Abstract
Bacteria are far more intelligent than we can think of. They adopt different survival strategies to make their life comfortable. Researches on bacterial communication to date suggest that bacteria can communicate with each other using chemical signaling molecules as well as using ion channel mediated electrical signaling. Though in past few decades the scopes of chemical signaling have been investigated extensively, those of electrical signaling have received less attention. In this article, we present a novel perspective on time-sharing behavior, which maintains the biofilm growth under reduced nutrient supply between two distant biofilms through electrical signaling based on the experimental evidence reported by Liu et al., in 2017. In addition, following the recent work by Humphries et al. Cell 168(1):200–209, in 2017, we highlight the consequences of long range electrical signaling within biofilm communities through spatially propagating waves of potassium. Furthermore, we address the possibility of two-way cellular communication between artificial and natural cells through chemical signaling being inspired by recent experimental observation (Lentini et al. 2017) where the efficiency of artificial cells in imitating the natural cells is estimated through cellular Turing test. These three spectacular observations lead us to envisage and devise new classical and quantum views of these complex biochemical networks that have never been realized previously.
Keywords: Quorum sensing; Artificial cells; Ion Channel; Electrical signaling; Time-sharing; Biofilm; Quantum biology, Quantum coherence
Introduction
Bacteria are unicellular organism in nature that survive everywhere in the world since the origin of life and their vast existence ranges from marine life to our every day life. An adult person has nearly ten trillion human cells in his body. But, the number of bacterial cells residing in a human body is ten times the number of human cells which ensures that the human kingdom is practically immersed in the bacterial world. This startling fact inspired the scientific community to search for bacterial intelligence. People are quite familiar with the bacteria called pathogen which creates disease. But, bacteria are not always harmful. They can as well be beneficial to their hosts. This mutually beneficial partnership between host (human body) and the guest (bacteria) is called symbiosis (Majumdar et al. 2012). As an example, bacteria that reside throughout the digestive tract of human body, can boost the human immune system. For these reasons, unicellular bacteria and its activity is one of the central research interests among the scientists. Based on Gram stain retention property, bacteria can be broadly classified into two classes: Gram- positive and Gram- negative. It has been found that both of them are capable of intra-species and interspecies communications using chemical signaling molecules (Waters and Bassler 2005) (Majumdar and Mondal 2016). For example Pseudomomas aeruginosa which is a Gram-negative bacteria, causes different types of infections especially in patients who have been hospitalized for a long time. Pseudomonal infections (nosocomial infection, urinary tract infection and many more) are very common and can be life threatening as well. Also, bacteria can make their optimal survival strategies using chemical signals (Quorum sensing molecules) which is directly proportional to the bacterial cell density and this cell density dependent collective behavior is formally known as “Quorum Sensing” (Majumdar and Pal 2016; 2017). In 2017, a group of researchers (Lentini et al. 2017), showed that artificial cells are also capable of sensing and sending quorum sensing molecules. On the other hand, (Liu et al. 2017) showed B. subtilis (Gram-positive bacteria) biofilm communities, which undergo metabolic oscillations, can become coupled through electrical signaling. But the coupling between artificial and natural systems which is focused not only on interaction within the community but also between communities is still left for future determination and can be a key to explore the utilization of time sharing in synthetic biology applications. Finally, we present a quantum viewpoint of these complex biological and electro-physical phenomena which can be of substantial importance in quantum computation.
Communication between artificial and natural cells and Turing test
Self- replication is one of the important features of life. But it is not sufficient to assess how lifelike a biochemical system is (Noireaux et al. 2011) (Szostak et al. 2001) (Luisi 2006). Cross- catalytic ribozyme ligases are capable of self-replication but they don’t alone constitute a living system (Lincoln and Joyce 2009). Rather it significantly deficits in some sort of metric by which progress can be measured. One can find the solution may narrate biochemical systems on a continuum where the binary categorization of alive not alive is replaced by states that are increasingly lifelike. Following this path, each iteration of constructing an artificial cell could be objectively and quantifiably evaluated in terms of likeness to a target natural cell. Recent experiment has attempted to reconstruct the well characterized quorum sensing pathways to build an artficial cells which can mimic the ability of the natural cells of V. fisheri, E. coli, P. aeruginosa. They show that these artificial cells are capable of sending and sensing the chemical signalls to the natural bacterial cells. This activity is assessed by luminescence, fluorescence, RT-qPCR and RNA-seq (Lentini et al. 2017).
Turing, in his seminal paper pointed out that the ability of a machine to deceive a judge through textual communication into believing that the machine is a person was used to circumvent the problem of defining intelligence (Turing 1950). Thus the ability of an artificial cell to deceive a natural cell can evaluate the proper artificial cell. A cellular Turing test is possible because all cells can communicate --- from quorum sensing pathways in bacteria to pheromone responses in higher organism (Lentini et al. 2016). Moreover these artificial cells containing DNA supports the transcription and translation prosesses which can express genes (Noireaux and Libchaber 2004) (Yu et al. 2001). Also they can send chemical signalls to bacteria (Gardner et al. 2009) (Lentini et al. 2014) and hence can interact with each other (Qiao et al. 2016).
The genetic constructs in water-in –oil emulsion droplets are able to either sense and send quorum sensing molecules. So, it is highly interesting that it should be possible to build genetically encoded artificial cells which can communicate with bacteria. Since quorum sensing is directly linked to gene expression, the next gereration sequencing technology can be used to quantifiably evaluate the extent of mimicry in a manner that is neither subjective nor binary. The repoted experimental evidence found in recent work (Lentini et al. 2017) is not sufficient to fully understand this complex biochemical process, Such an experimental approach can be extended beyond the charecterization of individual biomolecules and pathways leading to our present understanding of cellular life. A futher investigation is required for a much deeper understanding of life and the cellular Turing test can be a helful guide to achive this goal (Lentini et al. 2017).
Time sharing behavior
Limitation of resources in biological system occurs quite often (Hibbing et al. 2010). Time-sharing is a strategy, which is tropically employed in engineering and technological systems where users take turns consuming recourses. So the different systems are competing with each other. B. subtilis biofilm communities are engaged in collective growth-rate oscillations due to glutamate starvation (Liu et al. 2015). These oscillations are driven by a spatially extended negative feedback loop, where growth of the biofilms result in glutamate stress within interior and this stress in turn interfaced with biofilm growth. The ion channel mediated electrical signalings coordinate this phenomenon (Prindle et al. 2015). It has been reported that these biofilm communities undergoing metabolic oscillations become coupled through electrical signals which cause in synchronizing their growth dynamics. Also, it increases the competition by synchronizing demand for limited nutrients (Liu et al. 2017). They confirm that biofilms resolve this conflict by switching from in phase to anti- phase. Different biofilm communities take turns consuming nutrients. Thus distant biofilms can coordinate their behavior to resolve nutrient competition through time-sharing. This is a very intelligent and efficient strategy to share the limited resources (Liu et al. 2017).
In this recent report (Liu et al. 2017) showed that B. subtilis biofilm communities undergoing metabolic oscillation are also coupled through ion channels enable electrical communication and synchronize their growth dynamics. From theoretical and experimental point of view an in-phase and anti-phase oscillation is observed in the complex biological systems. They showed that pair of wild-type biofilms grown at 1× glutamate concentration (30 mM) starts oscillations slightly out of phase, but then become in-phase (approximately 0 phase difference) after one oscillation cycle (See the supplimentary and Movie S2 of Liu et al. 2017 ). Thus, one can find a constant phase difference in oscillation, which induce a “coherence” phenonenona. This is a remarkable achivement as coherence is observed in biological systems after quite a long time! We hope that in future this possibility of coherence can open a new avenue of research for biological systems.
Quantum effect in long-range communication
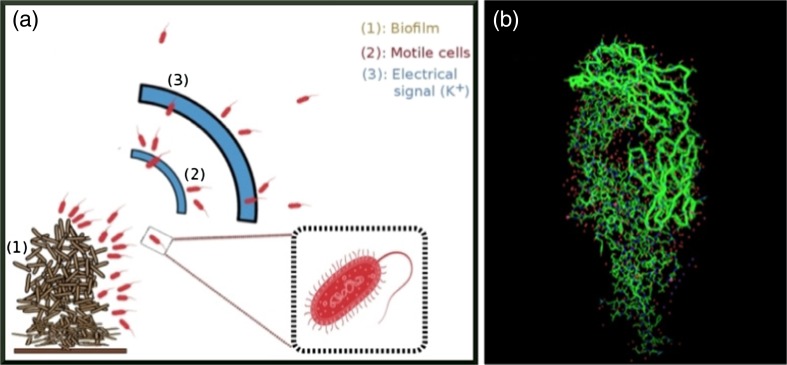
In 2017, Humphries et al. showed that electrical signaling can be another efficient way of cell – to – cell communication. Ion channel based electrical signaling attracts distant motile cells (see Fig. 1). This attraction depends on the membrane potential and its modulation of tumbling frequency. Moreover, this electrical signaling is generic in nature and it can attract the other bacterial cells (i.e., P. aeruginosa) also.
Fig. 1.
a Schematic diagram of electrical signaling within biofilms, which attract distant motile cells. b Schematic diagram of the KcsA potassium ion (K-ion) channel (from PDB 1K4C)
Cell – to- cell communications are subjected to random fluctuations in electrical signaling and interacting chemical signaling molecules. The role of noise is one of the major challenges for the robust function of natural cellular communications. The immediate question arises about the constructive and destruct role of noise (different types of noise) in bacterial communication systems. In addition, the origin of noise and its role to support cell-to-cell communication is one of the concerns to be investigated. Moreover, to gain insight into these issues, one needs to develop stochastic modeling approach, which can shed some light on questions regarding cell- to – cell communication that still remains unanswered.
The Quorum sensing mechanism is directly related to the activation of gene expression, virulence and metabolism. On the other hand, oscillations in biofilm growth is driven by metabolic co- dependence between peripheral and interior cells of the biofilm which in turn gives rise to synchronized collective oscillations of membrane potential and the bacterial ion channels activate the electrical communication (Liu et al. 2015)(Prindle et al. 2015). This electrical signal is generated from biofilm and can attract distant cells. This long range electrical signaling provides bacteria an advanced communication mechanism, which is completely generic. Consequently, it enables cross species communication in bacteria. As an example, quorum sensing bacteria P. aeruginosa cells are also attracted to electrical signalls released by B. subtills biofilm. The long range electrical signaling raises few important questions on the role of coherence in bacterial cell – to cell – communication. One may intend to investigate few of the obvious extensions of the above question ---- whether active electrochemical signaling could be responsible for the long range synchronization or not and what could be the effect of quorum sensing on electrical communication and vice versa? Is there any kind of condensation (Bose- Einstein like condensation) at room temperature? What are the most important parameters in cell communication?
The investigation on the role of quantum mechanics behind cellular communication is one of the fundamental priority of this century. Biological systems are composed of atoms and molecules and the quantum mechanics is the most effective in explaining unsolved questions in subatomic domain. Recent experiments already have shown some trivial and nontrivial quantum effects in biology. Motivated by these experiments, one can search for the interplay between noise and quantum coherence in this complex communication system. Moreover, the role of coherence in the communication dynamics has now become a major point to emphasize. Vaziri and Plenio 2010 suggest that the selectivity filter of the ion channels may exhibit quantum coherence. The dynamics inside the potassium ion channels are very complex due to the manifold interactions between ions and multiple degrees of freedom of the ion channel. Thus this selectivity filter can be able to explain the role of quantum coherence to give rise an important effects in conductance of the ion channels. Recently, Roy and Llinás 2009 have shown that the dynamics of K-ion channel can be explained using non-linear Schrödinger equation, which is compatible with the results of MacKinnons experimental observation (Doyle et al. 1998). The two K-ions may form an entangled state within a selectivity filter during a finite period of time. The temperature within the channel is generally considered to be high enough to destroy the coherence within a very short period.
On the other hand, this interdisciplinary research can have the significant impact on the implementation of new device to get rid of the bacterial infection that are usually hospital acquired and associated with catheterization, instrumentation and surgery. Indeed, the invention of biosensor can save millions of life. Besides, the study on bacterial ion channels will give significant insights into the structural basis of neuronal signaling and human brain. Though the quest for the quantum basis of the electrical signaling does not produce the benefit of mankind, but one can’t neglect the underlying theoretical aspect of the quantum viewpoint.
Contributor Information
Sarangam Majumdar, Email: majumdarsarangam@yahoo.in.
Sukla Pal, Email: sukla.ph10@gmail.com.
References
- Doyle DA, Cabral MJ, Pfuetzner AR, Kuo A, Gulbis MJ, Cohen LS, Chait TB, MacKinnon R (1998) The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280(5360):69–77 [DOI] [PubMed]
- Gardner PM, Winzer K, Davis BG. Sugar synthesis in a protocellular model leads to a cell signalling response in bacteria. Nat Chem. 2009;1:377–383. doi: 10.1038/nchem.296. [DOI] [PubMed] [Google Scholar]
- Hibbing EM, Fuqua C, Parsek MR, Peterson SB. Bacterial competition: surviving and thriving in the microbial jungle. Nat Rev Microbiol. 2010;8:15–25. doi: 10.1038/nrmicro2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries J, Xiong L, Liu J, Prindle A, Yuan F, Arjes AH, Tsimring L, Süel MG. Species-independent attraction to biofilms through electrical signaling. Cell. 2017;168(1):200–209. doi: 10.1016/j.cell.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luisi PL. The emergence of life: from chemical origins to synthetic biology. Cambridge: Cambridge University Press; 2006. [Google Scholar]
- Lincoln TA, Joyce GF. Self-sustained replication of an RNA enzyme. Science. 2009;323:1229–1232. doi: 10.1126/science.1167856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentini, R., Yeh Martín, N., Forlin, M., Belmonte, L., Fontana, J., Cornella, M., Martini, L., Tamburini, S., Bentley, E. W., Jousson, O. and Mansy, S. S. (2017) Two-way chemical communication between artificial and natural cells. ACS Central Science 3, (2): 117. [DOI] [PMC free article] [PubMed]
- Lentini R, Yeh Martín N, Mansy SS. Communicating artificial cells. Curr Opin Chem Biol. 2016;34:53–61. doi: 10.1016/j.cbpa.2016.06.013. [DOI] [PubMed] [Google Scholar]
- Lentini R, Santero SP, Chizzolini F, Cecchi D, Fontana J, Marchioretto M, Del Bianco C, Terrell JL, Spencer AC, Martini L, et al. Integrating artificial with natural cells to translate chemical messages that direct E coli behaviour Nat Commun. 2014;5:4012. doi: 10.1038/ncomms5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Corral MR, Prindle A, Dong-yeon LD, Larkin J, Sagarra GM, Ojalvo GJ, Süel MG (2017) Coupling between distant biofilms and emergence of nutrient time-sharing. Science 356(6338):638–642 [DOI] [PMC free article] [PubMed]
- Liu J, Prindle A, Humphries J, Sagarra MG, Asally M, Dong-yeon LD, Ly S, Ojalvo GJ, Süel MG. Metabolic co-dependence gives rise to collective oscillations within biofilms. Nature. 2015;523(7562):550–554. doi: 10.1038/nature14660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S, Mondal S. Conversation game: talking bacteria. J Cell Commun Signal. 2016;10(4):331–335. doi: 10.1007/s12079-016-0333-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S, Datta S, Roy S. Mathematical modelling of quorum sensing and bioluminescence in bacteria. Int J Adv Appl Sci. 2012;1(3):139–146. [Google Scholar]
- Majumdar S, Pal S. Quorum sensing: a quantum perspective. Journal of cell communication and signaling. 2016;10(3):173–175. doi: 10.1007/s12079-016-0348-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S, Pal S (2017) Cross-species communication in bacterial world. J Cell Commun Signal. doi:10.1007/s12079-017-0383-9 [DOI] [PMC free article] [PubMed]
- Noireaux V, Maeda YT, Libchaber A. Development of an artificial cell, from self-organization to computation and self- reproduction. Proc Natl Acad Sci U S A. 2011;108:3473–3480. doi: 10.1073/pnas.1017075108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noireaux V, Libchaber A. A vesicle bioreactor as a step toward an artificial cell assembly. Proc Natl Acad Sci U S A. 2004;101:17669–17674. doi: 10.1073/pnas.0408236101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prindle A, Liu J, Asally M, Ly S, Ojalvo GJ, Süel MG. Ion channels enable electrical communication in bacterial communities. Nature. 2015;527(7576):59–63. doi: 10.1038/nature15709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Y, Li M, Booth R, Mann S (2016) Predatory behaviour in synthetic protocell communities. Nat Chem. doi:10.1038/nchem.2617 [DOI] [PubMed]
- Roy S, Llinás R. Relevance of quantum mechanics on some aspects of ion channel function. Comptes rendus biologies. 2009;332(6):517–522. doi: 10.1016/j.crvi.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak JW, Bartel DP, Luisi PL. Synthesizing life. Nature. 2001;409:387–390. doi: 10.1038/35053176. [DOI] [PubMed] [Google Scholar]
- Turing AM. Computing machinery and intelligence. Mind. 1950;59:433–460. doi: 10.1093/mind/LIX.236.433. [DOI] [Google Scholar]
- Vaziri A, Plenio BM. Quantum coherence in ion channels: resonances, transport and verification. New J Phys. 2010;12(8) doi: 10.1088/1367-2630/12/8/085001. [DOI] [Google Scholar]
- Waters MC, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- Yu W, Sato K, Wakabayashi M, Nakaishi T, Ko-Mitamura EP, Shima Y, Urabe I, Yomo T. Synthesis of functional protein in liposome. J Biosci Bioeng. 2001;92:590–593. doi: 10.1016/S1389-1723(01)80322-4. [DOI] [PubMed] [Google Scholar]