Abstract
Deletion of the chromosome 5q [del(5q)] is one of the most common cytogenetic abnormalities observed in patients with de novo myelodysplastic syndromes (MDS) and therapy-related MDS or acute myeloid leukemia (t-MDS/tAML). Emerging evidence indicates that activation of the Wnt/β-catenin pathway contributes to the development of myeloid neoplasms with del(5q). Whether β-catenin is a potential therapeutic target for myeloid neoplasms with del(5q) has yet to be evaluated. Here we report that genetic deletion of a single allele of β-catenin rescues ineffective hematopoiesis in an Apc haploinsufficient mouse model, which recapitulates several characteristic features of the pre-leukemic stage of myeloid neoplasms with a -5/del(5q). In addition, loss of a single allele of β-catenin reversed the defective self-renewal capacity of Apc-haploinsufficient hematopoietic stem cells (HSC) and reduced the frequency of apoptosis induced by Apc haploinsufficiency. Suppression of β-catenin by indomethacin or β-catenin shRNA reduced proliferation and survival of human leukemia cell lines with del(5q) but not of control leukemia cell lines in vitro; β-catenin inactivation also inhibited leukemia progression in vivo in xenograft mice reconstituted with del(5q) leukemia cell lines. Inhibition of β-catenin also stunted growth and colony-forming abilities of primary bone marrow cells from del(5q) AML patients in vitro. Overall, our data support the idea that β-catenin could serve as a therapeutic target for the treatment of myeloid neoplasms with del(5q).
Keywords: β-catenin, Apc, MDS, AML, del(5q)
Introduction
Chromosome loss or deletions are common cytogenetic abnormalities in myeloid neoplasms. Deletion of the chromosome 5q (del 5q) is a recurring cytogenetic abnormality present in around 15% of de novo and more than 40% of therapy-related myelodysplastic syndrome or acute myeloid leukemia (t-MDS/t-AML) patients(1). There are two chromosomal regions located proximally at 5q33 or 5q31 on chromosome 5 in myeloid neoplasms(2). MDS patients with an isolated deletion of 5q are defined as having the 5q- syndrome, which is associated with loss of the 5q33 region. Patients with the 5q- syndrome are characterized by severe macrocytic anemia, normal or elevated platelet count, normal or slightly decreased neutrophil count, and a low rate of progression of AML(2,3). However, most advanced MDS/AML or t-MDS/t-AML patients have a large deletion of 5q that contains both loci. These patients often have additional cytogenetic abnormalities and have a poor prognosis(2,4). Deletions of chromosome 5q in MDS are somatically acquired, universally heterozygous and encompass multiple genes(2). Extensive sequencing has failed to identify other gene mutations on the remaining intact allele or uniparental disomy on 5q patients with MDS(5) Emerging data suggest that haplo-insufficiency of multiple genes including RPS14(6), miR-145&miR-146a(7), EGR1(8), CTNNA1(9), APC(10), NPM1(11), and CSNK1a(12), and TIFAB(13) could contribute to the development of the different phenotypes of del(5q) myeloid neoplasms(2).
The APC gene, which is located on 5q23, is deleted in more than 95% of cases with del (5q) MDS. APC is a negative regulator of the Wnt/β-catenin pathway by controlling β-catenin degradation (14,15). In a previous study of ours, we found that the loss of a single allele of Apc affects the function of HSCs and hematopoietic progenitor cells (HPCs) leading to an MDS-like syndrome in the mouse(10). Consistent with these findings, Gilliland and his colleagues found that mice with the Apc(min) allele that results in loss of function of Apc developed an MDS/myeloproliferative phenotype(16). Of interest, Apc haplo-insufficiency cooperates with Tp53 loss and Egr1 heterozygous deletion to induce AML(17). CSNK1a is also located on chromosome 5q and regulates the Wnt signaling pathway by controlling the stability of β-catenin. Most recently, Ebert et al. have shown that haplo-insufficiency of Csnk1a1 or heterozygous deletion of both Apc and Csnk1a1 in mice leads to HSC expansion via the β-catenin pathway. Together, these data are indicative of the critical role of activation of the Wnt/β-catenin pathway in the development of myeloid neoplasms with del(5q).
We have shown that APC regulates the function of hematopoietic stem/progenitor cells largely through a β-catenin dependent mechanism(18). In the present study, we have extended our efforts to evaluate the possibility that targeting β-catenin affords a potential new therapeutic approach for the treatment of del(5q) patients. We demonstrate that reduction of β-catenin by heterozygous deletion of β-catenin rescues abnormal hematopoiesis in the Apc haplo-insufficient mouse model. In addition, we show that inhibition of β-catenin by indomethacin or β-catenin-specific shRNAs reduces growth and/or survival of human leukemia cell lines and patient primary bone marrow cells with del(5q).
Methods
Mice and blood cell counts
Apc fl/fl Mx1-cre mice were crossed with β-cateninfl/fl transgenic mice (The Jackson Laboratory) to obtain Mx1-cre Apcfl/+β-cateninfl/+ , Mx1-creApcfl/+, Mx1-cre β-cateninfl/+. Deletion of Apc or β-catenin was induced by 3 doses of intraperitoneal (i.p.) injections of 6-10 μg polyI–polyC (pI-pC; GE Healthcare) per gram of body weight, and was confirmed by polymerase chain reaction (PCR) analysis as previously described (10,18).
Xenograft models were generated by injecting MDSL and UoCM1 cells (1 × 106) into the tail veins of 6-8 weeks old sub-lethally irradiated (2.5 Gy) NSG-hSCF/hGM-CSF/hIL3 (NSGS) mice (The Jackson Laboratory) as previously described(19). In vivo delivery of indomethacin (Sigma, St.Louis, MO, USA) was adapted from previous reports(20). Briefly, indomethacin was diluted in DMSO to a 1 mg/ul stock and further dissolved in sterile phosphate buffered saline (PBS; pH 7.2). Animals were given ip injections of indomethacin (4 mg/kg) every day for 14 days one day after transplantation of cell lines. DMSO was injected as a negative control. When the mice became moribund, bone marrow (BM), spleen (SP) and peripheral blood (PB) from all mice were collected and analyzed for engraftment of leukemia cells by flow cytometry using anti-human-CD45 and anti-mouse-CD45 (eBioscience). Complete blood counts (CBCs) and differentials were obtained with a Hemavet 950FS (Drew Scientific). All animal research was approved by the University of Illinois at Chicago Institutional Animal Care and Use Committee.
Flow cytometric analysis
Single-cell suspensions were prepared from BM (femurs and tibiae), SP, thymus and PB. Flow cytometric analyses of subsets of HPCs and HSCs have been described in our previous studies(21,22). Dead cells were excluded by DAPI staining. FACS analysis was performed using a CyAn ADP flow cytometer (Beckman Coulter). For erythoid differentiation assays, BM and SP cells were stained with anti-Ter119 and anti-CD71 (eBioscience). For the detection of apoptosis, BM cells were stained with antibody conjugates of Annexin V and DAPI (BD Biosciences). For the detection of cell cycle progression, cells were labeled with 30ug/ml BrdU, followed by fixation and permeabilization with Cytofix/Cytoperm (BD Biosciences), treatment with DNase I (Sigma), and staining with a BrdU-specific antibody according to the instruction manual of the BrdU flow kit (BD Pharmingen). All data were analyzed by FlowJo software (TreeStar, Inc).
Cell culture and patient samples
The 293T, KG1, Kasumi1, Kasumi3 and MV4-11 were originally obtained from ATCC and were cultured according to ATCC. 293T, KG1, Kusumi1 and MV4-11 cell lines were obtained from ATCC in 2009 while Kasumi3 was obtained from ATCC in 2013. These cell lines were expanded and cryogenically frozen upon acquisition to establish stocks that were stored in liquid nitrogen until use. The cell lines were cultured within in 3-6 months after resuscitation. The MDSL cell line, which was originally established by Dr. Tohyama's laboratory (Kawasaki Medical School, Japan), was kindly provided by Dr. Daniel Starczynowski (University of Cincinnati College of Medicine) in 2014. The MDSL cell line was authenticated by morphology check and in vivo growth as its ability to induce MDS-like disease in xenograft mice in vivo by Dr. Starczynowski's lab in 2014 as described (19). UocM1 was obtained from Dr. Michelle Le Beau's laboratory (University of Chicago)(23) in 2010. We authenticated this cell line by morphology check and growth curve analysis, tested free of mycoplasma and quantitative PCR analysis of the expression of genes including APC and Erg1 that are located on 5q31-32 regions. The patient samples were collected at the University of Illinois at Chicago Medical Center. This study was approved by the ethics committees at the University of Illinois at Chicago, and informed consent was obtained from each patient. The study protocols were in accordance with the Declaration of Helsinki. For human cell studies, frozen AML samples were thawed and cultured as described previously(24).
Primary BM cells from AML patients were included based on material availability. All patient samples were collected under University of Illinois Healthcare System Institutional Review Board policies and protocols. Samples of the BM were collected before initiation of chemotherapy. All patients provided written informed consent in accordance with the Declaration of Helsinki. Institutional Review Board approval was obtained at the University of Illinois Medical Center prior to patient enrollment in the clinical trial.
Lentivirus construct and packaging and lentiviral infection
β-catenin shRNAs targeting different specific sequences of β-catenin (shRNA1-1248, shRNA2-2279) expressed by a lentiviral vector were purchased from Addgene, cat. #19761 and 19762. Lentivirus production and cell infection has been described previously(22).
Luciferase reporter assay
The luciferase assay was performed with the Dual-GloTM luciferase assay system (Promega) as previously described(25).
Colony-forming unit (CFU) assay
Human cell lines or human primary AML cells were plated in methylcellulose (04434; Stemcell Technologies). Colony number was counted 7 days after plating.
For the indomethacin treatment experiments, methylceullulose was supplemented with either DMSO or 100μm indomethacin.
Western blotting
Equal amounts of protein were separated by SDS–PAGE and transferred onto PVDF membranes. Anti-β-catenin primary antibodies (BD Biosciences) and anti-tubulin were added and incubated at 4°C overnight with gentle shaking, followed by incubation for 1 hr with peroxidase-conjugated secondary antibodies (Cell Signaling Technology, USA) at room temperature. Immunoreactive bands were visualized using an ECL system (Amersham Pharmacia).
Statistics
In general, results are expressed as the mean ± standard deviation (SD) for triplicate experiments. The Student paired t test was used to compare differences between groups. P values of < 0.05 were regarded as statistically significant.
Results
Genetic deletion of a single allele of β-catenin prevents Apc-haplo-insufficiency induced MDS in mice
To determine whether Apc-haplo-insufficiency-induced MDS is mediated by β-catenin, we crossed β-cateninfl/fl mice with Mx1-Cre+ Apcfl/fl mice to generate cohorts of Mx1-Creβ-cateninfl/+, Mx1-Cre β-cateninfl/+ Apcfl/+, β-cateninfl/+ Apcfl/+, and Mx1-Cre Apcfl/+ mice. The deletion of Apc or β-catenin was induced by pI-pC injection as we previously described(18). We monitored cohorts of mice for up to 8 months by CBC analysis monthly. Consistent with our previous study(10), all Apc heterozygous mice died within 3-8 months due to severe anemia. In contrast, the Apc and β-catenin double heterozygous mutant mice and control mice all survived, and displayed normal red blood cell (RBC) counts and hemoglobin level (Fig. 1A,B).
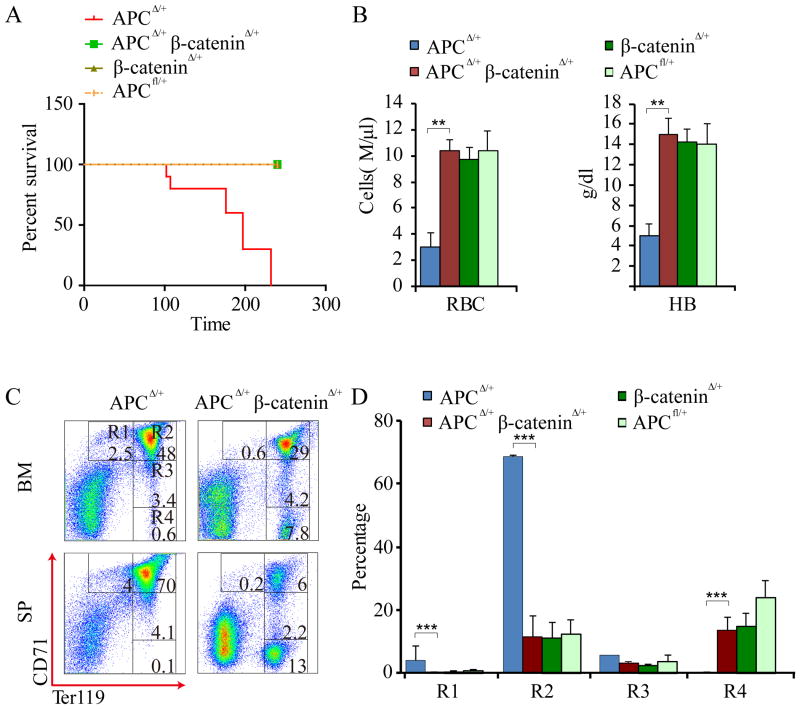
Figure 1. Heterozygous deletion of β-catenin inhibits Apc-haploinsufficiency induced MDS-like disease in mice.
(A) Kaplan-Meier survival curve of Mx1-Cre Apcfl/+, Mx1-Cre Apcfl/+β-cateninfl/+ (n=11) Mx1-Creβ-cateninfl/+, or β-cateninfl/+(n = 5-10) mice after 3 doses of pI-pC injection. P<0.01. (B) The number of red blood cells (RBC) and hemoglobin (Hb) levels in peripheral blood (PB) from the Apc Δ/+ and ApcΔ/+β-cateninΔ/+, β-catenin Δ/+, or β-cateninfl/+ mice (n=6-10). (C) Representative histogram for flow cytometric analysis of erythroblasts in BM and PB from ApcΔ/+ and ApcΔ/+ β-cateninΔ/+ mice. (D) Histograms showing the frequency of erythroblasts in spleen from the indicated mice.
Erythroblasts can be defined by expression of the Ter119 and CD71 cell surface markers. From least to most differentiated, there are 4 cell populations with specific staining characteristics: R1 (Ter119lowCD71hi), R2 (Ter119hiCD71hi), R3 (Ter119hiCD71med), and R4 (Ter119hiCD71low) respectively(26). We previously showed that heterozygous deletion of Apc blocked erythroid lineage differentiation at an early stage(4). We characterized the erythroblasts in the SP (Fig. 1C,D) from Apc+/Δ, Apc+/Δ β-catenin+/Δ and β-catenin+/Δ mice 6 weeks after induction of Apc and β-catenin by pI-pC. Our data showed that heterozygous deletion of β-catenin reversed Apc-loss induced blockage of erythroid lineage differentiation at early R1 and R2 stages.
Genetic deletion of a single allele of β-catenin prevents expansion of Apc heterozygous HSCs and rescues self-renewal capacity of Apc heterozygous HSCs
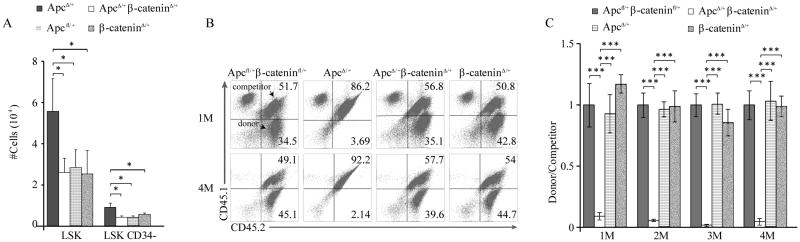
Next, we examined the hematopoietic stem/progenitor populations in Apc+/Δ, Apc+/Δ β-catenin+/Δ and control mice 6 months after induction of Apc and β-catenin by flow cytometric analysis. As shown in Fig. 2A, the number of LSKs and LSKCD34- was significantly lower in Apc+/Δβ-catenin+/Δ mice than the number of these cells in Apc+/Δ while Apc+/Δβ-catenin+/Δ, β-catenin+/Δ and Apcfl/+ had a comparable number of these cells. These results suggest that reduced expression of β-catenin suppresses expansion of Apc-haplo-insufficient stem/progenitor cells. To determine whether heterozygous deletion of β-catenin rescues the functional defects of Apc haplo-insufficient HSC, we performed a competitive repopulation assay. Eight months after induction of Apc and β-catenin deletions, the BM cells from Apc+/Δ, Apc+/Δβ-catenin+/Δ, β-catenin+/Δ and Apcfl/+β-cateninfl/+ were mixed with the same number of BM cells from 3 month-old wild type mice, followed by transplantation into lethally-irradiated recipients. Consistent with our previous results(10), Apc haplo-insufficient hematopoietic stem/progenitor cells had a significantly reduced cell repopulation capacity as compared to Apcfl/+β-cateninfl/+ (control) and β-catenin+/Δ mice (Fig. 2B-C). Notably, Apc+/Δβ-catenin+/Δ mice with heterozygous deletion of both Apc and β-catenin, Apc+/flβ-catenin+/fl mice and β-catenin+/Δ mice had a comparable cell repopulation capacity of HSCs (Fig. 2B-C), indicating that reduced expression of β-catenin rescues the repopulation capacity of Apc haplo-insufficient HSCs in vivo.
Figure 2. Reduced β-catenin expression suppresses expansion and rescues self-renewal capacity of ApcΔ/+ hematopoietic stem/progenitor cells in vivo.
(A) The total number of the LSK (Lin-Sca+c-Kit+) and LSKCD34+ from ApcΔ/+, ApcΔ/+β-cateninΔ/+ , β-cateninΔ/+ and Apcfl/+ mice (mean ± standard deviation [SD], n = 4-6). (B) Flow cytometric analysis of CD45.2+CD45.1− (donor cells) and CD45.1+CD45.2+ (competitor cells) PB cells from representative chimeric mice 1 or 4 months after transplantation. The numbers indicate the percentage of cells in each population. (C) Histogram shows the relative ratio of CD45.2+CD45.1− vs CD45.1+CD45.2+ PB cells in chimeric mice examined at 1, 2, 3 to 4 months after the transplantation (mean ± standard deviation [SD], n = 4-5). The mice were analyzed 6-7 months after pI-pC injection. *, P<0.05; **, P<0.01.
Inhibition of β-catenin by indomethacin suppresses the proliferation of human MDSL, UoCM1 and KG1 cell lines with del(5q)
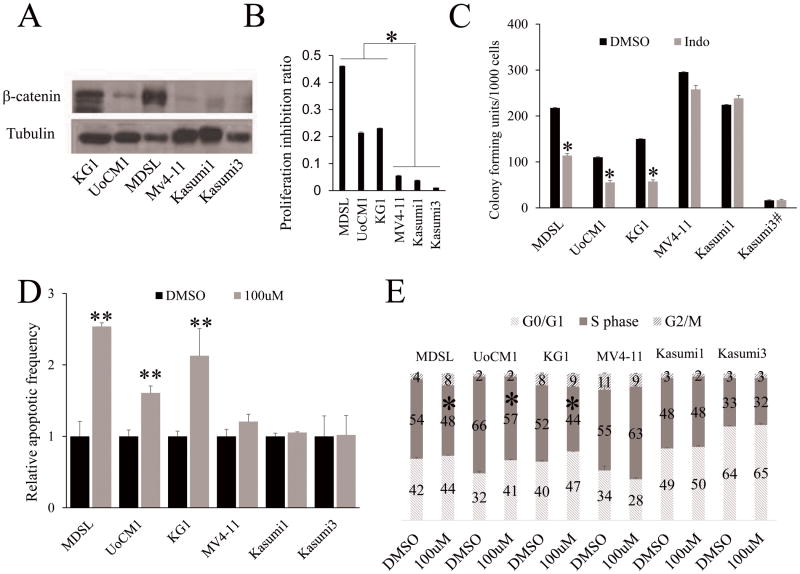
Next, we determined the effects of pharmacological inhibition of β-catenin on human leukemia cell lines derived from patients with myeloid neoplasms with del(5q). The UoCM1 cell line was derived from a patient with de novo AML with a del(5q) (23), while the MDSL line was derived from a MDS patient with del(5q)(27). The KG-1 cell line which was established from a patient with erythroleukemia evolving to AML harbored the deletion of 5q11-q31(28). In agreement with our previous finding that Wnt/β-catenin signaling is activated in both del(5q) MDS patients(29) and in t-MDS/t-AML patients with del(5q)(30), expression of β-catenin was significantly higher in MDSL, UoCM1 and KG1 cell lines than it was in Kasumi1, Kasumi3 and MV4-11 human leukemia cells, which do not have chromosome 5 deletion (Fig 3A). Indomethacin, a COX inhibitor, has been reported to inhibit β-catenin expression in vitro and in vivo(31,32). We confirmed that indomethacin antagonized lithium chloride (LiCl)-induced activation of β-catenin in 293T cells (Supplementary Fig. S1A), and inhibited β-catenin expression in MDSL and UoCM1 cells (Supplementary Fig. S1B), as determined by luciferase assay and Western Blot. We treated these leukemia cell lines with indomethacin. MDSL, UoCM1and KG1 cells grew more slowly than Kasumi1, Kusumi3 and MV4-11 cells did in medium containing indomethacin (Fig. 3B). In addition, indomethacin treatment significantly reduced the colony-forming ability of MDSL, UoCM1 and KG1 but not Kasumi1, Kasumi3 or MV4-11 cells (Fig. 3C). These data suggest that the MDSL, UoCM1 and KG1 cells were much more sensitive to indomethacin treatment as compared to Kasumi1, Kasumi3 and MV4-11 cells. We further analyzed apoptosis and cell cycle status of these cells with treatment of indomethacin. Of interest, β-catenin inhibition by indomethacin induces significant apoptosis and S phase arrest in MDSL, UoCM1 and KG1 cells but not in Kasumi1, Kasumi3 and MV4-11 cells (Fig. 3D-F & Supplementary Fig. S2), indicating that suppression of β-catenin inhibits proliferation and survival of leukemia cells with del(5q) but not leukemia cells without del(5q).
Figure 3. Indomethacin treatment inhibits the proliferation and survival of human MDSL, UoCM1 and KG1 cell lines with a del(5q).
(A) The expression of β-catenin in leukemia cell lines was determined by Western Blot analysis. (B) Analysis of cell growth of leukemia cell lines in presence of DMSO or 100μM indomethacin. Y axis denotes percentage of growth inhibition after treatment of indomethacin for 3 days. (C) The colony-forming ability of leukemia cell lines in methylcellulose containing DMSO or 100 μM indomethacin. # 6000 Kasumi3 cells were plated in methylcellulose medium. (D) Flow cytometric analysis of apoptosis (D) and cell cycle status (E) of leukemia cell lines. All data are representative of two-to-three independent experiments. The cells were treated with 100μM indomethacin or DMSO for 72 hours. *, P<0.05; **, P<0.01; ***, P<0.001.
shRNA against β-catenin induces apoptosis and blocks the cell cycle of human MDSL and UoCM1 cell lines with del(5q)
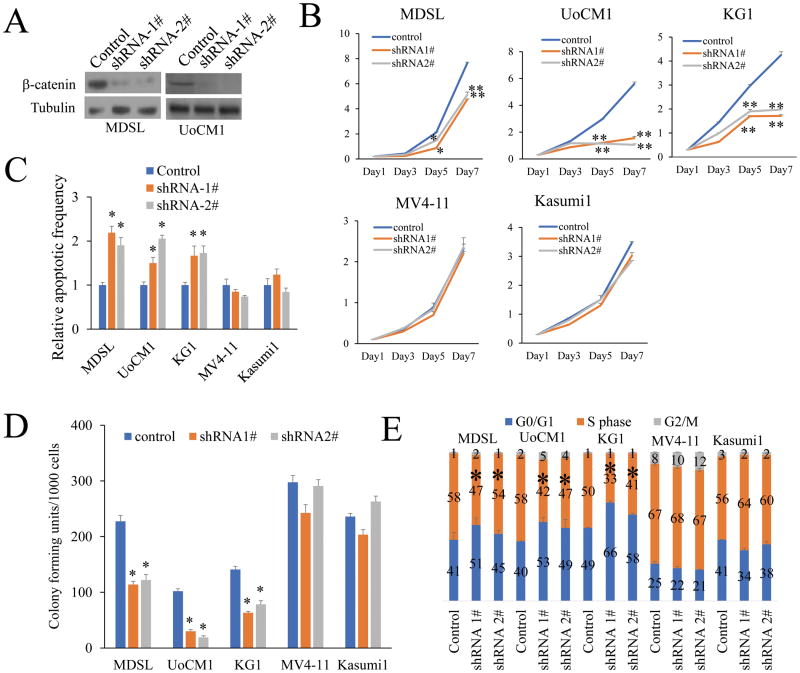
To exclude the possibility that inhibition of MDSL, UoCM1 and KG1 growth is mediated by some nonspecific activity of indomethacin, we determined whether suppression of β-catenin by β-catenin-specific shRNAs inhibits the survival and proliferation of MDSL, UoCM1 and KG1. β-catenin shRNAs targeting different specific sequences of β-catenin (shRNA1, shRNA2) were expressed by a lentiviral vector. As determined by Western blotting (Fig.4A), both β-catenin shRNAs markedly inhibited β-catenin expression, Notably, both β-catenin shRNAs significantly inhibited the growth (Fig. 4B) and colony-forming ability (Fig.4C) of MDSL, UoCM1 and KG1 but not Kasumi1, Kasumi3 and MV4-11 (Fig.4C). In addition, we showed that β-catenin inhibition by shRNAs induced significant apoptosis and S phase arrest in MDSL, UoCM1 and KG1 cells but not Kasumi1, Kasumi3 and MV4-11 cells (Fig. 4D, E & Supplementary Fig. S3).
Figure 4. β-catenin specific shRNAs inhibit the proliferation and survival of human MDSL and UoCM1 cell lines with a del(5q).
(A) Western blot analysis of β-catenin expression in MDSL and UoCM1 cell lines expressing PLKO or PLKO-β-catenin shRNAs. (B) Viable cell growth of leukemia cell lines expressing PLKO or PLKO-β-catenin shRNAs was determined by trypan blue exclusion in presence of DMSO or 100μM indomethacin. (C) The colony-forming ability of leukemia cell lines expressing PLKO or PLKO-β-catenin shRNAs D) Flow cytometric analysis of apoptosis (D) and cell cycle status (E) of leukemia cell lines expressing PLKO or PLKO-β-catenin shRNAs. All data are representative of two-to-three independent experiments. *, P<0.05; ***, P<0.001.
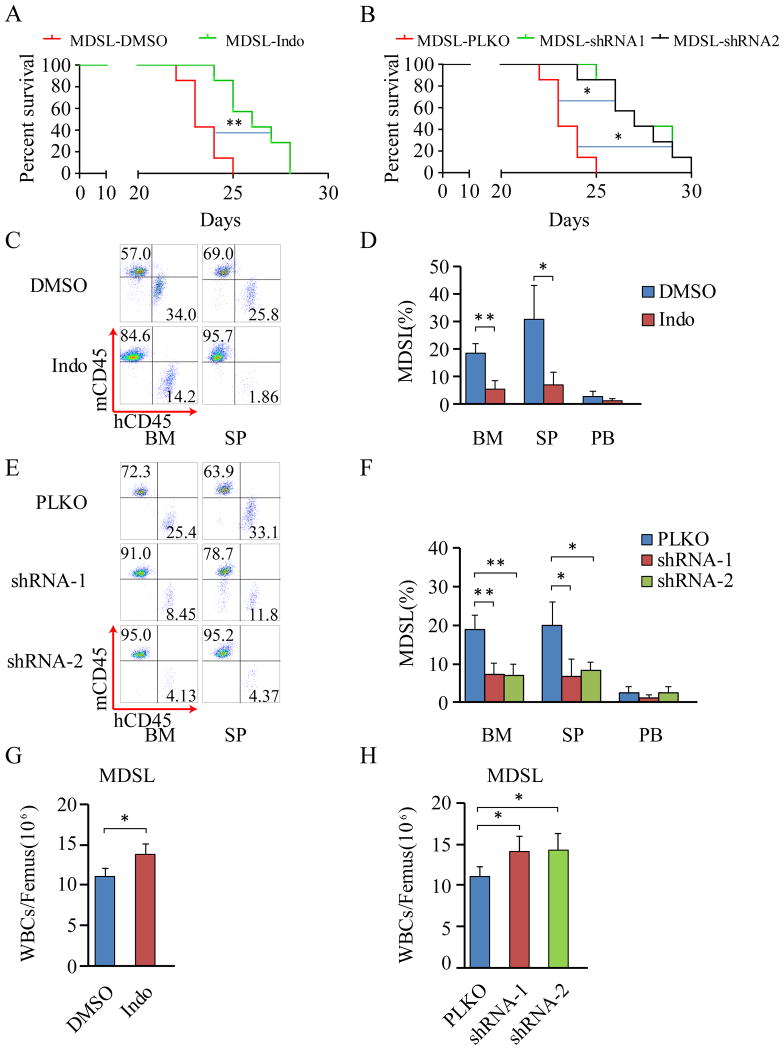
Inhibition of β-catenin by indomethacin or shRNAs prolongs survival of MDSL and UoCM1 xenograft mice and inhibits expansion of leukemia cells in SP and BM
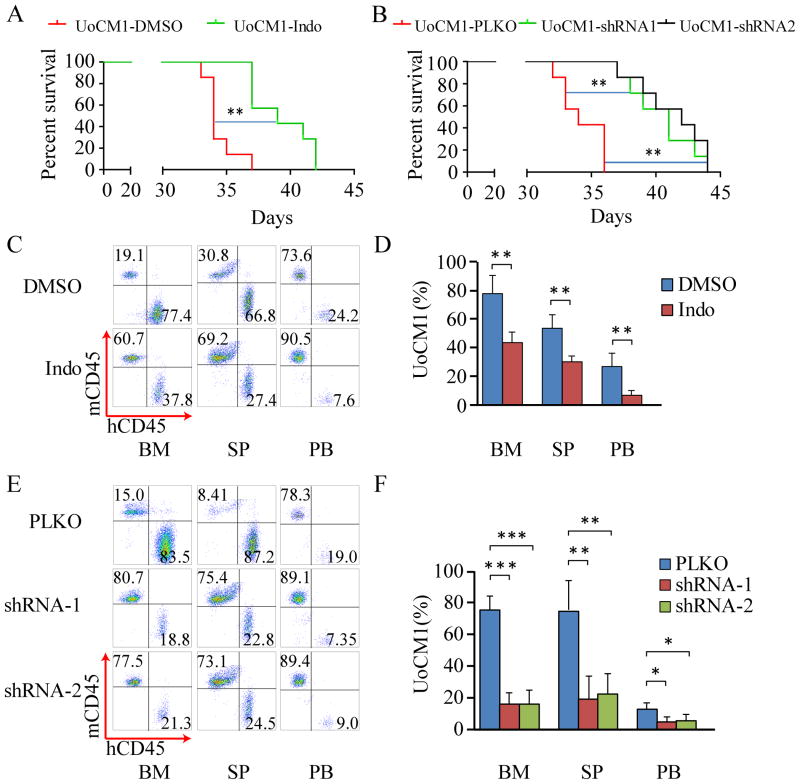
We next determined the effects of β-catenin inhibition on tumorigenic potential of del(5q) leukemia cells in vivo. MDSL cells were derived from an MDS patient in a non-leukemic phase(27,33). A recent report by Rhyasen et al. has shown that MDSL cells have the ability to engraft into NOD/SCID-IL2Rγ mice (NSG) and NSG-hSCF/hGM-CSF/HIL3 (NSGS) mice and initiate development of an MDS-like disease characterized by cytopenias, clonal expansion and suppression of host hematopoietic cells (19). We transplanted MDSL leukemia cells into sub-lethally irradiated NSGS mice and treated the xenografted mice with indomethacin or DMSO the next day after transplantation for 14 days. Similar to the results found by Rhyasen et al. (19), most MDSL xenograft mice without indomethacin treatment died within 25 days post transplantation (Fig. 5A). However, the disease latency of indomethacin-treated xenograft mice (median survival time of 27 days) was significantly prolonged compared to xenograft mice without drug treatment (median survival time of 22 days) (Fig. 5A). In addition, we expressed β-catenin-specific shRNA1, shRNA2 or control vector in MDSL cells, followed by transplantation into sub-lethally irradiated NSGS mice. Most of the MDSL xenograft mice carrying control vector died within 25 days of post transplantation. The disease latency of xenograft mice expressing β-catenin-specific shRNA1 or shRNA2 was prolonged (median survival time of 28 days as compared to the control xenograft mice (median survival time of 23 days) (Fig. 5B). Further, flow cytometric analysis of engrafted MDSL cells in hematopoietic tissues in moribund xenograft mice revealed a significantly reduced number of MDSL cells in BM and SP but not PB in MDSL xenograft mice treated with indomethacin or those mice expressing β-catenin-specific shRNA1 or shRNA2 as compared to control xenograft mice (Fig. 5C-F). Interestingly, indomethacin or β-catenin-specific shRNA treatment increased total white blood cell (WBC) counts in MDSL xenograft mice (Fig. 5G,H), suggesting that β-catenin inhibition reversed cytopenias induced by MDSL cells in recipient mice. To rule out the possibility that the effects of β-catenin inhibition on tumorigenic potential of MDSL cells in vivo are cell line dependent, we next determined the effects of β-catenin inhibition on xenograft mice reconstituted with UoCM1 cells with del(5q). We found that UoCM1 cells were able to engraft and induce disease in xenograft mice. Most UoCM1 xenograft mice survived longer (up to 37 days) as compared to MDSL xenograft mice (Fig. 6A,B). Notably, treatment with indomethacin delayed disease latency of UoCM1 xenograft mice by 5 days (median survival of time 39 days) whereas β-catenin-specific shRNAs delayed disease latency by 7-8 days (median survival of time 41-42 days) as compared to control xenograft mice (median survival of time 34 days) (Fig. 6A, B). In addition, indomethacin treatment and β-catenin specific shRNAs significantly reduced the number of engrafted UoCM1 cells in BM, SP and PB (Fig. 6C-F). Taken together, these results demonstrate that inhibition of β-catenin significantly improves survival of xenograft mice reconstituted with myeloid neoplasm cells with del(5q), and inhibits clonal expansion of del(5q) leukemic cells in vivo.
Figure 5. Characterization of MDSL xenograft model.
(A) Kaplan–Meier analysis of MDSL cell-bearing NSGS mice (n=7) with I.P. administration of DMSO or indomethacin. (B) Kaplan–Meier analysis of NSGS mice (n=7) reconstituted with MDSL cells expressing PLKO, PLKO-shRNA1 or shRNA2. (C-F) Flow cytometric analysis of percentage of MDSL cells in bone marrow (BM), spleen (SP) and peripheral blood (PB) from the xenograft mice. (G and H) BM cellularity was examined in MDSL xenograft mice. All analyses were performed when the xenograft mice became moribund. *, P <0.05; **, P<0.01; ***, P<0.001.
Figure 6. Characterization of UoCM1 xenograft model.
(A) Kaplan–Meier analysis of UoCM1 cell-bearing NSGS mice (n=7) with I.P. administration of DMSO or indomethacin. (B) Kaplan–Meier analysis of NSGS mice (n=7) reconstituted with UoCM1 cells expressing PLKO, PLKO-shRNA1 or shRNA2. (C-F) Flow cytometric analysis of percentage of UoCM1 cells in bone marrow (BM), spleen (SP) and peripheral blood (PB) from the xenograft mice. All analyses were performed when the xenograft mice became moribund. *, P <0.05; **, P<0.01; ***, P<0.001.
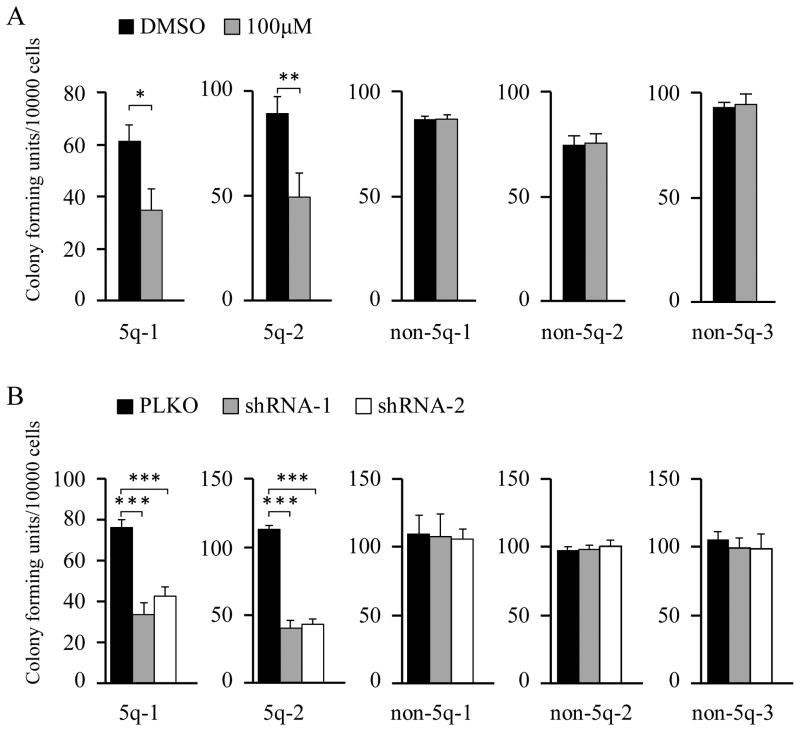
Suppression of β-Catenin impairs human AML cells with del(5q)
The results from mouse models and human leukemia cell lines provided strong evidence for a critical role of β-catenin in the maintenance of del(5q) leukemia cells. Thus, we examined the effects of inhibition of β-catenin by indomethacin and β-catenin specific shRNAs on tumorigenic potential of primary leukemia cells from del(5q) AML patients using a colony-forming assay. We found that indomethacin-treated or β-catenin-expressed primary human AML patients with del(5q) gave rise to significantly lower numbers of colonies in methocult medium than did cells from the same patients without treatment by indomethacin or expression of β-catenin-specific shRNAs (Fig. 7A,B). In contrast, the cells from primary human AML patients without del(5q) with or without indomethacin treatment or expression of β-catenin-specific shRNAs gave rise to a comparable number of colonies (Fig. 7A,B). These data show that β-catenin inhibition significantly inhibits the colony-forming ability of primary del(5q) AML cells but not primary AML cells without del(5q), indicating that inhibition of β-catenin suppresses tumorigenic potential of primary del(5q) AML cells.
Figure 7. β-Catenin inhibition suppresses tumorigenic potential of primary human AML Cells with a del(5q).
(A) Colony-forming ability of del(5q) and non- del(5q) primary AML cells in methylcellulose containing DMSO or 100 μM indomethacin. (B) Colony-forming ability of del(5q)and non- del(5q) primary AML cells expressing PLKO or PLKO-β-catenin shRNA1 or shRNA2. *, P <0.05; **, P<0.01; ***, P<0.001.
Discussion
MDS patients with del(5q) have high response rates to lenalidomide (LEN) treatment(34). Recent studies have revealed that LEN-induced promotion of CRBN-dependent degradation of CK1α and IKZF1 leads to induction of CAPN1 and cytosolic calcium flux, as well as increased p53 activity that mediates LEN-mediated cytotoxic effects on malignant cells with a del(5q)(35,36). However, MDS patients with a del(5q) who have durable hematologic and cytogenetic responses to LEN treatment often experience relapse(37). De novo AML patients or therapy-related MDS/AML patients with a del(5q) often have other complicated cytogenetic abnormalities. However, no curative therapy is available for these patients(4). Although the Wnt/β-catenin pathway has been implicated in the pathogenesis of myeloid neoplasms with a del(5q), it has not been determined whether β-catenin is a potential therapeutic target for this group of patients. Herein, we demonstrated that β-catenin inhibition either by indomethacin or β-catenin specific shRNAs induced apoptosis and blocked in vitro proliferation of MDSL, UoCM1 and KG1 leukemia cell lines that were derived from MDS and AML patients with del(5q) respectively(23,27), but not the non-del(5q) leukemia cell lines. We performed in vivo studies, and showed that reduced β-catenin expression by genetic deletion of a single allele of β-catenin reversed blockage of erythroid differentiation, thereby rescuing severe anemia induced by Apc haplo-insufficiency in mice. Additionally, we found that β-catenin inhibition delayed disease progression and significantly improved survival of MDSL and UoCM1 xenograft mice. The MDSL-xenograft mouse has been shown to be a useful preclinical model to evaluate therapeutic treatments for MDS(19,38). We showed that indomethacin treatment has a similar inhibitory effect as LEN treatment does on disease progression in MDSL xenograft mice(19). Additionally, we found that β-catenin inhibition significantly impaired the colony-forming ability of primary BM cells from AML patients with del(5q). Collectively, our studies showed that β-catenin inhibition is detrimental to del(5q) human MDS and AML cells. Of note, β-catenin is largely dispensable for normal HSCs in contrast to leukemic stem cells. Heterozygous and homozygous deletion of β-catenin does not have obvious effects on HSC function (39,40). Therefore, β-catenin inhibition may be a potential therapy for patients with del(5q) myeloid neoplasms.
MDS is considered a clonal hematopoietic stem cell disease(41). Del(5q) is detected in the HSCs with combined lympho-myeloid potential in patients with MDS(42,43). Del(5q) MDS stem cells gain clonal advantage and the del(5q) lesion remains present in BM cells when del(5q) MDS progresses to AML, indicating that deletion of 5q occurs in HSCs and is a driver genetic lesion that promotes clonal dominance of the malignant stem cells in del(5q) patients. Haplo-insufficiency of Apc and Csnk1a1 as a consequence of deletion of 5q in myeloid neoplasm patients could contribute to development of clonal dominance in these patients(10,12). Haplo-insufficiency of Apc and Csnk1a1 activates the Wnt/β-catenin signaling pathway in hematopoietic stem/progenitor cells (10,12). We previously showed that loss of both alleles of Apc leads to rapid exhaustion of HSCs while loss of a single allele of Apc leads to expansion of HSCs, gradually impairing the function of HSCs, indicating a dosage effect of the Wnt/ signaling pathway in maintaining the function of HSCs(10,44). Consistent with these data and using different hypomorphic Apc alleles leading to different Wnt signaling levels, Luis et al. showed that only slightly increased levels of canonical Wnt signaling have a positive effect on HSC repopulation capacity(45). Our recent studies showed that Apc function in hematopoietic stem/progenitor cells is largely dependent on β-catenin(18). Here, we provide additional evidence that reduced doses of β-catenin by heterozygous deletion is sufficient to reverse Apc-heterozygous-induced expansion of HSCs as well as the functional defects of Apc haplo-insufficient HSCs. β-catenin has been shown to be required for the self-renewal of murine leukemia stem cells initiated by MLL-AF9 and loss of Pten, and for the maintenance of chronic myeloid leukemia (CML) stem cells (32,46,47). Our studies suggest that β-catenin is likely required for the development of clonal dominance in del(5q) myeloid neoplasms by maintaining the self-renewal capacity of leukemia stem cells in patients with del(5q) MDS/AML. Thus, inhibition of β-catenin may benefit patients with del(5q) myeloid neoplasms by inhibiting expansion of or eliminating malignant stem cells. In addition, our data suggest that administration of LEN together with β-catenin inhibitors may be more effective for treatment of 5q MDS patients by potentially preventing or delaying disease relapse.
Supplementary Material
Acknowledgments
The MDSL cell line was kindly provided by Dr. Daniel Starczynowski at the University of Cincinnati College of Medicine.
Financial support: This work was supported in part by National Institute of Health grant RO1 CA140979, RO1 DK107615 and RO1 HL131444-01, Chicago Biomedical Consortium (Catalyst Award) (to Z. Qian), China Scholarship Council (To L. Li), Science and Technology Commission of Shanghai Municipality (15YF1408600, W. Li).
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Thirman MJ, Larson RA. Therapy-related myeloid leukemia. Hematol Oncol Clin North Am. 1996;10(2):293–320. doi: 10.1016/s0889-8588(05)70340-3. [DOI] [PubMed] [Google Scholar]
- 2.Ebert BL. Molecular dissection of the 5q deletion in myelodysplastic syndrome. Semin Oncol. 2011;38(5):621–6. doi: 10.1053/j.seminoncol.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giagounidis AA, Germing U, Aul C. Biological and prognostic significance of chromosome 5q deletions in myeloid malignancies. Clin Cancer Res. 2006;12(1):5–10. doi: 10.1158/1078-0432.CCR-05-1437. [DOI] [PubMed] [Google Scholar]
- 4.Qian Z, Joslin JM, Tennant TR, Reshmi SC, Young DJ, Stoddart A, et al. Cytogenetic and genetic pathways in therapy-related acute myeloid leukemia. Chem Biol Interact. 2010;184(1-2):50–7. doi: 10.1016/j.cbi.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heinrichs S, Kulkarni RV, Bueso-Ramos CE, Levine RL, Loh ML, Li C, et al. Accurate detection of uniparental disomy and microdeletions by SNP array analysis in myelodysplastic syndromes with normal cytogenetics. Leukemia. 2009;23(9):1605–13. doi: 10.1038/leu.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebert BL, Pretz J, Bosco J, Chang CY, Tamayo P, Galili N, et al. Identification of RPS14 as a 5q- syndrome gene by RNA interference screen. Nature. 2008;451(7176):335–9. doi: 10.1038/nature06494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Starczynowski DT, Kuchenbauer F, Argiropoulos B, Sung S, Morin R, Muranyi A, et al. Identification of miR-145 and miR-146a as mediators of the 5q- syndrome phenotype. Nat Med. 2010;16(1):49–58. doi: 10.1038/nm.2054. [DOI] [PubMed] [Google Scholar]
- 8.Joslin JM, Fernald AA, Tennant TR, Davis EM, Kogan SC, Anastasi J, et al. Haploinsufficiency of EGR1, a candidate gene in the del(5q), leads to the development of myeloid disorders. Blood. 2007;110(2):719–26. doi: 10.1182/blood-2007-01-068809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu TX, Becker MW, Jelinek J, Wu WS, Deng M, Mikhalkevich N, et al. Chromosome 5q deletion and epigenetic suppression of the gene encoding alpha-catenin (CTNNA1) in myeloid cell transformation. Nat Med. 2007;13(1):78–83. doi: 10.1038/nm1512. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Fernald AA, Anastasi J, Le Beau MM, Qian Z. Haploinsufficiency of Apc leads to ineffective hematopoiesis. Blood. 2010;115(17):3481–8. doi: 10.1182/blood-2009-11-251835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grisendi S, Bernardi R, Rossi M, Cheng K, Khandker L, Manova K, et al. Role of nucleophosmin in embryonic development and tumorigenesis. Nature. 2005;437(7055):147–53. doi: 10.1038/nature03915. [DOI] [PubMed] [Google Scholar]
- 12.Schneider RK, Adema V, Heckl D, Jaras M, Mallo M, Lord AM, et al. Role of casein kinase 1A1 in the biology and targeted therapy of del(5q) MDS. Cancer Cell. 2014;26(4):509–20. doi: 10.1016/j.ccr.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varney ME, Niederkorn M, Konno H, Matsumura T, Gohda J, Yoshida N, et al. Loss of Tifab, a del(5q) MDS gene, alters hematopoiesis through derepression of Toll-like receptor-TRAF6 signaling. J Exp Med. 2015;212(11):1967–85. doi: 10.1084/jem.20141898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281(5382):1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 15.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398(6726):422–6. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 16.Lane SW, Sykes SM, Al-Shahrour F, Shterental S, Paktinat M, Lo Celso C, et al. The Apc(min) mouse has altered hematopoietic stem cell function and provides a model for MPD/MDS. Blood. 2010;115(17):3489–97. doi: 10.1182/blood-2009-11-251728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stoddart A, Fernald AA, Wang J, Davis EM, Karrison T, Anastasi J, et al. Haploinsufficiency of del(5q) genes, Egr1 and Apc, cooperate with Tp53 loss to induce acute myeloid leukemia in mice. Blood. 2014;123(7):1069–78. doi: 10.1182/blood-2013-07-517953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li W, Hou Y, Ming M, Yu L, Seba A, Qian Z. Apc regulates the function of hematopoietic stem cells largely through beta-catenin-dependent mechanisms. Blood. 2013;121(20):4063–72. doi: 10.1182/blood-2012-12-473470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhyasen GW, Wunderlich M, Tohyama K, Garcia-Manero G, Mulloy JC, Starczynowski DT. An MDS xenograft model utilizing a patient-derived cell line. Leukemia. 2014;28(5):1142–5. doi: 10.1038/leu.2013.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heidel FH, Bullinger L, Feng Z, Wang Z, Neff TA, Stein L, et al. Genetic and pharmacologic inhibition of beta-catenin targets imatinib-resistant leukemia stem cells in CML. Cell Stem Cell. 2012;10(4):412–24. doi: 10.1016/j.stem.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou Y, Wang X, Li L, Fan R, Chen J, Zhu T, et al. FHL2 regulates hematopoietic stem cell functions under stress conditions. Leukemia. 2015;29(3):615–24. doi: 10.1038/leu.2014.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou Y, Li W, Sheng Y, Li L, Huang Y, Zhang Z, et al. The transcription factor Foxm1 is essential for the quiescence and maintenance of hematopoietic stem cells. Nat Immunol. 2015;16(8):810–8. doi: 10.1038/ni.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian Z, Fernald AA, Godley LA, Larson RA, Le Beau MM. Expression profiling of CD34+ hematopoietic stem/ progenitor cells reveals distinct subtypes of therapy-related acute myeloid leukemia. Proc Natl Acad Sci U S A. 2002;99(23):14925–30. doi: 10.1073/pnas.222491799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeung J, Esposito MT, Gandillet A, Zeisig BB, Griessinger E, Bonnet D, et al. beta-Catenin mediates the establishment and drug resistance of MLL leukemic stem cells. Cancer Cell. 2010;18(6):606–18. doi: 10.1016/j.ccr.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 25.Ming M, Wang S, Wu W, Senyuk V, Le Beau MM, Nucifora G, et al. Activation of Wnt/beta-catenin protein signaling induces mitochondria-mediated apoptosis in hematopoietic progenitor cells. J Biol Chem. 2012;287(27):22683–90. doi: 10.1074/jbc.M112.342089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Socolovsky M, Nam H, Fleming MD, Haase VH, Brugnara C, Lodish HF. Ineffective erythropoiesis in Stat5a(-/-)5b(-/-) mice due to decreased survival of early erythroblasts. Blood. 2001;98(12):3261–73. doi: 10.1182/blood.v98.12.3261. [DOI] [PubMed] [Google Scholar]
- 27.Matsuoka A, Tochigi A, Kishimoto M, Nakahara T, Kondo T, Tsujioka T, et al. Lenalidomide induces cell death in an MDS-derived cell line with deletion of chromosome 5q by inhibition of cytokinesis. Leukemia. 2010;24(4):748–55. doi: 10.1038/leu.2009.296. [DOI] [PubMed] [Google Scholar]
- 28.Mrozek K, Tanner SM, Heinonen K, Bloomfield CD. Molecular cytogenetic characterization of the KG-1 and KG-1a acute myeloid leukemia cell lines by use of spectral karyotyping and fluorescence in situ hybridization. Genes Chromosomes Cancer. 2003;38(3):249–52. doi: 10.1002/gcc.10274. [DOI] [PubMed] [Google Scholar]
- 29.Pellagatti A, Cazzola M, Giagounidis A, Perry J, Malcovati L, Della Porta MG, et al. Deregulated gene expression pathways in myelodysplastic syndrome hematopoietic stem cells. Leukemia. 2010;24(4):756–64. doi: 10.1038/leu.2010.31. [DOI] [PubMed] [Google Scholar]
- 30.Stoddart A, Nakitandwe J, Chen SC, Downing JR, Le Beau MM. Haploinsufficient loss of multiple 5q genes may fine-tune Wnt signaling in del(5q) therapy-related myeloid neoplasms. Blood. 2015;126(26):2899–901. doi: 10.1182/blood-2015-10-673228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goessling W, North TE, Loewer S, Lord AM, Lee S, Stoick-Cooper CL, et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136(6):1136–47. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Krivtsov AV, Sinha AU, North TE, Goessling W, Feng Z, et al. The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science. 2010;327(5973):1650–3. doi: 10.1126/science.1186624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tohyama K, Tsutani H, Ueda T, Nakamura T, Yoshida Y. Establishment and characterization of a novel myeloid cell line from the bone marrow of a patient with the myelodysplastic syndrome. Br J Haematol. 1994;87(2):235–42. doi: 10.1111/j.1365-2141.1994.tb04904.x. [DOI] [PubMed] [Google Scholar]
- 34.List A, Dewald G, Bennett J, Giagounidis A, Raza A, Feldman E, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355(14):1456–65. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 35.Fang J, Liu X, Bolanos L, Barker B, Rigolino C, Cortelezzi A, et al. A calcium- and calpain-dependent pathway determines the response to lenalidomide in myelodysplastic syndromes. Nat Med. 2016 doi: 10.1038/nm.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kronke J, Fink EC, Hollenbach PW, MacBeth KJ, Hurst SN, Udeshi ND, et al. Lenalidomide induces ubiquitination and degradation of CK1alpha in del(5q) MDS. Nature. 2015;523(7559):183–8. doi: 10.1038/nature14610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tehranchi R, Woll PS, Anderson K, Buza-Vidas N, Mizukami T, Mead AJ, et al. Persistent malignant stem cells in del(5q) myelodysplasia in remission. N Engl J Med. 363(11):1025–37. doi: 10.1056/NEJMoa0912228. [DOI] [PubMed] [Google Scholar]
- 38.Rhyasen GW, Bolanos L, Fang J, Jerez A, Wunderlich M, Rigolino C, et al. Targeting IRAK1 as a Therapeutic Approach for Myelodysplastic Syndrome. Cancer Cell. 2013;24(1):90–104. doi: 10.1016/j.ccr.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cobas M, Wilson A, Ernst B, Mancini SJ, MacDonald HR, Kemler R, et al. Beta-catenin is dispensable for hematopoiesis and lymphopoiesis. J Exp Med. 2004;199(2):221–9. doi: 10.1084/jem.20031615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koch U, Wilson A, Cobas M, Kemler R, Macdonald HR, Radtke F. Simultaneous loss of beta- and gamma-catenin does not perturb hematopoiesis or lymphopoiesis. Blood. 2008;111(1):160–4. doi: 10.1182/blood-2007-07-099754. [DOI] [PubMed] [Google Scholar]
- 41.Nimer SD. MDS: a stem cell disorder--but what exactly is wrong with the primitive hematopoietic cells in this disease? Hematology Am Soc Hematol Educ Program. 2008:43–51. doi: 10.1182/asheducation-2008.1.43. [DOI] [PubMed] [Google Scholar]
- 42.Nilsson L, Astrand-Grundstrom I, Arvidsson I, Jacobsson B, Hellstrom-Lindberg E, Hast R, et al. Isolation and characterization of hematopoietic progenitor/stem cells in 5q-deleted myelodysplastic syndromes: evidence for involvement at the hematopoietic stem cell level. Blood. 2000;96(6):2012–21. [PubMed] [Google Scholar]
- 43.Nilsson L, Eden P, Olsson E, Mansson R, Astrand-Grundstrom I, Strombeck B, et al. The molecular signature of MDS stem cells supports a stem-cell origin of 5q myelodysplastic syndromes. Blood. 2007;110(8):3005–14. doi: 10.1182/blood-2007-03-079368. [DOI] [PubMed] [Google Scholar]
- 44.Qian Z, Chen L, Fernald AA, Williams BO, Le Beau MM. A critical role for Apc in hematopoietic stem and progenitor cell survival. J Exp Med. 2008;205(9):2163–75. doi: 10.1084/jem.20080578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luis TC, Naber BA, Roozen PP, Brugman MH, de Haas EF, Ghazvini M, et al. Canonical wnt signaling regulates hematopoiesis in a dosage-dependent fashion. Cell Stem Cell. 2011;9(4):345–56. doi: 10.1016/j.stem.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 46.Guo W, Lasky JL, Chang CJ, Mosessian S, Lewis X, Xiao Y, et al. Multi-genetic events collaboratively contribute to Pten-null leukaemia stem-cell formation. Nature. 2008;453(7194):529–33. doi: 10.1038/nature06933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao C, Blum J, Chen A, Kwon HY, Jung SH, Cook JM, et al. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12(6):528–41. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.