Abstract
The aim of this research was to compare plasma pharmacokinetics of ceftiofur sodium (CS) in healthy calves, and in calves with experimentally induced endotoxemia. Six calves received CS (2.2 mg/kg, IM) 2 hr after intravenous administration of 0.9% NaCl (Ceft group). After a washout period, the same 6 calves received CS 2 hr after intravenous injection of lipopolysaccharide (LPS+Ceft group). Another group of 6 calves received a combination of drug therapies that included CS 2 hr after administration of 0.9% NaCl (Comb group). A third group of 6 calves received the same combination therapy regimen 2 hr after intravenous injection of lipopolysaccharide (LPS+Comb group). Plasma concentrations of CS and all desfuroylceftiofur-related metabolites were determined using HPLC, and its pharmacokinetic properties were determined based on a two-compartment model. The peak concentration of CS in the LPS+Comb group occurred the earliest, and the clearance rate of CS was the highest in the Comb and LPS+Comb groups (P<0.05). The elimination half-life of CS in the LPS+Ceft group was longer than that in the Ceft and Comb groups (P<0.05). The results of this study indicate that combined therapies and endotoxemic status may alter the plasma pharmacokinetics of CS in calves.
Keywords: calve, ceftiofur, endotoxemia, pharmacokinetic
Neonatal calf diarrhea is one of the main causes of mortality in calves. Diarrhea is triggered by lipopolysaccharide (LPS) produced by Escherichia coli (E. coli). Exposed calves can exhibit sepsis-related symptoms. The primary aims in treating sepsis in calves should be controlling infection, regulating the inflammatory response, replacing fluids, managing circulatory tension and relieving stress [1, 4, 5, 41].
Ceftiofur sodium (CS) is a broad-spectrum, third-generation cephalosporin that was developed for use in animals, and is the most widely recommended parenteral antibiotic for treatment of E. coli-induced diarrhea in calves [4, 5, 53]. CS is approved for the treatment of respiratory infections in cattle and pigs [14]. Although previous studies have shown that 2.2 mg/kg of CS is effective in animals, this dosage has not been approved for use in preruminating calves [4]. However, CS can be used for extra-label purposes. Pharmacokinetic studies of CS indicate that it is rapidly absorbed at the site of the injection, and is distributed throughout the body fluid compartments [2, 48]. CS is rapidly metabolized to desfuroylceftiofur (DFC) after administration. DFC, which contains an intact β-lactam ring, is the microbiologically active metabolite of CS [39].
Intravenous administration of LPS can experimentally induce symptoms similar to naturally occurring endotoxemia in animal models of septic shock [6, 54]. The use of intravenous LPS injections in experimental studies is useful for determining dosage regimens of therapeutic drugs for septic shock [12, 13]. Although previous studies have described the pharmacokinetics of various antibiotics for treatment of sepsis and septic shock in humans and in veterinary medicine [13, 38], the pharmacokinetics of CS in subjects with sepsis or septic shock have not been fully characterized.
In critically ill patients, the distribution of hydrophilic antibiotics including β-lactams can broaden, and the pharmacokinetic profiles of drugs can become unpredictable, requiring careful consideration of pharmacokinetic parameters when determining the dosage of β-lactam antibiotics [27, 35]. Animal experiments indicate that efficacies of β-lactams for treatment of systemic microbial infections are related to the duration a drug concentration remains above the minimum inhibitory concentration (T >MIC), and time-dependent killing is characteristic of β-lactams [29]. Although therapeutic drug level monitoring (TDM) methods are not frequently used for β-lactam treatments, TDM can be used to optimize the dosage of drugs, improve clinical outcomes, and prevent the development of antibiotic resistance when treating critically ill patients for sepsis or other systemic microbial infections requiring a high MIC (minimum inhibition concentration) [37, 44].
The basic aim of the current study was to determine the pharmacokinetic profile of CS with or without combined treatment in endotoxemic calves. We used LPS to experimentally induce endotoxemia in our calf model of septic shock, and monitored the pharmacokinetic parameters of CS and the improvements in clinical and physiological measures that occurred in endotoxemic calves treated with CS alone or CS combined with a vasoactive medication (dopamine), an anti-inflammatory drug (dexamethasone), or intense fluid replacement.
MATERIALS AND METHODS
Animals and materials
Eighteen healthy Holstein calves (<6 days old, 43.54 ± 5.21 kg) were used and experimental procedures were approved by the Ethics Committee of the Faculty of Veterinary Medicine at Selcuk University (Konya, Turkey). The animals were divided into two categories, with each category consisting of two groups.
The calves (n=6) in the first group of the first category received 2.2 mg/kg (IM) dose of CS (Excenel enj, Pfizer®, Istanbul, Turkey) 2 hr after administration of a 30-min intravenous infusion of 0.9% NaCl (Ceft group). After a 5-day washout period, the calves (n=6) in the first group of the first category (cross-over design) were treated with CS (2.2 mg/kg, IM) 2 hr after administration of a 30-min intravenous infusion of 2 µg/kg of LPS (E. coli 0111:B4, Sigma-Aldrich, Hamburg, Germany) dissolved in 100 ml 0.9% NaCl (LPS+Ceft group).
The calves in the first group (n=6) of the second category (parallel design) received combination drug therapy containing [Voluven (20 ml /kg, IV infusion), ringers lactate (20 ml/kg, IV), dexamethasone (10 mg/calf, IV), dopamine (30 µg/kg/min, IV), sodium bicarbonate (8.4%, IV), dextrose (5%, IV)] and CS (2.2 mg/kg, IM) 2 hr after administration of a 30-min intravenous infusion of 0.9% NaCl (Comb group). The calves in the second group (n=6) of the second category received the same treatment as the Comb group 2 hr after intravenous injection of 2 µg/kg of LPS (LPS+Comb group).
Parameters of endotoxemia model
Body temperature is a critical indicator of septic shock. The body temperature of the calves was measured rectally at 0.5, 1, 1.5 and 2 hr before and 0, 0.5, 1, 2, 4, 6, 8, 10 and 22 hr after administration of CS. Clinical and physiological indicators, including sucking reflex, capillary refill time, defecation interval, respiratory pattern, arterial blood pressure (auricular artery), heart rate (brachial artery), respiratory rate, and body temperature were monitored and recorded at all time points. The physiological parameters were measured using a BM5Vet Monitor (Bionet, Seoul, South Korea). Changes in the parameters observed were scored numerically.
Sample collection
The body temperature of the calves was measured rectally at 0.5, 1, 1.5 and 2 hr before and 0, 0.5, 1, 2, 4, 6, 8, 10 and 22 hr after the administration of CS. Other clinical and physiological indicators were recorded (Table 1). Physiological parameters were measured using a monitor (BM5Vet Monitor). Changes in parameters were scored numerically. Prior to drug administration, a jugular catheter was inserted into the jugular vein. Blood samples (4 ml) were collected from the jugular vein in lithium heparin vacuum tubes at 5, 10, 20, 30, 45, 60 and 90 min before and 2, 3, 4, 6, 8, 10, 22, 34 and 46 hr after administration of CS. Blood samples were centrifuged at 3,000 g for 10 min to obtain plasma, and plasma samples were stored at −70°C.
Table 1. HPLC-UV double gradient elution scheme.
| Time (min) | Flow rate (ml/min) | A (%) | B (%) |
|---|---|---|---|
| 0.01 | 0.3 | 10 | 90 |
| 4 | 0.3 | 30 | 70 |
| 6 | 0.3 | 25 | 75 |
| 8 | 0.3 | 25 | 75 |
| 11 | 0.3 | 18 | 82 |
| 16 | 0.3 | 10 | 90 |
| 20 | 0.3 | 10 | 90 |
A, acetonitrile; B, 0.1% formic acid in water.
CS and desfuroylceftiofur-related metabolite analysis
CS and all desfuroylceftiofur-related metabolites (DFC) in plasma samples were quantitated using high-pressure liquid chromatography-ultraviolet (HPLC-UV) spectrophotometry (Shimadzu, Tokyo, Japan). The HPLC system consisted of a low-pressure-gradient flow control valve (FCV-10AL), pump (LC-10AD), system control module (CBM 20A), degasser (DGU-14A), auto sampler (SIL-10AD), column oven (CTO-10A), and UV-VIS detector (SPD-10A). Separation was achieved using a PLRP-S column (150 × 2.1 mm; internal diameter, 3 µm; Agilent technologies). The mobile phase, which consisted of acetonitrile (Merck, Darmstadt, Germany) (A) in water (B) with 0.1% formic acid (Merck), was filtered through a 0.4-µm nylon filter (0.45 µm, Millipore, Bedford, MA, U.S.A.), and sonicated (Elma, Singen, Germany) for 30 min. A gradient elution was performed using a flow rate of 0.3 ml/min and an injection volume of 20 µl (Table 1). The column oven was set at 40°C, and the eluate was scanned at 266 nm for peak detection. The retention time was approximately 11 min, and the total analysis time was 20 min.
CS and DFC were quantitated according to a previously published method [50]. After administration of CS, it is rapidly converted to the biologically active metabolite, DFC. Therefore, plasma DFC concentrations were determined for the pharmacokinetics of CS. In this method, CS is extracted from plasma samples using a derivitization method that converts CS and all metabolites to DFC. In brief, about 200 µl of each plasma sample was brought to room temperature and transferred to 2 ml microcentrifuge tubes. Two-hundred microliters of methanol was added, and samples were vortexed for 30 sec. After centrifugation of the sample at 13,000 g for 10 min at 22°C, clear supernatant was transferred to 2 ml microcentrifuge tubes, 100 µl of 10% dithioerythritol in borate buffer was added to each tube, and each tube was placed in a water bath at 50°C for 15 min. Tubes were moved from the water bath and allowed to reach room temperature. Next, 100 µl of 23.3% iodoacetamide in phosphate buffer was added to each tube, tubes were wrapped in aluminum foil and shaken at 350 rpm for 45 min at room temperature. Twenty-five microliters of formic acid was added to each sample. Following derivatization, samples were vortexed for 30 sec after stirring at 22°C, and were centrifuged for 10 min at 13,000 rpm. An aliquot of the resulting supernatant (20 µl) was injected into the HPLC-UV system. All chemicals and solvents used in our experiments were HPLC-grade.
The CS standards were prepared from a 1 mg/ml stock solution of CS by diluting 0.05 and 20 µg/ml solutions of CS with 190 and 10 µl of water, respectively. The assay was linear over the concentration range of 0.05 to 20 µg/ml. The limits of quantification (LOQ) and detection (LOD) of the method were found to be 0.1 and 0.05 µg/ml in plasma, respectively. LOD was defined as the lowest amount of analyte detectable in the plasma at which the signal/noise ratio in a sample is at least 3. LOQ is defined as the lowest computable concentration of the analyte in the plasma with a signal/noise ratio of at least 10. Intraday percent coefficients of variation (CV) (n=18) were as follows: 0.5 µg/ml, 3.63%; 2 µg/ml, 2.81%; 10 µg/ml, 10.05%. Interday per cent CV for CS in plasma (n=54) were as follows: 0.5 µg/ml, 4.36%; 2 µg/ml, 1.70%; 10 µg/ml, 5.93%. The coefficient of variation was calculated as CV (%)=(standard deviation/mean) × 100.
Data processing
The plasma concentration-time curve was constructed for each calf using the software program (Phoenix WinNonLin 6.3, Pharsight, St. Louis, MO, U.S.A.). Individual plasma concentration-time curves and Akaike Information Criteria (AIC) were examined for evaluation of the pharmacokinetics of CS and DCF, as previously described [52]. The pharmacokinetic variables were analyzed based on a two-compartment model. The peak plasma concentration (Cmax), and time elapsed to reach peak plasma concentration (Tmax) were determined based on the plasma concentration-time curve for each calf. Other parameters were calculated using the pharmacokinetic software program (Phoenix WinNonLin 6.3, Pharsight).
Statistical analysis
The statistical differences among parameters were determined using SPSS, version 19.0, software. Differences in body temperature were assessed using analysis of variance (ANOVA) and Tukey tests. The mean harmonic value was calculated for the time parameters (t1/2a, t1/2α and t1/2β), which compared using the Mann-Whitney U test. Differences among the other parameters were evaluated using ANOVA and Tukey tests (SPSS 22.0). P<0.05 was accepted to represent statistical significance.
RESULTS
Parameters of endotoxemia model
The sucking reflex, respiratory activity and general health indicators of the animals changed significantly following treatment with LPS, with signs of septic shock detectable within 1 hr following LPS-induction. However, the calves had recovered from these pathophysiological changes by the last time point post LPS-induction. Following LPS treatment, capillary refill time increased, defecation interval decreased, and each animal’s overall condition markedly deteriorated. Irregular decreases in arterial blood pressure and erratic changes in pulse were observed in the LPS+Ceft group. Irregular increases in body temperature and respiration rate were observed in calves treated with LPS (Table 2).
Table 2. General clinical data for healthy calves and experimentally induced endotoxemic calves that received ceftiofur sodium treatment or combination therapy.
| Variables | Ceft | LPS+Ceft | Comb | LPS+Comb | |
|---|---|---|---|---|---|
| Clinical scoring a) | Sucking reflex | Normal | No | Normal | Improvement |
| Capillary refill | Normal | Cyanotic | Normal | Altered light | |
| Defecation interval | Normal | Short | Normal | Normal | |
| Respiration type | Normal | Changed | Normal | Altered light | |
| General health condition | Normal | Changed | Normal | Altered light | |
| Monitoring a) | Arterial blood | Normal | Irregular reduction | Normal | Normal |
| Pressures pulses | Normal | Irregular promotion | Normal | Irregular promotion | |
| Body temperature | Normal | Irregular promotion | Normal | Irregular promotion | |
| Respiratory rate | Normal | Irregular promotion | Normal | Irregular promotion | |
a) General group assessment was performed based on the results of the statistical analysis.
Pharmacokinetics
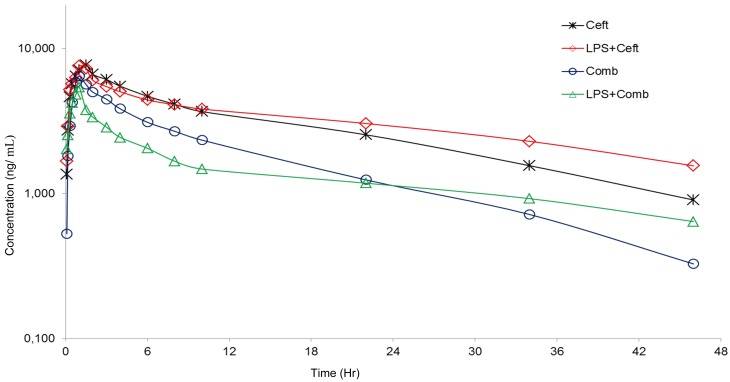
Figure 1 illustrates the plasma concentration-time curves of each group. Pharmacokinetic properties were calculated based on a two-compartment model. The pharmacokinetic parameters of CS and DFC are given in Table 3. A lower Cmax was observed (P<0.05) in the LPS+Comb group than in the Ceft and LPS+Ceft groups; Ka and t1/2a of the Comb group were statistically different (P<0.05) from those of the LPS treated groups and all other groups, respectively. The t1/2β of the LPS+Ceft group was longer (P<0.05) than that of the Ceft and Comb groups, while the CL/F values of the Comb and LPS+Comb groups were larger (P<0.05).
Fig. 1.
Semilogarithmic plots of mean plasma concentrations of ceftiofur and desfuroylceftiofur versus time after intramuscular administration of ceftiofur sodium (2.2 mg/kg) in healthy calves (ceftiofur and combined treatment) and experimentally induced endotoxemic calves (Lipopolysaccharide+ceftiofur and Lipopolysaccharide+combined treatment).
Table 3. Pharmacokinetic parameters of desfuroylceftiofur after ceftiofur sodium injection single dose (2.2 mg/kg, IM) in healthy calves and experimentally induced endotoxemic calves.
| Parameters | Ceft | LPS+Ceft | Comb | LPS+Comb |
|---|---|---|---|---|
| Ka (1/hr) | 1.86 ± 0.45ab) | 2.18 ± 0.66a) | 1.21 ± 0.25b) | 2.52 ± 0.78a) |
| t1/2a (hr) (HM) | 0.37 ± 0.09b) | 0.32 ± 0.17b) | 0.57 ± 0.12a) | 0.27 ± 0.10b) |
| Tmax (hr) | 1.5a) | 1.5a) | 1b) | 1b) |
| (Interval) | (1.5) | (1–1.5) | (1–1.5) | (0.75–1) |
| Cmax (µg/ml) | 7.73 ± 0.81a) | 7.86 ± 0.98a) | 6.54 ± 0.85ab) | 5.74 ± 1.47b) |
| AUC0-∞ (hr*µg/ml) | 153 ± 26b) | 223 ± 41a) | 100 ± 17c) | 86 ± 17c) |
| α (1/hr) | 0.59 ± 0.32 | 0.70 ± 0.48 | 0.98 ± 0.21 | 1.19 ± 0.59 |
| t1/2α (hr) (HM) | 1.17 ± 1.26 | 0.99 ± 0.69 | 0.71 ± 0.16 | 0.58 ± 0.48 |
| V1/F (ml/kg) | 212 ± 22ab) | 220 ± 56ab) | 189 ± 36b) | 281 ± 75a) |
| V2/F (ml/kg) | 182 ± 64b) | 254 ± 168b) | 342 ± 97b) | 568 ± 126a) |
| K10 (1/hr) | 0.07 ± 0.01bc) | 0.05 ± 0.02c) | 0.12 ± 0.03a) | 0.10 ± 0.03ab) |
| K12 (1/hr) | 0.26 ± 0.17b) | 0.38 ± 0.31ab) | 0.56 ± 0.11ab) | 0.77 ± 0.41a) |
| K21 (1/hr) | 0.30 ± 0.16 | 0.30 ± 0.18 | 0.34 ± 0.14 | 0.36 ± 0.18 |
| β (1/hr) | 0.04 ± 0.01a) | 0.02 ± 0.00b) | 0.04 ± 0.01a) | 0.03 ± 0.01ab) |
| t1/2β (hr) (HM) | 19.90 ± 3.46b) | 32.56 ± 6.11a) | 17.90 ± 2.54b) | 23.39 ± 5.88ab) |
| CL/F (ml/hr/kg) | 14.70 ± 2.57b) | 10.16 ± 2.24b) | 22.52 ± 4.19a) | 26.68 ± 7.81a) |
| K12/K21 | 0.86 ± 0.31b) | 1.25 ± 0.92ab) | 1.84 ± 0.55ab) | 2.12 ± 0.69a) |
| V1/F+V2/F (ml/kg) | 394 ± 67b) | 474 ± 162b) | 532 ± 112b) | 850 ± 169a) |
a, b, c) Varied characters in the same row are statistically significantly different (P<0.05). Ka; absorption rate constant, t1/2a; absorption half-life, Tmax; time to reach the maximum concentration, C max; peak plasma concentration, AUC; area under curve, α; distribution rate constant, t1/2α; distribution half-life, V1/F; central compartment volume of distribution, V2/F; peripheral compartment volume of distribution, K10; elimination rate constant of central compartment, K12; transfer rate constant from central compartment to peripheral compartment, K21; transfer rate constant from peripheral compartment to central compartment, β; elimination rate constant, CL/F; total clearance, t1/2β; elimination half-life, HM; harmonic means, Ceft; ceftiofur, LPS+Ceft; lipopolysaccharide + ceftiofur, Comb; combined treatments, LPS+Comb; lipopolysaccharide + combined treatments.
Pharmacodynamics
Bacterial isolates having an MIC value ˂2.0 µg/ml are considered sensitive to CS according to current CLSI guidelines (National Committee for Clinical Laboratory Standards, 1999).
DISCUSSION
Parameters of the endotoxemia model
Neonatal calf diarrhea is an important health problem in calves. In the current research, the calves in LPS-treated groups showed some of the clinical and physiological signs of endotoxemia (Table 1). Previous studies have shown that intravenous injection of E. coli toxin in calves leads to changes in sucking reflex, capillary refilling time, body temperature, respiration rate, and heart rate [23, 43]. In our current study, a combination therapy initiated 2 hr after LPS injection prevented frequent defecation, and decreased the adverse effects of endotoxemia on capillary refilling time, sucking reflex and general health indicators. Our results are consistent with those obtained in other studies of the treatment of sepsis in calves using various therapeutic agents [4, 28]. Similar results have been reported in previous studies [1, 6]. These results indicate that LPS treatment can mimic endotoxemia.
Pharmacokinetics/pharmacodynamics
In vivo, the systemic half-life of ceftiofur is shorter than 10 min due to rapid degradation of thioester bonds and rapid conversion into DFC, which is the active metabolite in the body. The beta-lactam ring belonging to ceftiofur remains unchanged in DFC [20, 39]. Conversion of ceftiofur to DFC is followed by derivatization, which stabilizes DFC. Therefore, total antibacterial activity related to ceftiofur and its metabolites correlates with DFC concentration in plasma samples.
The definition of a drug-drug interaction (DDI), affecting pharmacokinetics and/or pharmacodynamics, is a change in the effects of one drug induced by the presence of another drug [30]. Drug-drug interactions are more likely to be seen in patients with reduced absorption, disrupted metabolism, renal insufficiency and polymedication [31]. DDIs, which occur in pharmacokinetic stages such as absorption, distribution, metabolism and excretion, are caused by significant changes in pharmacokinetics of drugs [17, 19]. The liver is the most effective organ at biotransformation of drugs. Drugs metabolized via the liver can be affected by physiological processes such as liver blood flow rate, enzyme activity and binding to proteins [26]. Co-administered drugs have different effects on the activity of drug metabolizing enzymes. In addition, endotoxins produced in response to bacterial inflammatory mediators have different effects on these enzymes [9]. The inhibition or induction of enzymes responsible for chemical modification of drugs indirectly changes the pharmacokinetic profile of the drug [17]. In this study, changes in the pharmacokinetic processes of CS by LPS-induced endotoxicosis and combination treatment may be related to changes in metabolite composition of CS. However, determining the DDI in patients receiving combination drug therapy for LPS may be a very complex task.
In this study, we investigated the pharmacokinetics of intramuscularly administered CS in healthy calves and calves with experimentally induced septic shock (Table 3). The results of our analysis of the pharmacokinetic parameters of CS, as well as those of previous studies performed in healthy calves [2] were consistent with a two-compartment open model (Fig. 1). Pharmacokinetic properties of CS in healthy calves are consistent with Brown et al. [2]. Although the pharmacokinetics of CS have not been described in endotoxemic calves treated with CS alone or in combination therapy, another cephalosporin has been examined in calves [21]. The calves in the LPS+Comb group have a large CL/F and increased V1/F+V2/F compared to those in the LPS+Ceft group (P<0.05, Table 3). These results may indicate that combined therapy alters the pharmacokinetics of CS in endotoxemic calves.
In vivo, ceftiofur is rapidly metabolized to DFC, which is the microbiologically active metabolite of CS [20, 39]. In several other studies [3, 10, 18, 51] the dose values of ceftiofur used to calculate pharmacokinetic parameters did not use the total amount of DFC. In this method, conversion of ceftiofur to DFC is followed by derivatization to stabilize DFC. Therefore, total antibacterial activity related to ceftiofur and its metabolites is presented with DFC concentration in plasma samples. In this method, metabolites, including desfuroylceftiofur–protein conjugates, are converted into desfuroylceftiofuracetamide (DCA), which is then quantified by HPLC. Therefore, in this study, pharmacokinetic parameters were determined by DFC concentrations in plasma at different time points.
The Ka in the Comb group was significantly lower than that of the LPS+Ceft and LPS+Comb groups (P<0.05; Table 3). The t1/2a in the Comb group was significantly longer than that of the other groups (P<0.05; Table 3). The Tmax values in the Comb and LPS+Comb groups were significantly shorter (P<0.05), and the Cmax was significantly lower in the LPS+Comb group, compared with that in the other groups (P<0.05). In a previous study, the t1/2a of intramuscularly administered oxytetracycline was reduced in calves with experimentally induced endotoxemia [24], and the t1/2a of intramuscularly administered levofloxacin was prolonged, compared with the t1/2a for these drugs in healthy calves [25]. Absorption of CS in the Comb group was significantly slowed, because Ka was low and t1/2a was long. Our current findings suggest that, if CS is to exert its effect rapidly, it should not be administered as a component of combined therapy via a route that involves absorption processes. However, differences in CS absorption processes including Cmax between normal and other groups would not be of particular note.
The V1/F+V2/F in the LPS+Comb was significantly larger than that of the Ceft group (P<0.05; Table 3). In addition, the ratio of the rate constants for transfer from the central compartment to peripheral compartments over that for transfer from peripheral compartments to the central compartment (k12/k21) in the LPS+Comb group (2.12 ± 0.69) was higher than that of the Ceft group (0.86 ± 0.31, P<0.05; Table 3). Recent studies have reported that the distribution volume and the Cmax of meropenem, ceftazidime, cefepime, piperacillin-tazobactam, piperacillin, amikacin, and vancomycin increase and decline respectively in sepsis/septic shock patients receiving fluid therapy and catecholamine in intensive care units (ICU) [7, 42, 46]. Abundant intravenous fluid therapies that increase extracellular compartment fluid may cause a significant increase in distribution [40]. Elevated distribution volumes of CS and cefepime have been shown in experimentally infected pigs and calves [32, 47]. Combination therapies including vasopressor drugs and abundant intravenous fluid therapies may lead to an increase in distribution of CS by causing rapid transfer of the drug into the tissues and prolonging elimination in endotoxemic calves.
In our current study, the Ceft group was used as a control group. We observed that the t1/2β in the LPS+Ceft group was significantly longer (P<0.05) than that in the Ceft group, but was similar to that in the Comb and LPS+Comb groups (P<0.05, Table 3). In addition, the t1/2β in the LPS+Ceft group was significantly longer than in the other groups (P>0.05), and the CL/F in the LPS+Ceft group was meaningfully elevated, compared with that in the other groups (P>0.05). We also found that the CL/F in the Comb and LPS+Comb groups was significantly higher than that of the LPS+Ceft and Ceft groups (P<0.05; Table 3). Recent studies have shown that plasma half-life and clearance of CS in experimentally infected pigs were longer than in healthy pigs [47]. A study of septic patients treated with ceftriaxone showed that the clearance time of drug increased when used in combination with hemodynamically active drugs [22]. Also, the clearances of ceftazidime and cefepime were higher in patients with septic shock, compared with those in healthy subjects [34]. Blood pressure is increased by vasopressor drug application and fluid therapy, which are provided to achieve a perfusion pressure and maintain adequate blood flow [8]. Intravenously administered dopamine elevates mean arterial blood pressure of calves by increasing vascular resistance [16]. The use of high-dose dexamethasone has been shown to increase survival rate [15] by reducing oxidative stress and organ damage [54]. These findings indicate that CS was excreted faster in the combination therapy group, whereas in the LPS+Ceft group, the rate of excretion slowed down due to circulatory and renal failures that develop during septic shock and extend the half-life of drugs, particularly those that are excreted by the kidneys. However, the current study indicates that combination drug therapy can help regulate the half-life of CS in endotoxemic calves.
The activity of beta-lactams including CS is characterized by time-dependent killing, and the MIC is an indicator of the potency of an antimicrobial drug against a pathogen [11]. The most useful and predictable parameter for therapeutic efficacy of beta-lactams is the percentage of time its concentration remains above MIC (%T>MIC) [27, 38, 49]. It has been reported that%T >4xMIC for maximal bactericidal activity in beta-lactams is shown to be maintained longer (>100%) in critically ill patients and patients with compromised immune responses [33, 45]. Bacterial isolates having an MIC value ≤2.0 µg/ml are considered sensitive to CS according to current CLSI guidelines (National Committee for Clinical Laboratory Standards, 1999). Based on previous studies, a dose regimen of 6.6 mg/kg of CS administered subcutaneously is effective for treatment of severe septic disease in neonatal calves and foals according to the MIC ≤2.0 µg/ml for >90% of the 12-hr study duration [18, 51]. Therefore, the choice of 2.0 µg/ml as the most effective concentration of CS for endotoxemic shock resulting from E. coli appears to be suitable according to current data and CLSI. This pharmacodynamics target was achieved for the major pathogens identified in endotoxemic shock, with effective concentrations of CS observed in plasma for 22, 34, 10 and 6 hr intervals, respectively, in the Ceft, LPS+Ceft, Comb and LPS+Comb groups. In Ceft and LPS+Ceft groups, IM administration of CS at 2.2 mg/kg reached plasma concentrations ≥2.0 µg/ml for >80% of the 46 hr study duration, while T >MIC above 80% is not achieved with the 2.2 mg/kg body weight of CS applied with combined therapy for bacteria with MIC value ≤2.0 µg/ml at 46 hr interval (Fig. 1). However, the%T >MIC is shown to be maintained for 100% of the dosing interval during treatment of patients in critical condition with septic shock [33, 36]. In this instance, following administration at a dose of 2.2 mg/kg, CS does not maintain a T >MIC above 100% for bacteria with MIC value ≤2.0 µg/ml for a 46 hr interval in Comb and LPS+Comb groups. However, the same dose (2.2 mg/kg, IM) maintains this target dosage for 34 hr and 6 hr intervals, respectively, in LPS+Ceft and LPS+Comb groups (Fig. 1). The T ˃ 4×MIC of CS should be sustained across dosing intervals for effective treatment of sepsis in newborn calves. Therefore, the total plasma concentration of CS in this study did not achieve%T >4xMIC for bacteria with MIC value ≤2.0 µg/ml.
In conclusion, the present study indicates that combined therapy may alter the plasma pharmacokinetic of CS in healthy and endotoxemic calves and an experimentally induced model of shock can generate the same types of pathological changes observed during actual disease. The pharmacokinetic data of CS obtained from this study are applicable to the treatment of newborn calves with sepsis. The results of this study suggest that a 2.2 mg/kg (IM) dose of CS every 6 hr with combined therapy may provide plasma concentrations above the therapeutic target of 2.0 µg/ml in endotoxemic new born calves. However, 2.2 mg/kg (IM) dose of CS could not provide plasma concentrations above the therapeutic target of 2.0 µg/ml in critical patients with septic shock. The dose and dose interval of CS may be increased and reduced in newborn calves with combined therapy and endotoxemic status, respectively. The investigation of pharmacokinetics for farm animals in disease state and undergoing a course of medication may provides a rationale for practical adjustment of established dose regimes.
Acknowledgments
This manuscript summarizes the PhD thesis of Feray Altan. Research was supported by TUBITAK (110O404). The abstract was submitted to the 12th International Congress of the European Association for Veterinary Pharmacology and Toxicology (EAVPT), Amsterdam, Holland, July 2012.
REFERENCES
- 1.Akyuz E., Coskun A., Sen I.2016. The effects of fluid resuscitation on the hemodynamic parameters of experimental induced endotoxemia in the neonatal calves. Eurasian J. Vet. Sci. 32: 246–254. doi: 10.15312/EurasianJVetSci.2016422396 [DOI] [Google Scholar]
- 2.Brown S. A., Chester S. T., Robb E. J.1996. Effects of age on the pharmacokinetics of single dose ceftiofur sodium administered intramuscularly or intravenously to cattle. J. Vet. Pharmacol. Ther. 19: 32–38. doi: 10.1111/j.1365-2885.1996.tb00005.x [DOI] [PubMed] [Google Scholar]
- 3.Brown S. A., Hanson B. J., Mignot A., Millérioux L., Hamlow P. J., Hubbard V. L., Callahan J. K., Kausche F. M.1999. Comparison of plasma pharmacokinetics and bioavailability of ceftiofur sodium and ceftiofur hydrochloride in pigs after a single intramuscular injection. J. Vet. Pharmacol. Ther. 22: 35–40. doi: 10.1046/j.1365-2885.1999.00182.x [DOI] [PubMed] [Google Scholar]
- 4.Constable P. D.2004. Antimicrobial use in the treatment of calf diarrhea. J. Vet. Intern. Med. 18: 8–17. doi: 10.1111/j.1939-1676.2004.tb00129.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Constable P. D.2009. Treatment of calf diarrhea: antimicrobial and ancillary treatments. Vet. Clin. North Am. Food Anim. Pract. 25: 101–120, vi. doi: 10.1016/j.cvfa.2008.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coskun A., Sen I.2012. Haematological, biochemical and coagulation changes in calves with endotoxemia. Agric. J. 7: 37–41. [Google Scholar]
- 7.Delattre I. K., Musuamba F. T., Nyberg J., Taccone F. S., Laterre P. F., Verbeeck R. K., Jacobs F., Wallemacq P. E.2010. Population pharmacokinetic modeling and optimal sampling strategy for Bayesian estimation of amikacin exposure in critically ill septic patients. Ther. Drug Monit. 32: 749–756. doi: 10.1097/FTD.0b013e3181f675c2 [DOI] [PubMed] [Google Scholar]
- 8.Dellinger R. P., Levy M. M., Carlet J. M., Bion J., Parker M. M., Jaeschke R., Reinhart K., Angus D. C., Brun-Buisson C., Beale R., Calandra T., Dhainaut J. F., Gerlach H., Harvey M., Marini J. J., Marshall J., Ranieri M., Ramsay G., Sevransky J., Thompson B. T., Townsend S., Vender J. S., Zimmerman J. L., Vincent J. L.2008. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 34: 17–60. doi: 10.1007/s00134-007-0934-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di L., Feng B., Goosen T. C., Lai Y., Steyn S. J., Varma M. V., Obach R. S.2013. A perspective on the prediction of drug pharmacokinetics and disposition in drug research and development. Drug Metab. Dispos. 41: 1975–1993. doi: 10.1124/dmd.113.054031 [DOI] [PubMed] [Google Scholar]
- 10.Doré E., Angelos J. A., Rowe J. D., Carlson J. L., Wetzlich S. E., Kieu H. T., Tell L. A.2011. Pharmacokinetics of ceftiofur crystalline free acid after single subcutaneous administration in lactating and nonlactating domestic goats (Capra aegagrus hircus). J. Vet. Pharmacol. Ther. 34: 25–30. doi: 10.1111/j.1365-2885.2010.01187.x [DOI] [PubMed] [Google Scholar]
- 11.Drusano G. L.1998. Infection in the intensive care unit: beta-lactamase-mediated resistance among Enterobacteriaceae and optimal antimicrobial dosing. Clin. Infect. Dis. 27 Suppl 1: S111–S116. doi: 10.1086/514915 [DOI] [PubMed] [Google Scholar]
- 12.Elmas M., Yazar E., Uney K., Er Karabacak A.2006. Influence of Escherichia coli endotoxin-induced endotoxaemia on the pharmacokinetics of enrofloxacin after intravenous administration in rabbits. J. Vet. Med. A Physiol. Pathol. Clin. Med. 53: 410–414. doi: 10.1111/j.1439-0442.2006.00854.x [DOI] [PubMed] [Google Scholar]
- 13.Elmas M., Yazar E., Uney K., Er Karabacak A., Traş B.2008. Pharmacokinetics of enrofloxacin and flunixin meglumine and interactions between both drugs after intravenous co-administration in healthy and endotoxaemic rabbits. Vet. J. 177: 418–424. doi: 10.1016/j.tvjl.2007.05.021 [DOI] [PubMed] [Google Scholar]
- 14.EMEA.http://www.ema.europa.eu/docs/en_GB/document_library/Maximum_Residue_Limits_- _Report/2009/11/WC500011904.pdf (accessed December 15, 2016)
- 15.Er A., Uney K., Altan F., Cetin G., Yazar E., Elmas M.2009. Effects of different doses of dexamethasone plus flunixin meglumine on survival rate in lethal endotoxemia. Acta Vet. (Beogr.) 59: 47–51. doi: 10.2298/AVB0901047E [DOI] [Google Scholar]
- 16.Greiner A. S., Skeehan T. M., Larach D. R., Schuler H. G., Pierce W. S.1990. Vascular responses to dopamine and dobutamine in the awake calf during constant aortic flow or constant aortic pressure. J. Cardiovasc. Pharmacol. 15: 392–397. doi: 10.1097/00005344-199003000-00008 [DOI] [PubMed] [Google Scholar]
- 17.Giacomini K. M., Huang S. M., Tweedie D. J., Benet L. Z., Brouwer K. L., Chu X., Dahlin A., Evers R., Fischer V., Hillgren K. M., Hoffmaster K. A., Ishikawa T., Keppler D., Kim R. B., Lee C. A., Niemi M., Polli J. W., Sugiyama Y., Swaan P. W., Ware J. A., Wright S. H., Yee S. W., Zamek-Gliszczynski M. J., Zhang L., International Transporter Consortium2010. Membrane transporters in drug development. Nat. Rev. Drug Discov. 9: 215–236. doi: 10.1038/nrd3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall T. L., Tell L. A., Wetzlich S. E., McCormick J. D., Fowler L. W., Pusterla N.2011. Pharmacokinetics of ceftiofur sodium and ceftiofur crystalline free acid in neonatal foals. J. Vet. Pharmacol. Ther. 34: 403–409. doi: 10.1111/j.1365-2885.2010.01252.x [DOI] [PubMed] [Google Scholar]
- 19.Ito K., Iwatsubo T., Kanamitsu S., Ueda K., Suzuki H., Sugiyama Y.1998. Prediction of pharmacokinetic alterations caused by drug-drug interactions: metabolic interaction in the liver. Pharmacol. Rev. 50: 387–412. [PubMed] [Google Scholar]
- 20.Jacobson G. A., Martinod S., Cunningham C. P.2006. Determination of ceftiofur in bovine plasma by HPLC-DAD. J. Pharm. Biomed. Anal. 40: 1249–1252. doi: 10.1016/j.jpba.2005.09.010 [DOI] [PubMed] [Google Scholar]
- 21.Joshi B., Sharma S. K.2009. The pharmacokinetics of cefepime in E. coli lipopolysaccharide induced febrile buffalo calves. Vet. Arh. 79: 523–530. [Google Scholar]
- 22.Joynt G. M., Lipman J., Gomersall C. D., Young R. J., Wong E. L., Gin T.2001. The pharmacokinetics of once-daily dosing of ceftriaxone in critically ill patients. J. Antimicrob. Chemother. 47: 421–429. doi: 10.1093/jac/47.4.421 [DOI] [PubMed] [Google Scholar]
- 23.Kinsbergen M., Bruckmaier R. M., Blum J. W.1994. Metabolic, endocrine and haematological response to intravenous E. coli endotoxin administration in 1-week-old calves. J. Vet. Med. A Physiol. Pathol. Clin. Med. 41: 530–547. doi: 10.1111/j.1439-0442.1994.tb00121.x [DOI] [PubMed] [Google Scholar]
- 24.Kumar R., Malik J. K.2001. Effects of multiple injections of Escherichia coli endotoxin on the pharmacokinetics and dosage regimens of a long-acting formulation of oxytetracycline (OTC-LA) in cross-breed calves. Vet. Arh. 71: 245–263. [Google Scholar]
- 25.Kumar S., Kumar S., Kumar V., Singh K. K., Roy B. K.2009. Pharmacokinetic studies of levofloxacin after oral administration in healthy and febrile cow calves. Vet. Res. Commun. 33: 887–893. doi: 10.1007/s11259-009-9237-0 [DOI] [PubMed] [Google Scholar]
- 26.Martinez M., Modric S.2010. Patient variation in veterinary medicine: part I. Influence of altered physiological states. J. Vet. Pharmacol. Ther. 33: 213–226. doi: 10.1111/j.1365-2885.2009.01139.x [DOI] [PubMed] [Google Scholar]
- 27.McKinnon P. S., Davis S. L.2004. Pharmacokinetic and pharmacodynamic issues in the treatment of bacterial infectious diseases. Eur. J. Clin. Microbiol. Infect. Dis. 23: 271–288. doi: 10.1007/s10096-004-1107-7 [DOI] [PubMed] [Google Scholar]
- 28.Nagy D. W.2009. Resuscitation and critical care of neonatal calves. Vet. Clin. North Am. Food Anim. Pract. 25: 1–11, xi. doi: 10.1016/j.cvfa.2008.10.008 [DOI] [PubMed] [Google Scholar]
- 29.Nicolau D. P.2008. Pharmacodynamic optimization of β-lactams in the patient care setting. Crit. Care 12 Suppl 4: S2. doi: 10.1186/cc6818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palleria C., Di Paolo A., Giofrè C., Caglioti C., Leuzzi G., Siniscalchi A., De Sarro G., Gallelli L.2013. Pharmacokinetic drug-drug interaction and their implication in clinical management. J. Res. Med. Sci. 18: 601–610. [PMC free article] [PubMed] [Google Scholar]
- 31.Papadopoulos J., Smithburger P. L.2010. Common drug interactions leading to adverse drug events in the intensive care unit: management and pharmacokinetic considerations. Crit. Care Med. 38 Suppl: S126–S135. doi: 10.1097/CCM.0b013e3181de0acf [DOI] [PubMed] [Google Scholar]
- 32.Pawar Y. G., Sharma S. K.2008. Influence of E. coli lipopolysaccharide induced fever on the plasma kinetics of cefepime in cross-bred calves. Vet. Res. Commun. 32: 123–130. doi: 10.1007/s11259-007-9010-1 [DOI] [PubMed] [Google Scholar]
- 33.Pea F., Viale P.2009. Bench-to-bedside review: Appropriate antibiotic therapy in severe sepsis and septic shock--does the dose matter? Crit. Care 13: 214. doi: 10.1186/cc7774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pea F., Viale P., Furlanut M.2005. Antimicrobial therapy in critically ill patients: a review of pathophysiological conditions responsible for altered disposition and pharmacokinetic variability. Clin. Pharmacokinet. 44: 1009–1034. doi: 10.2165/00003088-200544100-00002 [DOI] [PubMed] [Google Scholar]
- 35.Roberts J. A., Lipman J.2009. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit. Care Med. 37: 840–851, quiz 859. doi: 10.1097/CCM.0b013e3181961bff [DOI] [PubMed] [Google Scholar]
- 36.Roberts J. A., Norris R., Paterson D. L., Martin J. H.2012. Therapeutic drug monitoring of antimicrobials. Br. J. Clin. Pharmacol. 73: 27–36. doi: 10.1111/j.1365-2125.2011.04080.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts J. A., Ulldemolins M., Roberts M. S., McWhinney B., Ungerer J., Paterson D. L., Lipman J.2010. Therapeutic drug monitoring of β-lactams in critically ill patients: proof of concept. Int. J. Antimicrob. Agents 36: 332–339. doi: 10.1016/j.ijantimicag.2010.06.008 [DOI] [PubMed] [Google Scholar]
- 38.Roos J. F., Bulitta J., Lipman J., Kirkpatrick C. M.2006. Pharmacokinetic-pharmacodynamic rationale for cefepime dosing regimens in intensive care units. J. Antimicrob. Chemother. 58: 987–993. doi: 10.1093/jac/dkl349 [DOI] [PubMed] [Google Scholar]
- 39.Salmon S. A., Watts J. L., Yancey R. J., Jr.1996. In vitro activity of ceftiofur and its primary metabolite, desfuroylceftiofur, against organisms of veterinary importance. J. Vet. Diagn. Invest. 8: 332–336. doi: 10.1177/104063879600800309 [DOI] [PubMed] [Google Scholar]
- 40.Scaglione F., Paraboni L.2008. Pharmacokinetics/pharmacodynamics of antibacterials in the Intensive Care Unit: setting appropriate dosing regimens. Int. J. Antimicrob. Agents 32: 294–301. doi: 10.1016/j.ijantimicag.2008.03.015 [DOI] [PubMed] [Google Scholar]
- 41.Sen I., Constable P. D.2013. General overview to treatment of strong ion (metabolic) acidosis in neonatal calves with diarrhea. Eurasian J. Vet. Sci 29: 114–120. [Google Scholar]
- 42.Seyler L., Cotton F., Taccone F. S., De Backer D., Macours P., Vincent J. L., Jacobs F.2011. Recommended β-lactam regimens are inadequate in septic patients treated with continuous renal replacement therapy. Crit. Care 15: R137. doi: 10.1186/cc10257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh D., Bansal S. K., Ghumman G. S.2011. Effect of flunixin meglumine alone and in combination on haemodynamics during bovine endotoxic shock and after treatment. JBISE 4: 29–33. doi: 10.4236/jbise.2011.41004 [DOI] [Google Scholar]
- 44.Sinnollareddy M. G., Roberts M. S., Lipman J., Roberts J. A.2012. β-lactam pharmacokinetics and pharmacodynamics in critically ill patients and strategies for dose optimization: a structured review. Clin. Exp. Pharmacol. Physiol. 39: 489–496. doi: 10.1111/j.1440-1681.2012.05715.x [DOI] [PubMed] [Google Scholar]
- 45.Sime F. B., Roberts M. S., Peake S. L., Lipman J., Roberts J. A.2012. Does beta-lactam pharmacokinetic variability in critically ill patients justify therapeutic drug monitoring? A systematic review. Ann. Intensive Care 2: 35. doi: 10.1186/2110-5820-2-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taccone F. S., Laterre P. F., Spapen H., Dugernier T., Delattre I., Layeux B., De Backer D., Wittebole X., Wallemacq P., Vincent J. L., Jacobs F.2010. Revisiting the loading dose of amikacin for patients with severe sepsis and septic shock. Crit. Care 14: R53. doi: 10.1186/cc8945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tantituvanont A., Yimprasert W., Werawatganone P., Nilubol D.2009. Pharmacokinetics of ceftiofur hydrochloride in pigs infected with porcine reproductive and respiratory syndrome virus. J. Antimicrob. Chemother. 63: 369–373. doi: 10.1093/jac/dkn496 [DOI] [PubMed] [Google Scholar]
- 48.Tell L., Harrenstien L., Wetzlich S., Needham M., Nappier J., Hoffman G., Caputo J., Craigmill A.1998. Pharmacokinetics of ceftiofur sodium in exotic and domestic avian species. J. Vet. Pharmacol. Ther. 21: 85–91. doi: 10.1046/j.1365-2885.1998.00124.x [DOI] [PubMed] [Google Scholar]
- 49.Toutain P. L., del Castillo J. R. E., Bousquet-Mélou A.2002. The pharmacokinetic-pharmacodynamic approach to a rational dosage regimen for antibiotics. Res. Vet. Sci. 73: 105–114. doi: 10.1016/S0034-5288(02)00039-5 [DOI] [PubMed] [Google Scholar]
- 50.Uney K., Altan F., Er A., Yazar E., Elmas M.2011. Determination of an HPLC-UV validation of ceftiofur and its active metabolite desfuroylceftiofur in rabbit plasma and its application to the pharmacokinetic study. SUBAPK: 11401102.(Un published data). [Google Scholar]
- 51.Woodrow J. S., Caldwell M., Cox S., Hines M., Credille B. C.2016. Comparative plasma pharmacokinetics of ceftiofur sodium and ceftiofur crystalline-free acid in neonatal calves. J. Vet. Pharmacol. Ther. 39: 271–276. doi: 10.1111/jvp.12275 [DOI] [PubMed] [Google Scholar]
- 52.Yamaoka K., Nakagawa T., Uno T.1978. Application of Akaike’s information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J. Pharmacokinet. Biopharm. 6: 165–175. doi: 10.1007/BF01117450 [DOI] [PubMed] [Google Scholar]
- 53.Yazar E.2016. Chemotherapeutics. pp: 25–198. In: Veterinary Drug, Ed: Yazar E, Olgun-Celik Press, Konya, Turkey. [Google Scholar]
- 54.Yazar E., Er A., Uney K., Bulbul A., Avci G. E., Elmas M., Traş B.2010. Effects of drugs used in endotoxic shock on oxidative stress and organ damage markers. Free Radic. Res. 44: 397–402. doi: 10.3109/10715760903513025 [DOI] [PubMed] [Google Scholar]