Abstract
Laparotomy remains the gold standard for diagnosis of acute mesenteric ischemia (AMI), but is often unhelpful or too late due to non-specific clinical and radiological signs. This systematic review and meta-analysis aims to evaluate the diagnostic accuracy of the novel serological biomarkers intestinal fatty acid-binding protein (I-FABP), α-glutathione S-transferase (α-GST), d-lactate, ischemia modified albumin (IMA), and citrulline to detect AMI. A systematic search of electronic databases was performed to identify all published diagnostic accuracy studies on I-FABP, α-GST, d-lactate, IMA, and citrulline. Articles were selected based on pre-defined inclusion and exclusion criteria. Risk of bias and applicability were assessed. Two-by-two contingency tables were constructed to calculate accuracy standards. Summary estimates were computed using random-effects models. The search yielded 1925 papers, 21 were included in the final analysis. Pooled sensitivity and specificity for investigated biomarkers were: I-FABP (Uden); 79.0 (95% CI 66.5–88.5) and 91.3 (87.0–94.6), I-FABP (Osaka); 75.0 (67.9–81.2) and 79.2 (76.2–82.0), d-lactate; 71.7 (58.6–82.5) and 74.2 (69.0–79.0), α-GST; 67.8 (54.2–79.5) and 84.2 (75.3–90.9), IMA; 94.7 (74.0–99.9) and 86.4 (65.1–97.1), respectively. One study investigated accuracy standards for citrulline: sensitivity 39% and specificity 100%. The novel serological biomarkers I-FABP, α-GST, IMA, and citrulline may offer improved diagnostic accuracy of acute mesenteric ischemia; however, further research is required to specify threshold values and accuracy standards for different aetiological forms.
Electronic supplementary material
The online version of this article (doi:10.1007/s11739-017-1668-y) contains supplementary material, which is available to authorized users.
Keywords: Acute abdomen, Intestinal fatty acid-binding protein, Glutathione S-transferases, d-Lactate, Ischemia modified albumin, Citrulline, Biomarker, Acute mesenteric ischemia, Non-occlusive mesenteric ischemia, Diagnostic accuracy
Introduction
Acute mesenteric ischemia (AMI) is a rare, but potentially catastrophic medical condition with mortality rates up to 58–80% in the critical care setting [1, 2]. Various mechanisms may provoke intestinal ischemia, either from vascular or obstructive origin, such as bowel strangulation [3–5]. Four aetiological forms of vascular AMI have been identified [6]: arterial embolism, arterial thrombosis, venous thrombosis, and non-occlusive mesenteric ischemia (NOMI). NOMI may be caused by profound and disproportionate splanchnic vasoconstriction during low flow states in critically ill patients, or perioperative during major aortic surgery when splanchnic blood flow is disrupted or mesenteric arteries are sacrificed [7–9]. Early diagnosis is pivotal for reversal of ischemic damage, whereas delayed intervention may result in intestinal necrosis, multiple organ dysfunction syndrome, and death. However, diagnosis is difficult, particularly in the early stages when treatment is most beneficial [10, 11]. Performance of currently available laboratory tests is suboptimal (e.g., the l-lactate sensitivity and specificity is 86 and 44% [12]). The best diagnostic test apart from diagnostic laparotomy remains contrast computed tomography (angiography), (sensitivity 94%, specificity 95% [12, 13]). Several new biomarkers may facilitate diagnostic accuracy and will be addressed in this article.
Intestinal fatty acid-binding protein (I-FABP) is a small cytosolic protein exclusively expressed by enterocytes and is rapidly released into the circulation in case of mesenteric cell damage [14, 15]. The short lifetime of plasma I-FABP (11 min) facilitates the tracking of ischemic enterocyte damage almost in real time [16]. The glutathione S-transferases (GSTs) are a family of enzymes involved in intracellular detoxification. The α-subunit of GST is present in the liver and small intestines. The plasma level of α-GST has been suggested to be a sensitive marker of small bowel ischemia [17, 18]. d-Lactate is the stereoisomer of l-lactate and is produced by colonic bacteria only as a product of fermentation. Elevated d-lactate levels have been associated with bacterial overgrowth due to infection [19], short bowel syndrome [20] and mesenteric infarction [21]. Ischemia modified albumin (IMA) is human serum albumin that is less capable of binding cobalt due to ischemia [22]. Elevated IMA plasma levels have been associated with myocardial ischemia [23], but may also be of value for the diagnosis of mesenteric ischemia. IMA is measured through the cobalt–albumin-binding assay (CABA) test. Citrulline is an amino acid produced in the mitochondria of mature enterocytes. It has been shown that plasma citrulline is an accurate biomarker of the functional enterocyte mass and a plasma concentration less than 20 μmol/L is a marker of enterocyte mass reduction [24]. Its circulating half-life is 3–4 h [25, 26].
The aim of the present study is to perform a systematic review and meta-analysis of the available literature concerning the diagnostic accuracy and predictability of I-FABP, α-GST, d-lactate, IMA, and citrulline as serological biomarkers for the early diagnosis of AMI.
Methods
Search strategy
A systematic search in Embase, PubMed, and the Cochrane Library was performed to identify all relevant literature published before November 2016 (Supplementary Appendix 1). Only studies written in English, Dutch, French, Spanish, or German were included. Duplicates were removed using Covidence® software (Melbourne, Australia, 2015) [27]. Two reviewers (NT, AP) screened potential relevant articles based on title and abstract, and according to pre-defined inclusion and exclusion criteria (Fig. 1). Cross-references of relevant reviews were screened.
Fig. 1.
Search strategy and flow chart. Some authors investigated multiple biomarkers
Selection criteria
Eligible studies were observational or case-controlled studies that assessed the diagnostic accuracy of the investigated serological biomarkers in patients with AMI suspected on clinical grounds. AMI was ideally confirmed by laparotomy, colonoscopy, or autopsy. A study was included in the meta-analysis if true positive, false positive, true negative, and false negative test results could be derived to pool and calculate diagnostic accuracy standards directly from published data.
Assessment of methodological quality
Using modified criteria based on the QUADAS-2 tool and the Cochrane checklist for diagnostic studies, two authors (NT, AP) independently critically appraised the selected articles for risk of bias (validity) and applicability [27–30]. Judgments were discussed after which consensus was reached. Risk of bias was considered high in case of a low score on ≥2 items, moderate in case of a low score in 1–2 items, and low when all items were scored moderate or high. Verification bias was considered of limited importance, as AMI will eventually be either diagnosed by laparotomy or autopsy. In case of full clinical recovery without invasive intervention, it was safely assumed that no mesenteric infarction of clinical importance was present. Applicability was considered low in case of absent extractable data or poor representation of domain.
Data synthesis and statistical analysis
Data from individual studies and pooled results are expressed as means with 95% confidence intervals (CI). Data to construct two-by-two contingency tables were retrieved to calculate diagnostic accuracy standards. Meta-DiSc® version 1.4 (Meta-DiSc Software, Madrid, Spain) [31] was used to calculate pooled sensitivity and specificity, and positive- and negative-likelihood ratios (LR). A random-effect model according to DerSimonian and Laird was used for meta-analysis [32]. When a two-by-two table included a zero cell, 0.5 was added [31, 33].
Data derived by meta-analyses are presented as forest plots. Forest plots display the diagnostic probabilities of individual studies and the corresponding 95% CI. Units for d-lactate were converted from mcg/mL to mmol/L using 90 g/mol as the molar mass for lactate. Study heterogeneity was determined by the χ 2 tests and I 2 measures. Studies with an I 2 value below 25% were considered homogeneous, 26–50 and 51–75% as low and moderate and over 75% as high heterogeneity, respectively [34]. A p value of <0.05 was considered statistically significant. Results were reported in accordance with the PRISMA recommendations [35]. The protocol for this systematic review and meta-analysis was registered on PROSPERO (CRD42016052163) [36].
Results
Search and selection criteria
The study includes results of electronic searches up to November 2016. Figure 1 depicts the selection of articles included in the analysis. A total of 1925 papers were identified of which 44 were retrieved for full-text review. A total of 15 papers on I-FABP, seven on d-lactate, three on α-GST, two on IMA, and one on citrulline were ultimately selected for final critical appraisal. In one article [37], I-FABP, d-lactate, and α-GST were studied simultaneously. In two papers, both I-FABP and d-lactate were studied [38, 39].
Critical appraisal
Results of critical appraisal are shown in Table 1. After critical appraisal, three papers were excluded from the final analysis [49, 50, 52]. Camkiran studied plasma I-FABP levels in 35 patients undergoing elective coronary artery bypass; however, none of the patients developed AMI. The study by Lieberman was excluded due to low applicability and high risk of bias. As for Collange, no AMI was observed in patients undergoing elective infrarenal aortic aneurysm surgery.
Table 1.
Critical appraisal
| References | Study design | Patient selection | Threshold | Blinded index test results | Valid reference test | Disease progression | Verification | Withdrawal | Risk of bias | Representative patient sample | Extractable data | Applicability | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I-FABP | Block et al. [37] | Cohort | ● | ○ | ● | ● | ● | ○ | ● | Low | ● | ◐ | Moderate |
| Cronk et al. [40] | Cohort | ● | ◐ | ● | ● | ● | ◐ | ● | Low | ● | ● | High | |
| Güzel et al. [41] | Case–control | ◐ | ● | ● | ● | ○ | ● | ● | Moderate | ● | ● | High | |
| Kanda et al. [15]a | Case–control | ◐ | ● | ● | ● | ○ | ○ | ● | Moderate | ● | ● | High | |
| Kanda et al. [42] | Cohort | ● | ● | ● | ● | ○ | ◐ | ● | Low | ● | ● | High | |
| Kittaka et al. [43] | Cohort | ● | ● | ● | ● | ● | ◐ | ● | Low | ● | ● | High | |
| Matsumoto et al. [44] | Cohort | ● | ● | ● | ● | ● | ◐ | ● | Low | ● | ● | High | |
| Matsumoto et al. [45] | Cohort | ● | ● | ● | ● | ○ | ● | ● | Low | ● | ● | High | |
| Shi et al. [38] | Cohort | ● | ● | ● | ● | ● | ◐ | ● | Low | ● | ● | High | |
| Thuijls et al. [46] | Cohort | ● | ● | ● | ● | ● | ◐ | ● | Low | ● | ● | High | |
| Uzun et al. [47] | Case–control | ◐ | ● | ● | ◐ | ○ | ○ | ● | High | ● | ● | High | |
| Vermeulen et al. [48] | Cohort | ● | ● | ● | ● | ● | ◐ | ● | Low | ● | ● | High | |
| Voort et al. [39]a | Cohort | ● | ○ | ● | ● | ● | ◐ | ● | Low | ● | ◐ | Moderate | |
| Camkiran et al. [49] | Cohort | ● | ○ | ● | ○ | ● | ○ | ● | High | ● | ○ | Low | |
| Lieberman et al. [50]a | Case–control | ◐ | ○ | ● | ○ | ○ | ○ | ● | High | ◐ | ◐ | Low | |
| d-Lactate | Assadian et al. [51] | Cohort | ● | ○ | ● | ● | ● | ● | ● | Low | ● | ◐ | High |
| Block et al. [37] | Cohort | ● | ◐ | ● | ● | ● | ◐ | ● | Low | ● | ● | High | |
| Collange et al. [52] | Cohort | ● | ◐ | ● | ● | ● | ● | ● | Low | ● | ○ | Low | |
| Murray et al. [53] | Case–control | ◐ | ◐ | ○ | ● | ○ | ● | ● | Moderate | ● | ● | High | |
| Poeze et al. [21] | Case–control | ◐ | ◐ | ● | ◐ | ○ | ◐ | ● | High | ● | ● | High | |
| Shi et al. [38] | Cohort | ● | ● | ● | ● | ● | ● | ● | Low | ● | ● | High | |
| Voort et al. [39]a | Cohort | ● | ○ | ● | ● | ● | ◐ | ● | Low | ● | ◐ | Moderate | |
| α-GST | Block et al. [37] | Cohort | ● | ◐ | ● | ● | ● | ◐ | ● | Low | ● | ● | High |
| Delaney et al. [54] | Case–control | ◐ | ● | ● | ● | ● | ● | ● | Low | ● | ● | High | |
| Gearhart et al. [55] | Cohort | ● | ◐ | ● | ● | ● | ◐ | ◐ | Low | ● | ● | High | |
| IMA | Gunduz et al. [56] | Case–control | ◐ | ● | ● | ● | ● | ● | ● | Low | ● | ● | High |
| Polk et al. [57] | Cohort | ● | ● | ● | ● | ● | ● | ● | Low | ● | ● | High | |
| C | Kulu et al. [58] | Case–control | ○ | ● | ● | ● | ○ | ● | ● | Low | ● | ● | High |
Patient selection: ● consecutive order, well described in- and exclusion criteria ◐ case–control with consecutive case selection ○ inappropriate exclusions. Threshold: ● based on ROC-analysis ◐ pre-specified ○ not reported. |Blinded index test results: ● yes ○ no/not reported. Valid reference standard: ● surgery, endoscopy, autopsy, full clinical recovery ◐ CT scanning, lab findings ○ none/not reported. Disease progression: ● < 12 h ○ ≥ 12 h/not reported. Verification: ●all patients received both index and reference test. Reference test was the same for all patients ◐ selected patients received equal reference tests ○ selected patients received different reference tests. Withdrawal: ● no loss to follow up ◐loss to follow up, reasons given ○ loss to follow up without reasons given/not reported. Representative patient sample: ● patients with suspected AMI ◐ healthy control group ○ non-matching domain. Extractable data: ● 2 × 2 table data extractable ◐ levels of biomarkers reported, no 2 × 2 data extractable ○ only correlation, no data on AMI
aArticles found by hand searching
Clinical results
Table 2 presents the characteristics of the included studies. The final analysis includes 21 studies evaluating 1670 patients for AMI. The pooled prevalence is 22.0%. The pre-test probability of AMI varied (4.1–53.7%) between studies, reflecting variations in domains. In 15 studies, patients presenting with an acute abdomen were studied (n = 1436, mean prevalence 21.4%). Two papers [40, 43] evaluated patients with bowel obstruction (n = 58, 41.4%). An additional four papers [21, 39, 48, 51] included patients at risk for NOMI. Table 3 presents the accuracy data extracted from each individual study. Table 4 presents pooled sensitivity and specificity for each biomarker.
Table 2.
Characteristics of included studies
| (A) Study | Country | No. of patients | Study population | Timing of blood sampling | Reference test | I-FABP measurement | Prevalence AMI (%) |
|---|---|---|---|---|---|---|---|
| I-FABP studies | |||||||
| Block et al. [37] | Sweden | 71 | Acute abdomen | At presentation | Laparotomy, histopathology, autopsy, clinical evaluation, radiological findings | ELISA (Hycult Biotechnology b.c., Uden, The Netherlands) | 14.1 |
| Cronk et al. [40] | USA | 21 | Mechanical bowel obstruction | At presentation | Laparotomy | ELISA (Hycult Biotechnology b.c., Uden, The Netherlands) | 14.3 |
| Güzel et al. [41] | Turkey | 57 | Acute abdomen | NR | Laparotomy and histopathology | ELISA (Hycult Biotechnology b.c., Uden, The Netherlands) | 47.4 |
| Kanda et al. [42] | Japan | 361 | Acute abdomen | Within 24 h after presentation | Laparotomy | ELISA, rabbit and mice anti-human I-FABP polyclonal antibodies | 14.4 |
| Kittaka et al. [43] | Japan | 37 | Small bowel obstruction | At presentation | Laparotomy | ELISA, rabbit and mice anti-human I-FABP polyclonal antibodies | 45.9 |
| Matsumoto et al. [44] | Japan | 146 | Acute abdomen | Directly after initial assessment | Laparotomy, autopsy, clinical evaluation | Recombinant I-FABP assay (Sumitomo Pharma Biomedical Centre, Osaka, Japan) | 16.4 |
| Matsumoto et al. [45] | Japan | 48 | Pneumatosis intestinalis | At presentation | Laparotomy | Osaka | 39.0 |
| Shi et al. [38] | China | 272 | Acute abdomen | At presentation | Laparotomy, autopsy, CT scanning, colonoscopy | Standard ELISA kits NOS | 14.3 |
| Thuijls et al. [46] | The Netherlands | 50 | Acute abdomen | At presentation | Laparotomy/autopsy with PA, consensus | ELISA (Hycult Biotechnology b.c., Uden, The Netherlands) | 47.8 |
| Uzun et al. [47] | Turkey | 171 | Acute abdomen | At presentation | NR | ELISA (Hycult Biotechnology b.c., Uden, The Netherlands) | 4.1 |
| Vermeulen Windsant et al. [48] | The Netherlands | 96 | Major aortic surgery | At 7 time points peri-operatively | Laparotomy | ELISA (Hycult Biotechnology b.c., Uden, The Netherlands) | 4.2 |
| Kanda et al. [15] | Japan | 61 | Acute abdomen | At presentation | Laparotomy | ELISA (Niigata University School of Medicine, Niigata, Japan) | 21.3 |
| van der Voort et al. [39] | The Netherlands | 44 | ICU patients | When AMI was considered in the diagnostic work up | Laparotomy, histopathology, endoscopy, CT scan | ELISA (Hycult Biotechnology b.c., Uden, The Netherlands) | 52 |
| (B) Study | Country | No. of patients | Study population | Timing of blood sampling | Reference test | Biomarker measurement | Prevalence AMI (%) |
|---|---|---|---|---|---|---|---|
| d-Lactate studies | |||||||
| Block et al. [37] | Sweden | 71 | Acute abdomen | At presentation | Laparotomy, histopathology, autopsy, clinical evaluation, radiological findings | Spectrophotometry using R-BIOPHARM AG, Darmstadt, Germany) | 14.1 |
| Assadian et al. [51] | Austria | 12 | Open aortic reconstruction | At 4 time points peri-operatively | Histopathology (biopsy during sigmoidoscopy) | Enzymatic reactions using d-Lactate dehydrogenase and alanine aminotransferase | 25 |
| Shi et al. [38] | China | 272 | Acute abdomen | At presentation | Laparotomy, autopsy, CT scanning, colonoscopy | Standard ELISA kits | 14.3 |
| Murray et al. [53] | USA | 31 | Acute abdomen scheduled for surgery | Preoperative | Laparotomy | Spectrophotometrically | 29.0 |
| Poeze et al. [21] | The Netherlands | 24 | Major emergency aortic surgery | Postoperatively at admission ICU | Colonoscopy | Enzymatic reactions using d-Lactate dehydrogenase and alanine aminotransferase | 45.8 |
| van der Voort et al. [39] | The Netherlands | 44 | ICU patients | When AMI was considered in the diagnostic work up | Laparotomy, histopathology, endoscopy, CT scan | Spectrophotometrically | 52 |
| α-GST studies | |||||||
| Block et al. [37] | Sweden | 71 | Acute abdomen | At presentation | Laparotomy, histopathology, autopsy, clinical evaluation, radiological findings | ELISA IHEPKIT, Biotrin International, Dublin, Ireland) | 14.1 |
| Gearhart et al. [55] | USA | 54 | Patients with clinical suspicion for AMI | At presentation | Colonoscopy, angiography, laparotomy, autopsy | ELISA IHEPKIT, Biotrin International, Dublin, Ireland) | 53.7 |
| Delaney et al. [54] | Ireland | 26 | Acute abdomen | At presentation | Autopsy, laparotomy, other definitive investigation, return to full health | ELISA IHEPKIT, Biotrin International, Dublin, Ireland) | 46.2 |
| IMA studies | |||||||
| Gunduz et al. [56] | Turkey | 14 | Thromboembolic occlusion SMA | On admission | Laparotomy | Cobalt–Albumin-binding assay (zie references #10) | 50.0 |
| Polk et al. [57] | Sweden | 26 | Possible AMI scheduled for laparotomy | Within 1 h preoperatively | Laparotomy | Cobalt–Albumin-binding assay (zie references #3) | 46.2 |
| Citrulline studies | |||||||
| Kulu et al. [58] | Turkey | 48 | Acute abdomen | At presentation | Laparotomy | Amino Acids LC–MS/MS analysis Kit, Zivak Technologies, Turkey | 47.9 |
NR not reported, ICU intensive care unit, NOS not otherwise specified, AMI acute mesenteric ischemia
Table 3.
Data analysis
| Mean controla | Mean AMIb | p value | Cut-off level | TP | FP | TN | FN | PPVc | NPVd | |
|---|---|---|---|---|---|---|---|---|---|---|
| I-FABP Uden kit (ng/mL) | ||||||||||
| Block et al. [37] | 0.050 (0.0–0.197) | 0.186 (0.0–0.613) | 0.58 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Cronk et al. [40] | 0.281 | 1.772 | N/A | 0.1 | 3 | 4 | 14 | 0 | 0.43 (0.10–0.82) | 1.00 (0.77–1.00) |
| Güzel et al. [41] | 0.08 (0.01–0.20) | 0.421 (0.040–5.0) | <0.001 | 0.09 | 24 | 0 | 30 | 3 | 1.00 (0.86–1.00) | 0.09 (0.02–0.24) |
| Thuijls et al. [46]f | 0.109 [0.04–1.691] | 0.653 [0.04–74.711] | 0.02 | 0.268 | 15 | 7 | 17 | 7 | 0.68 (0.45–0.86) | 0.71 (0.49–0.87) |
| Uzun et al. [47] | 0.170 ± 0.543 | 0.709 ± 0.669 | N/A | 0.145 | 5 | 9 | 155 | 2 | 0.36 (0.13–0.65) | 0.99 (0.95–1.00) |
| Vermeulen Windsant et al. [48] | N/A | N/A | N/A | 0.815 | 4 | 0 | 92 | 0 | 1.00 (0.40–1.00) | 1.00 (0.96–1.00) |
| van der Voort et al. [39]f | 1.020 | 2.872 | 0.98 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| I-FABP Osaka kit (ng/mL) | ||||||||||
| Kanda et al. [15] | 25.1 ± 3.6 | 265.8 ± 111.3 | <0.0 | 100 | 7 | 0 | 48 | 6 | 1.00 (0.59–1.00) | 0.89 (0.77–0.96) |
| Kanda et al. [42] | 5.8 ± 15.6 | 40.7 ± 117.9 | <0.0001 | 3.1 | 41 | 81 | 228 | 11 | 0.34 (0.25–0.43) | 0.95 (0.92–0.98) |
| Kittaka et al. [43] | 1.6 | 18.5 | <0.001 | 6.5 | 15 | 1 | 15 | 6 | 0.94 (0.70–1.00) | 0.71 (0.48–0.89) |
| Matsumoto et al. [44] | 2.5 (0.2–56.7) | 31.0 (1.1–498.4) | <0.01 | 9.1 | 20 | 13 | 109 | 4 | 0.61 (0.42–0.77) | 0.96 (0.91–0.99) |
| Matsumoto et al. [45] | 3.2 [1.7–6.7] | 15.5 [5.3–52.9] | <0.001 | 9.7 | 19 | 6 | 37 | 8 | 0.76 (0.59 – 0.87) | 0.82 (0.72 – 0.89) |
| Shi et al. [38] | 33.9 ± 12.6 | 113.8 ± 46.3 | <0.001 | 93.07 | 30 | 59 | 174 | 9 | 0.34 (0.24–0.45) | 0.95 (0.91–0.98) |
| d-Lactate (mmol/L) | ||||||||||
| Block et al. [37] | 0.03 (0.02–0.07) | 0.05 (0.03–0.10) | 0.20 | 0.20 | 9 | 47 | 14 | 1 | 0.16 (0.08–0.28) | 0.93 (0.68–1.00) |
| Shi et al. [38]f | 0.15 ± 0.06 | 0.66 ± 0.29 | <0.001 | 0.38 | 26 | 33 | 200 | 13 | 0.44 (0.31–0.58) | 0.94 (0.90–0.97) |
| van der Voort et al. [39]e | 0.65 [0.37–0.94] | 0.79 (0.49–1.16) | 0.003g | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 0.41 [0.11–0.75] | 0.56 (0.27–0.77) | 0.46h | ||||||||
| Murray et al. [53]f | 0.12 ± 0.04 | 0.36 ± 0.04 | <0.0005 | 0.22 | 8 | 1 | 19 | 3 | 0.89 (0.52–1.00) | 0.86 (0.65–0.97) |
| Assadian et al. [51] | 1.25 ± 0.61 | 3.03 ± 1.65 | 0.035 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Poeze et al. [21] | 0.21 ± 0.06 | 0.32 ± 1.0 | <0.01 | 0.2 | 9 | 3 | 10 | 2 | 0.75 (0.43–0.95) | 0.83 (0.52–0.98) |
| α-GST (ng/ml) | ||||||||||
| Block et al. [37] | 1.3 (1.1–2.8) | 1.7 (0.7–4.2) | 0.21 | 4 | 2 | 9 | 53 | 8 | 0.18 (0.02–0.52) | 0.87 (0.76–0.94) |
| Gearhart et al. [55] | 2.2 (1.0–3.0) | 22.2 (7.0–126.0) | 0.001 | 4 | 25 | 4 | 15 | 10 | 0.86 (0.68–0.96) | 0.60 (0.39–0.79) |
| Delaney et al. [54] | 1.6 (0.8–2.2) | 75.8 (22.4–153.0) | <0.0001 | 4 | 12 | 2 | 12 | 0 | 0.86 (0.57–0.98) | 1.00 (0.74–1.00) |
| IMA (ABSU) | ||||||||||
| Polk et al. [57] | 0.31 ± 0.02 | 0.52 ± 0.04 | <0.0002 | 0.35 | 12 | 2 | 12 | 0 | 0.86 (0.57–0.98) | 1.00 (0.74–1.00) |
| Gunduz et al. [56] | 0.163 ± 0.025 | 0.264 ± 0.057 | 0.003 | 0.188 | 6 | 1 | 7 | 0 | 0.86 (0.42–1.00) | 1.00 (0.59–1.00) |
| Citrulline (nmol/ml) | ||||||||||
| Kulu et al. [58] | 32.8 ± 3.0 | 21.7 ± 3.1 | 0.01 | 15.8 | 9 | 0 | 25 | 14 | 1 | 0.64 (0.56–0.71) |
N/A not applicable, TP true positive, TN true negative, FP false positive, FN false negative, ABSU absorbance units
aNumbers between brackets represent 95% confidence intervals. Means are presented with standard deviation
bAcute mesenteric ischemia
cPositive predictive value
dNegative predictive value
eMedian [IQR] are presented
fValues were converted to mmol/L by multiplying by 0.0111
gIschemia vs. non-ischemia
hIschemia-likely vs. ischemia-unlikely
Table 4.
Meta-analysis
| No. of studies | Sensitivity | I 2 (%) | p value | Specificity | I 2 (%) | p value | Positive LRa | I 2 (%) | p value | Negative LR | I 2 (%) | p value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I-FABP (Uden kit) | 4 | 0.790 (0.665–0.885) | 16 | 0.312 | 0.913 (0.870–0.946) | 82 | 0.001 | 6.368 (2.100–18.534) | 79 | 0.003 | 0.262 (0.130–0.543) | 41 | 0.146 |
| I-FABP (Osaka kit) | 6 | 0.750 (0.679–0.812) | 0 | 0.463 | 0.792 (0.762–0.820) | 87 | 0.000 | 4.577 (2.910–7.197) | 75 | 0.001 | 0.321 (0.249–0.413) | 0 | 0.629 |
| d-lactate | 3 | 0.717 (0.586–0.825) | 20 | 0.288 | 0.742 (0.690–0.790) | 98 | 0.000 | 3.621 (0.770–17.035) | 97 | 0.000 | 0.371 (0.249–0.552) | 0 | 0.845 |
| α-GST | 3 | 0.678 (0.542–0.795) | 88 | 0.000 | 0.842 (0.753–0.909) | 0 | 0.792 | 3.27 (1.50–7.16) | 27 | 0.252 | 0.40 (0.11–1.49) | 90 | 0.000 |
| IMA | 2 | 0.947 (0.740–0.999) | 0 | 0.739 | 0.864 (0.651–0.971) | 0 | 0.906 | 6.931 (2.37–24.24) | 0 | 0.935 | 0.064 (0.02–0.48) | 0 | 0.742 |
Numbers between brackets represent 95% confidence intervals
I 2 inconsistency (I-square)
aLikelihood ratio
I-FABP
There are 13 studies including 1435 patients that examine the performance of I-FABP for the diagnosis of intestinal ischemia. The overall prevalence is 18.3%. Laparotomy (or autopsy) was performed in 1099 patients, including all patients with AMI. Plasma I-FABP was measured using two different kits. Since cut-off values differed greatly between these groups, data were pooled per kit.
In seven studies, a human ELISA kit (HyCult Biotechnologie, Uden, The Netherlands) was used. The cut-off values of these studies vary between 0.09 and 0.815 ng/mL. The studies by Block [I-FABP difference non-significant between patients with and without AMI (p = 0.58)] and Van der Voort [I-FABP with AMI: 2.872 ng/mL (95% CI 0.229–4.340) vs. I-FABP without AMI: 1.020 ng/mL (95% CI 0.239–5.324), p = 0.98] were not included as calculation of diagnostic accuracy standards was not possible. In four studies that examined the accuracy of I-FABP in patients presenting with acute abdomen, two-by-two contingency tables could be derived [40, 41, 46, 47]. Pooled sensitivity and specificity are 79.0% (95% CI 66.5–88.5) and 91.3% (95% CI 87.0–94.6), respectively (Fig. 2). Vermeulen et al. studied patients after thoracic, thoracoabdominal or abdominal aneurysm repair. They find a sensitivity and specificity of both 100%, with a cut-off value of 0.815 ng/ml.
Fig. 2.
Forest plots and SROC curve of I-FABP (Uden kit) to detect acute mesenteric ischemia. SROC summary receiver-operating characteristic, AUC area under curve, SE sensitivity
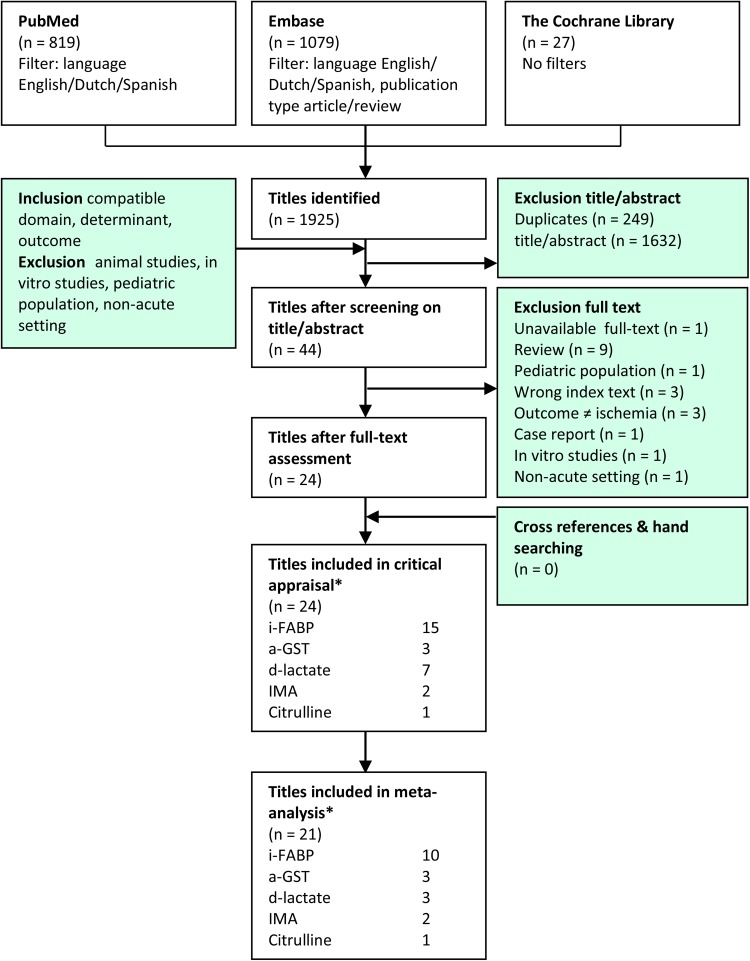
In six studies, plasma I-FABP levels were measured using a sandwich ELISA system with rabbit anti-human I-FABP polyclonal antibodies in the solid phase and mouse anti-human I-FABP monoclonal antibodies in the liquid phase (D.S. Pharma Biomedical Co., Ltd., Osaka, Japan). Although in one study [38], the exact ELISA test used was unclear; the reference values were comparable to the studies in which the Osaka kit was used. Therefore, we combined results from this study with the other Osaka kit studies. Pooled sensitivity and specificity are 75.0% (95% CI 67.9–81.2%) and 79.2% (95% CI 76.2–82.0), respectively (Fig. 3). The cut-off value varies from 3.1 and 100 ng/mL.
Fig. 3.
Forest plots and SROC curve of I-FABP (Osaka kit) to detect acute mesenteric ischemia. SROC summary receiver-operating characteristic, AUC area under curve, SE sensitivity
d-Lactate
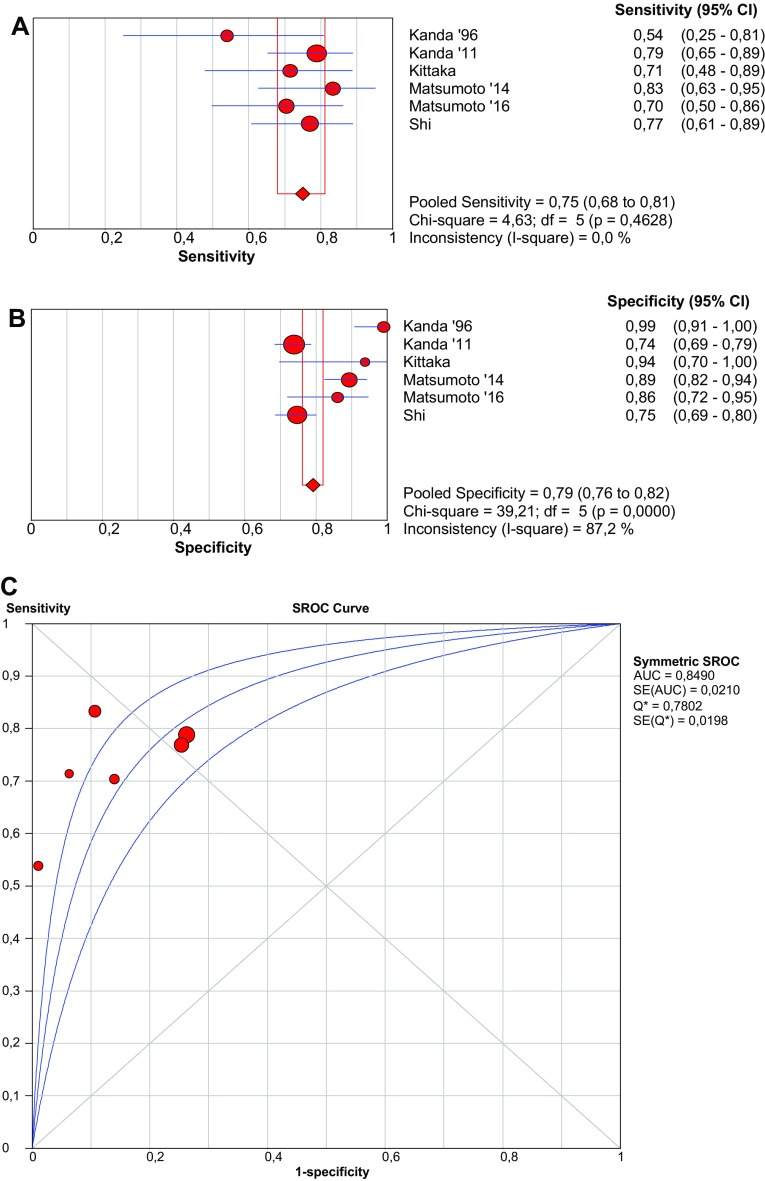
Six studies focused on d-lactate as a serological biomarker for AMI. Pooled prevalence of AMI is 17.3%. Three studies examined patients with an acute abdomen [37, 38, 53]. Pooled sensitivity and specificity are 71.7% (95% CI 58.6–82.5%) and 74.2% (95% CI 69.0–79.0%), respectively (Fig. 4).
Fig. 4.
Forest plots and SROC curve of d-lactate to detect acute mesenteric ischemia. SROC summary receiver-operating characteristic, AUC area under curve, SE sensitivity
In addition, three authors investigated patients at risk for NOMI. Poeze and colleagues studied the accuracy of d-lactate in patients after repair of ruptured abdominal aortic aneurysm (AAA), and find a sensitivity and specificity of 82 and 77%, respectively. Assadian et al. studied the presence of AMI after repair of ruptured or symptomatic AAA. A significant difference in serum d-lactate is found at 2, 24 and 48 h postoperatively (p = 0.045, p = 0.027 and p = 0.035, respectively). Van der Voort et al. calculated mean d-lactate levels in critically ill ICU patients suspected for AMI. A significant difference is found in d-lactate levels between patients with proven and likely AMI versus unlikely and non-ischemic patients (p = 0.003).
α-GST
Three studies, including 151 patients with suspected AMI, addressed the performance of α-GST for the diagnosis of AMI. The cut-off value of α-GST was pre-defined as 4 ng/mL in all studies. Pooled sensitivity and specificity are 67.8 (95% CI 54.2–79.5%) and 84.2% (95% CI 75.3–90.9%), respectively (Fig. 5).
Fig. 5.
Forest plots and SROC curve of alpha-GST to detect acute mesenteric ischemia. SROC summary receiver-operating characteristic, AUC area under curve, SE sensitivity
IMA
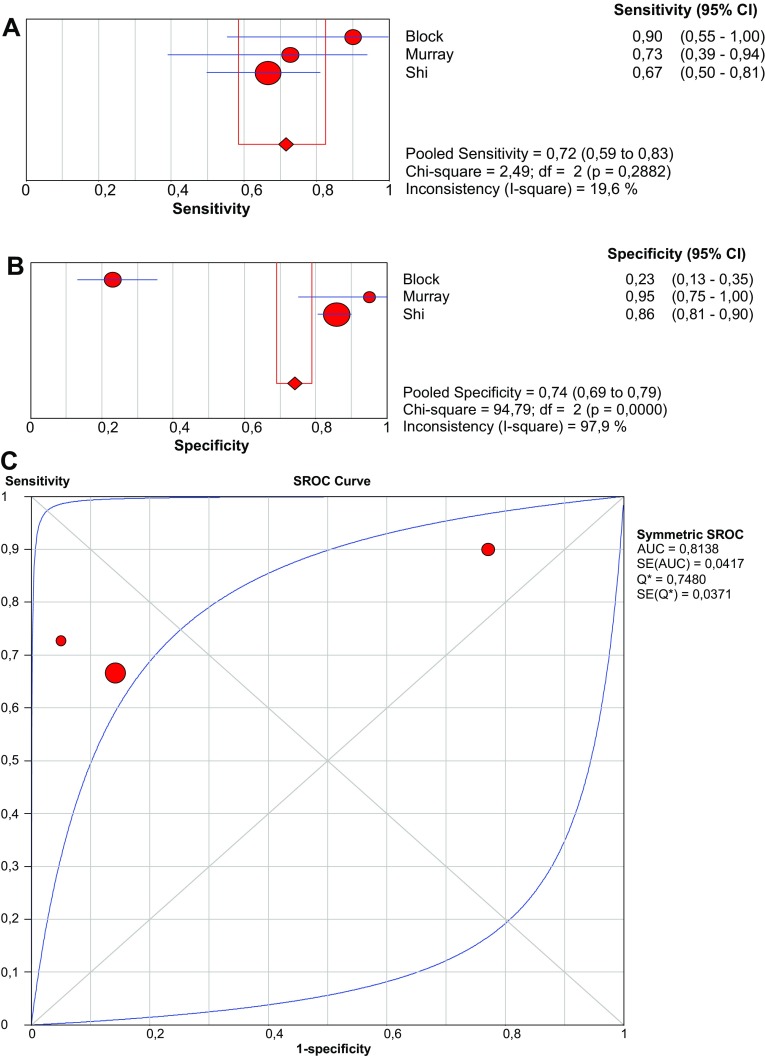
Gunduz et al. determined whether IMA is elevated in patients with AMI. In their case-controlled study of seven cases with thromboembolic occlusion of the superior mesenteric artery, they find a statistically different concentration of IMA compared to seven controls (p = 0.003). The cut-off value of 0.188 ABSU yields a positive predictive value (PPV) and negative predictive value (NPV) of 0.86 (95% CI 0.42–1.00) and 1.00 (95% CI 0.59–1.00). Polk et al. studied the value of IMA in patients presenting with an acute abdomen and calculate a PPV and NPV of 0.86 (95% CI 0.57–0.98) and 1.00 (0.74–1.00), respectively. Pooled sensitivity and specificity are 94.7 (95% CI 74.0–99.9%) and 86.4% (95% CI 65.1–97.1%), respectively (Fig. 6).
Fig. 6.
Forest plots and SROC curve of IMA to detect acute mesenteric ischemia
Citrulline
Kulu et al. investigated the diagnostic accuracy of citrulline for AMI in patients with acute abdomen. Specificity and sensitivity of 100 and 39% are found, respectively.
Discussion
Usage of serological markers as screening tools, either to contribute to the present diagnostic armamentarium, or to replace presently used diagnostic tests, should depend on the clinical setting and the pre-test probability. The incidence of AMI in patients presenting with acute abdominal pain at the emergency department is relatively low compared to patients in the ICU, and differential diagnosis is comprehensive. The CT (angiography) scan can be a valuable diagnostic tool in diagnosing occlusive AMI [59]. In NOMI, however, radiological findings are often less specific [60]. In addition, CT scanning can be contraindicated in patients with impaired kidney function or contrast allergy. Especially, in these patients, a screening serum test would be helpful. In this systematic review and meta-analysis, six serological biomarkers were analysed for their capability to diagnose AMI: I-FABP, d-lactate, α-GST and IMA, and citrulline. Citrulline (100%), I-FABP (Uden kit, 91%), and IMA (86%) demonstrate high specificity, suggesting that when the levels are below the defined cutoff, chances of AMI are low. However, false negative rates of 9–14% in I-FABP and IMA are still debatable considering the consequences of delaying laparotomy and the impact on the final outcome.
Compared to a meta-analysis performed by Evennett et al., we separated the different kits in our analyses and added eight new studies in the evaluation of I-FABP. In the follow-up of patients who were diagnosed with AMI and in whom a segment of questionable viable intestine was not resected, interval I-FABP levels can support the decision to perform a second-look operation [40]. Therefore, interval postoperative I-FABP measurement may be useful. Although the true incidence of clinically relevant AMI in patients presenting to the emergency department with acute abdominal pain is unknown [38], Thuijls et al. studied a population with a relatively high pre-test probability of AMI of 47.8%, compared to the other studies. This might have led to an overestimation of the predictive contribution of I-FABP in the diagnosis of AMI in these patients. A poor renal function delays the clearance of plasma I-FABP [43]. Except for Vermeulen, none of the selected studies excluded this group nor described renal function in baseline tables on patient characteristics. This may have led to information bias.
In d-lactate, summary sensitivity and specificity are relatively low with 71.7 (95% CI 58.6–82.5) and 74.2 (95% CI 69.0–79.0), respectively. Previously, a meta-analysis by Evennett [33] reports a sensitivity and specificity of 82 and 48%, respectively. The studies on d-lactate scored low on heterogeneity, because only studies with patients with an acute abdomen as a domain were pooled. Except for the results from Shi, none of the other studies calculate an optimal threshold according to the results. Therefore, the pooled sensitivity and specificity do not represent the most optimal values.
Although results are fairly promising (sensitivity 67.8%, specificity 84.2%), α-GST may be non-specific for AMI, as it may also be released by the liver during oxidative stress [33]. Plasma levels of α-GST may increase in patients with shock, acute, or chronic liver failure and hepatitis. These factors may influence the diagnostic accuracy in these specific patient groups, however, which have not yet been studied extensively. Since α-GST is especially specific for small bowel ischemia, isolated colonic ischemia may go underdiagnosed. This may explain the relatively low pooled sensitivity. Therefore, it seems attractive to combine α-GST with a marker more specific for the colon. Moreover, the pre-test prevalence of AMI was relatively high in two out of three studies on α-GST, leading to a limited external validity for patients with lower pre-test probabilities.
IMA demonstrated the highest sensitivity (94.7%). Nevertheless, the patient groups were small and pre-test probability was high (48.7%), because patients with a known thromboembolic occlusion were included as well. These factors may have led to an overestimation of the diagnostic accuracy. IMA levels may also be elevated in patients with cardiac ischemia. In patients in the ICU, cardiac ischemia may be present due to secondary ischemia caused by severe illness. In future research, it should be acknowledged that this might convey risk of confounding.
Only one study was found on citrulline. High specificity (100%) and positive predictive value are reported. These results should be interpreted with caution, since there was a high pre-test probability. Nevertheless, citrulline remains a potential accurate marker for AMI, since it has been shown to be a reliable marker of functional enterocyte mass [24], prognostic value of mortality in the ICU [60], and NOMI after cardiac arrest [61].
General strengths and limitations
Strengths of our review are the extensive search and critical review by independent authors. Moreover, cross-references of relevant reviews were checked to include all relevant articles. All studies on I-FABP have a low risk of interval bias because of a narrow interval between the diagnosis of AMI and the obtainment of blood samples for the determination of I-FABP levels. In addition, there is a low risk of review bias in studies on I-FABP, α-GST, and IMA, since all included studies blindly assessed the index test. Therefore, the decision whether or not to perform a laparotomy was not influenced by test results. Murray did not report blind assessment of index test results. AMI has multiple aetiologies. Therefore, in the analysis, data were pooled separately according to aetiology.
Several limitations should be mentioned. In the included studies not all patients underwent a laparotomy, which is regarded as the gold standard for AMI. Instead, diagnosis was based on combinations of clinical features, CT findings, colonoscopy, and regular laboratory findings. Partial verification bias may have been introduced. However, the effect will be limited, as clinically relevant AMI typically needs surgical intervention, or will result in a poor outcome that is detectable in the studies. Furthermore, this systematic review is limited by inter-study variation in cut-off values. An overall cut-off value could not be given for all biomarkers, except for α-GST. The serum values of the biomarkers are influenced by several factors. First, the severity of intestinal damage may result in more divergent plasma levels. The timing of sampling after the onset of symptoms varied among studies, potentially leading to interval bias. In addition, the previous colonic surgery or chronic kidney failure may affect the base-level and clearance of the investigated biomarkers. For example, in short bowel syndrome, the citrulline levels are generally lower than in the general population [62], which may bias test results. In addition, high levels of citrulline now reflect plasma clearance, and may overestimate functional enterocyte mass. In addition, the method of measurement may be of influence as well. For example, different ELISA kits were used in the studies on I-FABP. To circumvent the effect of bias caused by variation in I-FABP measurements, we considered the Uden and Osaka kits as different diagnostic tests and performed meta-analysis only on the separate groups. Variation within the same kits may be caused by inappropriate storage of samples, incorrect analyses, and inter-laboratory variation. However, no indications of variation within the same kits were found in the description of methods of these studies. Another limitation of this review is that studies with a small study population are also included. This may have incorrectly influenced the pooled diagnostic accuracy standards, leading to an over- or underestimation of results.
In conclusion, this systematic review and meta-analysis presents pooled estimates of I-FABP, d-lactate, α-GST, and IMA as serological biomarkers for the diagnosis of acute mesenteric ischemia. The best pooled performance is demonstrated for IMA and I-FABP (Uden kit). Citrulline is a promising marker as well with high reported specificity. Results should be interpreted with caution due to the heterogeneous and small patient populations studied. As both positive and negative predictive values do not demonstrate optimal performance, it is too early to consider them to replace other diagnostic modalities such as CT angiography. Possibly, combination of multiple biomarkers may lead to a synergistic diagnostic performance. Diagnostic models including both clinical, radiological, and laboratory tests may eventually facilitate identification of those patients with AMI who need urgent surgical treatment potentially reducing morbidity and mortality from this life-threatening disease.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- I-FABP
Intestinal fatty acid-binding protein
- AMI
Acute mesenteric ischemia
- OMI
Occlusive mesenteric ischemia
- NOMI
Non-occlusive mesenteric ischemia
- MODS
Multi-organ dysfunction syndrome
- α-GST
α-Glutathione S-transferase
- IMA
Ischemia-modified albumin
- ABSU
Absorbance units
- ELISA
Enzyme-linked immuno-sorbent assay
- CT
Computed tomography
- CABA
Cobalt–albumin-binding assay
- ICU
Intensive care unit
- LR
Likelihood ratio
- QUADAS
Quality assessment of diagnostic accuracy studies
- CI
Confidence interval
- NPV
Negative predictive value
- PPV
Positive predictive value
- AAA
Abdominal aortic aneurysm
- NR
Not reported
Author contributions
The research question was conceived by AvZ. NT and AP designed the study and constructed the search terms and selection criteria. Selection of eligible articles, data analysis, and interpretation were done by NT and AP. All work was drafted, critically reviewed, and edited by all authors. All authors have approved the final version.
Compliance with ethical standards
Conflict of interest
The authors declare they have no conflict of interests.
Statement of human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
None.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s11739-017-1668-y) contains supplementary material, which is available to authorized users.
Contributor Information
Nikki Treskes, Email: nikkitreskes@gmail.com.
Alexandra M. Persoon, Email: alexandrapersoon@gmail.com
Arthur R. H. van Zanten, Phone: +31-318-434115, Phone: +31-318-434116, Email: zantena@zgv.nl
References
- 1.Leone M, Bechis C, Baumstarck K, et al. Outcome of acute mesenteric ischemia in the intensive care unit: a retrospective, multicenter study of 780 cases. Intensive Care Med. 2015;41(4):667–676. doi: 10.1007/s00134-015-3690-8. [DOI] [PubMed] [Google Scholar]
- 2.Martin B. Prevention of gastrointestinal complications in the critically ill patient. Can Adv Crit Care. 2007;18:158–166. doi: 10.1097/01.AACN.0000269259.91546.d8. [DOI] [PubMed] [Google Scholar]
- 3.Reilly PM, Wilkins KB, Fuh KC, Haglund U, Bulkley GB. The mesenteric hemodynamic response to circulatory shock: an overview. Shock. 2001;15(5):329–343. doi: 10.1097/00024382-200115050-00001. [DOI] [PubMed] [Google Scholar]
- 4.Trompeter M, Brazda T, Remy CT, Vestring T, Reimer P. Non-occlusive mesenteric ischemia: etiology, diagnosis, and interventional therapy. Eur Radiol. 2002;12(5):1179–1187. doi: 10.1007/s00330-001-1220-2. [DOI] [PubMed] [Google Scholar]
- 5.Acosta S. Mesenteric ischemia. Curr Opin Crit Care. 2015;21(2):171–178. doi: 10.1097/MCC.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 6.Tilsed JV, Casamassima A, Kurihara H, et al. ESTES guidelines: acute mesenteric ischaemia. Eur J Trauma Emerg Surg. 2016;42(2):253–270. doi: 10.1007/s00068-016-0634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ceppa EP, Fuh KC, Bulkley GB. Mesenteric hemodynamic response to circulatory shock. Curr Opin Crit Care. 2003;9(2):127–132. doi: 10.1097/00075198-200304000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Lundberg J, Lundberg D, Norgren L, Ribbe E, Thörne J, Werner O. Intestinal hemodynamics during laparotomy: effects of thoracic epidural anesthesia and dopamine in humans. Anesth Analg. 1990;71(1):9–15. doi: 10.1213/00000539-199007000-00002. [DOI] [PubMed] [Google Scholar]
- 9.De Backer D, Creteur J, Silva E, Vincent JL. Effects of dopamine, norepinephrine, and epinephrine on the splanchnic circulation in septic shock: which is best? Crit Care Med. 2003;31(6):1659–1667. doi: 10.1097/01.CCM.0000063045.77339.B6. [DOI] [PubMed] [Google Scholar]
- 10.Nakashima K, Ishimaru H, Fujimoto T, et al. Diagnostic performance of CT findings for bowel ischemia and necrosis in closed-loop small-bowel obstruction. Abdom Imaging. 2015;40(5):1097–1103. doi: 10.1007/s00261-014-0335-2. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura Y, Urashima M, Toyota N, et al. Non-occlusive mesenteric ischemia (NOMI): utility of measuring the diameters of the superior mesenteric artery and superior mesenteric vein at multidetector CT. Jpn J Radiol. 2013;31:737. doi: 10.1007/s11604-013-0245-1. [DOI] [PubMed] [Google Scholar]
- 12.Cudnik MT, Darbha S, Jones J, Macedo J, Stockton SW, Hiestand BC. The diagnosis of acute mesenteric ischemia: a systematic review and meta-analysis. Acad Emerg Med. 2013;20(11):1087–1100. doi: 10.1111/acem.12254. [DOI] [PubMed] [Google Scholar]
- 13.Woo K, Major K, Kohanzadeh S, Allins AD. Laparotomy for visceral ischemia and gangrene. Am Surg. 2007;73(10):1006–1008. [PubMed] [Google Scholar]
- 14.Niewold TA, Meinen M, van der Meulen J. Plasma intestinal fatty acid binding proteitn (I-FABP) concentrations increase following intestinal ischemia in pigs. Res Vet Sci. 2004;77:89–91. doi: 10.1016/j.rvsc.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Kanda T, Fujii H, Tani T, et al. Intestinal fatty acid-binding protein is a useful diagnostic marker for mesenteric infarction in humans. Gastroenterology. 1996;110(2):339–343. doi: 10.1053/gast.1996.v110.pm8566578. [DOI] [PubMed] [Google Scholar]
- 16.Piton G, Capellier G. Biomarkers of gut barrier failure in the ICU. Curr Opin Crit Care. 2016;22(2):152–160. doi: 10.1097/MCC.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 17.Campbell JA, Corrigall AV, Guy A, Krisch RE. Immunological localization of α, μ and π class glutathione S-transferase in human tissues. Cancer. 1991;67:1608–1613. doi: 10.1002/1097-0142(19910315)67:6<1608::AID-CNCR2820670623>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 18.Mannervik B, Alin P, Guthenberg C, et al. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc Natl Acad Sci USA. 1985;82(21):7202–7206. doi: 10.1073/pnas.82.21.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith SM, Eng RH, Buccini F. Use of d-lactic acid measurements in the diagnosis of bacterial infections. J Infect Dis. 1986;154:658–664. doi: 10.1093/infdis/154.4.658. [DOI] [PubMed] [Google Scholar]
- 20.Oh MS, Phelps KR, Traube M, Barbosa-Saldivar JL, Boxhill C, Carroll HJ. d-Lactic acidosis in a man with the short-bowel syndrome. N Engl J Med. 1979;301:249–252. doi: 10.1056/NEJM197908023010505. [DOI] [PubMed] [Google Scholar]
- 21.Poeze M, Froon AH, Greve JW, Ramsay G. d-Lactate as an early marker of intestinal ischaemia after ruptured abdominal aortic aneurysm repair. Br J Surg. 1998;85(9):1221–1224. doi: 10.1046/j.1365-2168.1998.00837.x. [DOI] [PubMed] [Google Scholar]
- 22.Bar-Or D, Lau E, Winkler JV. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia-a preliminary report. J Emerg Med. 2000;19:311–315. doi: 10.1016/S0736-4679(00)00255-9. [DOI] [PubMed] [Google Scholar]
- 23.Sinha MK, Gaze DC, Tippins JR, Collinson PO, Kaski JC. Ischemia modified albumin is a sensitive marker of myocardial ischemia after percutaneous coronary intervention. Circulation. 2003;107:2403–2405. doi: 10.1161/01.CIR.0000072764.18315.6B. [DOI] [PubMed] [Google Scholar]
- 24.Crenn P, Coudray-Lucas C, Thuillier F, Cynober L, Messing B. Postabsorptive plasma citrulline concentration is a marker of absorptive enterocyte mass and intestinal failure in humans. Gastroenterology. 2000;119(6):1496–1505. doi: 10.1053/gast.2000.20227. [DOI] [PubMed] [Google Scholar]
- 25.Gondolesi G, Fishbein T, Chehade M, et al. Serum citrulline is a potential marker for rejection of intestinal allografts. Transplant Proc. 2002;34(3):918–920. doi: 10.1016/S0041-1345(02)02669-6. [DOI] [PubMed] [Google Scholar]
- 26.Ruiz P, Tryphonopoulos P, Island E, et al. Citrulline evaluation in bowel transplantation. Transplant Proc. 2010;42(1):54–56. doi: 10.1016/j.transproceed.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 27.Covidence. https://www.covidence.org/. Accessed 20 Dec 2015
- 28.QUADAS-2. http://www.bristol.ac.uk/social-community-medicine/projects/quadas/quadas-2/. Accessed 20 Dec 2015
- 29.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;10(3):25. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cochrane Checklist for Quality of Diagnostic Studies. http://netherlands.cochrane.org/sites/netherlands.cochrane.org/files/uploads/4.2%20Checklist%20Beoordeling%20diagnostisch%20onderzoek.pdf. Accessed 20 Dec 2015
- 31.Zamora J, Abraira V, Muriel A, et al. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;12:31. doi: 10.1186/1471-2288-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 33.Evennett NJ, Petrov MS, Mittal A, Windsor JA. Systematic review and pooled estimates for the diagnostic accuracy of serological markers for intestinal ischemia. World J Surg. 2009;33(7):1374–1383. doi: 10.1007/s00268-009-0074-7. [DOI] [PubMed] [Google Scholar]
- 34.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, John PA. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Treskes N, Persoon AM, Zanten ARH (2016) Diagnostic accuracy of novel serological biomarkers to detect acute mesenteric ischaemia: a systematic review and meta-analysis. CRD42016052163. https://www.crd.york.ac.uk/PROSPERO/register_new_review.asp. Accessed 6 Feb 2017 [DOI] [PMC free article] [PubMed]
- 37.Block T, Nilsson TK, Björck M, Acosta S. Diagnostic accuracy of plasma biomarkers for intestinal ischaemia. Scand J Clin Lab Investig. 2008;68(3):242–248. doi: 10.1080/00365510701646264. [DOI] [PubMed] [Google Scholar]
- 38.Shi H, Wu B, Wan J, Liu W, Su B. The role of serum intestinal fatty acid binding protein levels and d-lactate levels in the diagnosis of acute intestinal ischemia. Clin Res Hepatol Gastroenterol. 2015;2210–7401(14):00301–00305. doi: 10.1016/j.clinre.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 39.van der Voort PH, Westra B, Wester JP, et al. Can serum l-lactate, d-lactate, creatine kinase and I-FABP be used as diagnostic markers in critically ill patients suspected for bowel ischaemia. BMC Anesthesiol. 2014;2(14):111. doi: 10.1186/1471-2253-14-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cronk DR, Houseworth TP, Cuadrado DG, Herbert GS, McNutt PM, Azarow KS. Intestinal fatty acid binding protein (I-FABP) for the detection of strangulated mechanical small bowel obstruction. Curr Surg. 2006;63(5):322–325. doi: 10.1016/j.cursur.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 41.Güzel M, Sözüer EM, Salt Ö, İkizceli İ, Akdur O, Yazıcı C. The value of the serum I-FABP level for diagnosing acute mesenteric ischemia. Surg Today. 2014;44(11):2072–2076. doi: 10.1007/s00595-013-0810-3. [DOI] [PubMed] [Google Scholar]
- 42.Kanda T, Tsukahara A, Ueki K, et al. Diagnosis of ischemic small bowel disease by measurement of serum intestinal fatty acid-binding protein in patients with acute abdomen: a multicenter, observer-blinded validation study. J Gastroenterol. 2011;46(4):492–500. doi: 10.1007/s00535-011-0373-2. [DOI] [PubMed] [Google Scholar]
- 43.Kittaka H, Akimoto H, Takeshita H, et al. Usefulness of intestinal fatty acid-binding protein in predicting strangulated small bowel obstruction. PLoS One. 2001;9(6):e99915. doi: 10.1371/journal.pone.0099915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsumoto S, Sekine K, Funaoka H, et al. Diagnostic performance of plasma biomarkers in patients with acute intestinal ischaemia. Br J Surg. 2014;101(3):232–238. doi: 10.1002/bjs.9331. [DOI] [PubMed] [Google Scholar]
- 45.Matsumoto S, Sekine K, Funaoka H, et al. Diagnostic value of intestinal fatty acid-binding protein for pneumatosis intestinalis. Am J Surg. 2016;212(5):961–968. doi: 10.1016/j.amjsurg.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Thuijls G, van Wijck K, Grootjans J, et al. Early diagnosis of intestinal ischemia using urinary and plasma fatty acid binding proteins. Ann Surg. 2011;253(2):303–308. doi: 10.1097/SLA.0b013e318207a767. [DOI] [PubMed] [Google Scholar]
- 47.Uzun O, Turkmen S, Eryigit U, Mentese A, Turkyilmaz S, Turedi S, Karahan SC, Gunduz A. Can intestinal fatty acid binding protein (I-FABP) Be a marker in the diagnosis of abdominal pathology? Turkiye Acil Tip Dergisi. 2014;14(3):99–103. doi: 10.5505/1304.7361.2014.15679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vermeulen Windsant IC, Hellenthal FA, Derikx JP, Prins MH, Buurman WA, Jacobs MJ, Schurink GW. Circulating intestinal fatty acid-binding protein as an early marker of intestinal necrosis after aortic surgery: a prospective observational cohort study. Ann Surg. 2012;255(4):796–803. doi: 10.1097/SLA.0b013e31824b1e16. [DOI] [PubMed] [Google Scholar]
- 49.Camkıran A, Dönmez A, Aldemir D, Işgüzar RA, Gültekin B. Clinical significance of intestinal type fatty acid binding protein in patients undergoing coronary artery bypass surgery. Anadolu Kardiyol Derg. 2011;11(6):536–541. doi: 10.5152/akd.2011.139. [DOI] [PubMed] [Google Scholar]
- 50.Lieberman JM, Sacchettini J, Marks C, Marks WH. Human intestinal fatty acid binding protein: report of an assay with studies in normal volunteers and intestinal ischemia. Surgery. 1997;121(3):335–342. doi: 10.1016/S0039-6060(97)90363-9. [DOI] [PubMed] [Google Scholar]
- 51.Assadian A, Assadian O, Senekowitsch C, et al. Plasma d-lactate as a potential early marker for colon ischaemia after open aortic reconstruction. Eur J Vasc Endocvasc Surg. 2006;31:470–474. doi: 10.1016/j.ejvs.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 52.Collange O, Tamion F, Meyer N, Quillard M, Kindo M, Hue G, Veber B, Dureuil B, Plissonnier D. Early detection of gut ischemia-reperfusion injury during aortic abdominal aneurysmectomy: a pilot, observational study. J Cardiothorac Vasc Anesth. 2013;27(4):690–695. doi: 10.1053/j.jvca.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 53.Murray MJ, Gonze MD, Nowak LR, Cobb CF. Serum d(−)-lactate levels as an aid to diagnosing acute intestinal ischemia. Am J Surg. 1994;167(6):575–578. doi: 10.1016/0002-9610(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 54.Delaney CP, O’Neill S, Manning F, Fitzpatrick JM, Gorey TF. Plasma concentrations of glutathione S-transferase isoenzyme are raised in patients with intestinal ischaemia. Br J Surg. 1999;86(10):1349–1353. doi: 10.1046/j.1365-2168.1999.01245.x. [DOI] [PubMed] [Google Scholar]
- 55.Gearhart SL, Delaney CP, Senagore AJ, et al. Prospective assessment of the predictive value of alpha-glutathione S-transferase for intestinal ischemia. Am Surg. 2003;69(4):324–329. [PubMed] [Google Scholar]
- 56.Gunduz A, Turedi S, Mentese A, et al. Ischemia-modified albumin in the diagnosis of acute mesenteric ischemia: a preliminary study. Am J Emerg Med. 2008;26(2):202–205. doi: 10.1016/j.ajem.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 57.Polk JD, Rael LT, Craun ML, Mains CW, Davis-Merritt D, Bar-Or D. Clinical utility of the cobalt-albumin binding assay in the diagnosis of intestinal ischemia. J Trauma. 2008;64(1):42–45. doi: 10.1097/TA.0b013e31815b846a. [DOI] [PubMed] [Google Scholar]
- 58.Kulu R, Akyildiz H, Akcan A, et al. Plasma citrulline measurement in the diagnosis of acute mesenteric ischaemia. ANZ J Surg. 2016 doi: 10.1111/ans.13524. [DOI] [PubMed] [Google Scholar]
- 59.Mastoraki A, Mastoraki S, Tziava E, et al. Mesenteric ischemia: pathogenesis and challenging diagnostic and therapeutic modalities. World J Gastrointest Pathophysiol. 2016;7(1):125–130. doi: 10.4291/wjgp.v7.i1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piton G, Manzon C, Monnet E, Cypriani B, Barbot O, Navellou JC, Carbonnel F, Capellier G. Plasma citrulline kinetics and prognostic value in critically ill patients. Intensive Care Med. 2010;36(4):702–706. doi: 10.1007/s00134-010-1751-6. [DOI] [PubMed] [Google Scholar]
- 61.Piton G, Belin N, Barrot L, et al. Enterocyte damage: a piece in the puzzle of post-cardiac arrest syndrome. Shock. 2015;44(5):438–444. doi: 10.1097/SHK.0000000000000440. [DOI] [PubMed] [Google Scholar]
- 62.Papadia C, Sherwood RA, Kalantzis C, Wallis K, Volta U, Fiorini E, Forbes A. Plasma citrulline concentration: a reliable marker of small bowel absorptive capacity independent of intestinal inflammation. Am J Gastroenterol. 2007;102(7):1474–1482. doi: 10.1111/j.1572-0241.2007.01239.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.