ABSTRACT
Escherichia coli, an intestinal Gram-negative bacterium, has been shown to be associated with a variety of diseases in addition to intestinal infections, such as urinary tract infections (UTIs), meningitis in neonates, septicemia, skin and soft tissue infections (SSTIs), and colisepticemia. Thus, for nonintestinal infections, it is categorized as extraintestinal pathogenic E. coli (ExPEC). It is also an opportunistic pathogen, causing cross infections, notably as an agent of zoonotic diseases. However, comparative genomic data providing functional and genetic coordinates for ExPEC strains associated with these different types of infections have not proven conclusive. In the study reported here, ExPEC E. coli isolated from SSTIs was characterized, including virulence and drug resistance profiles, and compared with isolates from patients suffering either pyelonephritis or septicemia. Results revealed that the majority of the isolates belonged to two pathogenic phylogroups, B2 and D. Approximately 67% of the isolates were multidrug resistant (MDR), with 85% producing extended-spectrum beta-lactamase (ESBL) and 6% producing metallo-beta-lactamase (MBL). The blaCTX-M-15 genotype was observed in at least 70% of the E. coli isolates in each category, conferring resistance to an extended range of beta-lactam antibiotics. Whole-genome sequencing and comparative genomics of the ExPEC isolates revealed that two of the four isolates from SSTIs, NA633 and NA643, belong to pandemic sequence type ST131, whereas functional characteristics of three of the ExPEC pathotypes revealed that they had equal capabilities to form biofilm and were resistant to human serum. Overall, the isolates from a variety of ExPEC infections demonstrated similar resistomes and virulomes and did not display any disease-specific functional or genetic coordinates.
KEYWORDS: Escherichia coli, genomics, sepsis
IMPORTANCE
Infections caused by extraintestinal pathogenic E. coli (ExPEC) are of global concern as they result in significant costs to health care facilities management. The recent emergence of a multidrug-resistant pandemic clone, Escherichia coli ST131, is of primary concern as a global threat. In developing countries, such as India, skin and soft tissue infections (SSTIs) associated with E. coli are marginally addressed. In this study, we employed both genomic analysis and phenotypic assays to determine relationships, if any, among the ExPEC pathotypes. Similarity between antibiotic resistance and virulence profiles was observed, ST131 isolates from SSTIs were reported, and genomic similarities among strains isolated from different disease conditions were detected. This study provides functional molecular infection epidemiology insight into SSTI-associated E. coli compared with ExPEC pathotypes.
INTRODUCTION
Escherichia coli is one of the most important agents of extraintestinal infections, with the potential to cause infection in almost any anatomical site. It can be grouped into pathotypes, such as uropathogenic E. coli (UPEC), septicemia-associated E. coli (SePEC), skin and soft tissue infection (SSTI)-associated E. coli, neonatal-meningitis-causing E. coli (NMEC), and avian-pathogenic E. coli (APEC) (1, 2). Urinary tract infections (UTIs) alone account for about 25% of all cases of septic shock, while soft tissue infections represent 10% of reported severe sepsis cases (3, 4). Clinical stages of UTIs vary from nonsymptomatic acute infection to severe septic shock. In the latter case, the infection generally progresses from the urinary tract to the bladder (cystitis) and from the bladder to the kidneys (pyelonephritis) and finally into the bloodstream (urosepsis). Skin and soft tissue infections (SSTIs) are major bacterial infections, often self-limiting but in severe cases requiring hospitalization and parenteral antibiotic therapy. With the indiscriminate use of antibiotics and emergence of multidrug-resistant (MDR) organisms, such as sequence type 131 (ST131) and carbapenem-resistant strains, treatment has become quite challenging (5–7). Despite the fact that SSTI E. coli is an important pathotype, to our knowledge only UTI E. coli isolates isolated in India have been characterized (8, 9). This study provides a functional molecular infection insight for E. coli associated with SSTI. Phylogenetic relationships, virulence profiles, antibiotic resistance patterns, and functional attributes were determined, and whole genomes were sequenced and compared with septicemia and pyelonephritis isolates from the same setting of endemicity in India.
RESULTS
Phylogenetic groups and antimicrobial resistance.
The majority of the isolates included in this study were assigned to virulence B2 (36%) and D (29%) phylogroups, with 23% in the A group and 12% in the B1 group (Table 1). Of the three disease types, more than half of the isolates fell into B2 and D phylogroups. In total, 73 isolates (93%) were resistant to at least one of six antimicrobial agents, with highest resistance being shown to ciprofloxacin (86%), followed by tetracycline (77%), co-trimoxazole (72%), gentamicin (31%), chloramphenicol (23%), and fosfomycin (5%). Details of all bacterial isolates, including their antimicrobial resistance patterns, are provided in Table S1 in the supplemental material. Overall, 67% of the total E. coli isolates were found to be multidrug resistant (MDR), in which pyelonephritis, septicemia, and SSTI contributed fractions of 0.40, 0.32, and 0.26, respectively (Table 2). The extended-spectrum-beta-lactamase (ESBL)-producing E. coli strains were found to comprise as much as 85% of the isolates and were similar in all three disease types. Only 6% of the isolates were metallo-beta-lactamase (MBL) producers. Resistance gene profiling was compatible with the phenotypic observations, with blaCTX-M-15 being the predominant genotype and NDM-1 being a common occurrence among all MBL producers (Tables 1 and 2). The gene sul1 was detected in approximately 71% of the isolates in all three categories, conferring resistance to sulfonamides, while the plasmid-based fluoroquinolone resistance gene [aac(6′)-lb-cr] was detected in 30% and 71% of the pyelonephritis and SSTI isolates, respectively.
TABLE 1 .
Phylogenetic grouping, virulence, and antimicrobial resistance genotype of extraintestinal pathogenic E. coli included in this studya
| Characteristic | Gene or specific trait |
No. (%) of strains associated with infection: |
||
|---|---|---|---|---|
| Pyelonephritis (n = 30) |
Septicemia (n = 27) |
Skin and soft tissue infections (n = 21)b |
||
| Phylogenetic grouping, (no. [%] of strains) | ||||
| A (18 [23]) | 2 (7) | 8 (30) | 8 (38) | |
| B1 (9 [12]) | 3 (10) | 4 (15) | 2 (10) | |
| B2 (28 [36]) | 15 (50) | 7 (26) | 6 (29) | |
| D (23 [29]) | 10 (33) | 8 (30) | 5 (24) | |
| Virulence factors | ||||
| Toxins | sat | 16 (53) | 9 (33) | 6 (29) |
| usp | 10 (33) | 6 (22) | 6 (29) | |
| cvaC | 6 (20) | 6 (22) | 15 (71)* | |
| Adhesins | fimH | 27 (90) | 24 (89) | 13 (62)* |
| papC | 28 (93) | 25 (93) | 5 (24)* | |
| afaB/C | 8 (27) | 1 (4) | 1 (05) | |
| sfaD/E | 2 (7) | 2 (7) | 1 (05) | |
| Protectins | traT | 5 (17) | 4 (15) | 6 (29) |
| ibeA | 3 (10) | 3 (11) | 1 (5) | |
| Iron acquisition | iroN | 27 (90) | 21 (78) | 16 (76) |
| iucD | 19 (63) | 16 (59) | 15 (71) | |
| Antibiotic resistance profiling: antibiotic class | ||||
| ESBL | blaCTX-M-15 | 21 (70) | 24 (89) | 17 (81) |
| blaTEM | 15 (50) | 14 (52) | 4 (19)* | |
| blaCTX-M-15 + blaTEM | 11 (37) | 12 (44) | 4 (19) | |
| Metallo-beta-lactamase | blaNDM1 | 0 (0) | 1 (4) | 5 (24)* |
| Tetracyclines | tetA | 20 (67) | 17 (63) | 8 (38) |
| Aminoglycosides | strA | 14 (47) | 10 (37) | 9 (43) |
| Fluoroquinolone | aac(6′)-lb-cr | 9 (30) | 13 (48) | 15 (71) |
| Sulfonamides | sul1 | 25 (83) | 23 (82) | 15 (71) |
| sul2 | 12 (40) | 7 (26) | 6 (29) | |
| Trimethoprim | dhfr | 8 (27) | 9 (33) | 2 (10) |
| Integrin | int1 | 18 (60) | 15 (56) | 13 (62) |
Total number of strains was 78.
Asterisks denote a P value of <0.05 compared to the septicemia group of strains.
TABLE 2 .
Pathotypes and phenotypic drug resistance and multidrug resistance phenotypes
| Antimicrobial class or phenotype |
Specific drug | % overall prevalence of resistance phenotype in Indian population |
Estimated fraction in infection type: |
||
|---|---|---|---|---|---|
| Pyelonephritis (n = 30) |
Septicemia (n = 27) |
Skin and soft tissue infections (n = 21) |
|||
| Quinolone/fluoroquinolone | Ciprofloxacin | 86 | 0.37 | 0.34 | 0.28 |
| Sulfonamide/trimethoprim | Co-trimoxazole | 72 | 0.43 | 0.36 | 0.21 |
| Aminoglycosides | Gentamicin | 31 | 0.33 | 0.41 | 0.25 |
| Phenicols/phosphonic acid derivatives | Chloramphenicol | 23 | 0.39 | 0.50 | 0.11 |
| Fosfomycin | 5 | 0.00 | 0.25 | 0.75 | |
| Tetracyclines | Tetracycline | 77 | 0.43 | 0.35 | 0.22 |
| ESBL phenotype | Cefotaxime | 85 | 0.36 | 0.36 | 0.27 |
| MBL phenotype | Meropenem | 06 | 0.00 | 0.16 | 0.83 |
| Multidrug resistance | 67 | 0.40 | 0.32 | 0.26 | |
Phylogroup, extended-spectrum beta-lactamase (ESBL) production phenotype, and antimicrobial resistance patterns of ExPEC isolates against six non-beta-lactam antibiotics. Download TABLE S1, DOCX file, 0.03 MB (28KB, docx) .
Copyright © 2017 Ranjan et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Virulence genotypes.
Analysis of the virulence genotype fimH showed that it was present in ∼90% of the isolates in each category, with the exception of SSTI isolates (62%). Interestingly, papC was found to be prevalent in septicemia and pyelonephritis isolates. However, afaB/C and sfaD/E were present in various proportions in the three groups. Of the three toxin types, cvaC was detected, to a lesser extent, in pyelonephritis and septicemia isolates. The prevalence of protectin genes traT and ibeA was the same as that of iron acquisition genes iroN and iucD in all three groups (Table 1).
Whole-genome analysis of resistome and virulome.
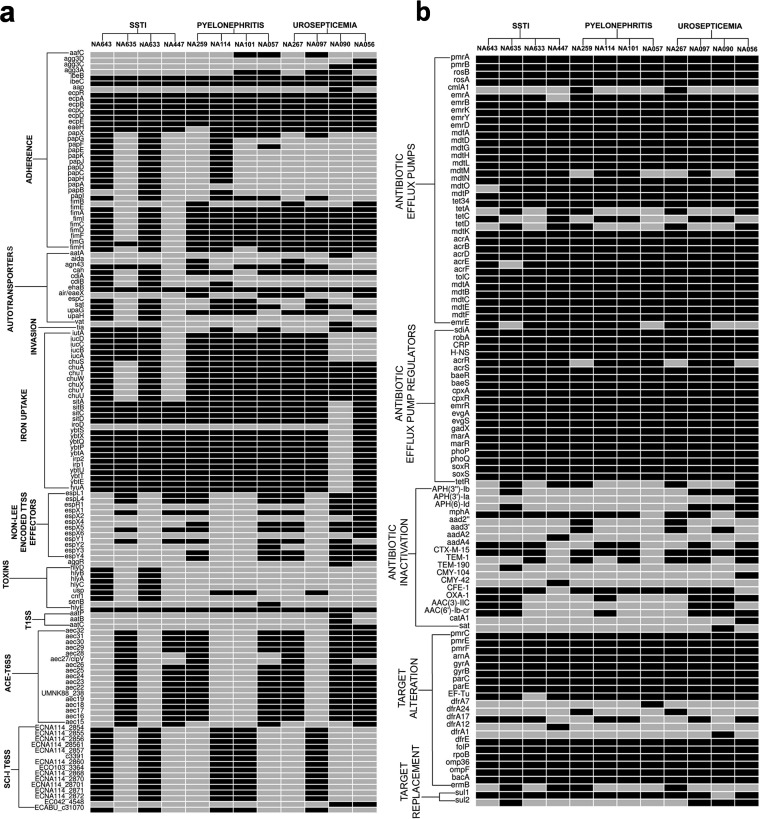
Twelve strains, comprising four from each of the three disease types, were subjected to comparative genomic analysis to obtain a broader perspective of the complexity of the E. coli disease types. Genome sequence data for four strains were also included from previous and ongoing studies, including reference strains Escherichia coli NA097 and NA114 (10, 11), and eight isolates of this study were subjected to whole-genome sequencing (WGS). Whole-genome-based resistome profiles confirmed the strains to be multidrug resistant. All strains demonstrated relatively similar combinations of resistance genes encoding antibiotic efflux pumps, regulators, antibiotic inactivation, and target modification (Fig. 1b). The virulomes also showed very little pathotype specificity (Fig. 1a). Based on in silico multilocus sequence typing (MLST) results, two SSTI strains (NA633 and NA643) were assigned to the pandemic ST131 clone, while the rest of the strains were assigned to different sequence types (STs), namely, ST38, ST68, ST405, and ST 617 (Table 3).
FIG 1 .
Heat map of virulome (a) and resistome (b) data generated from whole-genome comparative genomics for 12 strains that included four strains from each of the infection categories septicemia, pyelonephritis, and SSTI. Black boxes represent presence, and gray boxes represent absence.
TABLE 3 .
Genome statistics for eight strains sequenced in this study
| Strain | Accession no. | No. of raw reads |
Genome coverage (fold) |
No. of contigs (≥500 bp) |
Sequence type |
Genome size (bp) |
No. of CDSa |
Coding % | No. of rRNAs |
G+C content (%) |
N50 value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NA643 | MJDL00000000 | 2,136,944 | 108.5 | 112 | ST131 | 5,322,063 | 5,257 | 87.4 | 17 | 50.72 | 216,749 |
| NA635 | MJDK00000000 | 1,822,696 | 91.8 | 122 | ST617 | 4,948,859 | 4,769 | 87.2 | 12 | 50.78 | 164,858 |
| NA633 | MJDJ00000000 | 1,878,242 | 94.6 | 81 | ST131 | 5,275,425 | 5,212 | 87.7 | 17 | 50.68 | 265,266 |
| NA447 | JWHS00000000 | 1,500,566 | 45 | 239 | ST617 | 5,091,202 | 4,990 | 85.7 | 15 | 50.68 | 52,237 |
| NA267 | MJDI00000000 | 2,092,350 | 105 | 164 | ST405 | 5,393,308 | 5,229 | 86.8 | 13 | 50.51 | 118,812 |
| NA259 | MJGD00000000 | 1,758,550 | 88.6 | 162 | ST405 | 5,400,321 | 5,260 | 86.9 | 13 | 50.51 | 118,812 |
| NA056 | MKHD00000000 | 1,513,364 | 78 | 98 | ST68 | 5,322,471 | 5,099 | 87.6 | 12 | 50.54 | 143,398 |
| NA057 | JSXL00000000 | 1,913,156 | 57 | 150 | ST38 | 5,286,256 | 5,173 | 86.5 | 8 | 50.5 | 120,832 |
CDS, coding sequences.
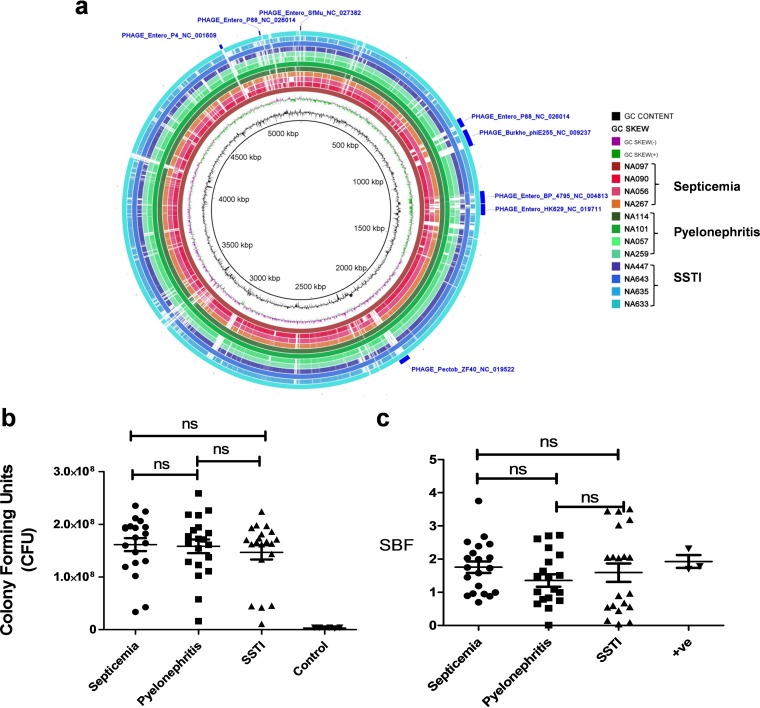
Results of comparative genomic analyses using the BLAST Ring Image Generator (BRIG) platform showed sequence-type-specific similarities, i.e., all five ST131 strains had similar arrangements of genomic regions, whereas isolates belonging to other STs had dissimilar regions (Fig. 2a). Clearly, disease-specific genomic features were not detected.
FIG 2 .
(a) BLAST Ring Image Generator (BRIG) images of 12 strains, including four from each infection category, where each concentric ring represents genomes sequentially in groups, e.g., septicemia, pyelonephritis, and SSTI, generated with the NA097 septicemia strain serving as a reference. (b) Serum resistance tested using 50% human serum. (c) Biofilm formation assay for 10 strains from each category, repeated twice in technical triplicate. ns, nonsignificant; SBF, specific biofilm formation; +ve, positive.
Functional virulence determination.
A total of 30 E. coli isolates, comprising 10 from each category, were analyzed for functional virulence determinants. Results revealed biofilm formation capabilities for all isolates when tested using M63 medium and incubation for 48 h at 28°C. Most were strong biofilm formers (Fig. 2c) and also able to grow in human serum (50%). Resistance to serum, i.e., demonstrating no deleterious effect, was variable among the isolates, but the difference was insignificant (Fig. 2b).
DISCUSSION
Infections caused by extraintestinal pathogenic E. coli (ExPEC) are common worldwide. In the present study, we observed that E. coli strains isolated from SSTI, septicemia, and pyelonephritis were predominately B2 and D phylogroups (Table 1), which are considered virulent E. coli (12, 13). The observations of this study are similar to those of Petkovsek et al. (14), who reported that SSTI E. coli possessed virulence factors (VFs). Ananias and Yano (15) also showed VFs to be common among different sepsis-associated E. coli (SePEC) strains notably able to invade Vero cell lines.
Recent studies reported a global increase in multidrug resistance (MDR) and extended-spectrum beta-lactamase (ESBL) genotypes among ExPEC strains associated with infections (16, 17). In the present study, the majority (67%) of ExPEC isolates were MDR, with CTX-M-15 being the predominant ESBL genotype (Tables 1 and 2). Furthermore, the combination of ESBL and fluoroquinolone resistance was the most frequent, followed by that of CTX-M-15 positivity (CTX-M-15+), fluoroquinolone resistance, and co-trimoxazole resistance and that of CTX-M-15+, fluoroquinolone resistance, co-trimoxazole resistance, and tetracycline resistance (Table 4). The resistome (Fig. 1b), based on WGS, showed the presence of multiple genes, whereas the MBL phenotype in all isolates was associated with the presence of the NDM gene, reflecting dissemination of NDM carbapenem resistance in India (7).
TABLE 4 .
Prevalence of combinations of ESBL, CTX-M-15, fluoroquinolone resistance, co-trimoxazole resistance, and tetracycline resistance among isolates included in this study (n = 78)
| Subset | Subset definitiona | Total no. (%) of Indian isolates |
No. of isolates (%) associated with infection type: |
||
|---|---|---|---|---|---|
| Pyelonephritis (n = 30) |
Septicemia (n = 27) |
Skin and soft tissue infections (n = 21) |
|||
| 1 | ESBL+ CTX-M-15+ | 60 (77) | 21 (70) | 23 (85) | 16 (76) |
| 2 | ESBL+ CTX-M-15+ Tem+ | 30 (38) | 11 (37) | 12 (43) | 7 (33) |
| 3 | CTX-M-15+, fluoroquinolone resistant | 57 (73) | 20 (67) | 22 (81) | 15 (71) |
| 4 | CTX-M-15+, fluoroquinolone resistant, co-trimoxazole resistant | 44 (56) | 17 (56) | 17 (63) | 10 (48) |
| 5 | CTX-M-15+, fluoroquinolone susceptible, co-trimoxazole resistant | 2 (3) | 1 (3) | 1 (4) | 0 (0) |
| 6 | CTX-M-15+, fluoroquinolone resistant, co-trimoxazole susceptible | 13 (17) | 3 (10) | 5 (18) | 5 (24) |
| 7 | CTX-M-15+, fluoroquinolone susceptible, co-trimoxazole susceptible | 3 (4) | 0 (0) | 1 (4) | 2 (10) |
| 8 | CTX-M-15+, fluoroquinolone resistant, co-trimoxazole resistant, tetracycline resistant | 39 (50) | 17 (56) | 16 (57) | 6 (29) |
| 9 | CTX-M-15+, fluoroquinolone resistant, co-trimoxazole resistant, tetracycline susceptible | 4 (5) | 0 (0) | 0 (0) | 4 (19) |
| 10 | CTX-M-15−, fluoroquinolone susceptible | 6 (8) | 4 (13) | 2 (7) | 0 (0) |
Tem, temoniera-beta-lactamase.
ExPEC is known to be evolving and disseminating globally via clonal expansion, with clones of similar genetic architectures but also demonstrating strain-specific features (11, 18). Here, we observed similarities in virulence and resistance coordinates of strains belonging to sequence types (STs) ST131, ST38, ST68, ST405, and ST617. Interestingly, ST-specific commonality but not disease-specific similarity was observed (Fig. 2a). These results support the conclusion that strains of diverse STs are capable of causing similar infections. We also report for the first time the occurrence in India of E. coli isolates from SSTIs that carry ST131.
In summary, the results of this study indicate that ExPEC isolates associated with pyelonephritis, septicemia, and SSTIs comprise overlapping phylogroups and patterns of virulence, drug resistance, genomic, and functional properties but are not specifically associated with pathotypes.
MATERIALS AND METHODS
Bacterial strains.
A total of 78 isolates, including 21 from patients suffering infections associated with skin and soft tissues, were obtained from the D. Y. Patil Hospital, Pune, India, during 2015. Also, 57 E. coli strains isolated during January 2009 to December 2012 were included in the study. All isolates were identified and preserved employing standard laboratory methods of the hospital as previously published (8) and the protocols were approved by Institutional Biosafety Committee (IBSC) of University of Hyderabad, Hyderabad, India.
Preparation of DNA template and E. coli phylogenetic grouping.
Template DNA was prepared using the boiling lysis method. In brief, 100 µl of the bacterial cultures was boiled at 95°C for 20 min and centrifuged at 6,000 rpm for 10 min. The supernatant obtained was used as the template. All E. coli isolates were classified into four major phylogenetic groups by multiplex PCR using three molecular markers: chuA, yjaA, and tspE4 (19). After electrophoresis (1.5% agarose), gel images were captured and isolates were assigned to phylogenetic groups based on the dichotomous decision tree of the work of Clermont et al. (19).
ESBL screening and antimicrobial resistance.
ESBL production was confirmed phenotypically, using guideline M31-A3 of the Clinical and Laboratory Standards Institute (CLSI) (20). Resistance to carbapenems was determined by using Etest (HiMedia), and susceptibility was defined by breakpoints per CLSI (20). All E. coli isolates were tested for resistance to six major classes of non-β-lactam antibiotics, employing standard disc agar diffusion on Muller-Hinton agar (HiMedia) (20). Six antimicrobial discs (HiMedia) were used: ciprofloxacin (30 µg), chloramphenicol (30 µg), fosfomycin (200 µg), gentamicin (10 µg), sulfamethoxazole-trimethoprim (25 µg), and tetracycline (30 µg). Isolates showing resistance to three or more antimicrobial drugs are considered multidrug resistant (MDR).
Antimicrobial resistance genotyping.
PCR was used to detect β-lactamase genes (blaTEM, blaSHV, and blaCTX-M-15) in ESBL-positive E. coli isolates (21, 22). The presence of genes encoding carbapenemase was detected using selected primers (23). Resistance genes {tetracycline resistance [tetA], sulfonamide resistance [sul1 and sul2], aminoglycoside resistance [strA], the aminoglycoside acetyltransferase [aac(6′)-lb-cr], and trimethoprim resistance [dhfr]} were determined by PCR using primers and programs reported elsewhere (24, 25). The gene int1, encoding class 1 integrase, was also detected by PCR (26).
Virulence genotyping.
The ExPEC isolates were tested for the presence of 11 E. coli virulence genes (VGs) associated with sepsis-related pathophysiology. The genes targeted were of the following four categories: (i) bacterial adhesins (papC, fimH, afaB/C, and sfaD/E) (27–30), (ii) toxins (usp, sat, and cvaC) (31–33), (iii) protectants (traT and ibeA) (33, 34), and (iv) the iron acquisition system (iroN and iucD) (30, 35).
Whole-genome sequencing and comparative genomics.
Eight of the isolates were sequenced, and comparative genomics analyses were done, including four strains from previous and ongoing studies, providing virulome, resistome, and whole-genome comparisons. Briefly, paired-end sequence data for eight strains were obtained using Illumina MiSeq for the following in silico analysis. The NGS QC Toolkit (v2.3.3) (36) was used to filter high-quality reads, followed by contig assembly using SPAdes Genome Assembler (v3.6.1) (37). Numbers of raw reads, respective read lengths, genome coverage obtained after filtering, and total number of contigs were computed. The generated de novo contigs were ordered and scaffolded using Contig-Layout-Authenticator (CLA) (38). Final draft genomes were obtained by merging scaffolds using a series of N’s. Draft genomes were submitted to the RAST (39) server for annotation, and genome statistics from the resulting file were extracted using Artemis (40). The sequence type (ST) of each strain was determined by submitting contigs to https://cge.cbs.dtu.dk/services/MLST/.
Twelve strains, including eight from the current study and four from earlier reported and ongoing studies, belonging to different pathotypes were used for the following comparative analysis. BRIG (41) was used to visualize genome variation of the 12 strains. GeneMarkS (42) was used to predict protein sequences. BLASTp (43) analysis of the putative protein sequences was performed against the database of E. coli virulence genes downloaded from the Virulence Factors Database (VFDB) (44), providing a virulence profile for each strain. A specific virulence gene was considered present only if the BLASTp hit had identity greater than 60% and query coverage greater than 85%. The presence-absence status of all virulence genes carried by each strain was represented in the form of a heat map using R. A similar heat map was generated for putative resistance-related genes from the Comprehensive Antibiotic Resistance Database (CARD) (45), using BLASTp.
Phenotypic virulence determination.
Biofilm formation was determined using M63 minimal medium as described earlier (13, 46). Briefly, bacteria grown overnight were diluted to an optical density at 600 nm (OD600) of 0.05 in M63 medium, and 200 μl of each was inoculated in triplicate in a 96-well microtiter plate. OD600 was measured, and the plate was covered with permeable sealing and incubated at 28°C for 48 h without shaking. Growth was measured after 48 h as OD600 using a microtiter plate reader. Medium was removed gently, washed three times with deionized water, and dried. Bacteria were fixed with methanol (99%) for 15 min and stained with 1% crystal violet (30 min) after air drying. Plates were washed three times with deionized water and air dried again. The fixed stained bacteria were resolubilized using 200 μl of ethanol-acetone (80:20), and absorbance was measured at 570 nm. Specific biofilm formation (SBF) was obtained using the formula SBF = (AB − CW)/G, where AB is absorbance at 570 nm, CW is OD570 of control well (without bacteria), and G is growth measured by the formula G = OD600(48 h) − OD600(0 h).
Serum bactericidal activity was assayed using 50% human serum as reported previously (13, 46). Briefly, 5 μl of overnight-grown culture was inoculated in 495 μl of fresh LB broth and allowed to grow for 1.5 h at 37°C at 200 rpm. The bacterial cells were pelleted and resuspended in 1 ml of sterile 1× phosphate-buffered saline (PBS). Thirty microliters of bacteria was added to 270 μl of 50% human serum in a 96-well microtiter plate in triplicates, and 30 μl of sample was taken out and plated on LB agar plates after dilution. The plate was covered and allowed to grow for 3 h at 37°C at 100 rpm. After 3 h of incubation, 30 μl of sample was collected again from each well, diluted, and plated on LB agar plates. The bacteria were enumerated after overnight incubation at 37°C, and growth in serum was calculated by subtracting the CFU of 0 h from that of 3 h. A graph was plotted, and results were analyzed. Both of the assays were performed twice and in triplicate on a subset of 10 isolates from each category.
Statistical analysis.
Statistical analysis of data for virulence and resistance genes was performed employing chi-square and Mann-Whitney tests for serum resistance and biofilm formation, respectively.
Accession number(s).
GenBank accession numbers of the eight genomes sequenced for this study are MJDL00000000 (NA643), MJDK00000000 (NA635), MJDJ00000000 (NA633), JWHS00000000 (NA447), MJDI00000000 (NA267), MJGD00000000 (NA259), MKHD00000000 (NA056), and JSXL00000000 (NA057). GenBank accession numbers of the other four genomes used are MIPU00000000 (NA114), JSXJ00000000 (NA097), JSXN00000000 (NA101), and MVIO00000000 (NA090).
ACKNOWLEDGMENTS
Funding provided by the Department of Biotechnology, Government of India [reference no. BT/HRD/NBA/34/01/2011(ix)] (National Bioscience Award to N.A.), and the support of the Indo-German International Research Training Group, Internationales Graduiertenkolleg (GRK1673)-Functional Molecular Infection Epidemiology, an initiative of the German Research Foundation (DFG) and the University of Hyderabad (India), of which N.A. and L.H.W. served as speakers, are gratefully acknowledged. N.A. is an Adjunct Professor, Academy of Scientific and Innovative Research (ACSIR), India. A.R. was the recipient of a fellowship provided by the Indian Council of Medical Research (ICMR).
Footnotes
Citation Ranjan A, Shaik S, Nandanwar N, Hussain A, Tiwari SK, Semmler T, Jadhav S, Wieler LH, Alam M, Colwell RR, Ahmed N. 2017. Comparative genomics of Escherichia coli isolated from skin and soft tissue and other extraintestinal infections. mBio 8:e01070-17. https://doi.org/10.1128/mBio.01070-17.
REFERENCES
- 1.Johnson JR, Russo TA. 2002. Extraintestinal pathogenic Escherichia coli: “the other bad E. coli.” J Lab Clin Med 139:155–162. doi: 10.1067/mlc.2002.121550. [DOI] [PubMed] [Google Scholar]
- 2.Smith JL, Fratamico PM, Gunther NW. 2007. Extraintestinal pathogenic Escherichia coli. Foodborne Pathog Dis 4:134–163. doi: 10.1089/fpd.2007.0087. [DOI] [PubMed] [Google Scholar]
- 3.Wagenlehner FM, Lichtenstern C, Rolfes C, Mayer K, Uhle F, Weidner W, Weigand MA. 2013. Diagnosis and management for urosepsis. Int J Urol 20:963–970. doi: 10.1111/iju.12200. [DOI] [PubMed] [Google Scholar]
- 4.Eckmann C, Dryden M. 2010. Treatment of complicated skin and soft tissue infections caused by resistant bacteria: value of linezolid, tigecycline, daptomycin and vancomycin. Eur J Med Res 15:554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petty NK, Ben Zakour NL, Stanton-Cook M, Skippington E, Totsika M, Forde BM, Phan MD, Gomes Moriel D, Peters KM, Davies M, Rogers BA, Dougan G, Rodriguez-Baño J, Pascual A, Pitout JD, Upton M, Paterson DL, Walsh TR, Schembri MA, Beatson SA. 2014. Global dissemination of a multidrug resistant Escherichia coli clone. Proc Natl Acad Sci U S A 111:5694–5699. doi: 10.1073/pnas.1322678111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Satlin MJ, Jenkins SG, Walsh TJ. 2014. The global challenge of carbapenem-resistant Enterobacteriaceae in transplant recipients and patients with hematologic malignancies. Clin Infect Dis 58:1274–1283. doi: 10.1093/cid/ciu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ranjan A, Shaik S, Mondal A, Nandanwar N, Hussain A, Semmler T, Kumar N, Tiwari SK, Jadhav S, Wieler LH, Ahmed N. 2016. Molecular epidemiology and genome dynamics of New Delhi metallo-β-lactamase-producing extraintestinal pathogenic Escherichia coli strains from India. Antimicrob Agents Chemother 60:6795–6805. doi: 10.1128/AAC.01345-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jadhav S, Hussain A, Devi S, Kumar A, Parveen S, Gandham N, Wieler LH, Ewers C, Ahmed N. 2011. Virulence characteristics and genetic affinities of multiple drug resistant uropathogenic Escherichia coli from a semi urban locality in India. PLoS One 6:e18063. doi: 10.1371/journal.pone.0018063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hussain A, Ewers C, Nandanwar N, Guenther S, Jadhav S, Wieler LH, Ahmed N. 2012. Multiresistant uropathogenic Escherichia coli from a region in India where urinary tract infections are endemic: genotypic and phenotypic characteristics of sequence type 131 isolates of the CTX-M-15 extended-spectrum-beta-lactamase-producing lineage. Antimicrob Agents Chemother 56:6358–6365. doi: 10.1128/AAC.01099-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avasthi TS, Kumar N, Baddam R, Hussain A, Nandanwar N, Jadhav S, Ahmed N. 2011. Genome of multidrug-resistant uropathogenic Escherichia coli strain NA114 from India. J Bacteriol 193:4272–4273. doi: 10.1128/JB.05413-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ranjan A, Shaik S, Hussain A, Nandanwar N, Semmler T, Jadhav S, Wieler LH, Ahmed N. 2015. Genomic and functional portrait of a highly virulent, CTX-M-15-producing H30-Rx subclone of Escherichia coli sequence type 131. Antimicrob Agents Chemother 59:6087–6095. doi: 10.1128/AAC.01447-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Picard B, Garcia JS, Gouriou S, Duriez P, Brahimi N, Bingen E, Elion J, Denamur E. 1999. The link between phylogeny and virulence in Escherichia coli extraintestinal infection. Infect Immun 67:546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussain A, Ranjan A, Nandanwar N, Babbar A, Jadhav S, Ahmed N. 2014. Genotypic and phenotypic profiles of Escherichia coli isolates belonging to clinical sequence type 131 (ST131), clinical non-ST131, and fecal non-ST131 lineages from India. Antimicrob Agents Chemother 58:7240–7249. doi: 10.1128/AAC.03320-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petkovsek Z, Elersic K, Gubina M, Zgur-Bertok D, Starcic Erjavec M. 2009. Virulence potential of Escherichia coli isolates from skin and soft tissue infections. J Clin Microbiol 47:1811–1817. doi: 10.1128/JCM.01421-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ananias M, Yano T. 2008. Serogroups and virulence genotypes of Escherichia coli isolated from patients with sepsis. Braz J Med Biol Res 41:877–883. [DOI] [PubMed] [Google Scholar]
- 16.Foxman B. 2010. The epidemiology of urinary tract infection. Nat Rev Urol 7:653–660. doi: 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- 17.Hawkey PM. 2008. Prevalence and clonality of extended-spectrum beta-lactamases in Asia. Clin Microbiol Infect 14(Suppl 1):159–165. doi: 10.1111/j.1469-0691.2007.01855.x. [DOI] [PubMed] [Google Scholar]
- 18.Banerjee R, Johnston B, Lohse C, Chattopadhyay S, Tchesnokova V, Sokurenko EV, Johnson JR. 2013. The clonal distribution and diversity of extraintestinal Escherichia coli isolates vary according to patient characteristics. Antimicrob Agents Chemother 57:5912–5917. doi: 10.1128/AAC.01065-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clermont O, Bonacorsi S, Bingen E. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl Environ Microbiol 66:4555–4558. doi: 10.1128/AEM.66.10.4555-4558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute 2008. M31 A3, 3rd ed Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 21.Monstein HJ, Ostholm-Balkhed A, Nilsson MV, Nilsson M, Dornbusch K, Nilsson LE. 2007. Multiplex PCR amplification assay for the detection of blaSHV, blaTEM and blaCTX-M genes in Enterobacteriaceae. APMIS 115:1400–1408. doi: 10.1111/j.1600-0463.2007.00722.x. [DOI] [PubMed] [Google Scholar]
- 22.Pitout JD, Hossain A, Hanson ND. 2004. Phenotypic and molecular detection of CTX-M-beta-lactamases produced by Escherichia coli and Klebsiella spp. J Clin Microbiol 42:5715–5721. doi: 10.1128/JCM.42.12.5715-5721.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nordmann P, Poirel L, Carrër A, Toleman MA, Walsh TR. 2011. How to detect NDM-1 producers. J Clin Microbiol 49:718–721. doi: 10.1128/JCM.01773-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park CH, Robicsek A, Jacoby GA, Sahm D, Hooper DC. 2006. Prevalence in the United States of aac(6′)-Ib-cr encoding a ciprofloxacin-modifying enzyme. Antimicrob Agents Chemother 50:3953–3955. doi: 10.1128/AAC.00915-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boerlin P, Travis R, Gyles CL, Reid-Smith R, Janecko N, Lim H, Nicholson V, McEwen SA, Friendship R, Archambault M. 2005. Antimicrobial resistance and virulence genes of Escherichia coli isolates from swine in Ontario. Appl Environ Microbiol 71:6753–6761. doi: 10.1128/AEM.71.11.6753-6761.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skurnik D, Le Menac’h A, Zurakowski D, Mazel D, Courvalin P, Denamur E, Andremont A, Ruimy R. 2005. Integron-associated antibiotic resistance and phylogenetic grouping of Escherichia coli isolates from healthy subjects free of recent antibiotic exposure. Antimicrob Agents Chemother 49:3062–3065. doi: 10.1128/AAC.49.7.3062-3065.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daigle F, Harel J, Fairbrother JM, Lebel P. 1994. Expression and detection of pap-, sfa-, and afa-encoded fimbrial adhesin systems among uropathogenic Escherichia coli. Can J Microbiol 40:286–291. doi: 10.1139/m94-046. [DOI] [PubMed] [Google Scholar]
- 28.Li G, Laturnus C, Ewers C, Wieler LH. 2005. Identification of genes required for avian Escherichia coli septicemia by signature-tagged mutagenesis. Infect Immun 73:2818–2827. doi: 10.1128/IAI.73.5.2818-2827.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weissman SJ, Johnson JR, Tchesnokova V, Billig M, Dykhuizen D, Riddell K, Rogers P, Qin X, Butler-Wu S, Cookson BT, Fang FC, Scholes D, Chattopadhyay S, Sokurenko E. 2012. High-resolution two-locus clonal typing of extraintestinal pathogenic Escherichia coli. Appl Environ Microbiol 78:1353–1360. doi: 10.1128/AEM.06663-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto S, Terai A, Yuri K, Kurazono H, Takeda Y, Yoshida O. 1995. Detection of urovirulence factors in Escherichia coli by multiplex polymerase chain reaction. FEMS Immunol Med Microbiol 12:85–90. doi: 10.1111/j.1574-695X.1995.tb00179.x. [DOI] [PubMed] [Google Scholar]
- 31.Nakano M, Yamamoto S, Terai A, Ogawa O, Makino SI, Hayashi H, Nair GB, Kurazono H. 2001. Structural and sequence diversity of the pathogenicity island of uropathogenic Escherichia coli which encodes the USP protein. FEMS Microbiol Lett 205:71–76. doi: 10.1111/j.1574-6968.2001.tb10927.x. [DOI] [PubMed] [Google Scholar]
- 32.Ewers C, Li G, Wilking H, Kiessling S, Alt K, Antáo EM, Laturnus C, Diehl I, Glodde S, Homeier T, Böhnke U, Steinrück H, Philipp HC, Wieler LH. 2007. Avian pathogenic, uropathogenic, and newborn meningitis-causing Escherichia coli: how closely related are they? Int J Med Microbiol 297:163–176. doi: 10.1016/j.ijmm.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Vandekerchove D, Vandemaele F, Adriaensen C, Zaleska M, Hernalsteens JP, De Baets L, Butaye P, Van Immerseel F, Wattiau P, Laevens H, Mast J, Goddeeris B, Pasmans F. 2005. Virulence-associated traits in avian Escherichia coli: comparison between isolates from colibacillosis-affected and clinically healthy layer flocks. Vet Microbiol 108:75–87. doi: 10.1016/j.vetmic.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Germon P, Chen YH, He L, Blanco JE, Brée A, Schouler C, Huang SH, Moulin-Schouleur M. 2005. ibeA, a virulence factor of avian pathogenic Escherichia coli. Microbiology 151:1179–1186. doi: 10.1099/mic.0.27809-0. [DOI] [PubMed] [Google Scholar]
- 35.Johnson JR, Russo TA, Tarr PI, Carlino U, Bilge SS, Vary JC Jr, Stell AL. 2000. Molecular epidemiological and phylogenetic associations of two novel putative virulence genes, iha and iroNE. coli, among Escherichia coli isolates from patients with urosepsis. Infect Immun 68:3040–3047. doi: 10.1128/IAI.68.5.3040-3047.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel RK, Jain M. 2012. NGS QC toolkit: a toolkit for quality control of next generation sequencing data. PLoS One 7:e30619. doi: 10.1371/journal.pone.0030619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaik S, Kumar N, Lankapalli AK, Tiwari SK, Baddam R, Ahmed N. 2016. Contig-layout-authenticator (CLA): a combinatorial approach to ordering and scaffolding of bacterial contigs for comparative genomics and molecular epidemiology. PLoS One 11:e0155459. doi: 10.1371/journal.pone.0155459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Overbeek R, Olson R, Pusch GD, Olsen GJ, Davis JJ, Disz T, Edwards RA, Gerdes S, Parrello B, Shukla M, Vonstein V, Wattam AR, Xia F, Stevens R. 2014. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res 42:D206–D214. doi: 10.1093/nar/gkt1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rutherford K, Parkhill J, Crook J, Horsnell T, Rice P, Rajandream MA, Barrell B. 2000. Artemis: sequence visualization and annotation. Bioinformatics 16:944–945. doi: 10.1093/bioinformatics/16.10.944. [DOI] [PubMed] [Google Scholar]
- 41.Alikhan NF, Petty NK, Ben Zakour NL, Beatson SA. 2011. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics 12:402. doi: 10.1186/1471-2164-12-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Besemer J, Lomsadze A, Borodovsky M. 2001. GeneMarkS: a self-training method for prediction of gene starts in microbial genomes. Implications for finding sequence motifs in regulatory regions. Nucleic Acids Res 29:2607–2618. doi: 10.1093/nar/29.12.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 44.Chen L, Yang J, Yu J, Yao Z, Sun L, Shen Y, Jin Q. 2005. VFDB: a reference database for bacterial virulence factors. Nucleic Acids Res 33:D325–D328. doi: 10.1093/nar/gki008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McArthur AG, Waglechner N, Nizam F, Yan A, Azad MA, Baylay AJ, Bhullar K, Canova MJ, De Pascale G, Ejim L, Kalan L, King AM, Koteva K, Morar M, Mulvey MR, O’Brien JS, Pawlowski AC, Piddock LJ, Spanogiannopoulos P, Sutherland AD, Tang I, Taylor PL, Thaker M, Wang W, Yan M, Yu T, Wright GD. 2013. The comprehensive antibiotic resistance database. Antimicrob Agents Chemother 57:3348–3357. doi: 10.1128/AAC.00419-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nandanwar N, Janssen T, Kühl M, Ahmed N, Ewers C, Wieler LH. 2014. Extraintestinal pathogenic Escherichia coli (ExPEC) of human and avian origin belonging to sequence type complex 95 (STC95) portray indistinguishable virulence features. Int J Med Microbiol 304:835–842. doi: 10.1016/j.ijmm.2014.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogroup, extended-spectrum beta-lactamase (ESBL) production phenotype, and antimicrobial resistance patterns of ExPEC isolates against six non-beta-lactam antibiotics. Download TABLE S1, DOCX file, 0.03 MB (28KB, docx) .
Copyright © 2017 Ranjan et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.