Abstract
Extragonadal tissues are known to produce estrogens. At these sites, the C19 precursor is important for aromatase expression for the production of estrogen. Aromatase expression is tissue-specific and is controlled by hormones. Recent studies have shown that rat gastric parietal cells expressed aromatase. Our first objective was to investigate steroidogenic enzyme expression in estrogen biosynthesis; the second objective was to investigate which site(s) of the GI tract expressed steroidogenic enzymes; and the third objective was to assess the effects of castration on steroidogenic enzyme expression. CYP19A1, 17β-HSD3, CYP17A1, 3β-HSD and P450scc were quantified in the GI tract by real-time PCR. CYP19A1 was detected mainly in the body and pyloric regions of the abomasum, while we detected weak expression of CYP19A1 in other parts of GI tract. In addition, the expression of 17β-HSD3 and CYP17A1 was detected in abomasum. 3β-HSD expression was observed in duodenum and jejunum, while P450scc was not detectable in any part of GI tract. Immunohistochemical results showed immunolocalization of aromatase in parietal cells. Aromatase expression was observed to increase after castration. Furthermore, immunohistochemical results demonstrated that parietal cells also produced luteinizing hormone receptor (LHR). These results indicate steroidogenic enzymes required for the biosynthesis of estrogen were expressed, and the abomasum appeared to be the responsible organ for estrogen biosynthesis in the goat GI tract. In addition, parietal cells were responsible for estrogen production and the expression of LHR. Castration increased aromatase expression in abomasum through LH mediation.
Keywords: abomasum, aromatase, estrogen, goat, LH
Estrogen is predominantly produced by granulosa cells of the ovary [8, 11]. In addition, it is known that Leydig and Sertoli cells of the testis produce estrogen [25]. Extragonadal tissues such as placenta [1], skin [9], vascular smooth muscle cell [6], adipose tissue [15, 28] and brain [17], are also known to produce estrogens. Estrogens have important physiological roles, such as cellular proliferation and regeneration via autocrine and paracrine roles, not only in reproductive organs but also in extragonadal tissues [19, 20]. Therefore, estrogen biosynthesis in extragonadal tissues is important for homeostasis. It is known that all steroid hormones are derived from cholesterol through metabolism by steroidogenic enzymes, and the last step in estrogen biosynthesis is the aromatization of androgens by aromatase (CYP19A1) [13, 22]. Unlike the gonads, extragonadal tissues are unable to produce the C19 (androgen) precursors. Therefore, provision of C19 is important for 17beta-estradiol (E2) biosynthesis in extragonadal tissues [19, 20]. Moreover, aromatase expression is tissue-specific and controlled by hormones. For examples, aromatase expression is induced by luteinizing hormones (LH) and follicle-stimulating hormone (FSH) in the ovary and testis [4, 10, 18]. Ueyama et al. (2002) have been shown that gastric parietal cells of rats produce E2 and secrete it into the portal vein [22]. The aims of this study were to examine which steroidogenic enzymes for estrogen production existed in the goat GI tract, and which organs were the primary sites for estrogen biosynthesis in the male goat GI tract. Additionally, we tried to explore the control mechanisms of steroidogenesis in the male GI tract by means of bilateral castration to evaluate contribution of testicular androgens for estrogen biosynthesis.
MATERIALS AND METHODS
Animals
In the present study, 7 male Shiba goats, 12–14 months of age and weighing 18–24 kg, were used. They were kept at goat barn belonging to the Laboratory of Reproductive Physiology, Department of Veterinary Medicine, Tokyo University of Agriculture and Technology, Tokyo, Japan under normal daylight and temperature. This breed shows nonseasonal breeding under natural photoperiod conditions in Tokyo. All procedures carried out in this experiments were in accordance with the guidelines established by the Tokyo University of Agriculture and Technology (29-5).
Experiment 1
Four male goats (12–14 months of age) were used for this experiment. The goats were sacrificed by an overdose of sodium pentobarbital (Somuno pentil injection®, Kyoritsu Seiyaku Corporation, Tokyo, Japan). Tissue samples were collected from the gastrointestinal (GI) tract from rumen to the rectum and kept on dry ice for gene analysis of steroidogenic enzyme mRNA expression of the GI tract. In addition, a portion of the tissues samples were also fixed in 4% paraformaldehyde in 0.05 M phosphate-buffered saline (PBS) for histological examination of steroidogenic enzymes and their distribution.
Experiment 2
Three male goats (12 months of age) were anesthetized with atropine sulfate (atropine sulphate monohydrate, 0.022 mg/kg; Tokyo Chemical Industry Co., Ltd., Tokyo, Japan), xylazine (xylazine hydrochloride, 0.05 mg/kg intramuscularly; Tokyo Chemical Industry Co., Ltd.) and pentobarbital (pentobarbital, 30 mg/kg intravenously, Somuno pentil injection®, Kyoritsu Seiyaku Corporation), the scrotum was cleaned and disinfected with alcohol, the bottom third was cut, and testes were removed. After castration, the animals were cared for through recovery. Two months after castration, goats were sacrificed by an overdose of pentobarbital. Tissue samples were then collected from the (GI) tract from the rumen to the rectum and kept on dry ice for gene analysis of steroidogenic enzyme mRNA expression of the GI tract. In addition, a portion of the tissues samples were also fixed in 4% paraformaldehyde in 0.05 M phosphate-buffered saline (PBS) for histological examination of steroidogenic enzymes and their distribution.
Tissue preparations
Tissues from rumen, reticulum, omasum, three parts of the abomasum (fundus, body and pyloric regions), duodenum, jejunum, ileum, cecum, colon and rectum were harvested. Samples were immediately fixed for 24 hr in 4% paraformaldehyde (Sigma Chemical, St. Louis, MO, U.S.A.) in 0.05 MPBS, pH 7.4. Samples were dehydrated by a series of ethanol and embedded in paraffin. The samples were sectioned at 4–6 µm and placed on APS (3-aminopropyl triethoxysilane)-coated slides (Matsunami Glass Ind., Ltd., Kishiwada, Japan).
Double-staining for aromatase and the hydrogen-potassium pump in the abomasum
In order to reduce background, sections were incubated with 10% normal goat serum for 60 min. For detection of co-localization of aromatase and hydrogen-potassium proton pump, sections were incubated with the both primary antibodies. Antibody dilutions were used as follows: anti-human placental cytochrome P450 aromatase at 1:2,000 and 1:4,000 (kindly provided by Dr. Y. Osawa, Medical foundation of Buffalo, Buffalo, NY, U.S.A.) raised in rabbit; and anti-hydrogen potassium-ATPase (H/K ATPase) beta Ab (1:200, [2G11] ab 2866, Abcam, Tokyo, Japan), raised in mouse overnight at 4○C. For co-localization of luteinizing hormone receptor (LHR) and H/K ATPase in abomasum, the sections were incubated with a mixture of primary antibodies as follows: anti-LHCGR (luteinizing hormone/choriogonadotropin receptor) (1:200, Proteinech, Chicago, IL, U.S.A.) and H/K ATPase together. After incubation of primary antibodies, the sections were incubated with Alexa flour 555 (A21428) and Alexa flour 488 (A11001) for 60 min. DAPI was used as a counterstain, and the normal rabbit serum and normal mice IgG were used as negative controls. All photomicrographs were captured by an immunofluorescence microscope BX-51 (Olympus, Osaka, Japan).
Real-time PCR for steroidogenic enzyme and LHCGR gene expression
Total RNA was extracted by TRIzol reagent (Invitrogen, Carlsbad, CA, U.S.A.) according to manufacturer’s instructions. Complementary deoxyribonucleic acid (cDNA) was synthetized for each sample by using PrimeScript 1st cDNA kit (Takara Bio Inc., Otsu, Japan). All oligonucleotide primers were designed using web-based Primer3 software (http://primer3.wi.mit.edu/). The sequences of primers are shown in Table 1. The PCR reactions were carried out in a 10 µl volume using Ex TaqR Hot Start version containing SYBR-Green I (Takara Bio) and performed with a chrome4 real-time PCR system (Bio-Rad, Hercules, CA, U.S.A.) using the following conditions: 95○C for 30 sec; followed by 40 cycles of 95○C for 5 sec, 60○C for 30 sec and then a dissociation protocol. The relative expression level of each target mRNA was calculated using the 2-ΔΔCT method in which all values were normalized to beta actin.
Table 1. Sequences of primers for real-time PCR.
| Target of gene | Primer sequence | |
|---|---|---|
| CYP19A1 | Forward: | AGCCAAGAGCAACAAGCAT |
| Reverse: | TGCATTTTTCCACGGTTACA | |
| 17β-HSD3 | Forward: | GTGATCACTGGAGCAGGAGATG |
| Reverse: | ATGGCCTGAAGTTTTTCCAGTG | |
| 17CYPA1 | Forward: | CATATTCCCTGCGCTGAAGATT |
| Reverse: | ATGGAGTCGCTGGTGAAGTTCT | |
| LHCGR | Forward: | AGGCATTCCGAAGGGATTTCT |
| Reverse: | GGGAGGGCTTATTTGATCCAG | |
| 3HSDB | Forward: | CGGCATCCTGACCAATTACT |
| Reverse: | TTTGGTGTGGTGTGTCGTCT | |
| P450scc | Forward: | AGACCCTGCCTTCTTCTCCAA |
| Reverse: | CGGAGTCAGGATGAGGTTGAA | |
| ACTB | Forward: | CTGCGGCATTCACGAAACTA |
| Reverse: | ATGCCAGGGTACATGGTGGT |
Statistical analysis
The data are presented as means ± SEM. Statistical analysis was performed with Student’s t-test, and one-way ANOVA followed by conservative Tukey’s test using GraphPad prism 5.01 (Graphpad Software Inc., San Deigo, CA, U.S.A.). A value of P<0.05 was used as an indication of statistical significance.
RESULTS
Estrogen-producing enzymes mRNA expression in male goat GI tract
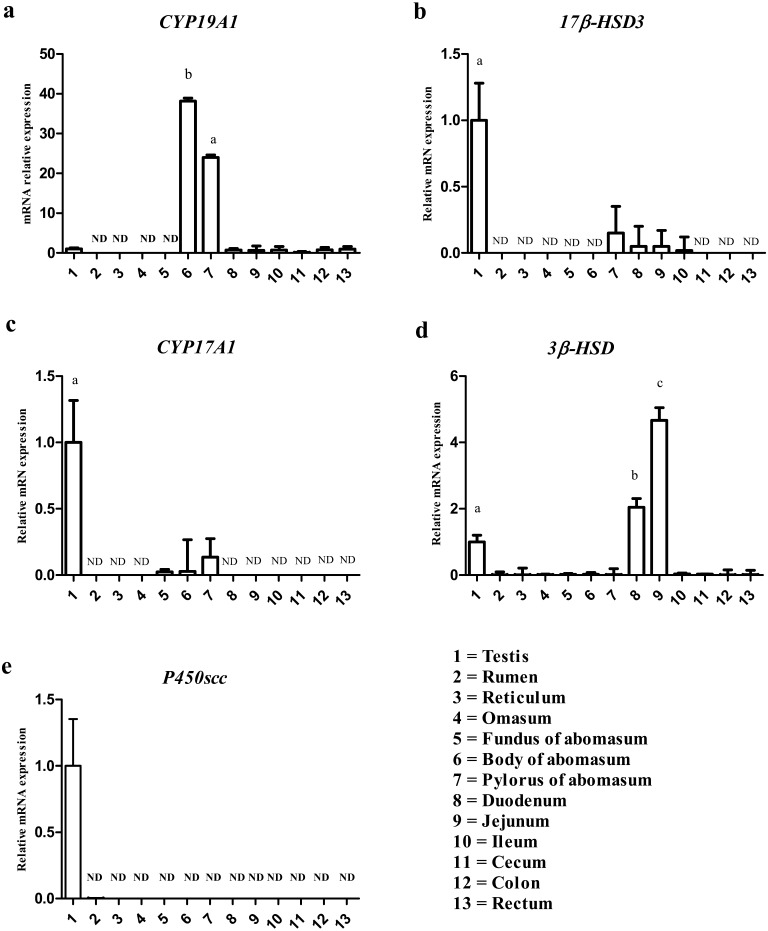
To explore which site of the male goat GI tract expresses steroidogenic enzymes involved in estrogen production (P450scc, 3β-HSD, CYP17A1, 17β-HSD3 and CYP19A1), we quantified their mRNA expression using real-time PCR (Fig. 1). CYP19A1 expression was detected in body and pyloric regions of the abomasum, testis, duodenum, jejunum, ileum, cecum, colon and rectum. CYP19A1 expression was significantly higher in the body and pylorus of the abomasum than testis. CYP19A1 was not detected in rumen, reticulum, omasum and fundus of abomasum (Fig. 1a). 17β-HSD3 mRNA was detected in testis, pyloric regions of abomasum, duodenum, jejunum and ileum. 17β-HSD3 expression in pyloric regions of abomasum, duodenum, jejunum and ileum was weaker in comparison with the testis. 17β-HSD3 expression was not detected in other parts of GI tract (Fig. 1b). CYP17A1 mRNA was only detected in testis, and fundus, body and pyloric regions of the abomasum. CYP17A1 mRNA expression was lower in the abomasum regions in comparison with testis (Fig. 1c). 3β-HSD expression was detected mainly in duodenum, jejunum and testis. In addition, 3β-HSD mRNA was detected only very weakly in other parts, while it was significantly higher in jejunum than in testis (Fig. 1d). P450scc expression was detected in testis while P450scc was not detected in GI tract organs.
Fig. 1.
The expressions of steroidogenic enzymes to produce estrogens in the goat male GI tract. CYP19A1 expression (Fig. 1a), 17β-HSD3 expression (Fig. 1b), 17CYPA1 expression, (Fig. 1c) 3β-HSD expression (Fig. 1d) and P450scc expression (Fig. 1e). The numbers indicate organ tissues quantified by real time PCR, as follows: 1=Testis, 2=Rumen, 3=Reticulum, 4=Omasum, 5=Fundus of abomasum, 6=Body of abomasum, 7=Pylorus of abomasum, 8=Duodenum, 9=Jejunum, 10=Ileum, 11=Cecum, 12=Colon, 13=Rectum. ND presents non detectable, a=significantly different (P<0.05), b=significantly different (P<0.01), c=significantly different (P<0.001).
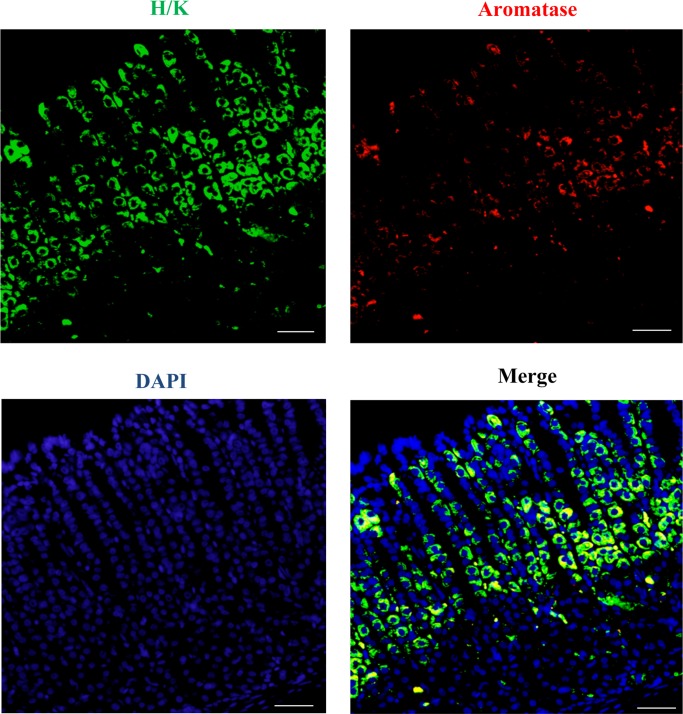
Co-localization of aromatase and H/K ATPase in abomasal gastric mucosa
Based on the real-time PCR results, abomasum was the main organ in the GI tract for aromatase mRNA expression. As a gastric mucosa is composed of different cell types, a double-staining was performed in order to ascertain which cells of the abomasum are responsible for synthesis of CYP19A1. It is known that parietal cells are characterized by H/K ATPase pump expression (Fig. 2). The green color represents the immunostained cells against H/K ATPase antibody, the red color shows the immunostained cells with anti-aromatase antibody, and the yellow color indicates the co-localization of H/K ATPase and aromatase. The majority of cells that stained for H/K ATPase expression co-expressed aromatase at the base of the gastric glands, while a few cells for H/K ATPase expression also co-expressed aromatase in the neck region of the gastric glands. Double immunofluorescence results showed that aromatase immunoreactivities were restrictedly localized in parietal cells.
Fig. 2.
Immunofluorescent images of H/K ATPase (green) and aromatase (red) in abomasum. The yellow color indicated co-localization of aromatase and H/K ATPase in abomasum. The cell nuclei were stained with DAPI (blue). Bar=50 µm.
The effect of castration on estrogen biosynthetic enzyme and LHCGR gene expression in abomasum
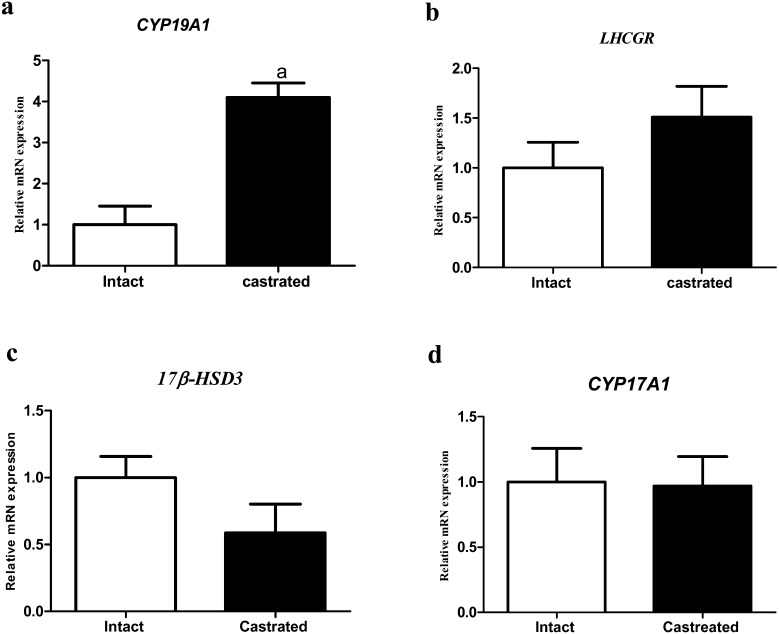
CYP19A1, LHCGR, 17β-HSD3 and CYP17A1 mRNA levels in abomasum were quantified in both intact and castrated groups by real-time PCR (Fig. 3). CYP19A1 mRNA expression was increased in the castrated group significantly (Fig. 3a). LHCGR mRNA expression appeared to increase in the castrated group (P=0.13; Fig. 3b). 17β-HSD3 expression tended to decrease in the castrated group (P=0.08; Fig. 3c). CYP17A1 mRNA expression did not change in the intact or castrated groups (Fig. 3d).
Fig. 3.
The effect of castration on CYP19A1, LHCGR, 17β-HSD3 and CYP17A1 mRNA levels in abomasum. CYP19A1 expression increased in castrated group significantly (Fig. 3a). LHCGR increased by castration (P=0.13; Fig. 3b). 17β-HSD3 expression was decreased in castrated group (P=0.08; Fig. 3c). CYP17A1 expression did not change between both groups (Fig. 3d). All data are presented as the mean ± SEM. a=significantly different (P<0.05).
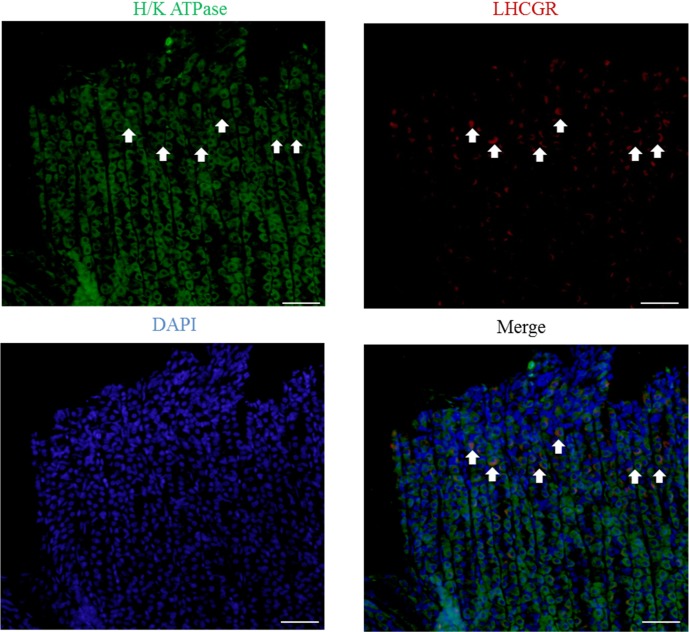
Co-localization of H/K ATPase and LHR in abomasal gastric mucosa
To reveal which cells of the abomasum were responsible for LHR expression in the abomasum, a double-staining was performed (Fig. 4). The green color represents the immunostained cells with H/K ATPase antibody, and the red color shows the immunostained cells with LHR antibody. The arrow indicates cells that co-localized both H/K ATPase and aromatase. Based on double-staining results, a portion of the cells positive for H/K ATPase in the neck of the gastric glands also co-expressed LHR.
Fig. 4.
Immunofluorescent images of H/K ATPase (green) and LHR (red) in abomasum. The arrow indicates co-localization of aromatase and H/K ATPase in abomasum. The cell nuclei were stained with DAPI (blue). Bar=50 µm.
DISCUSSION
This is the first report showing steroidogenic enzymes expression for estrogen biosynthesis in the male goat GI tract. The present study was also conducted to reveal the primary organ for steroidogenic enzymes that produce estrogens in the male goat GI tract, and the effect of castration. Our findings suggested that CYP19A1, 17β-HSD3 and CYP17A1 gene expression was higher in abomasum than in other parts of the GI tract. In addition, immunohistochemical results showed that parietal cells are responsible for aromatase (needed for E2 production) in the abomasum. Based on these data, the abomasum is the main organ for steroidogenic enzymes that produce estrogens in the male GI tract. In addition, castration increased aromatase expression in the abomasum.
CYP19A1 mRNA expression was quantified using real-time PCR in the GI tract. CYP19A1 mRNA expression was significantly higher in the abomasum than in other parts of male GI tract. Gastric mucosa consists of multiple cell types such as parietal cells that secrete hydrochloric acid and intrinsic factor, chief cells that secrete pepsinogen and surface epithelial cells that secrete mucus and bicarbonate [3]. We further identified cell types responsible for aromatase expression for E2 biosysnthesis in abomasum gastric mucosa. It is well known that parietal cells are characterized by H/K ATPase pump expression in the stomach [16], and our findings showed that parietal cells expressed aromatase in the male goat GI tract. Studies in rats and humans non-tumoral or tumoral stomach showed that the stomach expressed aromatase mRNA and gastric parietal cells produce aromatase in accordance with our findings [7, 12, 23]. Estrogens are known to be produced by extragonadal tissues, such as adrenal gland and adipose tissues. Estrogens exert their functions via their receptors. In addition, studies revealed presence of estrogen receptors (ERs) not only in the reproductive organs, but also in non-reproductive tissues, such as liver. It is known that estrogen involved in the process of liver regeneration through ERs [22]. Therefore, abomasal estrogen may play important roles in liver functions. Moreover, Pfaffl et al. (2003) reported all parts of bovine GI tract including abomasum expressed mRNA of both estrogen receptors, ERα and ERβ [14]. Furthermore, ERα and ERβ were localized in the parietal cells [5]. Abomasal estrogen may play important roles via autocrine or paracrine in abomasum, such as acid secretion and gut functions.
Extragonadal tissues are known to produce significant amounts of E2; for example, adipose tissue [15, 28], brain [17], skin [9] and vascular smooth muscle cells [6]. Local estrogen in these sites has numerous physiological functions, such as cell proliferation and regeneration. Moreover, estrogen biosynthesis in these sites is dependent on circulating precursor C19 steroid [19, 20, 26]. We further examined whether the GI tract can produce C19 steroids by its own tissues or whether it requires circulating C19. 17β-HSD3 was highly expressed in testis, whereas weak 17β-HSD3 was detected mainly in abomasum and small intestine (duodenum, jejunum and ileum). It is known that17β-HSD3 is responsible for converting androstenedione to testosterone (T) in testis or estrone to E2 in the stomach [24, 27]. Therefore, for estrogen production in the abomasum, small amounts of androstenedione might be converted to T and E2 by 17β-HSD3 and CYP19A1, respectively, or androstenedione might be converted to estrone and E2 by CYP19A1 and 17β-HSD3, respectively. In addition, abomasum also might be able to convert circulating androgens to estrogen by aromatase in abomasum. CYP17A1 was detected in abomasum, yet the expression level was lower than in testis. Progesterone and 17α-progesterone are converted to 17α-progesterone and androstenedione by CYP17A1 [23], and the expression of CYP17A1 was weak in abomasum, indicating that abomasum utilized little progesterone for steroid production. A study in rat showed that strong 17β-HSD3 staining was detected in stomach [22]. Strong expression of CYP19A1 in abomasum and weak expression of 17β-HSD3 and CYP17A1 in abomasum suggested that abomasal parietal cells may need circulating T for estrogen production, while the abomasum might be able to convert small amounts of progesterone and androstenedione for estrogen production. In the male goat GI, the abomasum may mainly utilize circulating testosterone and androstenedione for estrogen production.
We further investigated whether the GI tract can use cholesterol as a substrate for steroidogenic enzymes that produce estrogens. P450scc and 3β-HSD expression was examined in GI tract. P450scc was not detectable in abomasum and other parts of the GI tract. This suggested that all portions of GI tract cannot convert cholesterol for steroidogenesis. On the other hand, 3β-HSD expression was detected mainly in duodenum and jejunum, so that circulating pregnenolone may be converted to progesterone. Progesterone could be a source for further steroidogenesis in the GI tract or other organs. Studies in the rat showed that P450scc and 3β-HSD expression was not detectable in GI tract. Therefore, rat GI tract cannot utilize cholesterol as a substrate [23].
A final study was conducted in order to ascertain whether castration can influence steroidogenic enzyme expressions in abomasum. Our findings showed that aromatase mRNA expression in the abomasum was increased by castration. 17β-HSD3 expression appeared to decrease, and CYP17A1 expression did not change in abomasum. In addition, we showed the presence of LHR in abomasum, and LHCGR mRNA expression tended to increase with castration. A study in sheep showed that abomasum also expressed LHCGR mRNA [24]. In our study, LHR expression in abomasum appeared to increase in the castrated group. Since castration should lead to increment of circulating LH, and LH bind`s to their receptors, resulting in increased intracellular cyclic adenosine monophosphate (cAMP) concentration. Cyclic AMP activates gene transcription by binding to cAMP responsive elements present on the promoters of several genes, including aromatase [2, 18]. These collective data suggest that LH may increase aromatase expression in the male goat abomasum.
In conclusion, the abomasum was the main organ in the male goat GI tract for steroidogenic enzymes needed for estrogen production. Abomasal parietal cells also expressed aromatase and LHR. In addition, LH may be involved in this aromatase expression.
Acknowledgments
We express our gratitude to Dr. Reinhold J. Hutz (Department of Biological Science, University of Wisconsin-Milwaukee, Milwaukee, WI, U.S.A.) for reading the original manuscript and for valuable suggestions. We are grateful to Dr. Nobuyuki Shirasawa (Department of Anatomy and Structural Science, Yamagata University, Faculty of Medicine, Japan) for giving effective suggestions.
REFERENCES
- 1.Albrecht E. D., Pepe G. J.1990. Placental steroid hormone biosynthesis in primate pregnancy. Endocr. Rev. 11: 124–150. doi: 10.1210/edrv-11-1-124 [DOI] [PubMed] [Google Scholar]
- 2.Araki K., Arai K. Y., Watanabe G., Taya K.2000. Involvement of inhibin in the regulation of follicle-stimulating hormone secretion in the young adult male Shiba goat. J. Androl. 21: 558–565. [PubMed] [Google Scholar]
- 3.Beauchamp R. D., Barnard J. A., McCutchen C. M., Cherner J. A., Coffey R. J. R., Jr.1989. Localization of transforming growth factor alpha and its receptor in gastric mucosal cells. Implications for a regulatory role in acid secretion and mucosal renewal. J. Clin. Invest. 84: 1017–1023. doi: 10.1172/JCI114223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bulun S. E., Rosenthal I. M., Brodie A. M., Inkster S. E., Zeller W. P., DiGeorge A. M., Frasier S. D., Kilgore M. W., Simpson E. R.1993. Use of tissue-specific promoters in the regulation of aromatase cytochrome P450 gene expression in human testicular and ovarian sex cord tumors, as well as in normal fetal and adult gonads. J. Clin. Endocrinol. Metab. 77: 1616–1621. [DOI] [PubMed] [Google Scholar]
- 5.Campbell-Thompson M., Reyher K. K., Wilkinson L. B.2001. Immunolocalization of estrogen receptor alpha and beta in gastric epithelium and enteric neurons. J. Endocrinol. 171: 65–73. doi: 10.1677/joe.0.1710065 [DOI] [PubMed] [Google Scholar]
- 6.Harada N., Sasano H., Murakami H., Ohkuma T., Nagura H., Takagi Y.1999. Localized expression of aromatase in human vascular tissues. Circ. Res. 84: 1285–1291. doi: 10.1161/01.RES.84.11.1285 [DOI] [PubMed] [Google Scholar]
- 7.Izawa M., Inoue M., Osaki M., Ito H., Harada T., Terakawa N., Ikeguchi M.2008. Cytochrome P450 aromatase gene (CYP19) expression in gastric cancer. Gastric Cancer 11: 103–110. doi: 10.1007/s10120-008-0463-x [DOI] [PubMed] [Google Scholar]
- 8.Kandiel M. M., Watanabe G., Taya K.2010. Ovarian expression of inhibin-subunits, 3β-hydroxysteroid dehydrogenase, and cytochrome P450 aromatase during the estrous cycle and pregnancy of shiba goats (Capra hircus). Exp. Anim. 59: 605–614. doi: 10.1538/expanim.59.605 [DOI] [PubMed] [Google Scholar]
- 9.Leshin M., Baron J., George F. W., Wilson J. D.1981. Increased estrogen formation and aromatase activity in fibroblasts cultured from the skin of chickens with the Henny feathering trait. J. Biol. Chem. 256: 4341–4344. [PubMed] [Google Scholar]
- 10.Mahendroo M. S., Mendelson C. R., Simpson E. R.1993. Tissue-specific and hormonally controlled alternative promoters regulate aromatase cytochrome P450 gene expression in human adipose tissue. J. Biol. Chem. 268: 19463–19470. [PubMed] [Google Scholar]
- 11.McNatty K. P., Makris A., DeGrazia C., Osathanondh R., Ryan K. J.1979. The production of progesterone, androgens, and estrogens by granulosa cells, thecal tissue, and stromal tissue from human ovaries in vitro. J. Clin. Endocrinol. Metab. 49: 687–699. doi: 10.1210/jcem-49-5-687 [DOI] [PubMed] [Google Scholar]
- 12.Ozawa M., Takahashi K., Akazawa K. H., Takashima T., Nagata H., Doi H., Hosoya T., Wada Y., Cui Y., Kataoka Y., Watanabe Y.2011. PET of aromatase in gastric parietal cells using 11C-vorozole. J. Nucl. Med. 52: 1964–1969. doi: 10.2967/jnumed.110.087072 [DOI] [PubMed] [Google Scholar]
- 13.Payne A. H., Hales D. B.2004. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 25: 947–970. doi: 10.1210/er.2003-0030 [DOI] [PubMed] [Google Scholar]
- 14.Pfaffl M. W., Lange I. G., Meyer H. H.2003. The gastrointestinal tract as target of steroid hormone action: quantification of steroid receptor mRNA expression (AR, ERalpha, ERbeta and PR) in 10 bovine gastrointestinal tract compartments by kinetic RT-PCR. J. Steroid Biochem. Mol. Biol. 84: 159–166. doi: 10.1016/S0960-0760(03)00025-6 [DOI] [PubMed] [Google Scholar]
- 15.Polari L., Yatkin E., Martínez Chacón M. G., Ahotupa M., Smeds A., Strauss L., Zhang F., Poutanen M., Saarinen N., Mäkelä S. I.2015. Weight gain and inflammation regulate aromatase expression in male adipose tissue, as evidenced by reporter gene activity. Mol. Cell. Endocrinol. 412: 123–130. doi: 10.1016/j.mce.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 16.Prinz C., Kajimura M., Scott D., Helander H., Shin J., Besancon M., Bamberg K., Hersey S., Sachs G.1992. Acid secretion and the H,K ATPase of stomach. Yale J. Biol. Med. 65: 577–596. [PMC free article] [PubMed] [Google Scholar]
- 17.Roselli C. E., Abdelgadir S. E., Resko J. A.1997. Regulation of aromatase gene expression in the adult rat brain. Brain Res. Bull. 44: 351–357. doi: 10.1016/S0361-9230(97)00214-1 [DOI] [PubMed] [Google Scholar]
- 18.Shayu D., Rao A. J.2006. Expression of functional aromatase in the epididymis: role of androgens and LH in modulation of expression and activity. Mol. Cell. Endocrinol. 249: 40–50. doi: 10.1016/j.mce.2006.01.016 [DOI] [PubMed] [Google Scholar]
- 19.Simpson E., Rubin G., Clyne C., Robertson K., O’Donnell L., Davis S., Jones M.1999. Local estrogen biosynthesis in males and females. Endocr. Relat. Cancer 6: 131–137. doi: 10.1677/erc.0.0060131 [DOI] [PubMed] [Google Scholar]
- 20.Simpson E. R., Davis S. R.2001. Minireview: aromatase and the regulation of estrogen biosynthesis--some new perspectives. Endocrinology 142: 4589–4594. doi: 10.1210/endo.142.11.8547 [DOI] [PubMed] [Google Scholar]
- 21.Tuckey R. C.2005. Progesterone synthesis by the human placenta. Placenta 26: 273–281. doi: 10.1016/j.placenta.2004.06.012 [DOI] [PubMed] [Google Scholar]
- 22.Ueyama T., Shirasawa N., Numazawa M., Yamada K., Shelangouski M., Ito T., Tsuruo Y.2002. Gastric parietal cells: potent endocrine role in secreting estrogen as a possible regulator of gastro-hepatic axis. Endocrinology 143: 3162–3170. doi: 10.1210/endo.143.8.8974 [DOI] [PubMed] [Google Scholar]
- 23.Ueyama T., Shirasawa N., Ito T., Tsuruo Y.2004. Estrogen-producing steroidogenic pathways in parietal cells of the rat gastric mucosa. Life Sci. 74: 2327–2337. doi: 10.1016/j.lfs.2003.10.005 [DOI] [PubMed] [Google Scholar]
- 24.Wang L. H., Zhang W., Gao Q. X., Wang F.2012. Expression of the luteinizing hormone receptor (LHR) gene in ovine non-gonadal tissues during estrous cycle. Genet. Mol. Res. 11: 3766–3780. doi: 10.4238/2012.October.15.8 [DOI] [PubMed] [Google Scholar]
- 25.Weng Q., Medan M. S., Ren L., Watanabe G., Tsubota T., Taya K.2005. Immunolocalization of steroidogenic enzymes in the fetal, neonatal and adult testis of the Shiba goat. Exp. Anim. 54: 451–454. doi: 10.1538/expanim.54.451 [DOI] [PubMed] [Google Scholar]
- 26.Yue W., Wang J. P., Hamilton C. J., Demers L. M., Santen R. J.1998. In situ aromatization enhances breast tumor estradiol levels and cellular proliferation. Cancer Res. 58: 927–932. [PubMed] [Google Scholar]
- 27.Zhang Y., Word R., Fesmire S., Carr B. R., Rainey W. E.1996. Human ovarian expression of 17 beta-hydroxysteroid dehydrogenase types 1, 2, and 3. J. Clin. Endocrinol. Metab. 81: 3594–3598. [DOI] [PubMed] [Google Scholar]
- 28.Zhao Y., Nichols J. E., Bulun S. E., Mendelson C. R., Simpson E. R.1995. Aromatase P450 gene expression in human adipose tissue. Role of a Jak/STAT pathway in regulation of the adipose-specific promoter. J. Biol. Chem. 270: 16449–16457. doi: 10.1074/jbc.270.27.16449 [DOI] [PubMed] [Google Scholar]