Abstract
Low density lipoprotein receptor-related protein 1 (LRP1) C766T polymorphism (rs1799986) has been extensively investigated for Alzheimer’s disease (AD) susceptibility. However, results in different studies have been contradictory. Therefore, we conducted a meta-analysis containing 6455 AD cases and 6304 controls from 26 independent case–control studies to determine whether there was an association between the LRP1 C766T polymorphism and AD susceptibility. The combined analysis showed that there was no significant association between LRP1 C766T polymorphism and AD susceptibility (TT + CT versus CC: OR = 0.920, 95% CI = 0.817–1.037, P = 0.172). In subgroup analysis, significant decreased AD susceptibility was found among Asian population in allele model (T versus C: OR = 0.786, 95% CI = 0.635–0.974, P = 0.028) and dominant model (TT + CT versus CC: OR = 0.800, 95% CI = 0.647–0.990, P = 0.040). Moreover, T allele of LRP1 C766T was statistically associated with late onset of AD (LOAD) (T versus C: OR = 0.858, 95% CI = 0.748–0.985, P = 0.029; TT + CT versus CC: OR = 0.871, 95% CI = 0.763–0.994, P = 0.040). In conclusion, our meta-analysis suggested that LRP1 C766T polymorphism was associated with lower risk of AD in Asian, and could reduce LOAD risk especially. Considering some limitations of our meta-analysis, further large-scale studies should be done to reach a more comprehensive understanding.
Introduction
Alzheimer’s disease (AD), a progressive and lethal neurodegenerative disorder, has become a global challenge for the 21st century1, 2. It is essentially characterised by cerebral senile plaques laden with β-amyloid peptide (Aβ), dystrophic neurites in neocortical terminal fields as well as neurofibrillary tangles of hyperphosphorylated microtubule-associated protein tau3. Besides, loss of neurons and white matter, congophilic angiopathy, inflammation, and oxidative damage are also important pathological features of AD. It is believed that genetic factors, lifestyle and environmental factors synergistically give rise to AD. Variants associated with AD have been detected in more than 20 genes, which are involved in metabolism, inflammation, synaptic activity and intracellular trafficking4, 5.
Low density lipoprotein receptor-related protein 1 (LRP1) has been widely studied due to its pleiotropic roles in AD pathogenesis6. LRP1 is ubiquitously expressed in various tissues, especially high in liver, lung and brain7. In the central nervous system, LRP1 plays an important role in controlling Aβ metabolism and maintaining brain homeostasis. There are two forms of LRP1–soluble LRP1 and cell-surface LRP1. In plasma, soluble LRP1 binds to peripheral Aβ, and consequently prevents free Aβ access to the brain8. As a cell surface receptor, LRP1 can control the endocytosis of multiple ligands, mediate cell signaling transductions and regulate gene expression through its intracellular domain9–11. For instance, the interaction between amyloid precursor protein (APP) and cell-surface LRP1 leads to increased endosomal trafficking of APP, accelerating Aβ production. Besides that, Aβ can enter multiple cell types (eg. abluminal brain endothelial cell and hepatic cell) through cell-surface LRP1, in which the ubiquitous apolipoprotein E (APOE) and activated alpha-2-macroglobulins (A2M) are chaperones, and subsequently degraded by endopeptidase12. Therefore, LRP1 are involved in the bulk transport, primary production, brain and systemic clearance of AD toxin Aβ, and thus plays a critical role in AD pathogenesis.
The silent C766T polymorphism in exon 3 of LRP1 gene (rs1799986) has attracted extensive attention since first reported as a risk factor for AD13. However, results in different studies have been contradictory. The inconsistency is likely to relate with insufficient statistical power, racial differences or other demographic variables. Therefore, we conducted a comprehensive meta-analysis to determine whether there was an association between the LRP1 C766T polymorphism and AD susceptibility.
Results
Eligible studies
A total of 167 relevant studies were identified from initial database searching, of which 35 publications were included based on titles and abstracts (Fig. 1). Furthermore, 4 reviews, 1 duplicated publication and 3 studies with inadequate information were excluded after careful reading of the full text. Besides, manual search of references revealed 3 more articles. After primary data extracted from the 30 independent studies, 4 studies were excluded for genotype distribution of controls was not in Hardy-Weinberg equilibrium (HWE)14–17. Finally, 26 eligible studies containing 6455 AD cases and 6304 controls were included in our meta-analysis. The characteristics of the 26 studies on LRP1 C766T polymorphism and AD susceptibility was summarized in Table 1. The ethnicities of these subjects involved in the comparisons were diverse, including Caucasian (n = 16), Asian (n = 6), African (n = 1) and mixed (n = 3). Besides, LRP1 C766T genotype and allele distribution among AD cases and controls was summarized in Table 2, and the control group in all studies was in HWE.
Figure 1.
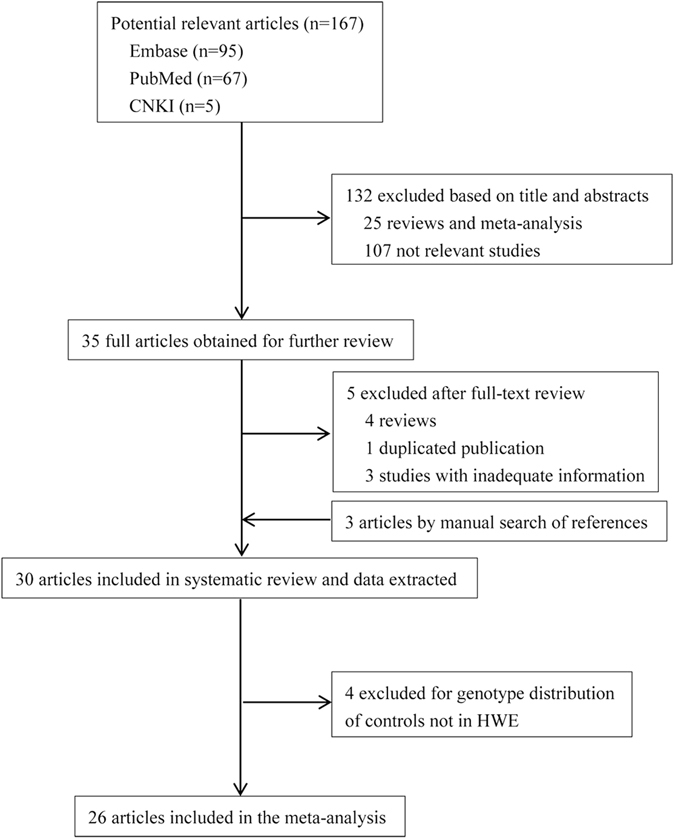
Flow chart of selection studies in our meta-analysis.
Table 1.
Characteristics of individual studies included in the meta-analysis.
| First author | Year | Country | Ethnicity | AD | Controls | Criteria for AD diagnosis | Genotyping method | Source of control | Time of AD onset | Quality score | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na | Ageb | Agec | Genderd | N | Ageb | Genderd | |||||||||
| Yuan, Q.50 | 2013 | China | Asian | 364 | 74.9 | 69.9 | 57% | 291 | 73.7 | 60% | NINCDS-ADRDA | PCR and Direct sequencing | HB | Mixed | 9 |
| Vargas, T.32 | 2010 | Spain | Caucasian | 746 | NA | 73.7 | 66% | 598 | 74.8 | 68% | NINCDS-ADRDA and DSM-IV | TaqMan SNP Genotyping Assays | PB | NA | 12 |
| Vazquez-Higuera, J. L.52 | 2009 | Spain | Caucasian | 246 | 76.6 | 72.9 | 65% | 237 | 81.2 | 69% | NINCDS-ADRDA | PCR-RFLP | PB | Mixed | 10 |
| Chen, Y.29 | 2009 | China | Asian | 67 | 71.9 | NA | 34% | 77 | 70.0 | 45% | NINCDS-ADRDA | PCR-RFLP | PB | NA | 8 |
| Bahia, V. S.33 | 2008 | Brazil | Mixed | 120 | 75.2 | 71.2 | 68% | 120 | 72.5 | 63% | NINCDS-ADRDA and DSM-IV | PCR-RFLP | PB | Mixed | 10 |
| Rodriguez, E.34 | 2006 | Spain | Caucasian | 274 | 75.4 | 71.6 | 68% | 283 | 80.5 | 71% | NINCDS-ADRDA | PCR-RFLP | PB | Mixed | 8 |
| Forero, D. A.35 | 2006 | Colombia | Mixed | 106 | 73.3 | 68.8 | 71% | 97 | 72.2 | NA | NINCDS-ADRDA | PCR-RFLP | NA | Mixed | 7 |
| Pritchard, A-136 | 2005 | UK | Caucasian | 250 | NA | 56.7 | 55% | 235 | 50.9 | 52% | NINCDS-ADRDA and DSM-III-R | PCR-RFLP | PB | Early | 9 |
| Pritchard, A-236 | 2005 | UK | Caucasian | 183 | NA | 73.8 | 65% | 220 | 76.8 | 44% | NINCDS-ADRDA and DSM-III-R | PCR-RFLP | PB | Late | 9 |
| Bian, L.60 | 2005 | China | Asian | 216 | NA | 74.7 | NA | 200 | 72.0 | NA | NINCDS-ADRDA and DSM-IV | PCR-RFLP | PB | Late | 11 |
| Panza, F.37 | 2004 | Italy | Caucasian | 166 | 69.4 | NA | 62% | 225 | 71.3 | 68% | NINCDS-ADRDA | Roche LightCycler Genotyping | PB | Mixed | 9 |
| Zheng, W. D.38 | 2004 | China | Asian | 79 | 72.8 | >65 | 49% | 156 | 71.2 | 41% | NINCDS-ADRDA | PCR-RFLP | PB | Late | 10 |
| Kolsch, H.31 | 2003 | Germany | Caucasian | 212 | 73.1 | NA | 71% | 337 | 73.2 | 61% | DSM-IV | PCR-RFLP | PB + HB | NA | 12 |
| Helbecque, N-153 | 2003 | France | Caucasian | 239 | 74.0 | NA | 65% | 232 | 79.0 | 68% | NINCDS-ADRDA and DSM-III-R | PCR-RFLP | HB | NA | 10 |
| Helbecque, N-253 | 2003 | France | Caucasian | 56 | 85.0 | NA | 80% | 180 | 79.0 | 51% | NINCDS-ADRDA and DSM-III-R | PCR-RFLP | HB | NA | 9 |
| Perry, R. T.39 | 2001 | USA | African | 111 | 71.3 | NA | 78% | 78 | 75.2 | 76% | NINCDS-ADRDA | PCR-RFLP | PB | NA | 11 |
| Bi, S.28 | 2001 | China | Asian | 38 | 70.2 | NA | 45% | 40 | 69.2 | 40% | NINCDS-ADRDA | PCR-RFLP | PB | NA | 8 |
| Sanchez-Guerra, M.40 | 2001 | Spain | Caucasian | 305 | 75.5 | 71.8 | 68% | 304 | 80.4 | 72% | NINCDS-ADRDA | PCR-RFLP | PB | Mixed | 12 |
| McIlroy, S. P.41 | 2001 | UK | Caucasian | 219 | 77.5 | >65 | 67% | 237 | 77.2 | 70% | NINCDS-ADRDA and DSM-IV | PCR-SSCP | PB | Late | 12 |
| Prince, J. A.42 | 2001 | Sweden | Caucasian | 204 | NA | NA | 61% | 171 | NA | 63% | NINCDS-ADRDA | PCR-SSCP | PB + HB | Late | 10 |
| Verpillat, P.43 | 2001 | France | Caucasian | 274 | NA | 65.5 | 56% | 290 | 67.4 | 57% | NINCDS-ADRDA | PCR-RFLP | PB | NA | 12 |
| Bullido, M. J.51 | 2000 | Spain | Caucasian | 199 | NA | 70.4 | 60% | 243 | 72.0 | 62% | NINCDS-ADRDA | PCR-SSCP | PB | Late | 10 |
| Hatanaka, Y.48 | 2000 | Japan | Asian | 100 | NA | 76.6 | 68% | 246 | 79.4 | NA | NINCDS-ADRDA and DSM-IV | PCR-RFLP | PB | Late | 8 |
| Bertram, L.45 | 2000 | USA | Mixed | 276 | NA | 71.7 | NA | 194 | NA | NA | NINCDS-ADRDA | PCR-SSCP | PB | NA | 11 |
| Beffert, U.44 | 1999 | Canada | Caucasian | 225 | NA | 70.9 | 48% | 187 | NA | 41% | NA | PCR-RFLP | PB + HB | NA | 9 |
| Kamboh, M. I.49 | 1998 | USA | Caucasian | 432 | 75.4 | 68.6 | 62% | 106 | 67.8 | 59% | NINCDS-ADRDA and DSM-III-R | PCR-SSCP | NA | NA | 9 |
| Lambert, J. -C.30 | 1998 | France | Caucasian | 558 | 71.8 | 68.6 | 62% | 596 | 72.7 | 63% | NINCDS-ADRDA and DSM-III-R | PCR-SSCP | NA | NA | 9 |
| Kang, D. E.13 | 1997 | USA | Caucasian | 157 | >65 | 73.2 | 53% | 102 | 77.1 | 53% | NINCDS-ADRDA | PCR-SSCP | PB | Late | 11 |
NINCDS: the National Institute of Neurological Disorders and Stoke; ADRDA: Alzheimer Diseases and Related Disorders Association; DSM: the Diagnostic and Statistical Manual of Mental Disorders; NA: not available; PB: population-based control; HB: hospital-based control. aNumber. bAge at survey. cAge at onset of Alzheimer’s disease. dPercentage of female.
Table 2.
LRP1 C766T genotype and allele distribution among AD cases and controls in the included studies.
| First author | AD | Control | HWE | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CC | CT | TT | C | T | CC | CT | TT | C | T | P a | |
| Yuan, Q.50 | 304 | 54 | 6 | 662 | 66 | 232 | 52 | 7 | 516 | 66 | 0.058 |
| Vargas, T.32 | 559 | 172 | 15 | 1290 | 202 | 442 | 138 | 18 | 1022 | 174 | 0.079 |
| Vazquez-Higuera, J. L.52 | 193 | 51 | 2 | 437 | 55 | 198 | 35 | 4 | 431 | 43 | 0.107 |
| Chen, Y.29 | 59 | 8 | 0 | 126 | 8 | 56 | 19 | 2 | 131 | 23 | 0.800 |
| Bahia, V. S.33 | 87 | 28 | 5 | 202 | 38 | 86 | 30 | 4 | 202 | 38 | 0.497 |
| Rodriguez, E.34 | 211 | NA | NA | NA | NA | 233 | NA | NA | NA | NA | 0.576 |
| Forero, D.A.35 | 84 | 22 | 0 | 190 | 22 | 78 | 18 | 1 | 174 | 20 | 0.972 |
| Pritchard, A.36 | 337 | 115 | 14 | 789 | 143 | 334 | 132 | 11 | 800 | 154 | 0.629 |
| Bian, L.60 | 189 | 26 | 1 | 404 | 28 | 179 | 21 | 0 | 379 | 21 | 0.433 |
| Panza F37 | 115 | 49 | 2 | 279 | 53 | 160 | 63 | 2 | 383 | 67 | 0.116 |
| Zheng, W. D.35 | 72 | 6 | 1 | 150 | 8 | 139 | 16 | 1 | 294 | 18 | 0.478 |
| Kolsch, H.31 | 145 | 59 | 8 | 349 | 75 | 250 | 84 | 3 | 584 | 90 | 0.156 |
| Helbecque, N.53 | 216 | 70 | 9 | 502 | 88 | 290 | 108 | 14 | 688 | 136 | 0.321 |
| Perry, R. T.39 | 97 | 14 | 0 | 208 | 14 | 74 | 4 | 0 | 152 | 4 | 0.816 |
| Bi, S.28 | 31 | 6 | 1 | 68 | 8 | 24 | 13 | 3 | 61 | 19 | 0.516 |
| Sanchez-Guerra, M.40 | 237 | 65 | 3 | 539 | 71 | 249 | 51 | 4 | 549 | 59 | 0.457 |
| McIlroy, S. P.41 | 193 | 24 | 2 | 410 | 28 | 198 | 37 | 2 | 433 | 41 | 0.852 |
| Prince, J. A.42 | 155 | 47 | 2 | 357 | 51 | 124 | 41 | 6 | 289 | 53 | 0.269 |
| Verpillat, P.43 | 198 | 71 | 5 | 467 | 81 | 214 | 66 | 10 | 494 | 86 | 0.092 |
| Bullido, M. J.51 | 151 | 47 | 1 | 349 | 49 | 173 | 66 | 4 | 412 | 74 | 0.417 |
| Hatanaka, Y.48 | 83 | 17 | 0 | 183 | 17 | 200 | 45 | 1 | 445 | 47 | 0.358 |
| Bertram, L.45 | 186 | 82 | 8 | 454 | 98 | 135 | 55 | 4 | 325 | 63 | 0.556 |
| Beffert, U.44 | 158 | 58 | 9 | 374 | 76 | 125 | 57 | 5 | 307 | 67 | 0.619 |
| Kamboh, M. I.49 | 310 | 111 | 11 | 731 | 133 | 71 | 29 | 6 | 171 | 41 | 0.205 |
| Lambert, J. -C.30 | 428 | 119 | 11 | 975 | 141 | 407 | 168 | 21 | 982 | 210 | 0.480 |
| Kang, D. E.13 | 127 | 26 | 4 | 280 | 34 | 65 | 34 | 3 | 164 | 40 | 0.563 |
HWE: Hardy-Weinberg equilibrium. a P value for HWE test in controls.
Meta-analysis and meta-regression results
The combined analysis showed that there was no significant association between LRP1 C766T polymorphism and AD susceptibility in any genetic model (T versus C: OR = 0.905, 95% CI = 0.813–1.008, P = 0.069; TT versus CC: OR = 0.791, 95% CI = 0.622–1.005, P = 0.055; CT versus CC: OR = 0.915, 95% CI = 0.813–1.030, P = 0.139; TT + CT versus CC: OR = 0.920, 95% CI = 0.817–1.037, P = 0.172; TT versus CC + CT: OR = 0.815, 95% CI = 0.640–1.037, P = 0.095) (Table 3 and Fig. 2).
Table 3.
Meta-analysis of LRP1 C766T polymorphism and AD susceptibility.
| Population | Comparison | Sample size | Na | Association | Model | Heterogeneity | Publication bias | |||
|---|---|---|---|---|---|---|---|---|---|---|
| AD | Control | OR (95% CI) | P | P | I 2 (%) | P | ||||
| Overall | T vs. C | 6181 | 6021 | 25 | 0.905 (0.813, 1.008) | 0.069 | Random | 0.013 | 43.0 | 0.849 |
| TT vs. CC | 6074 | 5943 | 24 | 0.791 (0.622, 1.005) | 0.055 | Fixed | 0.623 | 0 | 0.971 | |
| CT vs. CC | 6181 | 6021 | 25 | 0.915 (0.813, 1.030) | 0.139 | Random | 0.031 | 37.5 | 0.758 | |
| TT + CT vs. CC | 6455 | 6304 | 26 | 0.920 (0.817, 1.037) | 0.172 | Random | 0.008 | 44.7 | 0.829 | |
| TT vs. CC + CT | 6074 | 5943 | 24 | 0.815 (0.640, 1.037) | 0.095 | Fixed | 0.683 | 0 | 0.972 | |
| Caucasian | T vs. C | 4704 | 4522 | 15 | 0.905 (0.801, 1.022) | 0.107 | Random | 0.019 | 48.4 | 0.959 |
| TT vs. CC | 4704 | 4522 | 15 | 0.777 (0.595, 1.013) | 0.062 | Fixed | 0.329 | 11.1 | 0.901 | |
| CT vs. CC | 4704 | 4522 | 15 | 0.916 (0.795, 1.055) | 0.223 | Random | 0.021 | 47.7 | 0.950 | |
| TT + CT vs. CC | 4978 | 4805 | 16 | 0.926 (0.806, 1.065) | 0.281 | Random | 0.008 | 52.3 | 0.861 | |
| TT vs. CC + CT | 4704 | 4522 | 15 | 0.799 (0.612, 1.043) | 0.099 | Fixed | 0.353 | 8.9 | 0.941 | |
| Asian | T vs. C | 864 | 1010 | 6 | 0.786 (0.635, 0.974) | 0.028 | Fixed | 0.156 | 37.5 | 0.460 |
| TT vs. CC | 864 | 1010 | 6 | 0.642 (0.297, 1.386) | 0.259 | Fixed | 0.764 | 0 | 0.786 | |
| CT vs. CC | 864 | 1010 | 6 | 0.810 (0.648, 1.011) | 0.063 | Fixed | 0.351 | 10.1 | 0.279 | |
| TT + CT vs. CC | 864 | 1010 | 6 | 0.800 (0.647, 0.990) | 0.040 | Fixed | 0.232 | 27.0 | 0.388 | |
| TT vs. CC + CT | 864 | 1010 | 6 | 0.687 (0.315, 1.498) | 0.346 | Fixed | 0.825 | 0 | 0.732 | |
| EOAD | T vs. C | 355 | 300 | 3 | 0.966 (0.743, 1.257) | 0.799 | Fixed | 0.332 | 9.3 | 0.977 |
| TT vs. CC | 321 | 267 | 2 | 1.506 (0.477, 4.750) | 0.485 | Fixed | 0.719 | 0 | NA | |
| CT vs. CC | 355 | 300 | 3 | 0.906 (0.699, 1.174) | 0.454 | Fixed | 0.435 | 0 | 0.922 | |
| TT + CT vs. CC | 355 | 300 | 3 | 0.933 (0.727, 1.198) | 0.587 | Fixed | 0.363 | 1.2 | 0.947 | |
| TT vs. CC + CT | 321 | 267 | 2 | 1.536 (0.484, 4.873) | 0.467 | Fixed | 0.769 | 0 | NA | |
| LOAD | T vs. C | 1524 | 1832 | 10 | 0.858 (0.748, 0.985) | 0.029 | Fixed | 0.423 | 1.7 | 0.346 |
| TT vs. CC | 1524 | 1832 | 10 | 0.678 (0.374, 1.229) | 0.200 | Fixed | 0.889 | 0 | 0.994 | |
| CT vs. CC | 1524 | 1832 | 10 | 0.880 (0.767, 1.009) | 0.066 | Fixed | 0.176 | 29.2 | 0.702 | |
| TT + CT vs. CC | 1524 | 1832 | 10 | 0.871 (0.763, 0.994) | 0.040 | Fixed | 0.255 | 20.4 | 0.520 | |
| TT vs. CC + CT | 1524 | 1832 | 10 | 0.714 (0.394, 1.294) | 0.267 | Fixed | 0.875 | 0 | 0.861 | |
| APOE ε4+ | T vs. C | 924 | 308 | 6 | 0.706 (0.436, 1.145) | 0.158 | Random | 0.051 | 54.6 | 0.446 |
| TT vs. CC | 815 | 252 | 4 | 0.743 (0.320, 1.723) | 0.489 | Fixed | 0.532 | 0 | 0.378 | |
| CT vs. CC | 924 | 308 | 6 | 0.716 (0.407, 1.257) | 0.244 | Random | 0.048 | 55.2 | 0.683 | |
| TT + CT vs. CC | 1073 | 363 | 7 | 0.790 (0.475, 1.313) | 0.363 | Random | 0.030 | 57.1 | 0.683 | |
| TT vs. CC + CT | 815 | 252 | 4 | 0.770 (0.331, 1.791) | 0.544 | Fixed | 0.528 | 0 | 0.369 | |
| APOE ε4− | T vs. C | 819 | 1207 | 6 | 1.054 (0.894, 1.242) | 0.530 | Fixed | 0.591 | 0 | 0.546 |
| TT vs. CC | 819 | 1207 | 6 | 0.883 (0.475, 1.641) | 0.693 | Fixed | 0.924 | 0 | 0.776 | |
| CT vs. CC | 819 | 1207 | 6 | 1.095 (0.926, 1.295) | 0.288 | Fixed | 0.491 | 0 | 0.360 | |
| TT + CT vs. CC | 944 | 1435 | 7 | 1.120 (0.967, 1.298) | 0.130 | Fixed | 0.403 | 2.90 | 0.386 | |
| TT vs. CC+CT | 819 | 1207 | 6 | 0.876 (0.470, 1.632) | 0.677 | Fixed | 0.924 | 0 | 0.665 | |
OR: odds ratio; CI: Confidence interval; EOAD: early onset of AD; LOAD: late onset of AD. aNumber of comparisons.
Figure 2.
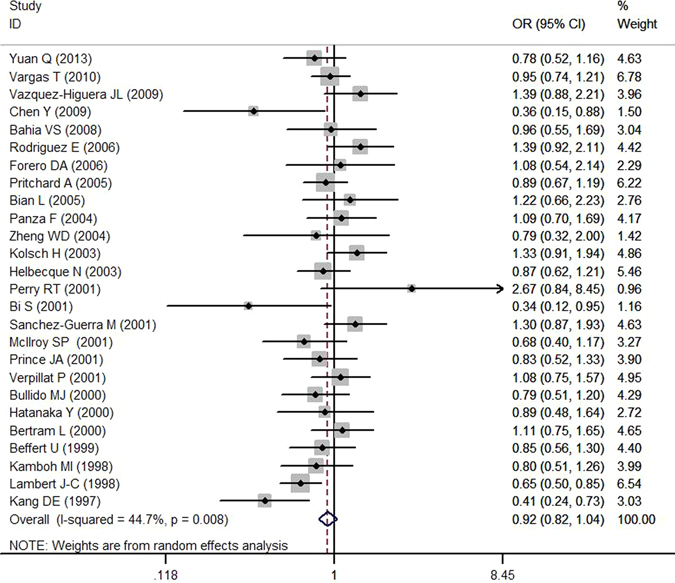
Forest plot of association between LRP1 C766T polymorphism (TT + CT vs. CC) and AD susceptibility.
In subgroup analysis by ethnicity, T allele of LRP1 C766T was found to be associated with decreased AD susceptibility among Asian population (T versus C: OR = 0.786, 95% CI = 0.635–0.974, P = 0.028; TT + CT versus CC: OR = 0.800, 95% CI = 0.647–0.990, P = 0.040) (Fig. 3). However, we did not observe any association for all comparisons in Caucasians. When stratified by time of AD onset, we found T allele of LRP1 C766T may act as a protective factor for late onset of AD (LOAD) (T versus C: OR = 0.858, 95% CI = 0.748–0.985, P = 0.029; TT + CT versus CC: OR = 0.871, 95% CI = 0.763–0.994, P = 0.040) (Fig. 4), but no significant association was observed for early onset of AD (EOAD). Furthermore, no significant interaction was observed for APOE ε4 status (P > 0.05).
Figure 3.
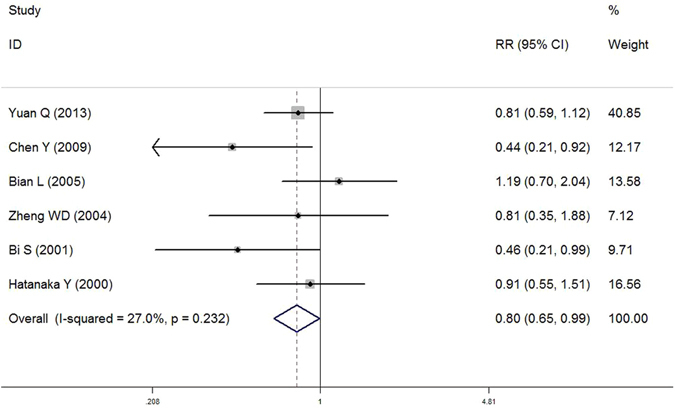
Forest plot of association between LRP1 C766T polymorphism (TT + CT vs. CC) and AD susceptibility in Asian population.
Figure 4.
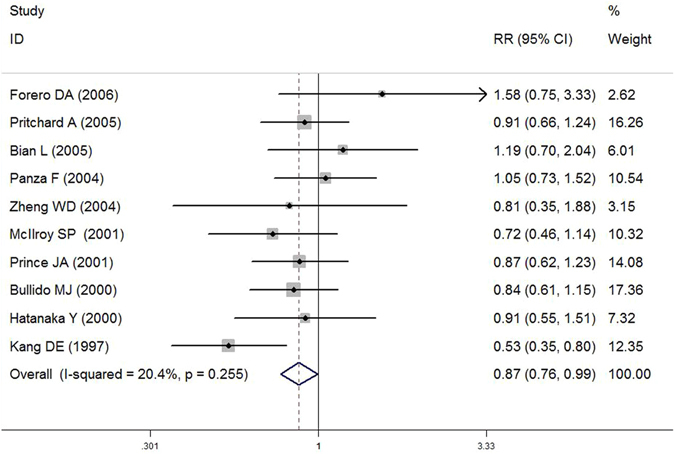
Forest plot of association between LRP1 C766T polymorphism (TT + CT vs. CC) and AD susceptibility in LOAD population.
The results of univariate and multivariate meta-regression analyses showed that age, MMSE and/or APOE ε4 were not potential factor(s) for heterogeneity among those studies, but gender might contributed to the heterogeneity (as shown in Table 4).
Table 4.
The potential sources of heterogeneity between LRP1 polymorphism and AD risk were evaluated by both of univariate and multivariate meta-regression analyses.
| Heterogeneity factors | Coefficient | 95% CI | SE | P |
|---|---|---|---|---|
| Age | ||||
| Univariate | 0.008 | (−0.027, 0.043) | 0.017 | 0.644 |
| Multivariate | −0.018 | (−0.051, 0.015) | 0.015 | 0.251 |
| Gender | ||||
| Univariate | 1.864 | (0.383, 3.345) | 0.712 | 0.016 |
| Multivariate | 2.193 | (0.233, 4.152) | 0.907 | 0.031 |
| MMSE | ||||
| Univariate | −0.081 | (−0.344, 0.182) | 0.127 | 0.532 |
| Multivariate | 0.004 | (−0.268, 0.277) | 0.126 | 0.975 |
| APOE ε4 status | ||||
| Univariate | −0.048 | (−0.440, 0.343) | 0.186 | 0.798 |
| Multivariate | 0.190 | (−0.252, 0.632) | 0.204 | 0.37 |
SE = standard error; 95%CI = 95% confidence interval.
Publication bias
Begg’s test and Egger’s test were performed to evaluate the publication bias of the included studies. The shape of Begg’s funnel plot appeared to be approximately symmetrical (Fig. 5). Besides, statistical significance was also not observed according to Egger’s test (P > 0.05, Table 3). In general, there was no publication bias in our included studies.
Figure 5.
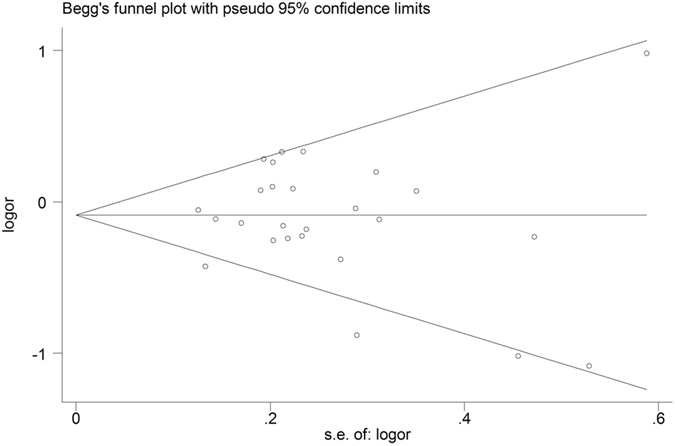
Funnel plot of association between LRP1 C766T polymorphism (TT + CT vs. CC) and AD susceptibility.
Discussion
AD, as a continuum, bring about serious threat to human health. Considering early detection and intervention at the asymptomatic stage may offer better chance of therapeutic success, it is urgent to identify early diagnostic biomarkers18, 19. LRP1, a member of the LDL receptor family, is an endocytic receptor for more than 40 structurally diverse ligands. The findings of previous studies indicate that LRP1 and many of its ligands (eg. APOE and A2M) are co-deposited with Aβ in senile plaques in AD brains20, 21. Subsequent studies demonstrated that LRP1 modulates the clearance of Aβ via receptor-mediated pathway in central nervous system22–24. Besides, soluble LRP1 provides an endogenous peripheral ‘sink’ activity for Aβ by preventing plasma free Aβ access to the brain25. It has also been reported that LRP1 is responsible for a rapid peripheral uptake of Aβ by the liver, which plays a key role in systemic clearance of Aβ26. On the other hand, endocytosis of LRP1 could modulate APP trafficking, and contribute to Aβ generation27. Interestingly, LRP1 can regulate Aβ metabolism in two contrary sides.
The association between LRP1 polymorphisms and AD susceptibility also has been described extensively, especially exon 3 C766T polymorphism. Kang et al. first reported the LRP1 C766T polymorphism, and found a positive association between C allele and AD susceptibility13. This finding was replicated in some following studies28–30, but Kolsch et al. found the opposite result that carriers of a C allele were at lower risk of AD31, while some failed to show any association between LRP1 C766T polymorphism and AD32–45. Previously, three meta-analysis have tried to clarify the relationship between LRP1 C766T polymorphism and AD susceptibility, which one revealed a weak correlation of LRP1 CC genotype with AD40, but other two separately studies showed that no positive evidence was involved in the relationship between this polymorphism and AD risk among overall36 and Chinese population46. Since several factors could be responsible for these discrepancies, such as inadequate sample size, variability in phenotype definition and allele frequency polymorphisms in different ethnic backgrounds47, we conducted a comprehensive meta-analysis with different genetic models in this study, to better clarify the association between LRP1 C766T polymorphism and AD susceptibility.
New results from our research did not show any association of LRP1 C766T polymorphism with AD susceptibility from 6455 AD cases and 6304 controls in overall population. This result is consistent with two published meta-analyses36, 46. Compared with the results from previous studies, our data from meta-analysis was relatively reliable to illustrate the association between LRP1 C766T polymorphism and AD susceptibility, because we used different genetic models with a larger number of case-controls.
Due to that people in different ethnic populations may have different allele frequency, and can affect the heterogeneity, we additionally conducted subgroup analysis by ethnicity, time of AD onset and APOE ε4 status. The outcomes by subgroups revealed that T allele of LRP1 C766T could reduce the risk of AD in allele model (T versus C) and dominant model (TT + CT versus CC) among Asian population, no significant role was found in Caucasian group. In terms of onset age, the results from subgroup analysis showed that T allele of LRP1 C766T could act as a protective factor for late onset of AD, but no significant association with early onset of AD. This is also consistent with previous report13.
It’s recognized that APOE ε4 is an important pathogenic factor for the development of AD. Several studies have revealed a possible protective effect of TT genotypes in carriers of APOE ε4 alleles48, 49. However, APOE ε4 status did not show that the influence of the association between LRP1 C766T polymorphism and AD susceptibility in our study. Moreover, our meta-regression analysis also showed that APOE ε4 status, age, and MMSE were not responsible for heterogeneity.
LRP1 C766T polymorphism is a silent mutation, which does not change the amino acid sequence or splice site. Therefore, it is unlikely to alter the biological function by a direct causal effect with the polymorphism. Some studies consider that the LRP1 C766T polymorphism might be responsible for susceptibility to AD by interact with other genes, such as APOE48–51, MAPT52, and MAPK8IP153. In addition, some speculated that LRP1 C766T may be in linkage disequilibrium with a deleterious mutation in the LRP1 gene, or with other biologically relevant mutation on neighbouring genes, which affected LRP1 expression44, 50. Besides, several studies have a hypothesis that the LRP1 C766T polymorphism might alter the secondary structure of the LRP mRNA to affect the translation and stability of the protein13, 48. To date, the conclusion with LRP1 C766T polymorphism with AD susceptibility is conflicting, further genetic analyses of this locus are needed to illuminate the potential mechanism and the functional interactions with AD.
Some limitations of our meta-analysis should be acknowledged. The sample size in some subgroup analysis was small, which may increase the risk of false negatives or false positives. Besides, we did not perform subgroup analysis based on other factors participated in the progression of AD, such as educational background, due to a lack of sufficient information. Larger and broader independent investigations are required to better understand the role of LRP1 C766T polymorphism in AD pathogenesis.
In conclusion, our meta-analysis suggested that LRP1 C766T polymorphism was associated with lower risk of AD in Asian, and could reduce LOAD risk especially. Furthermore, large-scale studies should be performed to reach more understanding of this association.
Materials and Methods
Search strategy
We searched electronic databases PubMed, Embase and CNKI (up to August 2016) using the following keywords: (“Alzheimer’s disease” or “Alzheimer disease” or “AD”) and (“low density lipoprotein receptor-related protein 1” or “LDL receptor-related protein 1” or “LRP1”) and (“polymorphism” or “SNP” or “variant” or “genotype”) without language restriction. The bibliographies of the retrieved studies were also screened to identify relevant publications.
Inclusion and exclusion criteria
The eligible studies had to meet all the following criteria: (1) a case–control study to evaluate the association between LRP1 C766T polymorphism and risk of AD; (2) useful data including sample size, allele or genotype distribution were given; (3) genotype distribution of controls followed the HWE. Accordingly, the exclusion criteria were as follows: (1) reviews, meta-analysis or editorial articles; (2) studies were provided with inadequate information; (3) for the studies with overlapping data, only the most relevant articles with the largest dataset were included in the final analysis.
The literature retrieval and inclusion were carried out in duplication by two independent reviewers.
Data extraction
Two reviewers independently extracted the following information: first author, year of publication, country, ethnicity, total number of cases and controls, mean age of cases and controls, proportion of female in cases and controls, AD diagnosis criteria, genotyping method, source of controls, time of AD onset, genotype or/and allele distribution in cases and controls. If conflicting results produced, two reviewers would review the publications again and reached a consensus by discussion.
Quality assessment
Two reviewers independently assessed the quality of each included studies in the meta-analysis according to the criteria of quality assessment (as referred in the Reference of 54, 55), and the disagreements were judged by the third reviewer to ensure a consistent outcome. Quality scores of studies ranged from 0 (the lowest) to 15 (the highest). Studies with quality scores among 10 to 15 were grouped into high quality studies and other studies scored between 0 and 9 were categorized into low quality studies.
Statistical analysis
HWE in controls was tested by a chi-square test. Summary odds ratio (OR) with confidence interval (95% CI) for genotypes and alleles were used to evaluate the strength of association between LRP1 C766T polymorphism and AD susceptibility. The significance of the pooled OR was measured using the Z-test. Four genetic models were performed in our meta-analysis: allele model (T versus C), codominant model [homozygote comparison (TT versus CC) and heterozygote comparison (CT versus CC)], dominant model (TT + CT versus CC), and recessive model (TT versus CC + CT). The heterogeneity was also quantified with I 2 statistics. If no significant heterogeneity was found between the studies, the pooled OR was calculated by using the fixed effects model (the Mantel-Haenszel method)56. Otherwise, the random effects model (the DerSimonian and Laird method) was applied57. Both of univariate and multivariate meta-regression analyses were also carried out to explore potential sources of heterogeneity among studies. The log of the ORs from involved studies was using as dependent variables, and age, gender, Mini-Mental State Exam (MMSE) and/or APOE ε4 status as covariates. Publication bias was tested by Begg’s test and Egger’s test58, 59. We also performed subgroup analysis according to ethnicity, time of AD onset and APOE ε4 status, respectively. Statistical analyses were conducted with Stata Version 11.0 (College Station, TX, USA), and a two-sided P < 0.05 was considered statistically significant.
Acknowledgements
This work was supported by Science and Technology project of Shenzhen (No. CXZZ20151117165117145) and Technology Research Project of Shenzhen (No. JSGG20160229154828546).
Author Contributions
Y.W. and S.L. contributed equally to this work, and they designed the study and wrote the main manuscript. J.W. and J.Z. collected the information of included articles. J.W. and Y.H. analyzed the data. H.L. and H.T. prepared figures and tables. B.K. and B.W. checked and revised the results. S.S. revised the manuscript. All authors reviewed and approved the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Yun Wang and Shengyuan Liu contributed equally to this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.2016 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia12, 459–509 (2016). [DOI] [PubMed]
- 2.Dartigues JF. Alzheimer’s disease: a global challenge for the 21st century. The Lancet Neurology. 2009;8:1082–1083. doi: 10.1016/S1474-4422(09)70298-4. [DOI] [PubMed] [Google Scholar]
- 3.Bettens K, Sleegers K, Van Broeckhoven C. Genetic insights in Alzheimer’s disease. The Lancet Neurology. 2013;12:92–104. doi: 10.1016/S1474-4422(12)70259-4. [DOI] [PubMed] [Google Scholar]
- 4.Farrer LA. Expanding the genomic roadmap of Alzheimer’s disease. The Lancet Neurology. 2015;14:783–785. doi: 10.1016/S1474-4422(15)00146-5. [DOI] [PubMed] [Google Scholar]
- 5.Gaiteri C, Mostafavi S, Honey CJ, De Jager PL, Bennett DA. Genetic variants in Alzheimer disease - molecular and brain network approaches. Nature reviews. Neurology. 2016;12:413–427. doi: 10.1038/nrneurol.2016.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sagare AP, Deane R, Zlokovic BV. Low-density lipoprotein receptor-related protein 1: a physiological Abeta homeostatic mechanism with multiple therapeutic opportunities. Pharmacology & therapeutics. 2012;136:94–105. doi: 10.1016/j.pharmthera.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiological reviews. 2008;88:887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zlokovic BV, Deane R, Sagare AP, Bell RD, Winkler EA. Low-density lipoprotein receptor-related protein-1: a serial clearance homeostatic mechanism controlling Alzheimer’s amyloid beta-peptide elimination from the brain. Journal of neurochemistry. 2010;115:1077–1089. doi: 10.1111/j.1471-4159.2010.07002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boucher P, Herz J. Signaling through LRP1: Protection from atherosclerosis and beyond. Biochemical pharmacology. 2011;81:1–5. doi: 10.1016/j.bcp.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu CC, et al. Neuronal LRP1 regulates glucose metabolism and insulin signaling in the brain. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2015;35:5851–5859. doi: 10.1523/JNEUROSCI.5180-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian X, et al. LRP-1-mediated intracellular antibody delivery to the Central Nervous System. Scientific reports. 2015;5:11990. doi: 10.1038/srep11990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanekiyo T, Bu G. The low-density lipoprotein receptor-related protein 1 and amyloid-beta clearance in Alzheimer’s disease. Frontiers in aging neuroscience. 2014;6:93. doi: 10.3389/fnagi.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang DE, et al. Genetic association of the low-density lipoprotein receptor-related protein gene (LRP), an apolipoprotein E receptor, with late-onset Alzheimer’s disease. Neurology. 1997;49:56–61. doi: 10.1212/WNL.49.1.56. [DOI] [PubMed] [Google Scholar]
- 14.Hollenbach E, Ackermann S, Hyman BT, Rebeck GW. Confirmation of an association between a polymorphism in exon 3 of the low-density lipoprotein receptor-related protein gene and Alzheimer’s disease. Neurology. 1998;50:1905–1907. doi: 10.1212/WNL.50.6.1905. [DOI] [PubMed] [Google Scholar]
- 15.Zhou YT, et al. Genetic association between low-density lipoprotein receptor-related protein gene polymorphisms and Alzheimer’s disease in Chinese Han population. Neuroscience letters. 2008;444:109–111. doi: 10.1016/j.neulet.2008.07.093. [DOI] [PubMed] [Google Scholar]
- 16.Zhou XH, Yue YH, Miao HJ, Hong Y, Ka-Bi N. Association of the low density lipoprotein receptor-related protein gene 766C/T polymorphism with Alzheimer’s disease in Xinjiang Uygurs and Hans. Zhonghua yi xue yi chuan xue za zhi = Zhonghua yixue yichuanxue zazhi = Chinese journal of medical genetics. 2008;25:455–458. [PubMed] [Google Scholar]
- 17.Feng YQ, et al. Correlation of the polymorphisms of apolipoprotein E gene and low-density lipoprotein receptor related protein gene with sporadic Alzheimer’s disease. Journal of International Neurology and Neuosurgery. 2006;33:9–12. [Google Scholar]
- 18.Chase A. Alzheimer disease: advances in imaging of AD biomarkers could aid early diagnosis. Nature reviews. Neurology. 2014;10:239. doi: 10.1038/nrneurol.2014.71. [DOI] [PubMed] [Google Scholar]
- 19.Dubois B, et al. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2016;12:292–323. doi: 10.1016/j.jalz.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Namba Y, Tomonaga M, Kawasaki H, Otomo E, Ikeda K. Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer’s disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain research. 1991;541:163–166. doi: 10.1016/0006-8993(91)91092-F. [DOI] [PubMed] [Google Scholar]
- 21.Rebeck GW, Harr SD, Strickland DK, Hyman BT. Multiple, diverse senile plaque-associated proteins are ligands of an apolipoprotein E receptor, the alpha 2-macroglobulin receptor/low-density-lipoprotein receptor-related protein. Annals of neurology. 1995;37:211–217. doi: 10.1002/ana.410370212. [DOI] [PubMed] [Google Scholar]
- 22.Arelin K, et al. LRP and senile plaques in Alzheimer’s disease: colocalization with apolipoprotein E and with activated astrocytes. Brain research. Molecular brain research. 2002;104:38–46. doi: 10.1016/S0169-328X(02)00203-6. [DOI] [PubMed] [Google Scholar]
- 23.Kang DE, et al. Modulation of amyloid beta-protein clearance and Alzheimer’s disease susceptibility by the LDL receptor-related protein pathway. The Journal of clinical investigation. 2000;106:1159–1166. doi: 10.1172/JCI11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanekiyo T, et al. Neuronal clearance of amyloid-beta by endocytic receptor LRP1. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:19276–19283. doi: 10.1523/JNEUROSCI.3487-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sagare A, et al. Clearance of amyloid-beta by circulating lipoprotein receptors. Nature medicine. 2007;13:1029–1031. doi: 10.1038/nm1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamaki C, et al. Major involvement of low-density lipoprotein receptor-related protein 1 in the clearance of plasma free amyloid beta-peptide by the liver. Pharmaceutical research. 2006;23:1407–1416. doi: 10.1007/s11095-006-0208-7. [DOI] [PubMed] [Google Scholar]
- 27.Cam JA, Zerbinatti CV, Li Y, Bu G. Rapid endocytosis of the low density lipoprotein receptor-related protein modulates cell surface distribution and processing of the beta-amyloid precursor protein. The Journal of biological chemistry. 2005;280:15464–15470. doi: 10.1074/jbc.M500613200. [DOI] [PubMed] [Google Scholar]
- 28.Bi S, Zhang Y, Wu J, Wang D, Zhao Q. Association between low-density lipoprotein receptor-related protein gene, butyrylcholinesterase gene and Alzheimer’s disease in Chinese. Chinese medical sciences journal = Chung-kuo i hsueh k’o hsueh tsa chih. 2001;16:71–75. [PubMed] [Google Scholar]
- 29.Chen Y, Zhang SL, Yue Y. Relationship between the polymorphism of low-density lipoprotein receptor-related protein gene, butyrylcholinesterase-K variant and Alzheimer’s disease. Practical Geriatrics. 2009;23:132–134. [Google Scholar]
- 30.Lambert J-C, Vrièze FW-D, Amouyel P, Chartier-Harlin M-C. Association at LRP gene locus with sporadic late-onset Alzheimer’s disease. The Lancet. 1998;351:1787–1788. doi: 10.1016/S0140-6736(05)78749-3. [DOI] [PubMed] [Google Scholar]
- 31.Kolsch H, et al. Association of the C766T polymorphism of the low-density lipoprotein receptor-related protein gene with Alzheimer’s disease. American journal of medical genetics. Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2003;121B:128–130. doi: 10.1002/ajmg.b.20043. [DOI] [PubMed] [Google Scholar]
- 32.Vargas T, et al. A megalin polymorphism associated with promoter activity and Alzheimer’s disease risk. American journal of medical genetics. Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2010;153B:895–902. doi: 10.1002/ajmg.b.31056. [DOI] [PubMed] [Google Scholar]
- 33.Bahia VS, et al. Polymorphisms of APOE and LRP genes in Brazilian individuals with Alzheimer disease. Alzheimer disease and associated disorders. 2008;22:61–65. doi: 10.1097/WAD.0b013e31815a9da7. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez E, et al. Genetic interaction between two apolipoprotein E receptors increases Alzheimer’s disease risk. Journal of neurology. 2006;253:801–803. doi: 10.1007/s00415-005-0063-1. [DOI] [PubMed] [Google Scholar]
- 35.Forero DA, Arboleda G, Yunis JJ, Pardo R, Arboleda H. Association study of polymorphisms in LRP1, tau and 5-HTT genes and Alzheimer’s disease in a sample of Colombian patients. Journal of neural transmission. 2006;113:1253–1262. doi: 10.1007/s00702-005-0388-z. [DOI] [PubMed] [Google Scholar]
- 36.Pritchard A, et al. Association study and meta-analysis of low-density lipoprotein receptor related protein in Alzheimer’s disease. Neuroscience letters. 2005;382:221–226. doi: 10.1016/j.neulet.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 37.Panza F, et al. Regional European differences in allele and genotype frequencies of low density lipoprotein receptor-related protein 1 polymorphism in Alzheimer’s disease. American journal of medical genetics. Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2004;126B:69–73. doi: 10.1002/ajmg.b.20146. [DOI] [PubMed] [Google Scholar]
- 38.Zheng, W. D. et al. A genetic association study between the cardiovascular risk factor and late-onset Alzheimer Disease in Guangxi Han Chinese, Chinese Journal of Neuroimmunology and Neurology11, 68–71+90 (2004).
- 39.Perry RT, Collins JS, Harrell LE, Acton RT, Go RC. Investigation of association of 13 polymorphisms in eight genes in southeastern African American Alzheimer disease patients as compared to age-matched controls. American journal of medical genetics. 2001;105:332–342. doi: 10.1002/ajmg.1371. [DOI] [PubMed] [Google Scholar]
- 40.Sanchez-Guerra M, et al. Case-control study and meta-analysis of low density lipoprotein receptor-related protein gene exon 3 polymorphism in Alzheimer’s disease. Neuroscience letters. 2001;316:17–20. doi: 10.1016/S0304-3940(01)02342-4. [DOI] [PubMed] [Google Scholar]
- 41.McIlroy SP, et al. Common polymorphisms in LRP and A2M do not affect genetic risk for Alzheimer disease in Northern Ireland. American journal of medical genetics. 2001;105:502–506. doi: 10.1002/ajmg.1474. [DOI] [PubMed] [Google Scholar]
- 42.Prince JA, et al. Lack of replication of association findings in complex disease: an analysis of 15 polymorphisms in prior candidate genes for sporadic Alzheimer’s disease. European journal of human genetics: EJHG. 2001;9:437–444. doi: 10.1038/sj.ejhg.5200651. [DOI] [PubMed] [Google Scholar]
- 43.Verpillat P, et al. Use of haplotype information to test involvement of the LRP gene in Alzheimer’s disease in the French population. European journal of human genetics: EJHG. 2001;9:464–468. doi: 10.1038/sj.ejhg.5200644. [DOI] [PubMed] [Google Scholar]
- 44.Beffert U, Arguin C, Poirier J. The polymorphism in exon 3 of the low density lipoprotein receptor-related protein gene is weakly associated with Alzheimer’s disease. Neuroscience letters. 1999;259:29–32. doi: 10.1016/S0304-3940(98)00888-X. [DOI] [PubMed] [Google Scholar]
- 45.Bertram L, et al. Candidate genes showing no evidence for association or linkage with Alzheimer’s disease using family-based methodologies. Experimental gerontology. 2000;35:1353–1361. doi: 10.1016/S0531-5565(00)00193-5. [DOI] [PubMed] [Google Scholar]
- 46.Yang L, et al. Association of PS1 1/2, ACE I/D, and LRP C/T polymorphisms with Alzheimer’s disease in the Chinese population: a meta-analysis of case-control studies. Genetics and molecular research: GMR. 2015;14:1017–1024. doi: 10.4238/2015.February.6.5. [DOI] [PubMed] [Google Scholar]
- 47.Greene CS, Penrod NM, Williams SM, Moore JH. Failure to replicate a genetic association may provide important clues about genetic architecture. PloS one. 2009;4:e5639. doi: 10.1371/journal.pone.0005639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hatanaka Y, et al. Low density lipoprotein receptor-related protein gene polymorphisms and risk for late-onset Alzheimer’s disease in a Japanese population. Clinical genetics. 2000;58:319–323. doi: 10.1034/j.1399-0004.2000.580410.x. [DOI] [PubMed] [Google Scholar]
- 49.Kamboh MI, Ferrell RE, DeKosky ST. Genetic association studies between Alzheimer’s disease and two polymorphisms in the low density lipoprotein receptor-related protein gene. Neuroscience letters. 1998;244:65–68. doi: 10.1016/S0304-3940(98)00141-4. [DOI] [PubMed] [Google Scholar]
- 50.Yuan Q, Wang F, Xue S, Jia J. Association of polymorphisms in the LRP1 and A2M genes with Alzheimer’s disease in the northern Chinese Han population. Journal of clinical neuroscience: official journal of the Neurosurgical Society of Australasia. 2013;20:253–256. doi: 10.1016/j.jocn.2012.01.052. [DOI] [PubMed] [Google Scholar]
- 51.Bullido MJ, et al. Alzheimer’s risk associated with human apolipoprotein E, alpha-2 macroglobulin and lipoprotein receptor related protein polymorphisms: absence of genetic interactions, and modulation by gender. Neuroscience letters. 2000;289:213–216. doi: 10.1016/S0304-3940(00)01304-5. [DOI] [PubMed] [Google Scholar]
- 52.Vazquez-Higuera JL, et al. Genetic interaction between tau and the apolipoprotein E receptor LRP1 Increases Alzheimer’s disease risk. Dementia and geriatric cognitive disorders. 2009;28:116–120. doi: 10.1159/000234913. [DOI] [PubMed] [Google Scholar]
- 53.Helbecque N, et al. Islet-brain1/C-Jun N-terminal kinase interacting protein-1 (IB1/JIP-1) promoter variant is associated with Alzheimer’s disease. Molecular psychiatry. 2003;8(413–422):363. doi: 10.1038/sj.mp.4001342. [DOI] [PubMed] [Google Scholar]
- 54.Liu S, Zeng F, Wang C, et al. The nitric oxide synthase 3 G894T polymorphism associated with Alzheimer’s disease risk: a meta-analysis. Scientific Reports. 2015;5:13598. doi: 10.1038/srep13598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He J, Liao XY, Zhu JH, et al. Association of MTHFR C677T and A1298C polymorphisms with non-Hodgkin lymphoma susceptibility: evidence from a meta-analysis. Scientific Reports. 2014;4:6159. doi: 10.1038/srep06159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute. 1959;22:719–748. [PubMed] [Google Scholar]
- 57.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemporary clinical trials. 2007;28:105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 58.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 59.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed.) 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bian L, et al. Association study of the A2M and LRP1 Genes with Alzheimer disease in the Han Chinese. Biological psychiatry. 2005;58:731–737. doi: 10.1016/j.biopsych.2005.05.013. [DOI] [PubMed] [Google Scholar]


