Abstract
Both PET/CT and breast MRI are used to assess pathological complete response to neoadjuvant chemotherapy (NAC) in patients with breast cancer. The aim is to compare the utility of PET/CT and breast MRI by using head-to-head comparative studies. Literature databases were searched prior to July 2016. Eleven studies with a total of 527 patients were included. For PET/CT, the pooled SEN was 0.87 (95% confidence interval (CI): 0.71–0.95) and SPE was 0.85 (95% CI: 0.70–0.93). For MRI, the pooled SEN was 0.79 (95% CI: 0.68–0.87) and SPE was 0.82 (95% CI: 0.72–0.89). In the conventional contrast enhanced (CE)-MRI subgroup, PET/CT outperformed conventional CE-MRI with a higher pooled sensitivity (0.88 (95% CI: 0.71, 0.95) vs. 0.74 (95% CI: 0.60, 0.85), P = 0.018). In the early evaluation subgroup, PET/CT was superior to MRI with a notable higher pooled specificity (0.94 (95% CI: 0.78, 0.98) vs. 0.83 (95% CI: 0.81, 0.87), P = 0.015). The diagnostic performance of MRI is similar to that of PET/CT for the assessment of breast cancer response to NAC. However, PET/CT is more sensitive than conventional CE-MRI and more specific if the second imaging scan is performed before 3 cycles of NAC.
Introduction
Neoadjuvant chemotherapy (NAC) followed by surgery has been used as a standard treatment and offers advantages over traditional adjuvant approaches in patients with locally advanced breast cancer1. It has been established that early response after NAC, achieving pathologic complete response (pCR) or a minimal residual tumour burden might be an optimal predictor of a favorable long-term outcome2. Early prediction of outcome and monitoring the response to NAC are important for optimal management by improving the ability to individualise therapies, such as by avoiding additional toxic therapy in non-responding patients3.
Various noninvasive imaging tools are used to follow tumour change after NAC, including mammography, ultrasound, and magnetic resonance imaging (MRI). Breast MRI has been increasingly shown to correlate better with pathologic breast tumour size4. With the development of quantitative perfusion MRI, diffusion-weighted MRI (DWI) and magnetic resonance spectroscopy (MRS), multiparametric MRI also has been recommended as an accurate biomarker for NAC response evaluation in patients with operable breast cancer5, 6. Positron emission tomography (PET) with 18F-fluorodeoxyglucose (FDG) is correlated with increased glucose metabolism in cancer. This correlation has been harnessed to evaluate the clinical response to NAC in patients with breast cancer. Metabolic reduction detected between baseline and the early phase of NAC can provide early information on the potential tumour response.
Several systematic reviews have reported the accuracy of breast MRI or 18F-FDG PET/CT alone in predicting pathological response to NAC in breast cancer5, 7, 8. In addition, a large number of studies6, 9–23 have compared the value of MRI and PET/CT directly for the assessment of breast cancer response to NAC against a reference standard of histopathologic analysis. However, the findings of these studies have been inconsistent, and most of their sample sizes were small. Therefore, we conducted a meta-analysis of the literature to estimate the diagnostic performance of breast MRI compared with PET/CT for monitoring response to NAC in breast cancer. To identify the best evidence of the diagnostic performance of these two methods, we restricted the scope of this meta-analysis to direct comparative diagnostic accuracy studies.
Results
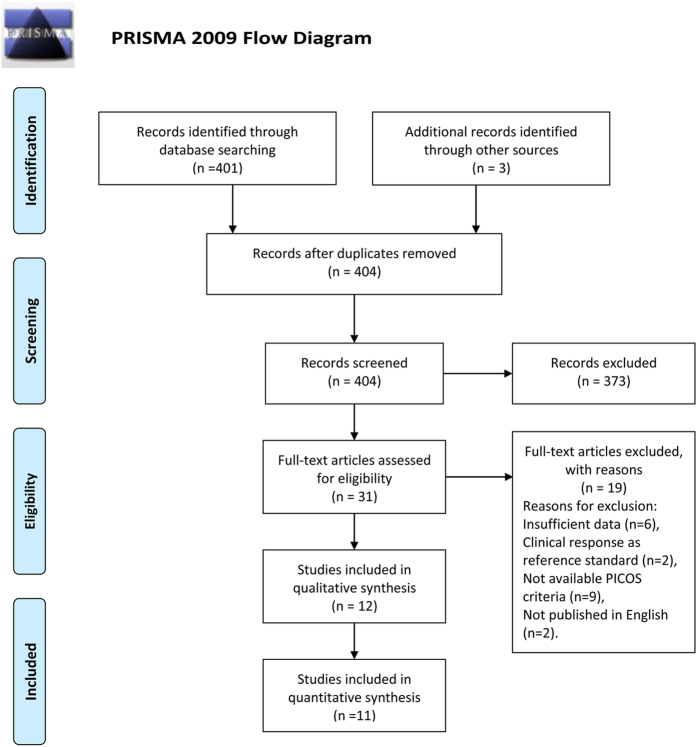
The database search initially identified 401 potential literature citations, and 3 additional records were obtained by searching the grey literature (Fig. 1). After reviewing the titles and abstracts, 373 of the studies were excluded as they were not relevant studies. After reading the full texts, we excluded 19 of the remaining 31 articles for the following reasons: 6 article lacked sufficient information to enable completion of a 2 × 2 contingency table, 9 article was not available, the reference standard in 2 articles was clinical response, and 2 article was not published in English. After this final screening, 12 published studies met our inclusion criteria. Ultimately, a total of 11 studies were included in our quantitative synthesis; 1 study was excluded because it assessed the axillary lymph node response to NAC. The data extracted from these individual studies are summarised in Table 1, Table 2, Table S1, and Table S2.
Figure 1.
Flowchart illustrating the selection of studies32.
Table 1.
Summary of the cohort, tumour, and treatment characteristics of the included studies.
| Variable | Number providing data | Median estimate | Range | ||
|---|---|---|---|---|---|
| Studies | Patients | ||||
| Cohort characteristics | |||||
| No., all tests | 12 | 641 | 53.4 | 16–142 | |
| Age (years) | 12 | 641 | 50.5 | 24–71 | |
| pCR (prevalence) | 12 | 245 | 40.7% | 16.9–85.0% | |
| non-pCR (prevalence) | 12 | 396 | 59.3% | 15.0–83.1% | |
| Tumour characteristics | |||||
| Stage | I | 2 | 20 | 11.9% | 6.3–17.5% |
| II | 7 | 235 | 42.2% | 10.0–68.3% | |
| III | 8 | 210 | 58.5% | 7.9–90.0% | |
| IVa | 3 | 9 | 4.3% | 3.4–6.3% | |
| Histology | IDC | 8 | 390 | 84.5% | 39.6–96.4% |
| ILC | 7 | 56 | 14.9% | 1.8–58.5% | |
| MC | 5 | 5 | 3.1% | 1.8–6.2% | |
| Other | 2 | 3 | 2.3% | 1.1–5.8% | |
| Receptor | ER (+) | 5 | 149 | 48.0% | 4.3–75% |
| PR (+) | 4 | 121 | 51.0% | 36.6–68.8% | |
| HER-2 (+) | 6 | 176 | 33.6% | 17.1–78.2% | |
| LA | 2 | 10 | 16.5% | 7.9–28.6% | |
| LB | 3 | 46 | 54.5% | 28.6–100% | |
| Triple (−) | 5 | 65 | 24.5% | 12.5–33.3% | |
ER = oestrogen receptor; PR = progesterone receptor; HER2 = human epidermal growth factor receptor 2; IDC = invasive ductal carcinoma; ILC = invasive lobular carcinoma; MC = mucinous carcinoma; LA = luminal A; LB = luminal B; NAC = neoadjuvant chemotherapy; NR = not reported; pCR = pathologic complete response; Triple (−) = Triple negative.
Table 2.
Absolute numbers of the included studies.
| Study | Patient (No.) | Design | Time of scan | MRI | PET/CT | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Cut-off | Sen | Spe | Parameter | Cut-off | Sen | Spe | ||||
| An, Y 2015 | 20 | Retro | B & A (preoperative) | ΔLD | Reduction > 30%a | 0.33 | 0.82 | ΔSUV | Reduction > 30%c | 0.33 | 0.88 |
| ΔLD | Increase > 88.7%b | 0.67 | 0.94 | ΔSUV | Reduction > 80.6%b | 0.67 | 0.88 | ||||
| ΔADC | Increase > 22.1%b | 0.67 | 0.71 | ||||||||
| Choi, J 2010 | 41 | Pro | B & A (3 or 8 cycles) | ΔLD | Reduction > 30%a | 0.71 | 0.95 | ΔSUV | Reduction > 50%d | 0.86 | 0.38 |
| Kim, T 2014 | 56 | Retro | B & A (3 or 6 cycles) | ΔLD | Reduction > 50%b | 0.91 | 0.77 | ΔSUV | Reduction > 60%b | 0.91 | 0.73 |
| Pahk, K 2015 | 21 | Retro | B & A (3 or 4 cycles) | ΔLD | Reduction > 38%b | 0.71 | 0.71 | ΔSUV | Reduction > 69%b | 0.86 | 1.00 |
| Park, J 2011 | 32 | Retro | B & A (18–22 days) | ΔLD | Reduction > 30%a | 0.63 | 0.96 | ΔSUV | Reduction > 50%d | 1.00 | 0.63 |
| Park, S 2012 | 34 | Retro | B & A (3 or 6 cycles) | ΔADC | Increase > 55%b | 1.00 | 0.70 | ΔSUV | Reduction > 64%b | 1.00 | 0.78 |
| Pengel 2014 | 93 | Pro | B & A (1 or 3 cycles) | ΔLD | Reduction > 50%b | 0.86 | 0.58 | ΔSUV | Reduction > 50%b | 0.47 | 0.94 |
| Tateishi 2012 | 142 | Retro | B & A (2 cycles) | ΔLD | Reduction > 30%a | 0.46 | 0.86 | ΔSUV | Reduction > 30%c | 0.67 | 0.96 |
| ΔKep | Reduction > 63%b | 0.52 | 0.92 | ΔSUV | Reduction > 80%b | 0.70 | 0.96 | ||||
| Cho, N 2016 | 35 | Pro | B & A (1 cycles) | ΔtCho | Reduction > 61%b | 1.00 | 0.76 | ΔSUV | Reduction > 62%b | 0.67 | 1.00 |
| Amioka 2016 | 63 | NR | B & A (NR) | ΔLD | Reduction > 30%a | 0.70 | 0.85 | ΔSUV | NR | 1.00 | 0.53 |
| Chen 2004 | 16 | Retro | B & A (NR) | ΔLD | Reduction > 30%a | 0.90 | 0.17 | ΔSUV | Reduction > 50%d | 0.90 | 0.83 |
| ΔLD | Reduction > 63%b | 0.90 | 0.50 | ΔSUV | Reduction > 50%b | 0.90 | 0.83 | ||||
| Hieken 2013† | 88 | Pro | B & A (NR) | ΔLD | NR | 0.61 | 0.59 | ΔSUV | NR | 0.63 | 0.85 |
aCut-off set by pre-specified RECIST criteria; bcut-off set by ROC analysis; ccut-off set by pre-specified PRECIST criteria; dcut-off set by pre-specified EORTC criteria; B & A, at baseline and after NAC; Pro, prospective; Retro, retrospective; NR, not reported; ΔLD, change in longest diameter; ΔADC, change in apparent diffusion coefficient; ΔKep, change in transfer constant; ΔtCho, change in total choline-containing compounds; ΔSUV, change in standardised uptake values. †Study assessed axillary lymph node response to NAC.
According to QUADAS-2, the quality assessment of the 12 studies was moderate. The results of the distribution of the study design are shown in Fig. 2.
Figure 2.
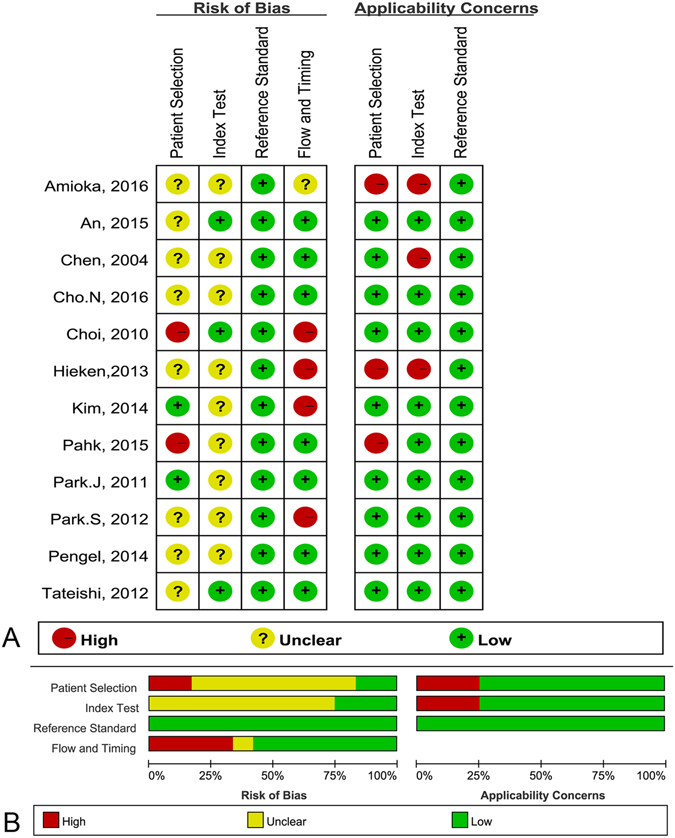
Methodological quality of the 12 included studies. (A) Risk of bias and applicability concerns summary; (B) risk of bias and applicability concerns graph.
As there was significant heterogeneity in both pooled analyses (MRI: I2 = 92.8%, P < 0.001; PET/CT: I2 = 97.2%, P < 0.001), we used a random-effects coefficient binary regression model. The pooled weighted values for MRI were sensitivity (SEN) 0.79 (95% CI: 0.68, 0. 88), sensitivity (SEN) 0.82 (95% CI: 0.72, 0.89), positive likelihood ratio (PLR) 4.29 (95% CI: 2.92, 6.30), negative likelihood ratio (NLR) 0.26 (95% CI: 0.18, 0.39), diagnostic odds ratio (DOR) 16.43 (95% CI: 10.05, 26.87), and the areas under the ROC curve (AUC) 0.87 (95% CI: 0.84, 0.90). The pooled weighted values for PET/CT were SEN 0.87 (95% CI: 0.71, 0. 95), SPE 0.85 (95% CI: 0.70, 0.93), PLR 5.76 (95% CI: 2.96, 11.12), NLR 0.16 (95% CI: 0.07, 0.34), DOR: 37.25 (95% CI: 17.00, 81.62), and AUC 0.93 (95% CI: 0.90, 0.95). The forest plots for the 11 studies are shown in Fig. 3. Hierarchical summary receiver operating characteristic (HSROC) curves are shown in Fig. 4.
Figure 3.
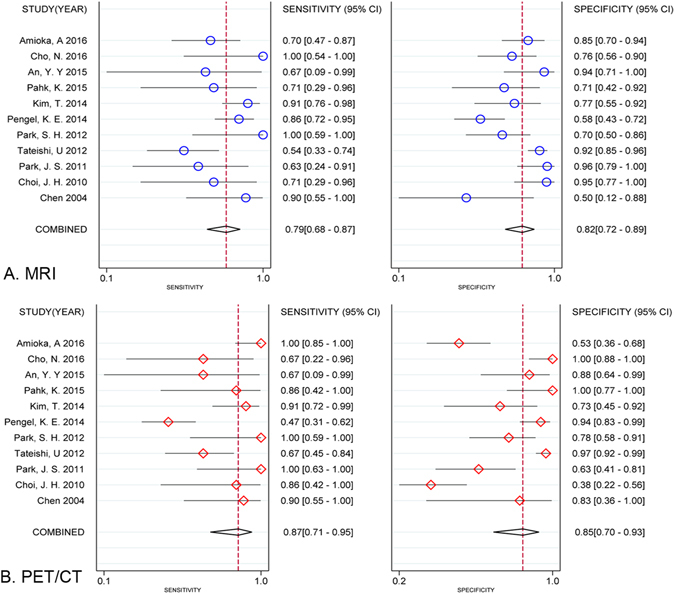
Forest plots of SEN and SPE with corresponding 95% CIs of MRI and PET/CT in assessing pathologic response to NAC. (A) MRI; (B) PET/CT.
Figure 4.
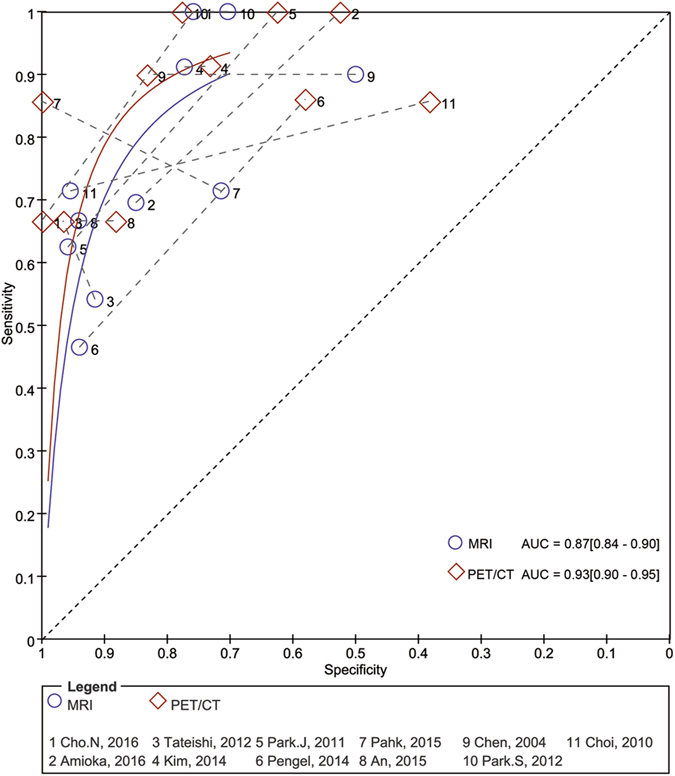
Pairs of observed values of sensitivity and specificity for MRI and PET/CT to assess pathologic response to NAC in HSROC curves.
The accuracy estimates for the different subgroups are presented in Table 3. In the pre-specified cut-off subgroup, PET/CT outperformed MRI in assessing the pathologic response to NAC, with a higher pooled sensitivity (0.79 [95% CI: 0.65, 0.89] vs. 0.61 [95% CI: 0.39, 0.79], P = 0.005) and a comparable pooled specificity (0.81 [95% CI: 0.75, 0.86] vs. 0.83 [95% CI: 0.54, 0.95], p = 0.713). However, in the cut-off obtained by ROC subgroup, the pooled sensitivity and pooled specificity of PET/CT were similar to those of MRI. In the conventional contrast enhanced (CE)-MRI subgroup, PET/CT was more effective than MRI in assessing the pathologic response to NAC, with a slightly higher pooled sensitivity (0.88 [95% CI: 0.71, 0.95] vs. 0.74 [95% CI: 0.60, 0.85], P = 0.018) and pooled specificity (0.82 [95% CI: 0.65, 0.92] vs. 0.82 [95% CI: 0.71, 0.89], P = 0.999). However, in the functional MRI subgroup, PET/CT appeared to have lower pooled sensitivity (0.78 [95% CI: 0.52, 0.92] vs. 0.88 [95% CI: 0.49, 0.98], P = 0.060) but higher pooled specificity (0.92 [0.82–0.98] vs. 0.82 [0.67–0.89], P = 0.057) than MRI. In the early evaluation subgroup, PET/CT was superior to MRI, with a similar pooled sensitivity (0.71 [95% CI: 0.35, 0.92] vs. 0.73 [95% CI: 0.53, 0.87], P = 0.753) and a notably higher pooled specificity (0.94 [95% CI: 0.78, 0.98] vs. 0.83 [95% CI: 0.64, 0.93], P = 0.015). By contrast, in the post evaluation subgroup, the pooled sensitivity and specificity of PET/CT were very similar to those of MRI.
Table 3.
Accuracy estimates for subgroup analyses.
| Factor | Subgroups | Imaging | No | pSEN (95% CI) | pSPE (95% CI) | AUC (95% CI) |
|---|---|---|---|---|---|---|
| Cut-off value | ROC analysis | MRI | 9 | 0.80 (0.73–0.86) | 0.80 (0.75–0.84) | 0.86 (0.83–0.89) |
| PET/CT | 9 | 0.74 (0.66–0.81) | 0.88 (0.84–0.91) | 0.92 (0.90–0.94) | ||
| P = 0.313 | P = 0.123 | P = 0.124 | ||||
| Pre-specified | MRI | 5 | 0.61 (0.39–0.79) | 0.83 (0.54–0.95) | 0.73 (0.69–0.76) | |
| PET/CT | 5 | 0.79 (0.65–0.89) | 0.81 (0.75–0.86) | 0.87 (0.82–0.92) | ||
| P = 0.005* | P = 0.713 | P = 0.022* | ||||
| MRI modality | Conventional CE-MRI | MRI | 9 | 0.74 (0.60–0.85) | 0.82 (0.71–0.89) | 0.84 (0.81–0.87) |
| PET/CT | 9 | 0.88 (0.71–0.95) | 0.82 (0.65–0.92) | 0.92 (0.89–0.94) | ||
| P = 0.018* | P = 0.999 | P = 0.104 | ||||
| Functional MRI | MRI | 4 | 0.87 (0.49–0.98) | 0.82 (0.67–0.89) | 0.89 (0.86–0.91) | |
| PET/CT | 4 | 0.78 (0.52–0.92) | 0.92 (0.82–0.98) | 0.93 (0.90–0.95) | ||
| P = 0.060 | P = 0.057 | P = 0.258 | ||||
| Evaluation time | Early evaluation | MRI | 4 | 0.73 (0.53–0.87) | 0.83 (0.64–0.93) | 0.85 (0.81–0.88) |
| PET/CT | 4 | 0.71 (0.35–0.92) | 0.94 (0.78–0.98) | 0.92 (0.89–0.94) | ||
| P = 0.753 | P = 0.015* | P = 0.163 | ||||
| Post evaluation | MRI | 5 | 0.85 (0.68–0.94) | 0.83 (0.70–0.92) | 0.91 (0.88–0.93) | |
| PET/CT | 5 | 0.89 (0.77–0.96) | 0.80 (0.53–0.93) | 0.90 (0.87–0.93) | ||
| P = 0.400 | P = 0.585 | P = 0.798 |
pSEN = pooled sensitivities; pSPE = pooled specificities; *P < 0.05.
The results of Deeks funnel plot asymmetry test (P = 0.160 and P = 0.804, respectively) showed no evidence of notable publication bias in the analysis of either MRI or PET/CT (Fig. 5).
Figure 5.
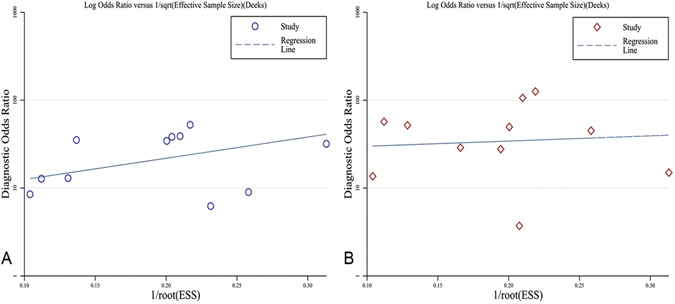
Funnel plot of publication bias. (A), MRI P = 0.160; (B) PET/CT P = 0.804.
Discussion
Although MRI and PET/CT already play daily clinical roles in determining whether to continue, change, or abandon NAC for breast cancer, previous meta-analyses and systematic reviews have yielded inconsistent findings7, 8, 24 when assessing these imaging modalities alone or together (Table 4). Several recent head-to-head comparative studies have also yielded inconsistent findings13, 17, 21, 22. Because head-to-head comparisons provide the best measurements of the diagnostic accuracy of two different techniques25, 26, we focused exclusively on direct comparative studies that evaluated both MRI and PET/CT in the same cohort of patients. Compared with the previous meta-analysis by Liu24, our research is strengthened by more careful selection of articles and the inclusion of two direct comparative studies6, 15 that might be missed in their analysis.
Table 4.
Summary of meta-analyses focused on MRI and PET/CT for the assessment of breast cancer response to NAC.
| Study | Search date | No. | Modality | PSEN (95% CI) | PSPE (95% CI) | DOR (95% CI) | AUC (95% CI) |
|---|---|---|---|---|---|---|---|
| Michae l7 | to 2011 | 44 | MRI | 0.92(0.85–0.97) | 0.60(0.39–0.96) | 17.89(11.45, 27.95) | 0.88(NR) |
| Mghanga8 | 2000–2012 | 15 | PET/CT | 0.81(0.76, 0.85) | 0.79(0.74, 0.83) | NR | 0.88(0.86–0.90) |
| Liu24 | 1992–2015 | 6 | MRI | 0.65(0.45, 0.80) | 0.88(0.75, 0.95) | NR | 0.84(0.80, 0.87) |
| 6 | PET/CT | 0.86(0.76, 0.93) | 0.72(0.49, 0.87) | NR | 0.88(0.85, 0.91) | ||
| Our | 2000–2016 | 11 | MRI | 0.79(0.76, 0.87) | 0.82(0.72, 0.89) | 16.43(10.05, 26.87) | 0.87(0.84, 0.90) |
| 11 | PET/CT | 0.87(0.71, 0.95) | 0.85(0.70, 0.93) | 37.25(17.01, 81.62) | 0.93(0.90, 0.95) |
PSEN = pooled sensitivities; PSPE = pooled specificities; DOR = diagnostic odds ratio; NR = not reported.
The results of our meta-analysis showed that MRI and PET/CT have similar high sensitivities (0.79 vs. 0.87) and specificities (0.82 vs. 0.85). However, among previous meta-analyses, the study focusing on MRI by Michael et al.8 had a much higher pooled sensitivity (0.92 vs. 0.81) than the study focusing on PET/CT by Mghanga et al.7, whereas completely opposite results were observed for pooled specificity (0.60 vs. 0.79). In addition, the AUCs of the two studies were identical (0.88 vs. 0.88). We speculate that the characteristic of high sensitivity with low specificity or vice versa may be caused by a threshold effect originating from the use of different diagnostic cut-off values in various studies. Due to this threshold effect, ROC curve and AUC analysis are more insightful approaches than evaluating the pooled sensitivity and pooled specificity. The AUC in our study (0.87 vs. 0.93) is consistent with these meta-analyses, which suggests that the diagnostic performance of MRI is similar to that of PET/CT for the assessment of breast cancer response to NAC.
Traditionally, tumour response has been monitored by conventional CE-MRI alone with standard anatomic response criteria (Response Evaluation Criteria in Solid Tumors (RECIST) and RECIST 1.1) during the course of NAC imaging4. Over the last decade, advances in functional imaging have enabled anatomic and functional information to be obtained, such as by PET/CT, DWI, perfusion MRI, and MRS. On the basis of PET, metabolic response criteria were established, including the European Organisation for Research and Treatment of Cancer (EORTC) criteria and PET Response Criteria in Solid Tumors (PERCIST)27. Several studies13, 14, 20, 23 attempted to compare the predictive roles of MRI and PET/CT during NAC using a pre-specified cut-off according to international standards (RECIST vs. PERCIST). Therefore, we performed subgroup analysis of different diagnostic cut-off values. In the pre-specified cut-off subgroup, PET/CT outperformed MRI in assessing pathologic response to NAC, with a higher pooled sensitivity (0.79 vs. 0.61) and a comparable pooled specificity (0.81 vs. 0.83). However, this trend was not observed in the cut-off obtained by ROC subgroup. We also performed subgroup analysis of different MRI modalities. In the conventional CE-MRI subgroup, PET/CT was more effective than MRI in assessing pathologic response to NAC, with a slightly higher pooled sensitivity (0.88 vs. 0.74) and pooled specificity (0.82 vs. 0.82). However, in the functional MRI (perfusion MR, DWI, or MRS) subgroup, PET/CT appeared to be equivalent to MRI, with lower pooled sensitivity (0.78 vs. 0.88), higher pooled specificity (0.92 vs. 0.82), and similar AUC (0.93 vs. 0.89). These results suggest that PET/CT is more accurate than conventional CE-MRI imaging and that PERCIST criteria may be more appropriate than RECIST criteria for monitoring breast cancer response to NAC. A possible explanation is the general limitation of anatomic MRI techniques, which are unable to distinguish potential residual tumour from fibrotic scar tissue in stable disease14.
Because the delay time between the initiation of therapy and changes in tumour size is usually longer than 2 cycles of NAC28, several studies18–20, 22 have attempted to investigate earlier predictors associated with angiogenesis, metabolism, or cellularity that may change before tumour shrinkage in the breast cancer response to NAC. Moreover, there is no consensus on the optimal timing of second imaging for evaluation of the response to NAC. Therefore, we performed a subgroup analysis of different evaluation time points of second imaging. In the early evaluation subgroup, PET/CT was superior to MRI in assessing pathologic response to NAC, with a notably higher pooled specificity (0.94 vs. 0.83) and a similar pooled sensitivity (0.71 vs. 0.73). By contrast, in the post evaluation subgroup, the pooled sensitivity, specificity and AUC of PET/CT were very similar to those of MRI. Our results support previous conjecture that PET/CT is superior to MRI in assessing response at times before 3 cycles of NAC but not at times after 3 cycles of NAC.
Although breast surgical resection after NAC is based on a combination of clinical and imaging assessments of the response to treatment, the axillary nodal stage continues to play a crucial role in clinical decisions. Hieken et al.15 reported that PET/CT has higher sensitivity (0.86 vs. 0.59) than MRI in assessing the axillary lymph node response to NAC. However, this result must be interpreted with caution because only one study of this type is available. More clinical studies are required to confirm this result, which would indicate that PET/CT has a greater advantage in assessing both breast cancer and axillary lymph node response to NAC than MRI.
The performance of either PET/CT or MRI alone was shown much different among breast cancer subtypes. Therefore, imaging techniques based on subtypes for personalizing may further improve their performance in NAC monitoring29. However, after reviewing the 12 included articles, only two studies with knowledge of the breast cancer subtypes were identified in our study (Table S2). One head-to-head comparative study revealed that it might be better to use PET/CT for early predicting pCR than conventional CE-MRI in luminal B subtype breast cancer17. The second study showed that pCR was associated with the reduction in SUVmax on PET/CT as well as the reduction in largest diameter on MRI in triple-negative tumours, but not in HER2-positive and ER-positive/HER2-negative tumours19. Although current evidence is not sufficient to draw recommendations, these results may be clinically useful and generate hypotheses for further research.
Some intrinsic disadvantages of our study should be considered when interpreting our results. First, the sample sizes of comparative studies available in the literature are relatively small, which may contribute to an overestimation of diagnostic accuracy26. However, a systematic review30 focused on meta-analysis studies from the Cochrane Database showed that the number of studies eligible for meta-analysis is typically small in all medical areas and for all outcomes and interventions covered by the Cochrane Reviews. Second, there may be publication bias in this meta-analysis. Our meta-analysis was based only on published and full-text articles, which tend to report positive or significant results rather than negative or not significant results. Although the quality of published data in peer-reviewed journals is generally considered superior to unpublished data31, the inclusion of only published studies may lead to reporting bias. Third, accuracy estimates are affected by various factors, such as the definition of pCR and the breast cancer phenotype8. As data are limited to investigate those factors, we did not assess these factors in our analyses.
In conclusion, a limited number of head-to-head studies indicates that the diagnostic performance of MRI is similar to that of PET/CT for the assessment of breast cancer response to NAC. However, for monitoring breast cancer response to NAC, PET/CT is more sensitive than anatomic MR imaging, and PERCIST criteria may be more appropriate than RECIST criteria. Moreover, PET/CT is superior to MRI in assessing response at times between 1–3 cycles of NAC but not at time after 3 cycles of NAC. In the future, large-scale, head-to-head, well-designed trials are necessary to compare the predictive value and consider more factors (such as the definition of pCR and phenotype of breast cancer) of these two imaging techniques.
Materials and Methods
We used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement32 to improve the reporting of our research (Fig. 1).
Search Strategy
A structured approach was followed to identify the patient population, interventions, comparators, outcomes, and study design (PICOS criteria)32. Two observers (Lihua Chen and Qifang Yang) performed the literature search of data sources independently (PUBMED, EMBASE, Web of Science, and the Cochrane Library). The search strategy (Appendix A) included both subject headings (MeSH terms) and keywords for the target condition (breast cancer), the imaging techniques under investigation (MRI and PET/CT), and the interventions (neoadjuvant therapy). We limited our search to publications with the search term in the title or abstract of the article and a publication date no later than July 2016. Review articles, letters, comments, case reports, and unpublished articles were excluded. Extensive cross-checking of the reference lists of all retrieved articles was performed.
Criteria for inclusion in the study
Studies were eligible if the following PICOS criteria were met. (a) The patient population consisted of primary breast cancer confirmed histologically; (b) the imaging response for pre-NAC and post-NAC was monitored with both MRI and FDG-PET; (c) histopathologic analysis was available as a reference standard; (d) the study outcome described pCR or near-pCR to NAC; and (e) the study design was described as a direct comparative study or randomised controlled trial.
Non-English and non-Chinese articles were excluded if a full-text translation or evaluation could not be obtained. Both prospective and retrospective studies were included.
We excluded studies if a 2 × 2 table could not be extracted from the data, if there were fewer than 10 patients, and if multiple reports were published for the same study population. In the latter case, the most detailed or recent publication was extracted.
Selection of Articles
Articles were selected by two authors (Lihua Chen and Qifang Yang) independently. The two authors initially screened the titles and abstracts of the search results and retrieved all potentially relevant reports in full. Next, they reviewed all relevant reports according to the predefined inclusion criteria. Disagreements were arbitrated by a third author (Jian Wang) who assessed all involved items.
Quality Assessment and Data Extraction
For each included study, the methodological quality was evaluated independently by two observers (Lihua Chen and Qifang Yang) using the standard quality assessment of diagnostic studies (QUADAS-2) checklist, which was specifically developed for systematic reviews of diagnostic accuracy studies33–35. In addition, the relevant information was also extracted from each study, including the author, year of publication, description of the study population, study nation, study design characteristics, therapeutic interventions, reference standard, evaluation time, and descriptions of the interpretations of the diagnostic tests. The true-positive (TP), false-positive (FP), true-negative (TN), and false-negative (FN) data were extracted or derived to construct 2 × 2 contingency tables.
Meta-analysis
We constructed forest plots to show the variations of the SEN and SPE estimates together with 95% confidence intervals (CI) for each imaging test in each study. We calculated the SEN, SPE, PLR, NLR and DOR values with their 95% CIs. We constructed HSROC curves to estimate SEN and SPE36.
Standard χ2-testing and the inconsistency index (I-squared, I2) were used to assess the heterogeneity of the individual studies using Stata software (Stata Corporation, College Station, TX, USA). P < 0.1 or I2 > 50% suggested notable heterogeneity37. If notable heterogeneities were detected, the test performance was summarised using a random-effects coefficient binary regression model; otherwise, a fixed-effects coefficient binary regression model was used25.
Subgroup analyses were performed as follows: (a) comparisons of studies using different cut-off values: ROC analysis subgroup (cut-off obtained by ROC analysis) or pre-specified subgroup (cut-off set by pre-specified criteria, MRI with anatomic response criteria, and PET/CT with metabolic response criteria); (b) comparisons of studies using different MRI modalities: conventional CE-MRI subgroup (longest diameter or tumour volume) or functional MRI subgroup (parameter of quantitative perfusion MR, DWI, or MRS); and (c) comparisons of studies with different evaluation time points of second imaging: early evaluation subgroup (second imaging scan before 3 cycles) or post evaluation subgroup (second imaging scan after 3 cycles).
The presence of publication bias was assessed by a Deeks funnel plot and an asymmetry test. Publication bias was considered present if there was a nonzero slope coefficient (P < 0.05), which suggests that only small studies reporting high accuracy had been published38, 39.
Electronic supplementary material
Author Contributions
Wang Jian and Huang Xuequan contributed to conception and design of the study; Chen Lihua and Yang Qifang contributed to data acquisition, analysis and interpretation of the data, design and editing the manuscript; Liu Daihong and Bao Jing contributed to statistical analysis and editing the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Lihua Chen and Qifang Yang contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-08852-8
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xuequan Huang, Email: hxuequan@163.com.
Jian Wang, Email: wangjian811@gmail.com.
References
- 1.Goldhirsch A, Glick JH, Gelber RD, Coates AS, Senn HJ. Meeting highlights: International Consensus Panel on the Treatment of Primary Breast Cancer. Seventh International Conference on Adjuvant Therapy of Primary Breast Cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2001;19:3817–3827. doi: 10.1200/JCO.2001.19.18.3817. [DOI] [PubMed] [Google Scholar]
- 2.von Minckwitz G, et al. Capecitabine in addition to anthracycline- and taxane-based neoadjuvant treatment in patients with primary breast cancer: phase III GeparQuattro study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:2015–2023. doi: 10.1200/JCO.2009.23.8303. [DOI] [PubMed] [Google Scholar]
- 3.Thomas, E. et al. The use of alternate, non-cross-resistant adjuvant chemotherapy on the basis of pathologic response to a neoadjuvant doxorubicin-based regimen in women with operable breast cancer: long-term results from a prospective randomized trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology22, 2294–2302, doi:10.1200/jco.2004.05.207 (2004). [DOI] [PubMed]
- 4.Marinovich ML, et al. Meta-analysis of agreement between MRI and pathologic breast tumour size after neoadjuvant chemotherapy. British journal of cancer. 2013;109:1528–1536. doi: 10.1038/bjc.2013.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu LM, et al. Can diffusion-weighted MR imaging and contrast-enhanced MR imaging precisely evaluate and predict pathological response to neoadjuvant chemotherapy in patients with breast cancer? Breast cancer research and treatment. 2012;135:17–28. doi: 10.1007/s10549-012-2033-5. [DOI] [PubMed] [Google Scholar]
- 6.Park SH, et al. Comparison of diffusion-weighted MR imaging and FDG PET/CT to predict pathological complete response to neoadjuvant chemotherapy in patients with breast cancer. European radiology. 2012;22:18–25. doi: 10.1007/s00330-011-2236-x. [DOI] [PubMed] [Google Scholar]
- 7.Mghanga FP, Lan X, Bakari KH, Li C, Zhang Y. Fluorine-18 fluorodeoxyglucose positron emission tomography-computed tomography in monitoring the response of breast cancer to neoadjuvant chemotherapy: a meta-analysis. Clinical breast cancer. 2013;13:271–279. doi: 10.1016/j.clbc.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Marinovich ML, et al. Meta-analysis of magnetic resonance imaging in detecting residual breast cancer after neoadjuvant therapy. Journal of the National Cancer Institute. 2013;105:321–333. doi: 10.1093/jnci/djs528. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs MA, et al. Monitoring of neoadjuvant chemotherapy using multiparametric, (2)(3)Na sodium MR, and multimodality (PET/CT/MRI) imaging in locally advanced breast cancer. Breast cancer research and treatment. 2011;128:119–126. doi: 10.1007/s10549-011-1442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Partridge SC, et al. Association between serial dynamic contrast-enhanced MRI and dynamic 18F-FDG PET measures in patients undergoing neoadjuvant chemotherapy for locally advanced breast cancer. Journal of magnetic resonance imaging: JMRI. 2010;32:1124–1131. doi: 10.1002/jmri.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semple SI, et al. Baseline MRI delivery characteristics predict change in invasive ductal breast carcinoma PET metabolism as a result of primary chemotherapy administration. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2006;17:1393–1398. doi: 10.1093/annonc/mdl136. [DOI] [PubMed] [Google Scholar]
- 12.Tozaki M, et al. Monitoring of early response to neoadjuvant chemotherapy in breast cancer with (1)H MR spectroscopy: comparison to sequential 2-[18F]-fluorodeoxyglucose positron emission tomography. Journal of magnetic resonance imaging: JMRI. 2008;28:420–427. doi: 10.1002/jmri.21454. [DOI] [PubMed] [Google Scholar]
- 13.An YY, Kim SH. Treatment Response Evaluation of Breast Cancer after Neoadjuvant Chemotherapy and Usefulness of the Imaging Parameters of MRI and PET/CT. Journal of Korean medical science. 2015;30:808–815. doi: 10.3346/jkms.2015.30.6.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi JH, et al. The role of PET CT to evaluate the response to neoadjuvant chemotherapy in advanced breast cancer: comparison with ultrasonography and magnetic resonance imaging. Journal of surgical oncology. 2010;102:392–397. doi: 10.1002/jso.21424. [DOI] [PubMed] [Google Scholar]
- 15.Hieken TJ, Boughey JC, Jones KN, Shah SS, Glazebrook KN. Imaging response and residual metastatic axillary lymph node disease after neoadjuvant chemotherapy for primary breast cancer. Annals of surgical oncology. 2013;20:3199–3204. doi: 10.1245/s10434-013-3118-z. [DOI] [PubMed] [Google Scholar]
- 16.Kim, T. et al. Utility of MRI and PET/CT after neoadjuvant chemotherapy in breast cancer patients: correlation with pathological response grading system based on tumor cellularity. Acta radiologica (Stockholm, Sweden: 1987) 55, 399–408, doi:10.1177/0284185113498720 (2014). [DOI] [PubMed]
- 17.Pahk K, Kim S, Choe JG. Early prediction of pathological complete response in luminal B type neoadjuvant chemotherapy-treated breast cancer patients: comparison between interim 18F-FDG PET/CT and MRI. Nuclear medicine communications. 2015;36:887–891. doi: 10.1097/MNM.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 18.Park JS, et al. The assessment of breast cancer response to neoadjuvant chemotherapy: comparison of magnetic resonance imaging and 18F-fluorodeoxyglucose positron emission tomography. Acta radiologica (Stockholm, Sweden: 1987) 2011;52:21–28. doi: 10.1258/ar.2010.100142. [DOI] [PubMed] [Google Scholar]
- 19.Pengel KE, et al. Combined use of (1)(8)F-FDG PET/CT and MRI for response monitoring of breast cancer during neoadjuvant chemotherapy. European journal of nuclear medicine and molecular imaging. 2014;41:1515–1524. doi: 10.1007/s00259-014-2770-2. [DOI] [PubMed] [Google Scholar]
- 20.Tateishi U, et al. Neoadjuvant chemotherapy in breast cancer: prediction of pathologic response with PET/CT and dynamic contrast-enhanced MR imaging–prospective assessment. Radiology. 2012;263:53–63. doi: 10.1148/radiol.12111177. [DOI] [PubMed] [Google Scholar]
- 21.Cho N, et al. Early prediction of response to neoadjuvant chemotherapy in breast cancer patients: comparison of single-voxel (1)H-magnetic resonance spectroscopy and (18)F-fluorodeoxyglucose positron emission tomography. European radiology. 2016;26:2279–2290. doi: 10.1007/s00330-015-4014-7. [DOI] [PubMed] [Google Scholar]
- 22.Amioka A, et al. Ability of contrast-enhanced ultrasonography to determine clinical responses of breast cancer to neoadjuvant chemotherapy. Japanese Journal of Clinical Oncology. 2016;46:303–309. doi: 10.1093/jjco/hyv215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X, et al. Combined use of MRI and PET to monitor response and assess residual disease for locally advanced breast cancer treated with neoadjuvant chemotherapy. Acad Radiol. 2004;11:1115–1124. doi: 10.1016/j.acra.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Liu Q, et al. The Role of (18)F-FDG PET/CT and MRI in Assessing Pathological Complete Response to Neoadjuvant Chemotherapy in Patients with Breast Cancer: A Systematic Review and Meta-Analysis. BioMed research international. 2016;2016 doi: 10.1155/2016/3746232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leeflang MM, Deeks JJ, Gatsonis C, Bossuyt PM. Systematic reviews of diagnostic test accuracy. Ann Intern Med. 2008;149:889–897. doi: 10.7326/0003-4819-149-12-200812160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brazzelli, M. et al. Magnetic resonance imaging versus computed tomography for detection of acute vascular lesions in patients presenting with stroke symptoms. Cochrane Database Syst Rev, CD007424, doi:10.1002/14651858.CD007424.pub2 (2009). [DOI] [PubMed]
- 27.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2009;50(Suppl 1):122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rieber A, et al. Breast MRI for monitoring response of primary breast cancer to neo-adjuvant chemotherapy. European radiology. 2002;12:1711–1719. doi: 10.1007/s00330-001-1233-x. [DOI] [PubMed] [Google Scholar]
- 29.Lindenberg MA, et al. Imaging performance in guiding response to neoadjuvant therapy according to breast cancer subtypes: A systematic literature review. Critical reviews in oncology/hematology. 2017;112:198–207. doi: 10.1016/j.critrevonc.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 30.Davey J, Turner RM, Clarke MJ, Higgins JP. Characteristics of meta-analyses and their component studies in the Cochrane Database of Systematic Reviews: a cross-sectional, descriptive analysis. BMC medical research methodology. 2011;11 doi: 10.1186/1471-2288-11-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McAuley L, Ba’Pham, Tugwell P, Moher D. Does the inclusion of grey literature influence estimates of intervention effectiveness reported in meta-analyses? Lancet. 2000;356:1228–1231. doi: 10.1016/S0140-6736(00)02786-0. [DOI] [PubMed] [Google Scholar]
- 32.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3 doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whiting PF, et al. Evaluation of QUADAS, a tool for the quality assessment of diagnostic accuracy studies. BMC Med Res Methodol. 2006;6 doi: 10.1186/1471-2288-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whiting PF, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 36.Menke J. Bivariate random-effects meta-analysis of sensitivity and specificity with SAS PROC GLIMMIX. Methods of information in medicine. 2010;49(54–62):62–54. doi: 10.3414/ME09-01-0001. [DOI] [PubMed] [Google Scholar]
- 37.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.StataCorp (2009). Stata Statistical Software: Release 11. College Station, TX: StataCorp LP. http://www.stata.com/.
- 39.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. Journal of Clinical Epidemiology. 2005;58:882–893. doi: 10.1016/j.jclinepi.2005.01.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.