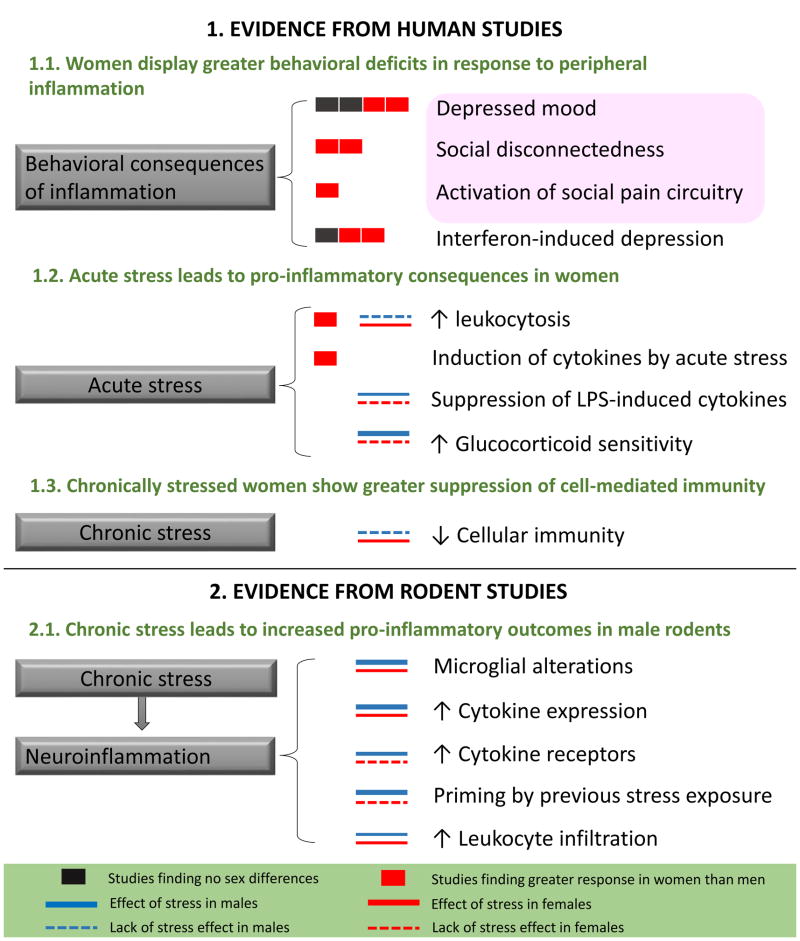
Figure 1.
Sex differences in stress-induced inflammation in humans and rodents. The number of the black and red boxes indicate the extent of data supporting each phenomenon based on human studies (referenced in sections 4.1.1 and 4.1.2) that directly compared men and women. The thickness of the blue and red lines corresponds to the extent of available data supporting each phenomenon based on studies (referenced in sections 4.1.1, 4.1.2, and 4.2.3) that included both sexes. The pink box represents the behavioral consequences of low-dose endotoxin administration in healthy humans. Thick line: documented by ≥ 2 studies, thin line: 1 study, dotted line: phenomenon was found not to occur in the indicated sex. LPS, lipopolysaccharide; GC, glucocorticoid. References: 1.1. Depressed mood: (Eisenberger et al 2009, Engler et al 2015, Lasselin et al 2016, Moieni et al 2015). Social disconnectedness: (Eisenberger et al 2009, Moieni et al 2015). Activation of social pain circuitry: (Eisenberger et al 2009). Interferon-induced depression: (Bonaccorso et al 2002, Koskinas et al 2002, Udina et al 2012b). 1.2. Leukocytosis: (Maes et al 1999, Pehlivanoglu et al 2012). Induction of cytokines by stress: (Endrighi et al 2016). Suppression of LPS-induced cytokines: (Prather et al 2009). Increased GC sensitivity: (Rohleder et al 2006, Rohleder et al 2001). 1.3. Chronic stress-driven decreases in cellular immunity: (Flynn et al 2009). 2.1. Microglial activation: (Caetano et al 2016, Diz-Chaves et al 2013, Diz-Chaves et al 2012). Increased cytokine expression: (Bronson & Bale 2014, Diz-Chaves et al 2013, Diz-Chaves et al 2012). Increased cytokine receptor expression: (Viviani et al 2014). Priming by previous stress exposure: (Hudson et al 2014, Pyter et al 2013). Leukocyte infiltration: (Ataka et al 2013, Brevet et al 2010).
In order to further understand sex differences in the neuro-immune consequences of psychosocial, physical, and/or emotional stress, many of the existing stress paradigms and immune measures need to be replicated in females. In particular, as this review indicates, there are a dearth of studies utilizing stress paradigms that take place in adulthood and during puberty. Part of the challenge in assessing sex differences in existing paradigms of stress-related inflammation is related to the lack of stress models in which male and female animals are subjected to equivalent stressors, fluctuations in ovarian hormone-mediated regulation of inflammatory processes, and differential kinetics displayed by males and females to antigen stimulation. Because non-lactating female rats and mice do not spontaneously display a similar range of aggressive behavior as their male counterparts, most of social defeat or social confrontation-based stress paradigms are exclusively performed in males (Solomon 2017). However, the lower incidence of physical injuries sustained in group-housed or socially-interacting females has been cited as a reason to use females in studies assessing inflammatory endpoints (Voorhees et al 2013). Importantly, sex-dependent behavioral phenotypes influence experimental designs and thereby the questions that can be addressed, but paradigms exist in which males and females can both be exposed to social stressors (Barnum et al 2012, Bourke & Neigh 2012, Burgado et al 2014). Furthermore, in cases such as social defeat in mice where a direct male-to-female comparison metrics have not been established within the stress paradigm, measuring “witness stress” as an alternative that could be equally experienced by animals of both sexes may help bridge the sex gap. For example, Ataka et al (2013) utilized a chronic psychological stress paradigm in which the experimental animal witnessed another, non-experimental animal undergoing footshock stress. It is imperative that researchers seek out and employ designs that facilitate assessment of sex differences in stress-induced inflammatory processes given that these preclinical models must inform translational research and ultimately clinical practice. Collectively, increased attention to comparison between the sexes in both rodent and human investigation and awareness regarding the differential function of the rodent and human immune systems will provide for advances in our understanding of stress-induced neuroimmune alterations and their relevance to clinical manifestation of affective disorders and potential novel therapeutic paths.
Highlights.
Women are more vulnerable to the depressogenic effects of inflammation than men.
Stress precipitates the development of mood disorders via neuroimmune alterations.
Sex differences in stress-related neuroinflammation are largely unknown.
Sex-specific neuroimmune effects of stress may explain the female bias in depression.
Replication of stress-induced neuroinflammation research in females is necessary.
Acknowledgments
The authors receive funding from NIH: NR014886 and MH110364.
Footnotes
Declaration of interest
The authors have no conflicting interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arakawa K, Arakawa H, Hueston CM, Deak T. Effects of the estrous cycle and ovarian hormones on central expression of interleukin-1 evoked by stress in female rats. Neuroendocrinology. 2014;100:162–77. doi: 10.1159/000368606. [DOI] [PubMed] [Google Scholar]
- Ataka K, Asakawa A, Nagaishi K, Kaimoto K, Sawada A, et al. Bone marrow-derived microglia infiltrate into the paraventricular nucleus of chronic psychological stress-loaded mice. PLoS One. 2013;8:e81744. doi: 10.1371/journal.pone.0081744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin DR, Wilcox ZC, Zheng G. The effects of voluntary exercise and immobilization on humoral immunity and endocrine responses in rats. Physiol Behav. 1997;61:447–53. doi: 10.1016/s0031-9384(96)00459-3. [DOI] [PubMed] [Google Scholar]
- Bale TL. Epigenetic and transgenerational reprogramming of brain development. Nat Rev Neurosci. 2015;16:332–44. doi: 10.1038/nrn3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, et al. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–73. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Beynon SB, Walker FR. Microglial activation in the injured and healthy brain: what are we really talking about? Practical and theoretical issues associated with the measurement of changes in microglial morphology. Neuroscience. 2012;225:162–71. doi: 10.1016/j.neuroscience.2012.07.029. [DOI] [PubMed] [Google Scholar]
- Bollinger JL, Bergeon Burns CM, Wellman CL. Differential effects of stress on microglial cell activation in male and female medial prefrontal cortex. Brain Behav Immun. 2016;52:88–97. doi: 10.1016/j.bbi.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaccorso S, Marino V, Biondi M, Grimaldi F, Ippoliti F, Maes M. Depression induced by treatment with interferon-alpha in patients affected by hepatitis C virus. J Affect Disord. 2002;72:237–41. doi: 10.1016/s0165-0327(02)00264-1. [DOI] [PubMed] [Google Scholar]
- Bourke CH, Harrell CS, Neigh GN. Stress-induced sex differences: adaptations mediated by the glucocorticoid receptor. Horm Behav. 2012;62:210–8. doi: 10.1016/j.yhbeh.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke CH, Neigh GN. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Horm Behav. 2011;60:112–20. doi: 10.1016/j.yhbeh.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke CH, Raees MQ, Malviya S, Bradburn CA, Binder EB, Neigh GN. Glucocorticoid sensitizers Bag1 and Ppid are regulated by adolescent stress in a sex-dependent manner. Psychoneuroendocrinology. 2013;38:84–93. doi: 10.1016/j.psyneuen.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevet M, Kojima H, Asakawa A, Atsuchi K, Ushikai M, et al. Chronic foot-shock stress potentiates the influx of bone marrow-derived microglia into hippocampus. J Neurosci Res. 2010;88:1890–7. doi: 10.1002/jnr.22362. [DOI] [PubMed] [Google Scholar]
- Bronson SL, Bale TL. Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology. 2014;155:2635–46. doi: 10.1210/en.2014-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Levine JD, Green PG. Sexual dimorphism in the effect of sound stress on neutrophil function. J Neuroimmunol. 2008;205:25–31. doi: 10.1016/j.jneuroim.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Caetano L, Pinheiro H, Patricio P, Mateus-Pinheiro A, Alves ND, et al. Adenosine A2A receptor regulation of microglia morphological remodeling-gender bias in physiology and in a model of chronic anxiety. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.173. [DOI] [PubMed] [Google Scholar]
- Chocyk A, Dudys D, Przyborowska A, Majcher I, Mackowiak M, Wedzony K. Maternal separation affects the number, proliferation and apoptosis of glia cells in the substantia nigra and ventral tegmental area of juvenile rats. Neuroscience. 2011;173:1–18. doi: 10.1016/j.neuroscience.2010.11.037. [DOI] [PubMed] [Google Scholar]
- Czlonkowska A, Ciesielska A, Gromadzka G, Kurkowska-Jastrzebska I. Gender differences in neurological disease: role of estrogens and cytokines. Endocrine. 2006;29:243–56. doi: 10.1385/ENDO:29:2:243. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine, sickness behavior, and depression. Neurol Clin. 2006;24:441–60. doi: 10.1016/j.ncl.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Coupade C, Gear RW, Dazin PF, Sroussi HY, Green PG, Levine JD. Beta 2-adrenergic receptor regulation of human neutrophil function is sexually dimorphic. Br J Pharmacol. 2004;143:1033–41. doi: 10.1038/sj.bjp.0705972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leon-Nava MA, Nava K, Soldevila G, Lopez-Griego L, Chavez-Rios JR, et al. Immune sexual dimorphism: effect of gonadal steroids on the expression of cytokines, sex steroid receptors, and lymphocyte proliferation. J Steroid Biochem Mol Biol. 2009;113:57–64. doi: 10.1016/j.jsbmb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Deak T, Quinn M, Cidlowski JA, Victoria NC, Murphy AZ, Sheridan JF. Neuroimmune mechanisms of stress: sex differences, developmental plasticity, and implications for pharmacotherapy of stress-related disease. Stress. 2015;18:367–80. doi: 10.3109/10253890.2015.1053451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DellaGioia N, Hannestad J. A critical review of human endotoxin administration as an experimental paradigm of depression. Neurosci Biobehav Rev. 2010;34:130–43. doi: 10.1016/j.neubiorev.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry HM, Padin AC, Kuo JL, Hughes S, Kiecolt-Glaser JK. Sex Differences in Depression: Does Inflammation Play a Role? Curr Psychiatry Rep. 2015;17:78. doi: 10.1007/s11920-015-0618-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS. Stress, leukocyte trafficking, and the augmentation of skin immune function. Ann N Y Acad Sci. 2003;992:205–17. doi: 10.1111/j.1749-6632.2003.tb03151.x. [DOI] [PubMed] [Google Scholar]
- Diz-Chaves Y, Astiz M, Bellini MJ, Garcia-Segura LM. Prenatal stress increases the expression of proinflammatory cytokines and exacerbates the inflammatory response to LPS in the hippocampal formation of adult male mice. Brain Behav Immun. 2013;28:196–206. doi: 10.1016/j.bbi.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Diz-Chaves Y, Pernia O, Carrero P, Garcia-Segura LM. Prenatal stress causes alterations in the morphology of microglia and the inflammatory response of the hippocampus of adult female mice. J Neuroinflammation. 2012;9:71. doi: 10.1186/1742-2094-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Gerpe L, Rey-Mendez M. Modulation of stress-induced murine lymphoid tissue involution by age, sex and strain: role of bone marrow. Mech Ageing Dev. 1998;104:195–205. doi: 10.1016/s0047-6374(98)00070-0. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. Neuroimage. 2009;47:881–90. doi: 10.1016/j.neuroimage.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endrighi R, Hamer M, Steptoe A. Post-menopausal Women Exhibit Greater Interleukin-6 Responses to Mental Stress Than Older Men. Ann Behav Med. 2016;50:564–71. doi: 10.1007/s12160-016-9783-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler H, Benson S, Wegner A, Spreitzer I, Schedlowski M, Elsenbruch S. Men and women differ in inflammatory and neuroendocrine responses to endotoxin but not in the severity of sickness symptoms. Brain Behav Immun. 2015 doi: 10.1016/j.bbi.2015.08.013. [DOI] [PubMed] [Google Scholar]
- Eyre HA, Air T, Proctor S, Rositano S, Baune BT. A critical review of the efficacy of non-steroidal anti-inflammatory drugs in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2015;57:11–6. doi: 10.1016/j.pnpbp.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Flynn SM, Schipper LJ, Roach AR, Segerstrom SC. Gender differences in delayed-type hypersensitivity response: effects of stress and coping in first-year law students. Brain Behav Immun. 2009;23:672–6. doi: 10.1016/j.bbi.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Gupta A, Labus J, Kilpatrick LA, Bonyadi M, Ashe-McNalley C, et al. Interactions of early adversity with stress-related gene polymorphisms impact regional brain structure in females. Brain Struct Funct. 2016;221:1667–79. doi: 10.1007/s00429-015-0996-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O’Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28:464–76. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Hao L, Huang H, Gao J, Marshall C, Chen Y, Xiao M. The influence of gender, age and treatment time on brain oxidative stress and memory impairment induced by D-galactose in mice. Neurosci Lett. 2014;571:45–9. doi: 10.1016/j.neulet.2014.04.038. [DOI] [PubMed] [Google Scholar]
- Haroon E, Miller AH, Sanacora G. Inflammation, Glutamate, and Glia: A Trio of Trouble in Mood Disorders. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpaz I, Abutbul S, Nemirovsky A, Gal R, Cohen H, Monsonego A. Chronic exposure to stress predisposes to higher autoimmune susceptibility in C57BL/6 mice: glucocorticoids as a double-edged sword. Eur J Immunol. 2013;43:758–69. doi: 10.1002/eji.201242613. [DOI] [PubMed] [Google Scholar]
- Hermes GL, Rosenthal L, Montag A, McClintock MK. Social isolation and the inflammatory response: sex differences in the enduring effects of a prior stressor. Am J Physiol Regul Integr Comp Physiol. 2006;290:R273–82. doi: 10.1152/ajpregu.00368.2005. [DOI] [PubMed] [Google Scholar]
- Howerton AR, Roland AV, Bale TL. Dorsal raphe neuroinflammation promotes dramatic behavioral stress dysregulation. J Neurosci. 2014;34:7113–23. doi: 10.1523/JNEUROSCI.0118-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson SP, Jacobson-Pick S, Anisman H. Sex differences in behavior and pro-inflammatory cytokine mRNA expression following stressor exposure and re-exposure. Neuroscience. 2014;277:239–49. doi: 10.1016/j.neuroscience.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Iwata M, Ota KT, Duman RS. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun. 2013;31:105–14. doi: 10.1016/j.bbi.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Marucha PT, Malarkey WB, Mercado AM, Glaser R. Slowing of wound healing by psychological stress. Lancet. 1995;346:1194–6. doi: 10.1016/s0140-6736(95)92899-5. [DOI] [PubMed] [Google Scholar]
- Klein SL. Immune cells have sex and so should journal articles. Endocrinology. 2012;153:2544–50. doi: 10.1210/en.2011-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71:1381–91. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- Koskinas J, Merkouraki P, Manesis E, Hadziyannis S. Assessment of depression in patients with chronic hepatitis: effect of interferon treatment. Dig Dis. 2002;20:284–8. doi: 10.1159/000067682. [DOI] [PubMed] [Google Scholar]
- Kubera M, Obuchowicz E, Goehler L, Brzeszcz J, Maes M. In animal models, psychosocial stress-induced (neuro)inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:744–59. doi: 10.1016/j.pnpbp.2010.08.026. [DOI] [PubMed] [Google Scholar]
- LaPlant Q, Chakravarty S, Vialou V, Mukherjee S, Koo JW, et al. Role of nuclear factor kappaB in ovarian hormone-mediated stress hypersensitivity in female mice. Biol Psychiatry. 2009;65:874–80. doi: 10.1016/j.biopsych.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasselin J, Elsenbruch S, Lekander M, Axelsson J, Karshikoff B, et al. Mood disturbance during experimental endotoxemia: Predictors of state anxiety as a psychological component of sickness behavior. Brain Behav Immun. 2016;57:30–7. doi: 10.1016/j.bbi.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Lenz KM, McCarthy MM. A starring role for microglia in brain sex differences. Neuroscientist. 2015;21:306–21. doi: 10.1177/1073858414536468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liukkonen T, Rasanen P, Jokelainen J, Leinonen M, Jarvelin MR, et al. The association between anxiety and C-reactive protein (CRP) levels: results from the Northern Finland 1966 birth cohort study. Eur Psychiatry. 2011;26:363–9. doi: 10.1016/j.eurpsy.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Loram LC, Sholar PW, Taylor FR, Wiesler JL, Babb JA, et al. Sex and estradiol influence glial pro-inflammatory responses to lipopolysaccharide in rats. Psychoneuroendocrinology. 2012;37:1688–99. doi: 10.1016/j.psyneuen.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucca G, Comim CM, Valvassori SS, Reus GZ, Vuolo F, et al. Increased oxidative stress in submitochondrial particles into the brain of rats submitted to the chronic mild stress paradigm. J Psychiatr Res. 2009;43:864–9. doi: 10.1016/j.jpsychires.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Maes M, Van Bockstaele DR, Gastel A, Song C, Schotte C, et al. The effects of psychological stress on leukocyte subset distribution in humans: evidence of immune activation. Neuropsychobiology. 1999;39:1–9. doi: 10.1159/000026552. [DOI] [PubMed] [Google Scholar]
- Maes M, Van der Planken M, Stevens WJ, Peeters D, DeClerck LS, et al. Leukocytosis, monocytosis and neutrophilia: hallmarks of severe depression. J Psychiatr Res. 1992;26:125–34. doi: 10.1016/0022-3956(92)90004-8. [DOI] [PubMed] [Google Scholar]
- Maggioli E, McArthur S, Mauro C, Kieswich J, Kusters DH, et al. Estrogen protects the blood-brain barrier from inflammation-induced disruption and increased lymphocyte trafficking. Brain Behav Immun. 2016;51:212–22. doi: 10.1016/j.bbi.2015.08.020. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–8. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- Marucha PT, Kiecolt-Glaser JK, Favagehi M. Mucosal wound healing is impaired by examination stress. Psychosom Med. 1998;60:362–5. doi: 10.1097/00006842-199805000-00025. [DOI] [PubMed] [Google Scholar]
- Merali Z, Lacosta S, Anisman H. Effects of interleukin-1beta and mild stress on alterations of norepinephrine, dopamine and serotonin neurotransmission: a regional microdialysis study. Brain Res. 1997;761:225–35. doi: 10.1016/s0006-8993(97)00312-0. [DOI] [PubMed] [Google Scholar]
- Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–8. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Pressman S, Barkin A, Rabin BS, Treanor JJ. Psychological Stress and Antibody Response to Influenza Vaccination: When Is the Critical Period for Stress, and How Does It Get Inside the Body? Psychosomatic Medicine. 2004;66:215–23. doi: 10.1097/01.psy.0000116718.54414.9e. [DOI] [PubMed] [Google Scholar]
- Miller GE, Murphy ML, Cashman R, Ma R, Ma J, et al. Greater inflammatory activity and blunted glucocorticoid signaling in monocytes of chronically stressed caregivers. Brain Behav Immun. 2014;41:191–9. doi: 10.1016/j.bbi.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza MA, Ritzel R, Xu Y, McCullough LD, Liu F. Sexually dimorphic outcomes and inflammatory responses in hypoxic-ischemic encephalopathy. J Neuroinflammation. 2015;12:32. doi: 10.1186/s12974-015-0251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moieni M, Irwin MR, Jevtic I, Olmstead R, Breen EC, Eisenberger NI. Sex differences in depressive and socioemotional responses to an inflammatory challenge: implications for sex differences in depression. Neuropsychopharmacology. 2015;40:1709–16. doi: 10.1038/npp.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganti JM, Nash KR, Grimmig BA, Ranjit S, Small B, et al. The soluble isoform of CX3CL1 is necessary for neuroprotection in a mouse model of Parkinson’s disease. J Neurosci. 2012;32:14592–601. doi: 10.1523/JNEUROSCI.0539-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munhoz CD, Lepsch LB, Kawamoto EM, Malta MB, de Lima LS, et al. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. J Neurosci. 2006;26:3813–20. doi: 10.1523/JNEUROSCI.4398-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, Dedovic K, Slavich GM, Jarcho MR, Breen EC, et al. Greater amygdala activity and dorsomedial prefrontal-amygdala coupling are associated with enhanced inflammatory responses to stress. Brain Behav Immun. 2015;43:46–53. doi: 10.1016/j.bbi.2014.06.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeman E, Shaashua L, Benish M, Page GG, Zmora O, Ben-Eliyahu S. Stress and skin leukocyte trafficking as a dual-stage process. Brain Behav Immun. 2012;26:267–76. doi: 10.1016/j.bbi.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth CL, Reddy R, Bekhbat M, Bailey J, Neigh GN. Microglial activation occurs in the absence of anxiety-like behavior following microembolic stroke in female, but not male, rats. J Neuroinflammation. 2014;11:174. doi: 10.1186/s12974-014-0174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertelt-Prigione S. The influence of sex and gender on the immune response. Autoimmun Rev. 2012;11:A479–85. doi: 10.1016/j.autrev.2011.11.022. [DOI] [PubMed] [Google Scholar]
- Panagiotakopoulos L, Neigh GN. Development of the HPA axis: where and when do sex differences manifest? Front Neuroendocrinol. 2014;35:285–302. doi: 10.1016/j.yfrne.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Pascuan CG, Simon EH, Genaro AM, Palumbo ML. Involvement of nitric oxide in improving stress-induced behavioural alteration by glatiramer acetate treatment in female BALB/c mice. Psychopharmacology (Berl) 2015;232:1595–605. doi: 10.1007/s00213-014-3791-z. [DOI] [PubMed] [Google Scholar]
- Pedersen AF, Zachariae R, Bovbjerg DH. Psychological stress and antibody response to influenza vaccination: a meta-analysis. Brain Behav Immun. 2009;23:427–33. doi: 10.1016/j.bbi.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Pehlivanoglu B, Bayrak S, Gurel EI, Balkanci ZD. Effect of gender and menstrual cycle on immune system response to acute mental stress: apoptosis as a mediator. Neuroimmunomodulation. 2012;19:25–32. doi: 10.1159/000327993. [DOI] [PubMed] [Google Scholar]
- Pitychoutis PM, Griva E, Ioannou K, Tsitsilonis OE, Papadopoulou-Daifoti Z. Chronic antidepressant treatment exerts sexually dimorphic immunomodulatory effects in an experimental model of major depression: do females lack an advantage? Int J Neuropsychopharmacol. 2009;12:1157–63. doi: 10.1017/S1461145709990502. [DOI] [PubMed] [Google Scholar]
- Prather AA, Carroll JE, Fury JM, McDade KK, Ross D, Marsland AL. Gender differences in stimulated cytokine production following acute psychological stress. Brain Behav Immun. 2009;23:622–8. doi: 10.1016/j.bbi.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribiag H, Stellwagen D. Neuroimmune regulation of homeostatic synaptic plasticity. Neuropharmacology. 2014;78:13–22. doi: 10.1016/j.neuropharm.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Pyter LM, Kelly SD, Harrell CS, Neigh GN. Sex differences in the effects of adolescent stress on adult brain inflammatory markers in rats. Brain Behav Immun. 2013;30:88–94. doi: 10.1016/j.bbi.2013.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH. The evolutionary significance of depression in Pathogen Host Defense (PATHOS-D) Mol Psychiatry. 2013;18:15–37. doi: 10.1038/mp.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey JM, Cooper JD, Bot M, Guest PC, Lamers F, et al. Sex Differences in Serum Markers of Major Depressive Disorder in the Netherlands Study of Depression and Anxiety (NESDA) PLoS One. 2016;11:e0156624. doi: 10.1371/journal.pone.0156624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles TF. Stress, social support, and delayed skin barrier recovery. Psychosom Med. 2007;69:807–15. doi: 10.1097/PSY.0b013e318157b12e. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Otto B, Wolf JM, Klose J, Kirschbaum C, et al. Sex-specific adaptation of endocrine and inflammatory responses to repeated nauseogenic body rotation. Psychoneuroendocrinology. 2006;31:226–36. doi: 10.1016/j.psyneuen.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Schommer NC, Hellhammer DH, Engel R, Kirschbaum C. Sex Differences in Glucocorticoid Sensitivity of Proinflammatory Cytokine Production After Psychosocial Stress. Psychosomatic Medicine. 2001;63:966–72. doi: 10.1097/00006842-200111000-00016. [DOI] [PubMed] [Google Scholar]
- Santos-Galindo M, Acaz-Fonseca E, Bellini MJ, Garcia-Segura LM. Sex differences in the inflammatory response of primary astrocytes to lipopolysaccharide. Biol Sex Differ. 2011;2:7. doi: 10.1186/2042-6410-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Sholar PW, Bilbo SD. Sex differences in microglial colonization of the developing rat brain. J Neurochem. 2012;120:948–63. doi: 10.1111/j.1471-4159.2011.07630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130:601–30. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein B. Gender difference in the prevalence of clinical depression: the role played by depression associated with somatic symptoms. Am J Psychiatry. 1999;156:480–2. doi: 10.1176/ajp.156.3.480. [DOI] [PubMed] [Google Scholar]
- Slavich GM, Way BM, Eisenberger NI, Taylor SE. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proc Natl Acad Sci U S A. 2010;107:14817–22. doi: 10.1073/pnas.1009164107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanski V, Gruner S. Gender difference in basal and stress levels of peripheral blood leukocytes in laboratory rats. Brain Behav Immun. 2006;20:369–77. doi: 10.1016/j.bbi.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Stephens MA, Mahon PB, McCaul ME, Wand GS. Hypothalamic-pituitary-adrenal axis response to acute psychosocial stress: Effects of biological sex and circulating sex hormones. Psychoneuroendocrinology. 2016;66:47–55. doi: 10.1016/j.psyneuen.2015.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez EC, Sundy JS, Erkanli A. Depressogenic vulnerability and gender-specific patterns of neuro-immune dysregulation: What the ratio of cortisol to C-reactive protein can tell us about loss of normal regulatory control. Brain Behav Immun. 2015;44:137–47. doi: 10.1016/j.bbi.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli LH, Holmes A, Postolache TT. Intranasal immune challenge induces sex-dependent depressive-like behavior and cytokine expression in the brain. Neuropsychopharmacology. 2008;33:1038–48. doi: 10.1038/sj.npp.1301488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udina M, Castellvi P, Moreno-Espana J, Navines R, Valdes M, et al. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry. 2012a;73:1128–38. doi: 10.4088/JCP.12r07694. [DOI] [PubMed] [Google Scholar]
- Udina M, Castellvi P, Moreno-Espana J, Navines R, Valdes M, et al. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry. 2012b:73. doi: 10.4088/JCP.12r07694. [DOI] [PubMed] [Google Scholar]
- Viviani B, Boraso M, Valero M, Gardoni F, Marco EM, et al. Early maternal deprivation immunologically primes hippocampal synapses by redistributing interleukin-1 receptor type I in a sex dependent manner. Brain Behav Immun. 2014;35:135–43. doi: 10.1016/j.bbi.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, et al. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31:6277–88. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Powell ND, Godbout JP, Sheridan JF. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci. 2013;33:13820–33. doi: 10.1523/JNEUROSCI.1671-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, et al. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]