Abstract
Background
Exposure to ethanol in utero alters the disposition of tangentially migrating GABAergic interneurons in the fetal brain. The medial ganglionic eminence (MGE) gives rise to a large portion of cortical GABAergic interneurons, including the parvalbumin-expressing interneurons that shape and contribute to inhibitory/excitatory (I/E) balance of the intracortical circuit. Here, we investigated in the mouse medial prefrontal cortex (mPFC) the hypothesis that low levels of maternal ethanol consumption from closure of the neural tube embryonic day(E) 9.5 until birth, results in an enduring interneuronopathy.
Methods
Pregnant mice were subjected to a 2% w/w ethanol consumption regimen starting at neural tube closure and ending at parturition. Neurogenesis in the MGE was assessed by BrdU-immunofluorescence at E12.5. The count and distribution of parvalbumin-expressing interneurons were determined in adult animals, and patch clamp electrophysiology was performed to determine GABAergic function and I/E balance. Open field behavior in adult mice was assessed to determine whether the ethanol-exposed cohort displayed a lasting alteration in exploratory behavior.
Results
In embryos exposed to ethanol in utero, we found increased BrdU labeling in the MGE, pointing to increased neurogenesis. Adult mice prenatally exposed to ethanol were hyperactive, and this was associated with an increase in parvalbumin-expressing GABAergic interneurons in the mPFC. In addition, prenatal ethanol exposure altered the balance between spontaneous inhibitory and excitatory synaptic input and attenuated GABAergic tone in layer V mPFC pyramidal neurons in juvenile mice.
Conclusion
These findings underscore that altered migration of GABAergic interneurons contributes to the ethanol-induced aberration of cortical development and that these effects persist into adulthood as altered cortical form and function.
Keywords: cortical development, medial prefrontal cortex, GABA, Inhibitory/excitatory balance, fetal alcohol spectrum disorders
Introduction
Preventable as it may be by abstinence, an alarming 7.6% of pregnant women in the United States self-report alcohol (ethanol) use in the last 30 days (Center for Disease Control and Prevention, 2012). Ethanol readily crosses the placenta – in mice, fetuses are exposed to levels of blood alcohol that are comparable to those of the ethanol-consuming mother (Cuzon et al., 2008), and develop long-lasting behavioral deficits, many of which are attributable to cortical dysfunction. Thus, elucidating how ethanol affects the developing cortex is key to advancing our understanding of the mechanisms underlying the enduring behavioral impairments that are the cardinal hallmarks of fetal alcohol spectrum disorders (FASD).
While it is abundantly clear that excessive consumption of ethanol causes brain damage, it is also clear that the specifics of the damage may be dependent on the drinking pattern (Astley and Clarren, 2000). The pattern of maternal ethanol consumption during embryonic development of the fetus may be particularly impactful, since this is when the immature fetal brain is exquisitely vulnerable to teratogenic insult by ethanol. In this light, our recent study demonstrated in mice that treatment with a binge-type exposure to ethanol in utero accelerates the tangential migration of primordial GABAergic interneurons from their subpallial origin to the embryonic medial prefrontal cortex (mPFC), and that this manifests in the young adult as altered mPFC form, function, and behavior (Skorput et al., 2015). An earlier study demonstrated a similar accelerated tangential migration into the developing cortex following chronic gestational exposure to a low dose of ethanol in utero (Cuzon et al., 2008). However, not examined was whether such a chronic prenatal ethanol exposure-induced aberrant pattern of migration might also be associated with enduring cortical abnormalities later in life. Additionally, our more recent work investigating the teratogenic effects of in utero ethanol exposure on Cajal-Retzius cells during corticogenesis (Skorput and Yeh, 2015) further supports a role for aberrant cortical development in the etiology of FASD. In the present study, we build and expand on these initial studies and ask whether chronic low-level ethanol exposure in utero can lead to FASD-relevant behavioral abnormalities and enduring aberrances in cortical form and function into adulthood.
We administered a low-dose chronic maternal ethanol consumption regimen consisting of 2% w/w ethanol in liquid food to pregnant mice from the start of corticogenesis to parturition. This regimen standardly yielded a relatively low blood alcohol level (BAL) of ~30 mg/dL in pregnant mice (Cuzon et al., 2008). We focused on a dose of ethanol that yielded a low BAL because the majority of patients diagnosed with FASD present with a spectrum of symptoms with varying degrees of severity that are more consistent with having been exposed prenatally to low or moderate doses of ethanol (May et al., 2013, 2014). In addition, perhaps because the effects on brain development are subtler, the potential long-term effects of gestational exposure to low-moderate doses of ethanol have been more challenging to uncover and investigate (e.g., Astley and Clarren, 2000; Farag, 2014; Fox et al., 2015). We report here that adult mice chronically exposed to a low dose of ethanol in utero display a hyperactive phenotype. In the mPFC, we report altered distribution of parvalbumin-expressing GABAergic interneurons commensurate with an inhibitory/excitatory (I/E) synaptic imbalance.
Materials and Methods
Animals
All procedures involving mice were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Dartmouth Institutional Animal Care and Use Committee. This study employed the Ebf2-EGFP BAC transgenic mouse line generated by Gene Expression Nervous System Atlas (GENSAT) and obtained from Dr. Portera-Cailliau (UCLA) (Gong et al., 2003; Chowdhury et al., 2010). It should be noted that Ebf2-EGFP BAC mice were used here because the present study was conducted in parallel with another study that focused on investigating Cajal-Retzius cells in the marginal zone of the developing cortex (Skorput and Yeh, 2015). In this mouse line, EGFP selectively labels Cajal-Retzius cells, which have undergone developmental apoptosis and are all but absent in the adult cortex, when the neuroanatomical analyses were performed. Nonetheless, to avoid any possible contamination by endogenously-expressed EGFP, all immunofluorescence assays were done with red-fluorescent Alexa Fluor 555-conjugated secondary antibody.
To obtain time-pregnant dams, pairs of male and female mice were housed overnight for mating; the following day was designated as embryonic day(E) 0.5. Embryos and postnatal pups of either sex were included in this study. The day of birth was defined as postnatal day(P) 0. This study employed postnatal day(P) 80–90 adult mice and P20 juvenile mice. The experiment assessing neurogenesis in the medial ganglionic eminence (MGE) employed E12.5 embryos harvested from pregnant dams.
Maternal ethanol consumption
Our previous work investigating the effects of in utero ethanol exposure on tangential migration of cortical interneurons employed a liquid diet regimen containing a low dose of ethanol that the pregnant dams consumed throughout gestation (Cuzon et al., 2008). In the present study, we intentionally limited ethanol exposure to the period of in utero corticogenesis in mouse in order to avoid potentially confounding effects of ethanol exposure prior to closure of the neural tube (E9.5), and to avoid direct administration of ethanol to neonatal animals (Fig. 1A).
Figure 1.

Offspring exposed to ethanol in utero spanning the period of embryonic corticogenesis are hyperactive as adults. (A): Experimental timeline highlighting the period of in utero ethanol exposure (red bar) and experimental endpoints. Time pregnant dams were fed a liquid diet without (control) or with 2% ethanol w/w (EtOH) from E9.5-birth. (B): Quantitative analysis of open field exploratory behavior; distance traveled (left) by adult offspring, each given 30mins to explore the 25cm×25cm open field arena, time spent in the center potion of the arena (center), and the number of stereotypic movements (right). Unpaired student’s t-test, *=P<0.05, **=P<0.01.
Pregnant dams were individually housed and assigned to one of two groups: ethanol-fed, or control-fed. Mice were maintained under normal 12/12hr light/dark cycle on a liquid diet (Research Diets, New Brunswick, NJ) supplemented with ethanol (2% w/w; ethanol-fed group), or an isocaloric control diet containing maltose (control-fed group); water was available ad libitum. The liquid food was replenished daily between 3:00 and 5:00PM. Pregnant mice were maintained on their respective diets until parturition, when they were returned to standard chow (Fig. 1A). Dam BAL was assessed by blood collected via the tail vein at 11:30PM on E15.5 using an Analox Instruments GM7 series analyzer (Lunenburg, MA). Litter size was not affected (Control pups; EtOH pups; Unpaired t-test P>0.05).
Histology, immunofluorescence, fluorescence microscopy and image analysis
In one set of experiments, ethanol-consuming and control dams were injected with 5-bromo-2-deoxyuridine (BrdU; 100mg/kg i.p.) at 12AM on E12.5 (Fig. 1A). Two hours later, time-pregnant dams were sacrificed by CO2 asphyxiation, the embryos were removed, their brains were dissected, immerse-fixed in 4% paraformaldehyde(PFA)/0.1M phosphate-buffered saline(PBS) overnight (~14hrs), and cryoprotected in 30% sucrose/0.1M PBS. Cryosections (30μm) were obtained using a sliding microtome and collected into PBS, processed for immunofluorescence by a 1-hour block at room temperature in PBS containing 10% normal goat serum (NGS) and 0.01% triton X-100, and incubated overnight at 4°C with a 1:10 dilution of mouse anti-BrdU primary antibody (G3G4 hybridoma). Sections were subsequently incubated overnight with a 1:1000 dilution of Alexa Fluor 555 conjugated goat-anti-mouse secondary antibody (Invitrogen, Grand Island, NY). Negative control with primary antibody omitted was routinely processed in parallel.
Images of the MGE from 10 sections per embryo were captured digitally using a CCD camera (Hamamatsu, Hamamatsu city, Japan) fitted on a spinning disk confocal microscope (BX61WI; Olympus, Melville, NY) controlled by IPLab 4.0 software (BD Biosciences, San Jose, CA). In each image, the MGE was manually delineated as the region of interest, and a quantification vector defined by pixel intensity over background intensity was applied using IPLab 4.0 software to yield a percent area of the MGE covered by immunofluorescent signal (Alexa 555).
Adult (P80–P90) mice were perfused trans-cardially with cold PBS and then with 4% PFA/0.1M PBS (Fig. 1A). The brains were removed and immerse fixed in 4% PFA/0.1M PBS overnight at 4°C. Following cryoprotection in 30% sucrose, 30μm coronal sections were cut on a sliding microtome, and collected into PBS. The tissue sections were blocked for 1-hour at room temperature in PBS containing 10% NGS and 0.05% Triton X-100. They were then incubated with mouse anti-parvalbumin (Millipore: MAB1572) primary antibody, which has been characterized by McKenna et al., 201, at a dilution of 1:1000. Sections were subsequently incubated overnight with a 1:1000 dilution of Alexa Fluor 555 conjugated goat anti-mouse secondary antibody (Invitrogen, Grand Island, NY).
Sections containing the mPFC were defined beginning with the most rostral section in which all five cortical layers were visible, and extending caudally to the decussation of the corpus callosum, and were inspected to assure the same rostral-caudal location between groups. 2 -3 images were stitched together to form a montage of the complete region of interest for each histological section (Image J). Images from 10 sections within the mPFC were analyzed per animal. The subdivisions and layers of the mPFC were delineated manually using the cytoarchitecture visualized by DAPI staining of the same sections, following the guidelines of Van De Werd et al. (2010). Parvalbumin positive cells per subdivision and per layer were manually counted by trained experimenters blinded to the treatment conditions using Image J software.
Electrophysiology
Mice (P20) were scarified by CO2 asphyxiation and their brains were dissected into ice-cold oxygenated (95% O2, 5% CO2) aCSF containing (in mM): NaCl 124; KCl 5.0; MgCl2 2.0; CaCl2 2.0; NaH2PO4 1.25; NaHCO3 26; D-glucose 10 (pH = 7.4, adjusted with 1N NaOH). Recordings of layer five pyramidal neurons of the mPFC were made in 200μm coronal slices cut using a vibrating microtome (Electron Microscopy Sciences, Hatfield, PA) in ice-cold oxygenated-aCSF, and then incubated in oxygenated-aCSF at room temperature for at least 60min before electrophysiological recording.
Brain slices were transferred to a 32°C recording chamber, mounted on a fixed-stage upright fluorescence microscope (Olympus BX51WI) equipped with Hoffman Modulation Contrast optics and perfused with oxygenated-aCSF (0.5 ml/min). Layer V pyramidal neurons were identified by location in the mPFC, morphology and orientation. They were held at −70mV and inhibitory and excitatory synaptic currents were isolated by focal application of the GABAA receptor (GABAAR) antagonist bicuculline (20μM) or CNQX/APV (20μM each) delivered via regulated air pressure (<3p.s.i.) through a multibarrel drug pipet consisting of six independently controlled barrels with a combined outer tip diameter of 10–15μm. A typical recording session consisted of a 3-minute baseline, 3-minute focal application of APV/CNQX, 1-minute wash, and 3-minute focal application of bicuculline. Borosilicate glass patch electrodes (4–6 MΩ in recording solution) were filled with recording solution composed of (in mM): KCl 120; MgCl2 2.0; EGTA 1.0; HEPES 10; Mg2+ ATP 3.0 (pH= 7.3, adjusted with 1N KOH). Alexa Fluor 555 (0.02mM) was added to the recording solution for intracellular filling to confirm the morphology of the recorded pyramidal cells. Whole cell recordings were performed using an AxoPatch 700B amplifier (Molecular Devices Inc., Sunnyvale, CA). Membrane currents were filtered at 10 kHz digitized (Digidata1320A; Molecular Devices Inc.) and analyzed using Clampex v9.0 (Molecular Devices Inc.). Electrophysiological recordings were performed in slices derived from juvenile animals in order to demonstrate altered intracortical microcircuitry at a developmental time point that is relevant to reports of altered frontal cortical function in humans with FASD (Fryer et al., 2007; Astley et al., 2009).
Open field-testing
Adult mice (P80–P90) were allowed to explore a novel moderately lit open field environment (25cm × 25cm) for 30 minutes. The chamber (Colburn instruments, Whitehall, PA) was equipped with infrared beams and sensors to detect activity, and was connected to a computer to record data using TruScan software (Colburn instruments, Whitehall, PA). The data was analyzed for the total distance traveled, the number of stereotypic movements performed (≥ 3 movements with coordinate change less than ±2.88cm and back to the original point within a 2-second interval), and the amount of time animals spent in the center portion of the arena (>4cm from the surrounding walls).
Statistics
Histological analyses included averaged data from the region of interest from 10 30μm tissue sections for each animal (n=1: 1 animal =10 sections). For comparisons of electrophysiological group data, n denotes the number of cells recorded. The electrophysiological data were also analyzed using the mean data for all cells from an individual animal, with the number of animals as the n value. This analysis revealed that all differences reported using the number of cells as n remained significant. As the variability (standard deviation) of all electrophysiological end points was larger between cells than between animals within a given treatment group, we reported the mean data per cell ± standard error. Overall, reporting the more variable unit of determination is more representative of the true biological variability (Brien et al., 2006). All groups consisted of data acquired from a minimum three individual animals from multiple litters. Group means were compared by unpaired t-test or two-way ANOVA with Bonferroni post-hoc test as indicated, and reported as mean ( ) ± standard error.
RESULTS
The chronic maternal ethanol consumption regimen produces adult offspring with FASD-relevant behavior
It was important to establish first that our chronic maternal ethanol consumption regimen (Fig. 1A) produced offspring that displayed FASD-relevant behavior. Children diagnosed with FASD are hyperactive (Ornoy and Ergaz, 2010; Steinhausen et al., 1993). This hallmark feature can be assessed by comparing the exploratory behavior of adult mice without and with ethanol exposure in utero in an open field arena (Skorput et al., 2015; Skorput and Yeh, 2015). As summarized in Figure 1B, in the prenatal ethanol-exposed adult cohort, we found an increase in the total distance traveled (Fig 1B, left; Control , 11 animals from 3 litters; EtOH , 15 animals from 3 litters; Unpaired t test, P<0.01), amount of time spent in the center of the arena (Fig. 1B, middle; Control ; EtOH ; Unpaired t test, P<0.05), and the number of stereotypic movements (Fig. 1B, right; Control moves; EtOH moves; Unpaired t test, P<0.01). In line with our investigation of open field exploratory activity at adolescent ages (Skorput and Yeh, 2015), this hyperactive phenotype confirms that our chronic maternal ethanol consumption regimen is a viable model for investigating the enduring aberrances in cortical form and function associated with FASD.
Chronic exposure to ethanol in utero increases parvalbumin-expressing interneurons in the adult mPFC
Chronic ethanol exposure in utero accelerates tangential migration and promotes the recruitment of MGE-derived GABAergic interneurons into the embryonic cortex (Cuzon et al., 2008). We tested the hypothesis that this effect persists into adulthood and manifests itself as more MGE-derived interneurons in the mPFC. To this end, we compared the number and position of parvalbumin immunopositive interneurons in the mPFC of prenatally ethanol-exposed mice with controls at P90. In adult cortex, parvalbumin expression is a reliable marker for GABAergic interneurons derived from the MGE that sort primarily to the infragranular deeper cortical layers V and VI (Kawaguchi and Kubota, 1997; Otsuka and Kawaguchi, 2009; Xu et al., 2008).
Parvalbumin-expressing cells in coronal mPFC sections were labeled by immunofluorescence and counted in bins delineated into subregions of the anterior cingulate (ACC), prelimbic (PL) and infralimbic (IL) (solid white lines Fig. 2), as well as by cortical layers I, II/III, V, VI in each mPFC subregion (dotted white lines in Fig. 2). Figure 2A and B illustrate representative images from the mPFC of control and in utero ethanol-exposed adult offspring, respectively. The mPFC of offspring exposed to ethanol in utero showed a significant overall increase in the total number of parvalbumin-expressing interneurons within the mPFC (Control cells 4 brains from 2 litters; EtOH cells 4 brains from 2 litters; Two-way ANOVA main effect, P<0.001). Post hoc analysis by cortical layer indicated that this increase resided mostly in the deeper cortical layers, primarily in layer V (Control cells; EtOH cells; Bonferroni post test, P<0.001) and layer VI (Control cells; EtOH cells; Bonferroni post test, P<0.01). As illustrated in Figure 2C–E, this layer-selectivity was most evident in the prelimbic (Fig. 2D; Control cells; EtOH cells; Two-way ANOVA main effect, P<0.001) and infralimbic (Fig. 2E; Control cells; EtOH cells; Two-way ANOVA main effect, P<0.0001) subregions, with the ACC showing a trend for increase in both layers V and VI that turned out not to be statistically significant (Fig. 2C; Control cells; EtOH cells; Two-way ANOVA main effect, P>0.05). Overall, our results pointed to altered distribution of parvalbumin-expressing interneurons in the adult mPFC following low dose ethanol exposure in utero.
Figure 2.

Adult offspring exposed to ethanol in utero display more parvalbumin positive interneurons in the mPFC. Coronal section of adult control (A) and ethanol-exposed (B) offspring processed for parvalbumin-immunofluorescence. The three functional regions of the mPFC are outlined in solid white lines; anterior cingulate cortex (ACC), prelimbic (PL), infralimbic (IL) regions. The 4 cortical layers are outlined in dotted white lines. (C): The number of parvalbumin-positive cell numbers are comparable in the ACC but increase in layer V in the PL region (D), and layers V and VI of the IL region (E). Scale bar = 300μm. Cell counts were compared between groups by two-way ANOVA per functional region, ****=P<0.0001, ***=P<0.001.
We conducted a 2-hour pulse labeling experiment by injecting pregnant dams with BrdU at E12.5 to ask whether the observed increase in parvalbumin-expressing interneurons in the adult mPFC could be accounted for at least in part by ethanol exposure in utero increasing neurogenesis of parvalbumin expressing interneurons, as was seen for Cajal-Retzius cells in the cortical hem under this same exposure paradigm (Skorput and Yeh, 2015). The vast majority of parvalbumin expressing interneurons originate in the MGE. We first standardized the criteria for defining its margin in DAPI-counterstained sections (Fig. 3A), and then quantified the percentage area of the region of interest occupied by BrdU-immunofluorescence. Analysis of BrdU-immunofluorescence in the control vis-à-vis the ethanol-exposed groups indicated that the area of MGE populated by BrdU-immunofluorescence was significantly greater in embryos exposed to ethanol in utero (Fig. 3B–C; Control 4 brains from 2 litters; EtOH 4 brains from 2 litters; Unpaired t test, P<0.01). This increase was observed with no change in the total area of the MGE (Fig. 3D; Control 4 brains from 2 litters; EtOH 4 brains from 2 litters; Unpaired t test, P>0.05). Thus, enhanced neurogenesis could indeed contribute to the persistent increase in parvalbumin-expressing interneurons observed in the mPFC of adult mice exposed to ethanol in utero.
Figure 3.

Exposure to maternal consumption of 2% ethanol increases neurogenesis in the MGE of E12.5 embryos. (A): Coronal section of E12.5 brain stained for BrdU and counterstained with DAPI, dotted white line outlines the MGE, scale bar = 100μm. (B): Representative isolated MGE regions of interest from control (left) and ethanol-exposed (right) embryos; scale bar = 100μm. (C): Quantification of percentage area positive for BrdU-immunofluorescence in the MGE of control (black dots) and ethanol-exposed (red dots) embryos. (D) Total area of E12.5 MGE in control (black dots) and ethanol-exposed (red dots) embryos. Unpaired student’s t-test, **=P<0.01.
Layer V pyramidal neurons in the mPFC of adult offspring exposed to ethanol in utero exhibit I/E synaptic imbalance
Regulating the number and position of inhibitory interneurons within the cortical circuit is necessary for proper I/E balance and circuit function (Kato and Dobyns, 2005; Jones and Rakic, 2010). Given the increase in parvalbumin-expressing interneurons in the mPFC, we reasoned that the I/E synaptic balance would be shifted to favor inhibition, as we observed in another recent study (Skorput et al., 2015). Whole cell voltage clamp experiments were conducted in layer V mPFC pyramidal neurons in acute coronal slices derived from P20 control and in utero ethanol-exposed offspring. The morphological identity of the pyramidal cells recorded was confirmed by filling with Alexa Fluor 555 (Fig. 4A). The continuous current trace in Figure 4B illustrates the monitoring of a mix of spontaneous postsynaptic currents (sPSCs) that could be demonstrated pharmacologically to be mediated by GABA and glutamatergic synaptic transmission. In a routine experiment, the GABAergic contingent of the compound synaptic events was first isolated by focal application of CNQX/APV. Following washout of CNQX/APV, bicuculline was focally applied to the cell to monitor glutamatergic synaptic events. Figures 4C and 4D illustrate respectively the frequency and amplitude of representative control and ethanol-exposed cells over the course of the recording epochs. The spontaneous postsynaptic currents were compared during application of the GABAA or AMPA/NMDA receptor antagonists in order to determine I/E balance.
Figure 4.

Offspring exposed to ethanol in utero exhibit inhibitory/excitatory imbalance of sPSCs in layer V pyramidal neurons of the mPFC. (A): Experimental setup for assessing the balance between sIPSC and sEPSC. A layer V pyramidal neuron is held in whole-cell voltage clamp (Vhold=−70mV) while antagonists of synaptic transmission are applied focally via a multi-barrel drug pipet. Scale bar = 25μm. (B): Voltage clamp recording to investigate sPSC I/E balance. Scale = 50pA × 1min, insets scale = 50pA × 1s). Following baseline recording (left inset) focal application of CNQX/APV (black bar) isolates GABAergic events (middle inset). Following washout of CNQX/APV with bath solution (upper black bar), focal application of bicuculline (black bar) isolates glutamatergic events (right inset). The mean frequency (C), and amplitude (D) of sPSCs recorded from representative control (black) and ethanol-exposed (red) layer V pyramidal neurons over the course of the recording epochs. (E): The frequency of bicuculline-insensitive sPSCs recorded from ethanol-exposed offspring is decreased in layer V pyramidal neurons of the mPFC. (F): The amplitude of bicuculline-insensitive sPSCs recorded from ethanol-exposed offspring is elevated in layer V pyramidal neurons of the mPFC. (G): The ratio of inhibition to excitation is elevated in layer V PFC pyramidal neurons of ethanol-exposed offspring compared with controls. Between group comparisons were made by unpaired students t-test per experimental epoch of baseline or antagonist application (CNQX/APV, bicuculline), **=P<0.01, *=P<0.05.
The overall mean frequency of the mixed sPSCs was similar between layer V pyramidal neurons recorded from ethanol-exposed and control offspring (Fig. 4C, Baseline; Control 12 cells from 3 brains from 2 litters; EtOH 11 cells from 3 brains from 2 litters; Unpaired t test, P>0.05), or during the wash period (Unpaired t test, P>0.05). Contrary to our hypothesis, we did not find any difference in the mean frequency of spontaneous inhibitory postsynaptic currents (sIPSCs) (Fig. 4C, CNQX/APV; Control ; EtOH ; Unpaired t test, P>0.05). Instead, the mean frequency of the isolated spontaneous excitatory postsynaptic currents (sEPSCs) was reduced in the prenatal ethanol-exposed cohort (Fig. 4C Bicuculline; Control ; EtOH ; Unpaired t test, P<0.05).
The mean amplitude of the mixed sPSCs was unchanged between groups (Fig. 4D Baseline; Control ; EtOH ; Unpaired t test, P>0.01), nor was that of the sIPSCs (Fig. 4D CNQX/APV; Control ; EtOH ; Unpaired t test, P>0.05). However, there was a significant increase in the mean amplitude of sEPSCs in the prenatal ethanol-exposed cohort (Fig. 4D Bicuculline; Control ; EtOH ; Unpaired t test, P<0.05).
To calculate the balance of spontaneous inhibitory to excitatory synaptic input, we determined the ratio of the charge transfer (pA•ms) between the CNQX/APV insensitive sIPSCs over that of the bicuculline insensitive sEPSCs and revealed a clear increase in the spontaneous I/E balance in layer V pyramidal neurons recorded in mPFC slices from offspring exposed to ethanol in utero (Fig. 4F; Control ; EtOH ; Unpaired t test, P<0.05). Thus, we confirmed our hypothesis of increased I/E ratio, albeit by uncovering an unanticipated mechanism of decreased sEPSC frequency.
Layer V pyramidal neurons in the mPFC of adult offspring exposed to ethanol in utero exhibit decreased GABAergic tone
In addition to mediating phasic inhibitory synaptic transmission, GABA also influences neuronal excitability non-synaptically via extrasynaptic GABAA receptors that are constantly activated by an ambient level of GABA to generate a tonic GABAergic conductance that maintains an inhibitory tone (Farrant and Nusser, 2005; Glykys and Mody, 2007). Here we asked whether the observed increase in parvalbumin-expressing interneurons in the mPFC would augment GABAergic tone, presumably due to an increase in the extrasynaptic ambient level of the neurotransmitter. Figure 5 (A, B) illustrates that, in mPFC slices derived from P20 mice, a tonic GABA-mediated conductance can be revealed in layer V pyramidal neurons under whole-cell voltage clamp as a shift in the baseline current in response to focal application of the GABAA receptor antagonist bicuculline. Approximately 50% of the layer V pyramidal neurons exhibited a measurable tonic GABA conductance (Fig. 5C; Control 7/16 cells out of 3 brains from 2 litters; EtOH 8/17 cells out of 3 brains from 2 litters; Chi-square, P>0.05). As summarized in Figure 5D, the results revealed that the tonic GABAergic conductance recorded in mPFC layer V pyramidal neurons from offspring exposed to ethanol in utero were consistently and significantly lower in amplitude (Control , 7 cells from 3 brains from 2 litters; EtOH , 8 cells from 3 brains from 2 litters; Unpaired t test, P<0.01). Thus, an enduring consequence of ethanol exposure in utero on GABA-mediated neurotransmission in the mPFC is reflected in a diminished extrasynaptically-mediated GABAergic tone.
Figure 5.
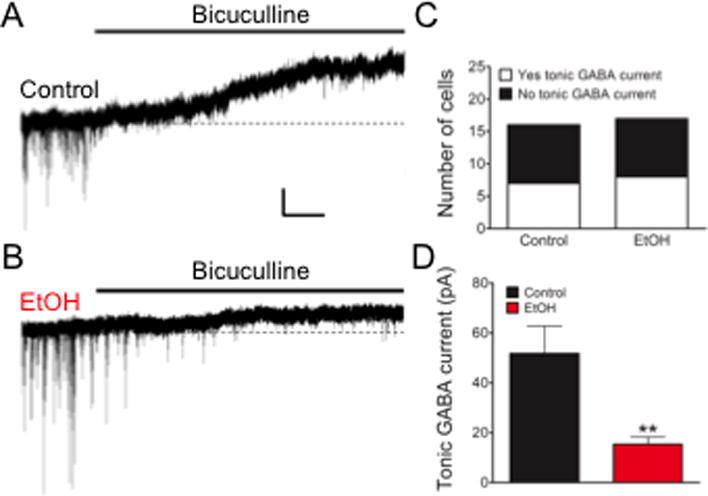
Tonic GABA current is attenuated in mPFC layer V pyramidal neurons of in utero ethanol-exposed offspring. (A): Tonic GABA current is uncovered by focal application of bicuculline. Scale = 20pA × 10s. (B): While not all cells recorded exhibited a tonic GABA-mediated conductance, the incidence of encountering such cells was comparable between groups based on Chi-Squared analysis (C). In cases where GABA-mediated tonic conductance was observed, those recorded from offspring exposed to ethanol in utero were consistently of lower amplitude (D). **=P<0.01, Unpaired student’s t-test.
Discussion
Chronic gestational ethanol exposure interferes with normal tangential migration of primordial GABAergic cortical interneurons (Cuzon et al., 2008). The present study focused on investigating the parvalbumin-expressing GABAergic interneurons, and extended our previous work to demonstrate aberrant cortical form and function as part of the long-term consequence of a prenatal ethanol exposure-induced “interneuronopathy”.
Maternal consumption of low-dose ethanol liquid diet produces FASD-relevant behavior in adult offspring
The Lieber-DeCarli diet is a standard liquid diet regimen for alcohol-related studies (Lieber and DeCarli, 1982). However, applied to our work, it is prudent to acknowledge potential confounding factors that may affect data interpretation. For example, the ethanol-containing liquid diet may directly affect pregnancy itself. In this regard, the control and ethanol-exposed pregnant dams did not exhibit notable differences in body weight (not shown), both cohorts routinely carried their pregnancies to full term and, as reported here, gave birth to normal litter sizes, and to pups that grew up to adulthood. Another potential confounding factor may be maternal stress, given that the chronic liquid diet regimen is arguably a forced feeding paradigm. In our hands, other approaches such as ethanol administration by gavage or vapor exposure early in pregnancy tended to be much more stressful and often incompatible with carrying fetuses to term. We have not assessed systematically other potentially less stressful gestational ethanol consumption models (e.g., Allan et al., 2003; Brady et al., 2012). Overall, the utility of the low-dose chronic ethanol consumption regimen employed in this study is demonstrated by the consistent and low maternal BAL, as well as the hyperactive phenotype in the adult offspring exposed to ethanol in utero that are also seen clinically in FASD and in preclinical studies that model FASD and ADHD (e.g., Riley et al., 1979, 1986; Steinhausen et al., 1993; Max et al., 2005; Ornoy and Ergaz, 2010; Schneider et al., 2011). As this developmentally delineated exposure is not common clinically, direct extrapolation of these studies to the human population with FASD is limited. In addition, the relatively low number of animals utilized in this study and focus on parvalbumin interneurons leaves room for continued work in determining the consistency and specificity of these ethanol induced effects on GABAergic cortical interneurons.
Increased neurogenesis in the MGE following ethanol exposure in utero manifests as a buildup of parvalbumin interneurons in the adult mPFC
We found enhanced neurogenesis at E12.5 in the MGE of fetuses exposed to low-dose ethanol in utero and interpreted this to persist and contribute to our finding of more parvalbumin-expressing interneurons in the adult mPFC. An enhancing effect of prenatal ethanol exposure on neurogenesis has also been demonstrated in the cortical hem (Skorput and Yeh, 2015) and in the hypothalamus following another low-dose model of ethanol exposure (Chang et al., 2012). However, other studies have reported reduced neurogenesis and/or neuronal survival; these studies typically employed developmental models with relatively high levels of alcohol exposure (Farber et al., 2010; Smiley et al., 2015). Indeed, the apparent discrepant outcomes underscore that dose-dependence is a key factor in considering the effects of ethanol on neurogenesis and subsequent neuronal survival.
Another factor critical to the outcome of ethanol exposure in utero is the timing of its administration. Our chronic ethanol exposure regimen was started at E9.5 and ended at parturition, so it targeted the entire period of embryonic corticogenesis in mice and, thus, spanned the genesis and tangential migration of the MGE-derived GABAergic interneurons (Myoshi and Fishell, 2011). In this regard, two key observations are noteworthy. Firstly, since the MGE-derived parvalbumin-expressing interneurons are found preferentially in the deeper cortical layers vis-à-vis their somatostatin-expressing counterparts, and since the number of parvalbumin-expressing interneurons remains the highest in layers V and VI following ethanol exposure in utero, sorting as a process of ushering interneurons to their designated laminar positions seems to be unperturbed. This study focused on assessing the parvalbumin-expressing subpopulation of cortical interneurons. Within this sorting scheme, and considering inside-out sequence by which the MGE-derived GABAergic interneurons are generated (Miyoshi and Fishell, 2011), our observation of a deep cortical layer-selective increase in parvalbumin-expressing interneurons also suggests that the earliest born MGE-derived interneurons are more sensitive to ethanol than subsequent waves that become sorted to the upper cortical layers. Due to the targeted innervation of layer V pyramidal neurons by cortical interneurons the majority of GABAergic input recorded at the soma are derived from synapses expressing parvalbumin. The inhibitory input to neocortical pyramidal cells has been extensively investigated in rodent and monkey across development (Kawaguchi and Kubota, 1997; Kawaguchi and Kondo, 2002; Erickson and Lewis, 2002; Otsuka and Kawaguchi, 2009).
Altered I/E synaptic balance and GABAergic tone in the mPFC following ethanol exposure in utero
This study uncovered an increase in the I/E ratio of spontaneous synaptic activity in mPFC layer V pyramidal neurons. Based on the observed layer V-selective increase in parvalbumin-expressing GABAergic interneurons, we had expected a commensurate increase in sIPSC frequency and/or amplitude to account for the change in I/E ratio. In contrast, we found a significant decrease in the mean frequency of sEPSCs recorded from ethanol-exposed offspring. This was accompanied by an increase in sEPSC amplitude. We speculate that the reduction in sEPSCs observed in Layer V pyramidal neurons might be polysynaptic in nature, due in part to increased inhibitory drive mediated by parvalbumin-expressing interneurons in the other cortical layers. Thus, increased inhibition of layer II/III pyramidal neurons may in turn lead to attenuated excitatory input to layer V pyramidal neurons. Indeed, layer II/III pyramidal neurons provide the major presynaptic source of sEPSCs recorded in layer V pyramidal neurons (Qiu et al., 2011).
Extrasynaptic GABAARs also contribute to mediating the inhibitory drive on neurons (Farrant and Nusser, 2005). They maintain a GABAergic tone that exerts a robust shunting inhibition (Tang et al., 2011). While not all mPFC layer V pyramidal neurons exhibited a tonic GABA conductance, in those that did, the amplitude was reduced in the ethanol-exposed cohort. The net effect of an increased I/E synaptic ratio and a decreased tonic GABAergic inhibition in layer V pyramidal neurons following ethanol exposure in utero remains to be elucidated. It may be that a decreased tonic inhibition may compensate for the decrease in synaptic excitation. Alternatively, a decreased GABA-mediated tonic current may reflect the dynamics in the expression of extrasynaptic receptors following long-term exposure to ethanol during corticogenesis. In layer V pyramidal neurons, both α5 subunit- and δ subunit-containing GABAA receptors mediate tonic inhibition (Yamada et al., 2007; Centanni et al., 2016). The δ subunit-containing GABAA receptors are sensitive to ethanol at concentrations as low as 3mM (Hanchar et al., 2005), a concentration consistent with our exposure paradigm. Further, numerous studies have reported altered GABAAR subunit composition following ethanol exposure (Matthews et al., 1998; Liang et al., 2007; Lindemeyer et al., 2014; Devaud et al., 1997). This is speculative at this point, since we have no data to substantiate this hypothesis.
Summary
This study addressed the issue of whether chronic exposure of mice to a low dose of ethanol in utero can be demonstrated to lead to enduring aberrances in cortical form and function. By combining a behavioral assay, immunohistochemistry and electrophysiology, we report aberrances in the mPFC of offspring exposed prenatally to chronic ethanol that can be attributed to an enduring interneuronopathy hallmarked by abnormalities in the number and distribution of parvalbumin-expressing GABAergic interneurons as well as functionally by distorted I/E synaptic balance in the mPFC.
Acknowledgments
The authors thank Pamela W.L. Yeh for expert technical assistance, Laurie C. Delatour and Steven L. Miller for critical reading of the manuscript.
Support: PHS grants R01 AA023410 and R21 AA024036
Contributor Information
Alexander G.J. Skorput, Department of Neuroscience, University of Minnesota Medical School Twin Cities, 321 Church Street, Minneapolis, MN 55455.
Hermes H. Yeh, Department of Physiology and Neurobiology, Geisel School of Medicine at Dartmouth Medical School, Dartmouth-Hitchcock Medical Center, One Medical Center Drive, Lebanon, NH 03756.
References
- Allan AM, Chinoweth J, Tyler LA, Caldwell KK. A mouse model of prenatal ethanol exposure using a voluntary drinking paradigm. Alcohol Clin Exp Res. 2003;27:2009–2016. doi: 10.1097/01.ALC.0000100940.95053.72. [DOI] [PubMed] [Google Scholar]
- Astley SJ, Clarren SK. Diagnosing the Full Spectrum of Fetal Alcohol-Exposed Individuals: Introducing the 4-Digit Diagnostic Code. Alcohol and Alcoholism. 2000;4:400–410. doi: 10.1093/alcalc/35.4.400. [DOI] [PubMed] [Google Scholar]
- Astley SJ, Aylward EH, Olson HC, Kerns K, Brooks A, Coggins TE, Davies J, Dorn S, Gendler B, Jirikowic T, Kraegel P, Maravilla K, Richards T. Magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2009;10:1671–89. doi: 10.1111/j.1530-0277.2009.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady ML, Allan AM, Caldwell KK. A limited access mouse model of prenatal alcohol exposure that produces long-lasting deficits in hippocampal-dependent learning and memory. Alcohol Clin Exp Res. 2012;36:457–466. doi: 10.1111/j.1530-0277.2011.01644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brien JF, Chan D, Green CR, Iqbal U, Gareri J, Kobus SM, McLaughlin BE, Klein J, Rao C, Reynolds JN, Bocking AD, Koren G. Chronic prenatal ethanol exposure and increased concentration of fatty acid ethyl esters in Mmeconium of term fetal guinea pig. Ther Drug Monit. 2006;28:345–50. doi: 10.1097/01.ftd.0000211819.35182.82. [DOI] [PubMed] [Google Scholar]
- Centanni SW, Burnett EJ, Trantham-Davidson H, Chandler LJ. Loss of δ-GABAA receptor-mediated tonic currents in the adult prelimbic cortex following adolescent alcohol exposure. Addiction Biology. 2016 doi: 10.1111/adb.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Alcohol use and binge drinking among women of childbearing age–United States, 2006–2010. Morb Mortal Wkly Rep. 2012;61:534–538. [PubMed] [Google Scholar]
- Chang G-Q, Karatayev O, Liang SC, Barson JR, Leibowitz SF. Prenatal ethanol exposure stimulates neurogenesis in hypothalamic and limbic peptide systems: possible mechanism for offspring ethanol overconsumption. Neuroscience. 2012;222:417–428. doi: 10.1016/j.neuroscience.2012.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury TG, Jimenez JC, Bomar JM, Cruz-Martin A, Cantle JP, Portera-Cailliau C. Fate of Cajal-Retzius neurons in the postnatal mouse neocortex. Front Neuroanat. 2010;4:10. doi: 10.3389/neuro.05.010.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzon VC, Yeh PWL, Yanagawa Y, Obata K, Yeh HH. Ethanol Consumption during Early Pregnancy Alters the Disposition of Tangentially Migrating GABAergic Interneurons in the Fetal Cortex. J Neurosci. 2008;28:1854–1864. doi: 10.1523/JNEUROSCI.5110-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaud LL, Fritschy JM, Sieghart W, Morrow AL. Bidirectional Alterations of GABA(A) Receptor Subunit Peptide Levels in Rat Cortex during Chronic Ethanol Consumption and Withdrawal. J Neurochem. 1997;69:126–30. doi: 10.1046/j.1471-4159.1997.69010126.x. [DOI] [PubMed] [Google Scholar]
- Erickson SL, Lewis DA. Postnatal development of parvalbumin- and GABA transporter-immunoreactive axon terminals in monkey prefrontal cortex. J Comp Neurol. 2002;448:186–202. doi: 10.1002/cne.10249. [DOI] [PubMed] [Google Scholar]
- Farag M. Diagnostic issues affecting the epidemiology of fetal alcohol spectrum disorders. J Popul Ther Clin Pharmacol J Thérapeutique Popul Pharamcologie Clin. 2014;21:e153–e158. [PubMed] [Google Scholar]
- Farber NB, Creeley CE, Olney JW. Alcohol-induced neuroapoptosis in the fetal macaque brain. Neurobiol Dis. 2010;40:200–206. doi: 10.1016/j.nbd.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nature Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fox DJ, Pettygrove S, Cunniff C, O’Leary LA, Gilboa SM, Bertrand J, Druschel CM, Breen A, Robinson L, Ortiz L, Frías JL, Ruttenber M, Klumb D, Meaney FJ, Centers for Disease Control and Prevention (CDC) Fetal alcohol syndrome among children aged 7–9 years – Arizona, Colorado, and New York, 2010. MMWR Morb Mortal Wkly Rep. 2015;64:54–57. [PMC free article] [PubMed] [Google Scholar]
- Fryer SL, Tapert SF, Mattson SN, Paulus MP, Spadoni AD, Riley EP. Prenatal alcohol exposure affects frontal-striatal BOLD response during inhibitory control. Alcohol Clin Exp Res. 2007;8:1415–24. doi: 10.1111/j.1530-0277.2007.00443.x. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I. The main source of ambient GABA responsible for tonic inhibition in the mouse hippocampus. J Physiol. 2007;582:1163–1178. doi: 10.1113/jphysiol.2007.134460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M. Alcohol-induced motor impairment caused by increased extrasynaptic GABA(A) receptor activity. Nature Neuroscience. 2005;8:339–45. doi: 10.1038/nn1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG, Rakic P. Radial Columns in Cortical Architecture: It Is the Composition That Counts. 2010;20:2261–2264. doi: 10.1093/cercor/bhq127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Dobyns WB. X-linked lissencephaly with abnormal genitalia as a tangential migration disorder causing intractable epilepsy: proposal for a new term, “interneuronopathy. J Child Neurol. 2005;20:392–397. doi: 10.1177/08830738050200042001. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kondo S. Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex. J Neurocytol. 2002;31:277–87. doi: 10.1023/a:1024126110356. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Liang J, Suryanarayanan A, Abriam A, Snyder B, Olsen RW, Spigelman I. Mechanisms of reversible GABAA receptor plasticity after ethanol intoxication. J Neurosci. 2007;27:12367–77. doi: 10.1523/JNEUROSCI.2786-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber CS, DeCarli LM. The feeding of alcohol in liquid diets: two decades of applications and 1982 update. Alcohol Clin Exp Res. 1982;4:523–31. doi: 10.1111/j.1530-0277.1982.tb05017.x. [DOI] [PubMed] [Google Scholar]
- Lindemeyer AK, Liang J, Marty VN, Meyer EM, Suryanarayanan A, Olsen RW, Spigelman I. Ethanol-induced plasticity of GABAA receptors in the basolateral amygdala. Neurochem Res. 2014;39:1162–70. doi: 10.1007/s11064-014-1297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DB, Devaud LL, Fritschy JM, Sieghart W, Morrow AL. Differential regulation of GABA(A) receptor gene expression by ethanol in the rat hippocampus versus cerebral cortex. J Neurochem. 1998;70:1160–66. doi: 10.1046/j.1471-4159.1998.70031160.x. [DOI] [PubMed] [Google Scholar]
- Max JE, Manes FF, Robertson BAM, Mathews K, Fox PT, Lancaster J. Prefrontal and executive attention network lesions and the development of attention-deficit/hyperactivity symptomatology. J Am Acad Child Adolesc Psychiatry. 2005;44:443–450. doi: 10.1097/01.chi.0000156661.38576.0f. [DOI] [PubMed] [Google Scholar]
- May PA, Blankenship J, Marais AS, Gossage JP, Kalberg WO, Joubert B, Cloete M, Barnard R, De Vries M, Hasken J, Robinson LK, Adnams CM, Buckley D, Manning M, Parry CDH, Hoyme HE, Tabachnick B, Seedat S. Maternal alcohol consumption producing Fetal Alcohol Spectrum Disorders (FASD): quantity, frequency, and timing of drinking. Drug Alcohol Depend. 2013;2:502–12. doi: 10.1016/j.drugalcdep.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Baete A, Russo J, Elliott AJ, Blankenship J, Kalberg WO, Buckley D, Brooks M, Hasken J, Abdul-Rahman O, Adam MP, Robinson LK, Manning M, Hoyme HE. Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics. 2014;134:855–866. doi: 10.1542/peds.2013-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JT, Yang C, Franciosi S, Winston S, Abarr KK, Rigby MS, Yanagawa Y, McCarley RW, Brown RE. Distribution and intrinsic membrane properties of basal forebrain GABAergic and parvalbumin neurons in the mouse. J Comp Neurol. 2013;521:1225–50. doi: 10.1002/cne.23290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi G, Fishell G. GABAergic interneuron lineages selectively sort into specific cortical layers during early postnatal development. Cereb Cortex. 2011;21:845–852. doi: 10.1093/cercor/bhq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornoy A, Ergaz Z. Alcohol abuse in pregnant women: effects on the fetus and newborn, mode of action and maternal treatment. Int J Environ Res Public Health. 2010;7:364–379. doi: 10.3390/ijerph7020364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka T, Kawaguchi Y. Cortical inhibitory cell types differentially form intralaminar and interlaminar subnetworks with excitatory neurons. J Neurosci. 2009;29:10533–10540. doi: 10.1523/JNEUROSCI.2219-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S, Anderson CT, Levitt P, Shepherd GMG. Circuit-specific intracortical hyperconnectivity in mice with deletion of the autism-associated Met receptor tyrosine kinase. J Neurosci. 2011;31:5855–5864. doi: 10.1523/JNEUROSCI.6569-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley EP, Barron S, Driscoll CD, Hamlin RT. The effects of physostigmine on open-field behavior in rats exposed to alcohol prenatally. Alcohol Clin Exp Res. 1986;10:50–53. doi: 10.1111/j.1530-0277.1986.tb05613.x. [DOI] [PubMed] [Google Scholar]
- Riley EP, Lochry EA, Shapiro NR. Lack of response inhibition in rats prenatally exposed to alcohol. Psychopharmacology. 1979;62:47–52. doi: 10.1007/BF00426034. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Adkins MM. The effects of prenatal alcohol exposure on behavior: rodent and primate studies. Neuropsychol Rev. 2011;21:186–203. doi: 10.1007/s11065-011-9168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorput AGJ, Vivek PG, Yeh PWL, Yeh HH. Persistent interneuronopathy in the prefrontal cortex of young adult offspring exposed to ethanol in utero. J Neurosci. 2015;31:10977–88. doi: 10.1523/JNEUROSCI.1462-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorput AGJ, Yeh HH. Effects of ethanol exposure in utero on Cajal-Retzius cells in the developing cortex. Alcohol Clin Exp Res. 2015;5:853–62. doi: 10.1111/acer.12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley JF, Saito M, Bleiwas C, Masiello K, Ardekani B, Guilfoyle DN, Gerum S, Wilson DA, Vadasz C. Selective reduction of cerebral cortex GABA neurons in a late gestation model of fetal alcohol spectrum disorder. Alcohol. 2015;6:571–80. doi: 10.1016/j.alcohol.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhausen HC, Willms J, Spohr HL. Long-term psychopathological and cognitive outcome of children with fetal alcohol syndrome. J Am Acad Child Adolesc Psychiatry. 1993;32:990–994. doi: 10.1097/00004583-199309000-00016. [DOI] [PubMed] [Google Scholar]
- Tang Z-Q, Dinh EH, Shi W, Lu Y. Ambient GABA-activated tonic inhibition sharpens auditory coincidence detection via a depolarizing shunting mechanism. J Neurosci. 2011;31:6121–6131. doi: 10.1523/JNEUROSCI.4733-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Werd HJJM, Rajkowska G, Evers P, Uylings HBM. Cytoarchitectonic and chemoarchitectonic characterization of the prefrontal cortical areas in the mouse. Brain Struct Funct. 2010;214:339–353. doi: 10.1007/s00429-010-0247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Tam M, Anderson SA. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J Comp Neurol. 2008;506:16–29. doi: 10.1002/cne.21529. [DOI] [PubMed] [Google Scholar]
- Yamada J, Furukawa T, Ueno S, Yamamoto S, Fukuda A. Molecular basis for the GABAA receptor-mediated tonic inhibition in rat somatosensory cortex. Cerebral Cortex. 2007;17:1782–87. doi: 10.1093/cercor/bhl087. [DOI] [PubMed] [Google Scholar]


