Abstract
Re-strengthening of the residual language network is likely crucial for speech recovery in post-stroke aphasia. Eight participants with chronic aphasia received intensive speech therapy for three weeks, with standardized naming tests and brain MRIs before and after therapy. Kurtosis-based diffusion tensor tractography was used to measure mean kurtosis (MK) along a segment of the inferior longitudinal fasciculus (ILF). Therapy related reduction in the number of semantic but not phonemic errors was associated with strengthening (renormalization) of ILF MK (r=-0.90, P<0.05 corrected), suggesting that speech recovery is related to structural plasticity of linguistic specific components of the residual language network.
Introduction
Stroke is the leading cause of neurological disability and acquired language problems (aphasia)1. For survivors with chronic aphasia, speech therapy can lead to language improvements, but the response is highly variable2. The neurobiological bases of therapy-mediated recovery are not completely understood and it remains unclear why some individuals benefit while other exhibit little response.
A leading hypothesis suggests that re-strengthening of the residual language network is crucial for recovery in post-stroke aphasia3. The dual stream model of language suggests that ventral (parietal – temporal) networks are responsible for integrating the lexical-semantic system, whereas dorsal (parietal – frontal) networks are related to the motor-articulatory system4. In a pioneering work, Schlaug et al. demonstrated non-specific structural changes associated with chronic aphasia improvement5, 6; subsequently, Van Hees et al. showed renormalization of the dorsal stream related to phonemic improvement7. However, it is unclear if semantic improvements are supported by structural plasticity of the ventral stream. This knowledge could help guide therapy approaches targeting residual brain integrity.
We tested if structural plasticity of the ventral stream, represented by a segment of the inferior longitudinal fasciculus (ILF), was related to linguistic improvements by examining a cohort of individuals with chronic aphasia who underwent speech therapy. We applied diffusional kurtosis imaging (DKI)8, a diffusion MRI technique that provides more comprehensive characterization of tissue microstructure, and improves the assessment of white matter tractography9. In accordance with the dual stream model, we hypothesized that re-strengthening of the residual ILF would be associated with semantic, but not phonemic, therapy related improvements in naming.
Material and Methods
We recruited eight participants, (52±7 years, 3 women) with a history of post-stroke aphasia due to a single left hemisphere stroke at least 12 (50.3±29.8) months prior to the study. The participants had no history of other neurological diseases and were all right-handed. This study was approved by Institutional Review Boards at our institutions.
The participants received group-based Intensive Language Action Therapy (constraint induced)10 for three weeks (five therapy sessions per week lasting four hours each). They were tested for confrontational naming using a short version of the Philadelphia Naming Test11 within one week prior and post therapy.
MRI data was collected using a Siemens 3T TIM Trio (12-channel head coil) at the University of South Carolina. DKI data: two b-values (1000 and 2000s/mm2), 30 diffusion-encoding directions, 45 slices, voxel size=(2.7mm)3, TR=6100ms, TE=101ms, FOV=222×222mm2, two averages and 11 non-diffusion weighted images. T1-weighted images: turboflash sequence, FOV=256×256mm2, 160 sagittal slices, 9-degree flip angle, TR=2250ms, TE=4.5ms, voxel size=1mm3. All subjects underwent four MRI sessions, two before and two after treatment, within one week prior and post therapy.
The image analysis pipeline was optimized to quantify diffusion (fractional anisotropy (FA), mean diffusivity (MD) and mean kurtosis (MK)) along a representative segment of the ILF as defined by the probabilistic JHU white matter atlas12, which travels from the coronal plane in the posterior edge of the cingulum to the temporal pole. Data from both pre-treatment and post-treatment sessions were combined into a set of 121 diffusion-weighted images, linearly registered to the initial scan using SPM8 to locate the ILF for each subject. Diffusional Kurtosis Estimator was used for deterministic kurtosis-based tractography (https://www.nitrc.org/projects/dke/). A white matter seeding mask was created with SPM's Clinical Toolbox (https://www.nitrc.org/projects/clinicaltbx/), which was normalized to diffusion space by cost function masking with the stroke lesion (drawn on T1 images). Individual whole brain tractography maps were analyzed using Automated Fiber Quantification (AFQ)13, customized to perform analysis in diffusion space. AFQ and DKI were combined as described previously14. AFQ results in a set of ILF fibers, which ultimately is abridged to one centroid. One hundred equidistant measurements along the centroid were obtained for each metric before and after therapy. Since no significant differences were revealed between the pre- or post-treatment scans, they were averaged to reduce noise. To assess the ILF's weakest segment, the location with the highest diffusion abnormality (minima MK or FA, maximum MD) was determined between nodes 20-80 of the core ILF. We called this highest abnormality the bottleneck, and a 6-node smoothing kernel was applied in this neighborhood to reduce contribution of outliers. All further analyses were carried out in the bottleneck.
Pre- to post-treatment structural changes in the ILF were examined in relationship to therapy related improvements in both semantic and phonemic paraphasias using linear regression. Baseline metrics were also related to baseline performance. Corresponding p-values are adjusted for multiple comparisons (n=12) using Bonferroni correction.
Results
As a group, subjects showed significant improvement in the number of correctly named items with therapy (paired t-test, p=0.002), which was driven by fewer semantic errors (p=0.01) and a decrease of no responses (p=0.03).
The left ILF was significantly different (p<0.001) from the right ILF for each metric. Compared to the contralateral side, the ipsilateral ILF had a higher MD, lower FA and lower MK (Figure 1).
Figure 1.
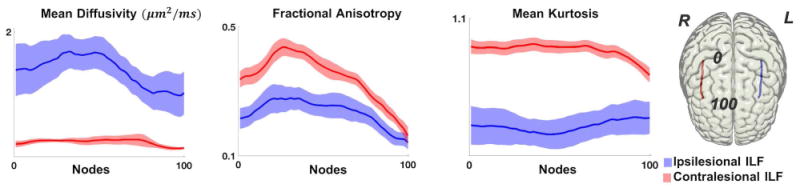
Along-tract diffusion metrics (y-axis) are demonstrated along equally spaced measurement points in the ILF (0 to 100, anterior to posterior) (x-axis). The solid line represents the average patient value, with the standard error of the mean shown as the shaded area. Ipsilesional ILF values are shown in blue, and contralesional ILF values are shown in red. The rightmost image illustrates an example of a participant's core right and left ILF.
Individualized perilesional changes in ILF microstructure in relationship with its proximity to the stroke lesion were also noted. MK values at greater distances from the lesion are higher, gradually decreasing when closer to the lesion (Figure 2). Overlap between the lesion core and the left ILF ranged from 0.4% to 94.7% (Figure 3a).
Figure 2.
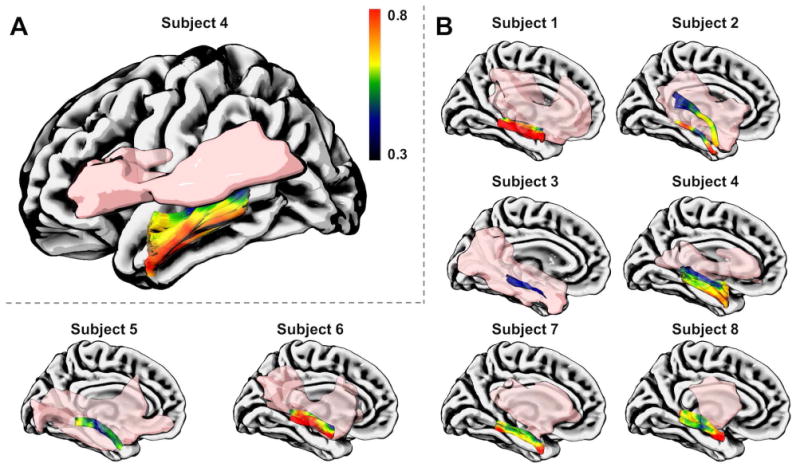
The relationship between along-tract ILF MK values and the chronic stroke lesion (in pink). A. The data from one representative participant is shown in a lateral view to demonstrate the lowest MK in perilesional areas (color bar). B. The ILF and the lesion are shown for this subject (panel A) and all others using medial views to illustrate their anatomical relationship. Note that the lesion was excluded from the seeding mask during ILF tractography.
Figure 3.

A. (top) The scatter plot demonstrates the relationship between pre- to post-therapy changes in MK measured at the ILF bottleneck, and pre- to post-therapy changes in semantic paraphasias (r=-0.90 and p<0.05 corrected). A. (bottom) Table summarizing MK values for the four MRI acquisitions for all subjects. The table also includes individual changes in the number of semantic paraphasias with treatment (in number of words) and the percent overlap between the stroke lesion and the ILF. The scatterplot in A (top) depicts the relationship between change MK (second to last column) and change in semantic paraphasias (last column). (BL=baseline; FU=follow-up; MK=mean kurtosis) B. Pre- and post-treatment MK values along the ILF from a representative participant are shown anatomically. The ILF bottleneck, which is marked with a black arrow, demonstrates an increase in MK towards normal values from before to after therapy. The stroke lesion is demonstrated in pink. This participant demonstrated a 55% improvement in semantic errors.
There was a strongly significant correlation between pre- to post-therapy increment in MK (renormalization towards normal values15) in the left ILF (at the bottleneck) and therapy-related improvement in semantic paraphasias (r=-0.90, p<0.05; Figure 3a). No relationships were observed for pre- to post-therapy MK changes and phonemic errors (r=-0.11) (semantic vs. phonemic R to Z comparison [Fisher transformation], p<0.05) or for right ILF changes and improvement in semantic paraphasias (left-ILF vs. right-ILF R to Z, p<0.05). The correlations with FA and MD did not reach significance level at p<0.05. Bottleneck increases in MK with therapy are shown in the perilesional space of a representative patient (Figure 3b; note MK color-code changes from blue to green). There was a trend towards statistical significance in the relationship between ILF MK pre-treatment and the number of semantic paraphasias prior to treatment (r=-0.82, p=0.15), this association did not increase with treatment.
To investigate the effect of lesion burden on recovery, we evaluated the number of residual fibers in each patient. The number of semantic paraphasias prior to therapy was related to ILF lesion burden (r=-0.65, p=0.07). However, lesion burden (or track integrity) was not associated with semantic recovery (r=0.19, p=0.65).
Discussion
The present study evaluated the relationship between structural plasticity of the ventral stream and therapy-related improvements in naming in individuals with chronic aphasia. We observed that pre- to post-treatment increases in ILF MK towards normal values15, specifically within the areas along the ILF with the highest degree of baseline structural compromise (the diffusion bottleneck), were strongly associated with semantic improvements.
These results leverage recent advancements in DWI and image analysis, which enable the investigation of white matter microstructure with higher sensitivity to microstructural changes16. MK is a biophysical measure less affected by partial volume, which can be higher in the proximity of a stroke lesion17. In this study, MK was the only diffusion metric that reached statistical significance suggesting that conventional diffusion measures may be less sensitive to structural changes associated with recovery, and MK may be optimally suited for assessing post-stroke neuroplasticity. Larger studies are needed to replicate these results.
The neurobiology underlying MK changes is likely due to a combination of factors that are known to occur after strokes. Namely, axonal sprouting, changes in axon thickness or neurogenesis, can contribute to an increase in complexity in perilesional tissues, which has been demonstrated in post-stroke experimental studies18. However, further specific biophysical tissue models are needed to completely elucidate the basis of post-stroke plasticity.
Our findings provide preliminary, but theory-driven, evidence of semantic improvements being supported by structural plasticity of the ventral language processing stream. This knowledge can be used to guide therapies to recruit ventral processing pathways in individuals with residual ILF, or direct stimulation to the ILF for semantic improvement. Of note, Language Action Therapy focuses on the improvement of communication skills in general, and future studies with a larger sample could address whether impairment based interventions (i.e., semantic based treatments for semantic paraphasias) could lead to further enhanced structural neuroplasticity.
Moreover, the residual integrity of the language network could help improve the predictions of recovery potential, together with other predictors such as lesion site, lesion load19, as well as the right language network, specifically the arcuate fasciculus, which has been implicated in recovery by previous studies5, 20.
In conclusion, therapy-related ventral stream plasticity, quantified by MK changes within a bottleneck of damage in the ILF, is related to semantic, but not phonemic, improvements due to therapy. These results are in accordance with the theoretical dual stream model of language, which predicts the involvement of the ILF in semantic processing. Furthermore, kurtosis-based tractography is a promising tool for the study of the neurobiology of stroke recovery. Understanding language network integrity and its relationship with clinical performance could advance our knowledge of stroke recovery mechanisms and the basic neurobiology of language.
Acknowledgments
This study was supported by research grants from the National Institutes of Health / National Institute on Deafness and Other Communication Disorders (NIDCD): DC014021 (PI: Bonilha), DC011739 (PI: Fridriksson), DC014664 (PI: Fridriksson), T32 DC0014435 (Trainee: EM); National institute of general medical sciences: T32GM008716 (Trainee: RG) and from the American Heart Association: SFDRN26030003 (PI: Bonilha) and The Litwin Foundation (PI: Helpern).
Footnotes
Author Contributions: The study was jointly designed by EM, LB, JJ, JH and JF. EM, RG, CR, AB, AS and VS aided in data collection and analysis. EM, LB wrote the manuscript, all authors commented on the manuscript at all stages.
Conflicts of Interest: Drs. Helpern and Jensen are co-inventors on patents related to diffusional kurtosis imaging.
References
- 1.Wade DT, Hewer RL, David RM, et al. Aphasia after stroke: natural history and associated deficits. J Neurol Neurosurg Psychiatry. 1986 Jan;49(1):11–6. doi: 10.1136/jnnp.49.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holland AL, Fromm DS, DeRuyter F, et al. Treatment efficacy: aphasia. J Speech Hear Res. 1996 Oct;39(5):S27–36. doi: 10.1044/jshr.3905.s27. [DOI] [PubMed] [Google Scholar]
- 3.Heiss WD, Thiel A. A proposed regional hierarchy in recovery of post-stroke aphasia. Brain Lang. 2006 Jul;98(1):118–23. doi: 10.1016/j.bandl.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007 May;8(5):393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- 5.Schlaug G, Marchina S, Norton A. Evidence for plasticity in white-matter tracts of patients with chronic Broca's aphasia undergoing intense intonation-based speech therapy. Ann N Y Acad Sci. 2009 Jul;1169:385–94. doi: 10.1111/j.1749-6632.2009.04587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wan CY, Zheng X, Marchina S, et al. Intensive therapy induces contralateral white matter changes in chronic stroke patients with Broca's aphasia. Brain Lang. 2014 Sep;136:1–7. doi: 10.1016/j.bandl.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Hees S, McMahon K, Angwin A, et al. Changes in white matter connectivity following therapy for anomia post stroke. Neurorehabil Neural Repair. 2014 May;28(4):325–34. doi: 10.1177/1545968313508654. [DOI] [PubMed] [Google Scholar]
- 8.Jensen JH, Helpern JA. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR in biomedicine. 2010 Aug;23(7):698–710. doi: 10.1002/nbm.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glenn GR, Kuo LW, Chao YP, et al. Mapping the Orientation of White Matter Fiber Bundles: A Comparative Study of Diffusion Tensor Imaging, Diffusional Kurtosis Imaging, and Diffusion Spectrum Imaging. AJNR Am J Neuroradiol. 2016 Jul;37(7):1216–22. doi: 10.3174/ajnr.A4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pulvermuller F, Neininger B, Elbert T, et al. Constraint-induced therapy of chronic aphasia after stroke. Stroke. 2001 Jul;32(7):1621–6. doi: 10.1161/01.str.32.7.1621. [DOI] [PubMed] [Google Scholar]
- 11.Walker GM, Schwartz MF. Short-form Philadelphia naming test: rationale and empirical evaluation. Am J Speech Lang Pathol. 2012 May;21(2):S140–53. doi: 10.1044/1058-0360(2012/11-0089). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hua K, Zhang J, Wakana S, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. NeuroImage. 2008 Jan 01;39(1):336–47. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeatman JD, Dougherty RF, Myall NJ, et al. Tract profiles of white matter properties: automating fiber-tract quantification. PloS one. 2012;7(11):e49790. doi: 10.1371/journal.pone.0049790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glenn GR, Jensen JH, Helpern JA, et al. Epilepsy-related cytoarchitectonic abnormalities along white matter pathways. J Neurol Neurosurg Psychiatry. 2016 Sep;87(9):930–6. doi: 10.1136/jnnp-2015-312980. [DOI] [PubMed] [Google Scholar]
- 15.Latt J, Nilsson M, Wirestam R, et al. Regional values of diffusional kurtosis estimates in the healthy brain. Journal of magnetic resonance imaging : JMRI. 2013 Mar;37(3):610–8. doi: 10.1002/jmri.23857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Umesh Rudrapatna S, Wieloch T, Beirup K, et al. Can diffusion kurtosis imaging improve the sensitivity and specificity of detecting microstructural alterations in brain tissue chronically after experimental stroke? Comparisons with diffusion tensor imaging and histology. NeuroImage. 2014 Aug 15;97:363–73. doi: 10.1016/j.neuroimage.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Yang AW, Jensen JH, Hu CC, et al. Effect of cerebral spinal fluid suppression for diffusional kurtosis imaging. Journal of magnetic resonance imaging : JMRI. 2013 Feb;37(2):365–71. doi: 10.1002/jmri.23840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dancause N, Barbay S, Frost SB, et al. Extensive cortical rewiring after brain injury. J Neurosci. 2005 Nov 2;25(44):10167–79. doi: 10.1523/JNEUROSCI.3256-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Marchina S, Norton AC, et al. Predicting speech fluency and naming abilities in aphasic patients. Front Hum Neurosci. 2013;7:831. doi: 10.3389/fnhum.2013.00831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forkel SJ, Thiebaut de Schotten M, Dell'Acqua F, et al. Anatomical predictors of aphasia recovery: a tractography study of bilateral perisylvian language networks. Brain. 2014 Jul;137(Pt 7):2027–39. doi: 10.1093/brain/awu113. [DOI] [PubMed] [Google Scholar]


