Abstract
Cyanobacteria can generate molecules hazardous to human health, but production of the known cyanotoxins is taxonomically sporadic. For example, members of a few genera produce hepatotoxic microcystins, whereas production of hepatotoxic nodularins appears to be limited to a single genus. Production of known neurotoxins has also been considered phylogenetically unpredictable. We report here that a single neurotoxin, β-N-methylamino-l-alanine, may be produced by all known groups of cyanobacteria, including cyanobacterial symbionts and free-living cyanobacteria. The ubiquity of cyanobacteria in terrestrial, as well as freshwater, brackish, and marine environments, suggests a potential for wide-spread human exposure.
Keywords: biomagnification, neurotoxin, symbiosis, amyotrophic lateral sclerosis/parkinsonism–dementia complex
Cyanotoxin production by cyanobacteria appears to be of wide, but irregular, occurrence throughout the five principal classification sections of cyanobacteria based on axenic strains (1). For example, the hepatotoxic cyclic heptapeptide microcystins are produced by members of the genera Microcystis, Anabaena, Planktothrix, Hapalosiphon, Anabaenopsis, and Nostoc. In contrast, production of the cyclic pentapeptide nodularins appears to be limited to Nodularia among the free-living cyanobacterial genera, with indications of nodularin production by cyanobacterial symbionts in a sponge (2). Phylogenetic investigations have indicated an early and widespread occurrence of genes involved in nonribosomal peptide synthesis, including microcystin synthesis. Sporadic distribution of microcystin synthesis in extant cyanobacteria appears to have occurred because of repeated losses of genes for microcystin biosynthesis in the later-derived lineages of these organisms (3). This pattern appears to have arisen because of an early development of microcystin production followed by repeated gene loss in cyanobacterial evolution. The consequences of cyanobacterial toxins on human health, water-based industries, recreation, and wildlife are of increasing concern as eutrophication and rising global temperatures trigger increases in the geographical extent, population densities, and duration of cyanobacterial blooms in fresh, brackish, and marine waters. Human poisonings from cyanobacterial blooms can be serious; 150 persons who drank cyanobacteria-contaminated water in Australia were hospitalized, and >50 kidney dialysis patients at a Brazilian clinic who were exposed to microcystins died (4–7).
β-N-methylamino-l-alanine (BMAA), a nonprotein amino acid, was found to be produced (8) by cyanobacterial root symbionts of the genus Nostoc (9). Originally discovered in cycad seeds (10), BMAA was suggested as a possible cause of the amyotrophic lateral sclerosis/parkinsonism–dementia complex (ALS/PDC) that has an extremely high rate of incidence among the Chamorro people of Guam compared with incidence rates of ALS elsewhere (11, 12). Although BMAA as a putative cause of ALS/PDC was initially disputed (13), this hypothesis has recently regained attention when it was discovered that BMAA is biomagnified within the Guam ecosystem (8, 14–16) and is found in the brain tissues of Chamorros who died of ALS/PDC, but not in patients who died of causes unrelated to neurodegenerative disease (8, 16, 17).
BMAA accumulates in ascending trophic levels within the Guam ecosystem. Axenic cultures of Nostoc (isolated from the coralloid roots of the cycad tree Cycas micronesica) produce BMAA at 0.3 μg/g. BMAA occurs at 37 μg/g within mildly infected C. micronesica coralloid roots, and at 3,556 μg/g in the flying foxes that forage on the sarcotesta of C. micronesica seeds, a roughly 100-fold increase for each trophic level (8, 15, 18). Because flying foxes are a traditional delicacy (19) of the indigenous Chamorro people, this 10,000-fold increase in BMAA concentration from cyanobacteria to flying foxes suggests that the Chamorros may unwittingly ingest high levels of BMAA in their traditional diet. Flying foxes are not the only BMAA source in the Chamorro diet: cycad seed flour, which shows little free BMAA after washing, releases up to 169 μg/g BMAA on acid hydrolysis (16). Other protein-associated fractions from each organism in the Guam food chain typically exhibit a 100-fold BMAA increase over the amount of free BMAA (16). Once ingested, BMAA can be bound by proteins within the body, resulting in a slow release of free BMAA over years as contaminated proteins are metabolized. Demonstration, through enzymatic cleavage, that BMAA is incorporated within the actual amino acid sequence of the protein would add weight to this hypothesis. Such a mechanism may explain the observed long latency period of ALS/PDC among the Chamorros (16). We suggest that the Chamorro people of Guam may not be the only human population exposed to this cyanobacteria-produced “slow toxin” (20).
BMAA was recently discovered in the brain tissues of nine Canadian Alzheimer's patients, but it was not detected in the brain tissues of 14 other Canadians who died of causes unrelated to neurodegeneration (8, 16, 17). Because cycads are not part of the Canadian flora, we suggested that cyanobacteria might be the ultimate source of the BMAA in the Canadian Alzheimer's patients (8). We also found BMAA in other cyanobacterial–plant symbioses (Azolla filiculoides, 2 μg/g; Gunnera kauaiensis, 4 μg/g) (8). These new findings raised additional questions. Is BMAA produced by other taxa of cyanobacteria? Is the biomagnification of cyanobacteria-produced BMAA unique to the Guam ecosystem or can it occur elsewhere?
Because molecular phylogenies unite the cyanobacteria into a single monophyletic group (21), we decided to examine BMAA production in representative free-living and morphological genera from all five of the principal sections (Fig. 1) of cyanobacteria (1) as well as in cyanobacterial symbionts isolated from lichen and a diversity of plant species. We analyzed cyanobacterial cultures maintained at the University of Dundee in Scotland, Stockholm University in Sweden, and the University of Hawaii in the United States, as well as natural bloom samples, by using a fluorescent derivatization of amino acids coupled with HPLC to quantify free and protein-associated BMAA for each sample (Fig. 2). We then confirmed selected BMAA peaks through liquid chromatography-MS.
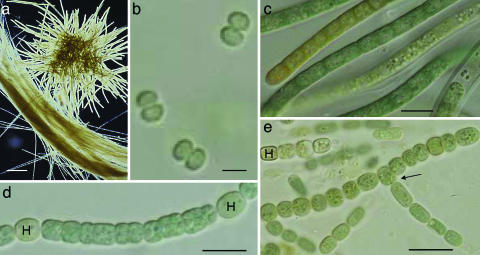
Fig. 1.
Cyanobacterial strains that produce BMAA representing different morphological sections. (a) The bloom-forming, filamentous, and colony-forming Trichodesmium thiebautii (section III). (Scale bar: 100 μm.) (b) The unicellular Synechococcus PCC 6301 (section I). (Scale bar: 1 μm.) (c) The filamentous Symploca PCC 8002 (section III). (Scale bar: 10 μm.) (d) The filamentous, nonbranching, and heterocystous (H) Nostoc PCC 7107 (section IV). (Scale bar: 10 μm.) (e) The filamentous, heterocystous (H) and branching (arrow) Fischerella PCC 7521 (section V). (Scale bar: 15 μm.)
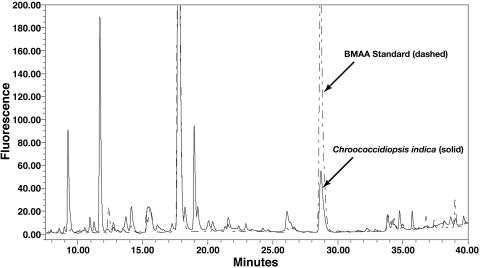
Fig. 2.
HPLC chromatograph of BMAA peak in Chroococcidiopsis indica GT-3–26 (section II) (solid line) and BMAA authenticated standard (dashed line) obtained by using fluorescence detection.
Materials and Methods
Samples of cyanobacterial cultures maintained at the Universities of Stockholm, Hawaii, and Dundee were analyzed for BMAA production. Some cultures had been isolated from symbioses with species of the flowering plant Gunnera or from lichens, hornworts, and liverworts; others came from marine, brackish, and freshwater environments collected throughout the world. Lyophilized samples (5–20 μg dry weight) of cyanobacterial cultures were extracted with 0.1 M trichloroacetic acid and centrifuged at 15,800 × g for 3 min to precipitate proteins. Bound BMAA was released from the protein pellets by hydrolysis (6 M HCl at 110°C for 24 h). Extracts underwent ultrafiltration before derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate. BMAA was quantified by using a validated HPLC separation. Free amino acids were separated by reverse-phase separation on a gradient HPLC system (Waters 717 Automated Injector and 1525 Binary Solvent Delivery System) and Waters Nova-Pak C18 column at 37°C. Individual compounds were eluted from the column with a gradient elution of 140 mM sodium acetate, 5.6 mM triethylamine (pH 5.2), and 60% or 52% acetonitrile (15). The identity of the BMAA peak was confirmed by comparison with an authenticated standard (Sigma) and was reverified by a modified gradient elution. The concentration of BMAA in samples was determined by fluorescence detection (Waters 2487 Dual-Fluorescence Detector) with excitation at 250 nm and emission at 395 nm. Detection of the 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate-derivatized BMAA depended on concentration and comparison of equal amounts of BMAA and a norleucine internal standard, resulting in a mean response of 51.2%. The lower limits of detection and lower limits of quantification were determined by a concentration gradient of an authenticated standard. The lower limits of detection was 0.000065 mol per injection for all analyses. The lower limits of quantification was 0.0065 mol per injection for all analyses. For data interpretation, all sample analyses were quantified within the range of the lower limits of quantification or were reported as not detected (Tables 1 and 2).
Table 1. BMAA in cyanobacteria isolated from symbioses.
| Cyanobacteria | Host | Symbiont | Free BMAA, μg/g | Protein BMAA, μg/g |
|---|---|---|---|---|
| Nostoc Pc | Lichen | Peltigera | ND | 1,671 |
| Nostoc PCC 9305 | Hornwort | Anthoceros | 156 | 1,400 |
| Nostoc LBG1 | Liverwort | Blasia | ND | ND |
| Nostoc PCC 73102 | Cycad | Macrozamia | ND | ND |
| Nostoc PCC 7422 | Cycad | Cycas | ND | 962 |
| Nostoc enc | Cycad | Encephalartos | 137 | ND |
| Nostoc PCC 9229 | Flowering plant | Gunnera monoica | 110 | ND |
| Nostoc 8001 | Flowering plant | Gunnera monoica | 203 | 664 |
| Nostoc 9401 | Flowering plant | Gunnera perpensa | ND | ND |
| Nostoc 8963 | Flowering plant | Gunnera prorepens | ND | 58 |
| Nostoc 8964 | Flowering plant | Gunnera macrophylla | 9 | ND |
ND, not detected.
Table 2. BMAA in free-living cyanobacteria.
| Cyanobacterial species/strain | Section* | Habitat | Origin | Free BMAA, μg/g | Protein BMAA, μg/g |
|---|---|---|---|---|---|
| Microcystis PCC 7806 | I | Freshwater | The Netherlands | 4 | 6 |
| Microcystis PCC 7820 | I | Freshwater | Scotland | 6 | 12 |
| Prochlorococcus marinus CCMP 1377 | I | Marine | Sargasso Sea | 32† | 57† |
| Synechocystis PCC 6308 | I | Freshwater | U.S.A. | ND | ND |
| Synechococcus PCC 6301 | I | Freshwater | U.S.A. | 25 | ND |
| Chroococcidiopsis indica GQ2-7 | II | Marine coral | Unknown | 435 | 76 |
| Chroococcidiopsis indica GT-3-26 | II | Marine rock | Unknown | 1,306 | 5,415 |
| Myxosarcina burmensis GB-9-4 | II | Marine coral | Marshall Islands | 79 | 1,943 |
| Myxosarcina concinna GT-7-6 | II | Marine coral | Unknown | 1,501 | 960 |
| Lyngbya majuscula | III | Marine | Zanzibar | 32 | 4 |
| Planktothrix agardhii NIES 595 | III | Freshwater | Northern Ireland | 318 | 30 |
| Plectonema PCC 73110 | III | Unknown | Unknown | 155 | 150 |
| Phormidium | III | Unknown | Unknown | 11 | 270 |
| Symploca PCC 8002 | III | Marine, intertidal | U.K. | 3 | 262 |
| Trichodesmium thiebautii | III | Marine | Caribbean | 145 | 8 |
| Trichodesmium CCMP 1985 | III | Marine, coastal | North Carolina | 13† | 17† |
| Anabaena PCC 7120 | IV | Unknown | U.S.A. | 32 | ND |
| Anabaena variabilis ATCC 29413 | IV | Freshwater | U.S.A. | 35 | ND |
| Aphanizomenon flos-aquae | IV | Marine | Baltic Sea | ND | 866 |
| Cylindrospermopsis raciborskii CR3 | IV | Freshwater | Australia | 6,478 | 14 |
| Nodularia spumigena | IV | Brackish water | Baltic Sea | 16‡ | 50‡ |
| Nodularia harveyana CCAP 1452/1 | IV | Marine | Unknown | 20 | 11 |
| Nostoc 268 | IV | Brackish Water | Baltic Sea | 34 | 274 |
| Nostoc PCC 6310 | IV | Freshwater | Israel | 42 | ND |
| Nostoc PCC 7107 | IV | Freshwater | U.S.A. | 27 | 1,772 |
| Nostoc sp. CMMED 01 | IV | Marine | Hawaiian Islands | 1,243 | 1,070 |
| Calothrix PCC 7103 | IV | Unknown | Unknown | 13‡ | 92‡ |
| Chlorogloeopsis PCC 6912 | V | Soil | India | 758 | ND |
| Fischerella PCC 7521 | V | Yellowstone, hot spring | U.S.A. | 44 | 175 |
| Scytonema PCC 7110 | V | Limestone cave | Bermuda | ND | 1,733 |
ND, not detected.
Morphological groupings are as defined in ref.1. Section I, unicellular cyanobacteria that reproduce by binary fusion or budding; section II, unicellular cyanobacteria that reproduce by multiple fission or by both multiple fission and binary fission; section III, filamentous, nonheterocystous cyanobacteria that divide in one plane; section IV, filamentous, heterocystous cyanobacteria that divide in only one plane; section V, heterocystous, filamentous cyanobacteria that divide in more than one plane.
Estimate of concentration based on <1 mg dry weight of sample.
BMAA was not detected in all samples of this isolate.
The presence of BMAA in the samples as well as the identity and purity of the BMAA peak in the HPLC separations was confirmed by liquid chromatography-MS by using the same HPLC system in-line with a Micromass ZQ/EMD1000 MS (Waters) mass spectrometer, single quadrapole MS with an atmospheric pressure ionization source by using the electrospray ionization interface all controlled by the same computer. Compounds were separated on a reverse-phase column (Nova-Pak C18, 4 Fm, 3.9 × 300 mm, Waters) heated at 30°C with a linear gradient elution of acetonitrile (10–60% over 25 min) in water. Nitrogen gas was purified and supplied to the electrospray ionization interface with a nebulizing pressure of 100 psi. BMAA was identified through selective ion monitoring in both positive and negative ion modes with detection of the derivatized parent molecule and two daughter ions. All BMAA ions were detected with a dwell time of 1 sec and a cone voltage of 35 V.
Results
Analysis of Nostoc strains isolated from symbiotic relationships with lichen and host plants of broad taxonomic diversity indicated that 73% (8/11) of these strains produced BMAA (Table 1).
For free-living cyanobacteria, we found that BMAA is produced by members of all five cyanobacterial sections, as well as in 95% (20/21) of the genera tested, and 97% (29/30) of the strains we tested, representing a wide phylogenetic and ecological diversity (Table 2). Because we found BMAA in an isolate of bloom-forming Nodularia from the Baltic Sea (Table 2), we have now begun testing samples from freshwater and marine cyanobacterial blooms. Our preliminary results are intriguing. For example, Trichodesmium sp. concentrated from two separate frozen 500-ml samples of seawater from a Hawaiian marine bloom, collected August 18, 2004, had 0.0079 μg/g and 0.0071 μg/g BMAA wet weight, respectively. The supernatant from the pelleted Trichodesmium in seawater had detectable BMAA; however, it was below our lower limits of quantification.
Discussion
Because cyanobacteria are arguably among the most widespread, abundant, and ancient organisms on planet Earth, our finding that all five sections and 95% of all genera of cyanobacteria tested produce the neurotoxin BMAA is of both ecological and evolutionary significance. For example, Trichodesmium blooms are known to cover thousands of square kilometers of the Earth's oceans (Fig. 3). They are observable by satellite from earth orbit and reflect light frequencies that allow taxonomic determination of the cyanobacterium (22). Our detection of BMAA in samples of Baltic Sea and oceanic blooms suggest that significant quantities of BMAA may be released into the world's oceans. Previous analysis of marine blooms of Trichodesmium thiebautii and Trichodesmium erythraeum have revealed high neurotoxicity to mice, but the low molecular weight neurotoxic entitity remained a mystery because peptide toxins and anatoxin-a could not be detected in the bloom extracts (23); if this work is repeated, we suggest that an analysis of BMAA be conducted. Similarly, the occurrence of BMAA in Prochlorococcus (Table 2), the most abundant oxyphototroph found in tropical and subtropical waters, could indicate a significant input of neurotoxin at the lowest trophic level of such marine ecosystems (24).
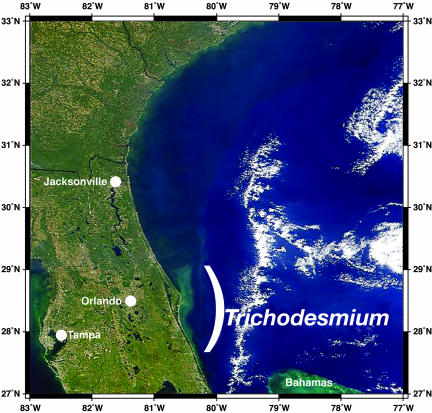
Fig. 3.
Satellite photograph of a Trichodesmium bloom by using SeaWiFS imagery for spectral imaging at 443, 490, and 550 nm off the eastern coast of Florida on October 30, 1998.
Although we found 95% of cyanobacterial genera and 97% of cyanobacterial strains in our samples to produce BMAA, it is possible that given the right conditions, all cyanobacteria are capable of producing BMAA. Our data (Table 2) show significant variance in the range of BMAA content between genera and species, and also within the ratio of free to protein-bound BMAA. This variance suggests that BMAA production and storage is a function of growth conditions and/or life cycle stages. For example, samples of a single Calothrix culture taken 3 months apart showed no BMAA in one sample but quantifiable amounts in the second, and a similar situation was found in Nodularia spumigena. This finding is unlike the situation for other known cyanotoxins (neuro- and hepatotoxins), which seem to be consistently produced by individual toxigenic strains, but with a relatively uneven occurrence among genera (2). Additionally, as our analysis shows that free-living (terrestrial, as well as freshwater, brackish, and marine) cyanobacteria produce BMAA, symbiosis is not a prerequisite for cyanobacterial BMAA production; instead, BMAA production appears to be a common trait within the entire cyanobacterial radiation.
The widespread occurrence of BMAA in our survey suggests that production of this unusual nonprotein amino acid may be a synapomorphy, a common conserved evolutionary feature that unites the cyanobacteria as a group. Because cyanobacteria were among the earliest biological entities to develop on Earth, with Precambrian fossil records conservatively stretching to 1.5 billion years B.P. (25), BMAA is likely to have been produced long before the evolution of organisms with neuronal systems. If so, any selective value of BMAA for the cyanobacteria must have been initially unrelated to its neurotoxicity. Because BMAA likely alters the tertiary conformation of proteins, and because incorporation of BMAA into cyanobacterial peptides may occur as the result of nonribosomal peptide synthesis (26), it is possible that the function of certain aspects of cyanobacterial biochemistry may depend on the presence of this nonprotein amino acid.
Our analysis greatly expands the potential distribution of BMAA in nature as well as the total quantity of BMAA released to the environment. Before 2003, BMAA was known to occur only in cycads, limiting potential human exposure to tropical and subtropical terrestrial environments where cycads grow and then only to indigenous peoples who consume cycad products or animals that feed on cycads. However, these new data demonstrate that BMAA is produced by cyanobacteria from geographical regions and diverse environments throughout the world (Table 2). Because cyanobacteria function as primary producers in many food chains, it is likely that human populations far from Guam may be exposed to this environmental neurotoxin. This suggestion is partially corroborated by the discovery of BMAA in brain tissues of Canadian Alzheimer's patients (16, 17).
In Guam, human exposure to high quantities of BMAA results from unique components of the traditional Chamorro diet including cycad tortillas, flying foxes, and possibly other feral animals (8). Here, we show that cyanobacterial symbionts of other plants also contain BMAA. Symbioses between cyanobacteria and plants are taxonomically uncommon but can be of ecological importance. For example, ungulates that graze on cyanolichen genera such as Peltigera (9, 27, 28) may ingest BMAA at certain seasons of the year (Table 1). The ubiquity of cyanobacteria in diverse terrestrial and aquatic environments suggests that ingestion of BMAA may occur through even less esoteric routes, including direct consumption of cyanobacteria or cyanobacterial hosts, bioaccumulation in additional food chains, or exposure to cyanobacteria-contaminated water supplies.
The recent hypothesis that BMAA accumulates in proteins, which collectively function as an endogenous neurotoxic reservoir within the human body, and then is slowly released through time as these proteins are metabolized (16) suggests that possible health consequences of chronic exposure to low doses of BMAA deserve further investigation. It may now be prudent to monitor BMAA concentrations in drinking waters contaminated by cyanobacterial blooms. BMAA concentrations should also be monitored within invertebrates, fish, or grazing animals used for human consumption that either directly consume cyanobacteria or forage on plants or prey that may have accumulated cyanobacteria-produced BMAA. Given the global importance of marine cyanobacterial blooms, such as those generated by iron-laden dust in the Atlantic and Pacific Oceans (29–31), a broader analysis of the production and fate of BMAA in marine ecosystems is also needed.
Acknowledgments
We thank M. K. Asay and H. Johnson at the Institute for Ethnomedicine for technical support and R. Honegger of the University of Zurich for useful discussions of cyanolichens. We thank the Acacia Foundation (Larkspur, CA), B. and J. Lane, P. and H. Henry, and C. Childs for laboratory equipment, and N. Kuring of the National Aeronautics and Space Administration (Washington, DC) and A. Subaramaniam of the LaMont Doherty Observatory (Columbia University, NY) for satellite imagery. This work was funded by grants from the Harold K. L. Castle Foundation (Kailua, HI) (to P.A.C.); the Swedish Research Council (Stockholm) (to B.B.); the European Commission (Brussels) (to G.A.C.); the Swedish International Development Cooperation Agency (Stockholm) (to U.R.); National Institute for Environmental Health Sciences (Research Triangle Park, NC) (to R.R.B.); and the National Science Foundation (Arlington, VA) (to R.R.B.).
Author contributions: P.A.C., S.A.B., S.J.M., U.R., G.T., R.R.B., J.S.M., L.F.M., G.A.C., and B.B. designed research; P.A.C., S.A.B., S.J.M., U.R., G.T., R.R.B., J.S.M., L.F.M., G.A.C., and B.B. performed research; P.A.C., S.A.B., S.J.M., U.R., G.T., R.R.B., J.S.M., L.F.M., G.A.C., and B.B. analyzed data; and P.A.C., S.A.B., U.R., G.T., R.R.B., J.S.M., L.F.M., G.A.C., and B.B. wrote the paper.
Abbreviations: BMAA, β-N-methylamino-l-alanine; ALS, amyotrophic lateral sclerosis; PDC, parkinsonism–dementia complex.
References
- 1.Rippka, R., Deruelles, J. B., Waterbury, J. B., Herdman, M. & Stanier, R. Y. (1979) J. Gen. Microbiol. 111, 1–61. [Google Scholar]
- 2.Sivonen, K. & Jones, G. (1999) in Toxic Cyanobacteria in Water, eds. Chorus, I. & Bartram, J. (E & F. N. Spon, London), pp. 41–111.
- 3.Rantala, A., Fewer, D. P., Hisbergues, M., Rouhiainen, L., Vaitomaa, J., Börner, T. & Sivonen, K. (2004) Proc. Natl. Acad. Sci. USA 101, 568–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuiper-Goodman, T., Falconer, I. & Fitzgerald, J. (1999) in Toxic Cyanobacteria in Water, eds. Chorus, I. & Bartram, J. (E & F. N. Spon, London), pp. 113–153.
- 5.Pouria, S., de Andrade, A., Barbosa, J., Cavalcanti, R. L., Barreto, V. T. S., Ward, C. J., Preiser, W., Poon, G. K., Neild, G. H. & Codd, G. A. (1998) Lancet 352, 21–26. [DOI] [PubMed] [Google Scholar]
- 6.Carmichael, W. W. (1994) Sci. Am. 270, 78–86. [DOI] [PubMed] [Google Scholar]
- 7.Codd, G. A., Bell, S. G., Kaya, K., Ward, C. J., Beattie, K. A. & Metcalf, J. S. (1999) Eur. J. Phycol. 34, 405–415. [Google Scholar]
- 8.Cox, P. A., Banack, S. A. & Murch, S. J. (2003) Proc. Natl. Acad. Sci. USA 100, 13380–13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vessey, J. K., Pawlowski, K. & Bergman, B. (2005) Plant Soil 266, 205–230. [Google Scholar]
- 10.Vega, A. & Bell, E. A. (1967) Phytochemistry 6, 759–762. [Google Scholar]
- 11.Spencer, P. S., Nunn, P. B., Hugan, J., Ludolph, A. & Roy, D. N. (1986) Lancet i, 965. [DOI] [PubMed] [Google Scholar]
- 12.Spencer, P. S., Nunn, P. B., Hugon, J., Ludolph, A. C., Ross, S. M., Roy, D. N. & Robertson, R. C. (1987) Science 237, 517–522. [DOI] [PubMed] [Google Scholar]
- 13.Duncan, M. W., Steele, J. C., Kopin, I. J. & Markey, S. P. (1990) Neurology 40, 767–772. [DOI] [PubMed] [Google Scholar]
- 14.Cox, P. A. & Sacks, O. W. (2002) Neurology 58, 956–959. [DOI] [PubMed] [Google Scholar]
- 15.Banack, S. A. & Cox, P. A. (2003) Neurology 61, 387–389. [DOI] [PubMed] [Google Scholar]
- 16.Murch, S. J., Cox, P. A. & Banack, S. A. (2004) Proc. Natl. Acad. Sci. USA 101, 12228–12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murch, S. J., Cox, P. A., Banack, S. A., Steele, J. C. & Sacks, O. W. (2004) Acta Neurol. Scand. 110, 267–269. [DOI] [PubMed] [Google Scholar]
- 18.Banack, S. A. & Cox, P. A. (2003) Bot. J. Linn. Soc. 143, 165–168. [Google Scholar]
- 19.Monson, C. S., Banack, S. A. & Cox, P. A. (2003) Conserv. Biol. 17, 678–686. [Google Scholar]
- 20.Spencer, P. S., Kisby, G. E. & Ludolph, A. C. (1991) Neurology 41, Suppl. 2, 41–68. [DOI] [PubMed] [Google Scholar]
- 21.Turner, S. (1988) Plant Syst. Evol. 11, 13–52. [Google Scholar]
- 22.Subramaniam, A., Brown, C. W., Hood, R. R., Carpenter, E. J. & Capone, D. G. (2002) Deep-Sea Res. 49, 107–121. [Google Scholar]
- 23.Hawser, S. P. & Codd, G. A. (1992) in Marine Pelagic Cyanobacteria: Trichodesmium and Other Diazotrophs, eds. Carpenter, E. J., Capone, D. G. & Rueter, J. G. (Kluwer, Dordrecht, The Netherlands), pp. 319–329.
- 24.Rocap, G., Alrimer, F. W., Lamerdin, J., Malfatti, S., Chain, P., Ahlgren, N. A., Arellano, A., Coleman, M., Hauser, L., Hess, W. R., et al. (2003) Nature 424, 1042–1047. [DOI] [PubMed] [Google Scholar]
- 25.Schopf, J. W. (1996) Nova Hedwigia 112, 12–32. [Google Scholar]
- 26.Neilan, B. A., Dittmann, E., Rouhiainen, L., Bass, R. A., Schaub, V., Sivonen, K. & Börner, T. (1999) J. Bacteriol. 181, 4089–4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rai, A. N., Bergman, B. & Rasmussen U. (2002) Cyanobacteria in Symbiosis (Kluwer, Dordrecht, The Netherlands).
- 28.Bergman, B., Matveyev, A. & Rasmussen, U. (1996) Trends Plant Sci. 1, 191–197. [Google Scholar]
- 29.Capone, D. G., Zehr, J. P., Paerl, H. W., Bergman, B. & Carpenter, E. J. (1997) Science 276, 1221–1229. [Google Scholar]
- 30.Karl, D., Michaels, A., Bergman, B., Capone, D., Carpenter, E., Letelier, R., Lipschultz, F., Paerl, H., Sigma, D. & Stal, L. (2002) Biogeochemistry 57/58, 47–98. [Google Scholar]
- 31.Lenes, J. M., Darrow, B. P., Cattrell, C., Heil, C. A., Callahan, M., Vargo, G. A., Byrne, R. H., Prospero, J. M., Bates, D., Farning, K. A., et al. (2001) Limnol. Oceanogr. 46, 1261–1277. [Google Scholar]