Abstract
Many diseases and disease symptoms still lack effective treatment. At the same time, certain controversial Schedule I drugs, such as heroin and cannabis, have been reputed to have considerable therapeutic potential for addressing significant medical problems. Yet, there is a paucity of U.S. clinical studies on the therapeutic uses of controlled drugs. For example, people living with HIV/AIDS experience a variety of disease- and medication-related symptoms. Their chronic pain is intense, frequent, and difficult to treat. Nevertheless, clinical trials of compassionate management for their chronic symptoms that should be a research priority, is stymied.
We employed qualitative methods to develop an understanding of the barriers to research on potential therapeutic uses of Schedule I drugs so that they might be addressed. We elicited the perspectives of key stakeholder groups that would be involved in such studies: people living with HIV/AIDS, clinicians, and Institutional Review Board members. As we identified obstacles to research, we found all stakeholder groups to arrive at the same conclusion, that clinical research on the therapeutic potential of these drugs is ethically required.
Background
Certain controversial controlled drugs can have considerable therapeutic potential. Heroin, cannabis and other illegal drugs are potent analgesics, some prescribed routinely in Europe1. Animal experiments, as well as clinical data and trials, suggest that cocaine, ketamine2 and methylenedioxy-methamphetamine (MDMA)3 may have a role in the treatment of a variety of physical and mental illnesses and ailments. Randomized controlled trials and systematic reviews have documented therapeutic benefits of heroin, cannabis, ketamine and cocaine for selected indications, even for children, women in labor, and the elderly.4 Some have hypothesized that cannabis or ibogaine might even have therapeutic benefits as treatment of HIV.5 Yet the evidence is sparse; there is a paucity of U.S. studies on the therapeutic uses of controlled drugs. Clearly, we need more high quality studies to assess the efficacy of these drugs and determine the risks that would be associated with their medicinal use.6 The limited literature examining barriers to such research suggests that strict regulations, fear of legal consequences, stigma associated with abuse and populations using illicit drugs, and lack of funding could act as barriers to research on controlled drugs.7 Indeed, in the United States, drugs such as heroin and cannabis, are classified as Schedule I. That classification defines them as having “no currently accepted medical use in treatment.”8 Research on the therapeutic potential of Schedule I drugs has therefore been difficult, even though pain and other symptoms remain undertreated. The regulatory roadblocks, coupled with the need for effective treatment, create problematic situations. For example, the off label use has recently increased. Although Ketamine may be a promising drug for the treatment of depression, we still lack rigorous evidence of its efficacy and the risks associated with sustained use.9 Although the conduct of clinical studies with controversial controlled substances may raise unique ethical issues,10 these impediments are barriers to developing effective treatments to alleviate chronic suffering and exploring harm reduction, and thus have significant implications for clinical ethics.11
People living with HIV/AIDS illustrate the effects of today’s restrictive policies on research with Schedule I drugs. They experience a variety of symptoms related to their disease and the long-term use of prescribed medications. Their chronic pain is intense, frequent, and difficult to treat.12 Chronic pain associated with HIV/AIDS affects one third of the HIV+ population, and amounts to a significant disease burden.13 Chronic pain persists despite attempts at management with opioids, NSAID, anti-inflammatory agents, tricyclic antidepressants, and pain modifying therapies.14 In addition to pain, people living with HIV/AIDS (PLWHA) endure multiple chronic, systemic, neurological, oral, respiratory, musculoskeletal, ophthalmological, dermatological, genitourinary, and psychological symptoms.15 For many patients, their symptoms do not respond to currently available treatments. When legal drugs are ineffective, PLWHA often self-treat with illicit drugs that can produce medical complications and involve a risk of disease transmission.16 It therefore seems reasonable to conclude that research on compassionate management and effective treatment for the chronic symptoms of this population should be a research priority, even if the potentially effective agents include substances that we now classified as Schedule I.
In order to explore the actual social, ethical, legal or regulatory barriers that inhibit such research on the effectiveness of Schedule I drugs, we elicited the perspectives of the key stakeholder groups that would be involved if studies were to be undertaken. Because of the relevance of such studies to PLWHA, we selected them to represent the viewpoints of potential research participants. In addition, we sought the input of clinicians working with PLWHA, and Institutional Review Board (IRB) members.
Our pilot study had three immediate goals. One was to develop the tools for identifying actual and perceived barriers to clinical research with controversial Schedule I drugs and pilot our methodology. Another was to enable a rational discussion of permissible research with such drugs and elicit stakeholder views on the core issues that would be raised by such studies. Our broader aim was to use the insights from our study to develop an empirical basis for drug research policy reform and practical guidance for future research design. Most specifically, we wanted to elicit stakeholders’ opinions and experiences related to two key issues:
Do the risks of physical harm associated with controlled substances constitute an insurmountable barrier to research on their medicinal value?
Are social stigmas associated with controlled drugs, drug users, and/or PLWHA critical barriers to research?
Methods
Study design and recruitment
To distinguish contrasting stakeholder views on standardly prescribed and illicit drugs, we chose heroin, marijuana, and gabapentin as examples of possible study drugs for treatment of symptoms associated with HIV/AIDS. Gabapentin, heroin, and cannabis differ in legal status, addiction potential, and associated stigma. Gabapentin, our “inert comparator,” is approved by the Food and Drug Administration (FDA), and is commonly prescribed to treat general pain as an off-label use. No stigma is associated with its name, and its addiction potential is generally dismissed.17 Cannabis may be neither highly addictive nor harmful.18 Support of research and legalization of medicinal and recreational cannabis use is becoming more popular as public perceptions of marijuana shift. The FDA still, however, classifies marijuana as Schedule I. Heroin is also Schedule I, with a high level of street value and potential for abuse.
Using purposive sampling, we enrolled participants for this study from three stakeholder groups:
People living with HIV/AIDS (PLWHA) were recruited at inner city clinics serving predominantly under-resourced patients in NYC. They are potential research participants and beneficiaries of new therapeutics.
HIV clinicians were recruited at an HIV update conference. They can appreciate the need for new therapies. They also have medically informed concerns about research on Schedule I drugs and potential obstacles to their use in the community.
Institutional Review Board (IRB) members were recruited from an annual bioethics meeting. IRB members serve their institutions by determining whether proposed studies comply with federal and institutional guidelines. They have a distinct perspective on concerns in the research process.
As a formative study based in qualitative methods, we formed homogeneous focus groups discussion (FGDs) with 6–13 participants in each.19 FGDs allowed us to elicit predominant group views and also to identify novel and unexpected perspectives. In FGDs participants are able to challenge each other on controversial topics and analyze ideas together. We also conducted four in-depth interviews so as to triangulate the data and check for differences between responses in the group discussions as compared with the private interviews.20 All participants provided verbal consent to participate and be recorded. We compensated participants with cash: $100 for clinicians and IRB members, and $40 for PLWHA (a restriction imposed by the outpatient clinics). The study was approved by the Icahn School of Medicine at Mount Sinai IRB.
Data collection and management
Without existing models in the literature that were compatible with our research goals, we devised our own moderator guides, survey instruments, and clinical vignettes. We piloted them in one group and revised them after the pilot group discussion. To ensure anonymity, we did not request or record identifying data beyond those used for analysis purposes. We observed similar participant contributions from the FGDs and individual interviews.
Before the discussions began, participants in each FGD and individual interview completed identical anonymous surveys. The surveys collected basic demographic information, information related to personal experience, and opinions about illicit substances and HIV. At the end of each survey, participants were asked to list barriers to conducting research testing Schedule I drugs as treatment of HIV symptoms and complications.
Interviewers and focus group facilitators were trained to frame open ended questions so as to limit their impact on respondents. Note takers were trained to identify other unspoken or unintentional influences during the FGDs.21 FGD note takers were also instructed to record descriptions of interactions and personal characteristics that would not be captured by the audio recorder. Because group discussions can tend to discourage dissent, facilitators were trained to challenge opinions in order to evoke further conversation. We also chose to keep groups homogeneous so as to insure that the dialogues could delve into specific experiences, and so that PLWHA were not identified outside of their clinic.
For the group and individual interviews alike, we asked the same foundational questions, albeit with more focus on personal information in the latter. Questions elicited experience with HIV, views on the need for research, and opinions on participant willingness to participate in research using Schedule I drugs. Respondents were also asked about how the drug histories of study participants would impact their ability to provide informed consent. Finally, all stakeholders were asked for their opinions on measures to enable the study of therapeutic uses of Schedule I drugs and the necessary social or legal changes that would be needed in order for those trials to proceed.
All FGD were audiotaped and professionally transcribed verbatim, including emotional expressions, pauses, emphasis and laughter.22 Researchers reviewed the transcripts and recordings to ensure accuracy of the transcribed data.
Coding and analysis
Using inductive and deductive coding, six members of our research team independently read through at least one transcript from each stakeholder group and each individual set of interviews to formulate the codes based on relevant, emergent themes that participants mentioned (we only had focus group discussion data for HIV patients). The group discussed codes to find consensus, grouped themes, and generated axial codes.23 Once the codebook was finalized, we applied it to the remaining transcripts, and embedded comments and memos to highlight key sections, codes, and quotations. We used multiple coders for a representative sample of the data to ensure inter-rater reliability. We extrapolated a comprehensive codebook from the data and used the codebook as our conceptual framework for analyzing the FGD data.
Using a grounded theory approach, we developed themes and uncovered important new constructs from the data. We performed qualitative thematic analysis based in a critical realist epistemological position, the understanding that our findings, while real, are impacted by situational variables and the observer’s perspective.24 We compared data from individual interviews and FGDs to triangulate the data and discern if speaking in a private interview significantly altered the responses.
To analyze the free-list exercise at the end of the survey, we combined similar responses and generated a condensed, comprehensive list of reported barriers to research. We measured the frequencies and Smith’s Salience Index (SSI) for each listed barrier.25
Results
Participant characteristics
We conducted interviews with two clinicians and two IRB members. For the FGDs, we recruited two groups with IRB members (7–8 participants in each), two groups with clinicians (6–8 participants in each), and four groups with patients (9–13 participants in each): for a total of 15 IRB members, 14 clinicians, and 40 individuals living with HIV. Over 60% of the respondents living with HIV did not complete the free-listing exercise. In total, we collected free-listing data from 14 IRB members, 13 clinicians, and 15 individuals living with HIV.
Survey data
Tables 1 and 2 summarize the demographic data from the surveys completed at the FGDs by the 69 participants who completed them. The average ages were similar between groups, but most other characteristics were different. The overwhelming majority (73%) of PLWHA were Black, while clinicians and IRB members were predominantly White (56% and 93% respectively). All (100%) clinicians and IRB members had graduate school level educations, while most (66%) PLWHA achieved no more than high school level. The clinicians and IRB members had comparable average reported annual incomes over $100,000, while the average patient reported income was less than $20,000 annually. The self-described political affiliations of the groups also differed. IRB members and PLWHA described themselves as predominantly liberal (70% and 45% respectively) and clinicians as moderate (42%).
Table 1.
Demographic characteristics of survey respondents in study about barriers to researching tightly controlled substances for HIV, 2014
| Characteristic | Patient (n=40) | Clinician (n=14) | IRB Member (n=15) | Total (N=69) |
|---|---|---|---|---|
| Age (years) | 54.9 | 46.2 | 49.8 | 50.3 |
|
|
||||
| Sex | No.(%) | No.(%) | No.(%) | No.(%) |
| Male | 13(33) | 1(7) | 7(47) | 21(30) |
| Female | 16(40) | 10(71) | 6(40) | 32(47) |
| NA | 11(27) | 3(22) | 2(13) | 16(23) |
|
|
||||
| Race | No.(%) | No.(%) | No.(%) | No.(%) |
| Black | 29(73) | 1(7) | 1(7) | 31(45) |
| Black/Latino | 1(3) | 1(7) | 14(93) | 2(3) |
| Black/NatAm | 2(6) | 1(7) | 0(0) | 3(4) |
| White | 2(6) | 8(56) | 0(0) | 24(35) |
| Latino | 5(13) | 2(14) | 0(0) | 7(10) |
| Latino/NatAm | 1(3) | 0(0) | 0(0) | 1(1) |
| Asian | 0(0) | 1(7) | 0(0) | 1(1) |
|
|
||||
| Education | No.(%) | No.(%) | No.(%) | No.(%) |
| < High School | 5(13) | 0(0) | 0(0) | 5(7) |
| Some HS | 12(30) | 0(0) | 0(0) | 12(17) |
| High School | 9(23) | 0(0) | 0(0) | 9(13) |
| Some college | 13(33) | 0(0) | 0(0) | 13(19) |
| College | 1(3) | 0(0) | 0(0) | 1(3) |
| Grad School | 0(0) | 14(100) | 15(100) | 29(42) |
|
|
||||
| Income (Avg) | $18,528 | $120, 714 | $134,666 | $91,303 |
Table 2.
Experiences and opinions of respondents on survey for study about barriers to researching tightly controlled substances for HIV, 2014
| Experience* | Patient (n=40) | Clinician (n=14) | IRB Member (n=15) | Total (N=69) |
|---|---|---|---|---|
| HIV | No.(%) | No.(%) | No.(%) | No.(%) |
| Yes | 37(93) | 12(86) | 5(33) | 54(78) |
| No | 3(7) | 2(14) | 10(66) | 15(22) |
|
|
||||
| MSM | No.(%) | No.(%) | No.(%) | No.(%) |
| Yes | 13(33) | 13(93) | 9(60) | 35(51) |
| No | 27(67) | 1(7) | 6(40) | 34(49) |
|
|
||||
| Transgender | No.(%) | No.(%) | No.(%) | No.(%) |
| Yes | 11(27) | 10(71) | 5(33) | 26(37) |
| No | 29(73) | 4(29) | 10(66) | 43(63) |
|
|
||||
| IVDU | No.(%) | No.(%) | No.(%) | No.(%) |
| Yes | 10(25) | 10(71) | 6(40) | 26(37) |
| No | 30(75) | 5(29) | 9(60) | 43(63) |
|
|
||||
| Drug use | No.(%) | No.(%) | No.(%) | No.(%) |
| This month | 11(27) | 3(22) | 4(27) | 18(26) |
| Past-regularly | 12(30) | 3(22) | 2(13) | 17(25) |
| Past-rarely | 6(16) | 5(34) | 3(20) | 14(22) |
| Never | 3(8) | 3(22) | 5(33) | 11(16) |
| None of your business | 7(18) | 0(0) | 1(7) | 8(11) |
|
|
||||
| Legalize? | No.(%) | No.(%) | No.(%) | No.(%) |
| Yes | 32(80) | 9(64) | 12(80) | 53(75) |
| No | 4(10) | 2(14) | 0 (0) | 6(10) |
| No opinion | 2(5) | 3(22) | 3(20) | 8(12) |
| NA | 2(5) | 0(0) | 0(0) | 2(3) |
Experienced by participant or someone close to participant
Table 2 demonstrates the range of personal experiences and opinions that respondents associated with illicit substances, PLWHA, and vulnerable populations. All questions that asked about familiarity defined it as having personal experience or knowing someone who fit the category. We asked the questions in this way so as to encourage participants to feel comfortable in answering. PLWHA and clinicians treating PLWHA indicated having familiarity with people who were HIV+ (93% and 86% respectively), but only one third of IRB members reported personal experience with people who lived with the illness. The overwhelming majority (71%) of participants indicated familiarity with recreational drug use: Smoking recreational drugs was the most frequent method for all stakeholder groups. Few participants reported personal experience with injection drug use of any kind (16%), but many referenced first and second hand experiences during the discussion. Three quarters of participants (75% of total sample) responded that they would support the legalization of marijuana.
Free-list reports of barriers to research
Table 3 provides the data from the free-listing exercise. Of the seventy participants, 25 of the 40 HIV patients, one of the 14 medical providers, and one of the 15 IRB members did not complete the free list exercise. Those participants were excluded from the frequency and salience analysis of free-list exercises. We noted marked differences in the lists by stakeholder groups. Reporting bias was the most salient barrier identified by the PLWHA group (SSI=0.43). In their words, they were concerned that study participants would display a “lack of honesty” and that “most people just want the money.” They predicted participants lying about their comorbidities or consumption habits in order to gain access to the Schedule I drug (for personal use or sale). Few clinicians mentioned dishonesty as a salient barrier, and none of the IRB members included it on their lists. Clinicians most frequently listed legal (SSI=0.43), reputational and political barriers. IRB members also listed legal and reputational barriers, but they ranked ethical concerns most frequently (SSI=0.33), including issues such as informed consent, undue inducement, decisional capacity, and protecting vulnerable populations. Neither the clinician nor patient respondents listed any of these research ethical concerns as barriers to research.
Table 3.
Free list of barriers to researching tightly controlled substances for HIV in the USA, 2014
| Barrier** | Patient (n=15) | Clinician (n=13) | IRB Member (n=14) | |||
|---|---|---|---|---|---|---|
| Frequency | SSI* | Frequency | SSI* | Frequency | SSI* | |
| Legal/regulatory | 1 | 0.07 | 6 | 0.35 | 5 | 0.32 |
| Stigma | 1 | 0.07 | 7 | 0.22 | 4 | 0.22 |
| Politics | 0 | 0.0 | 6 | 0.29 | 1 | 0.07 |
| Ethics (informed consent, undue inducement, capacity, vulnerable population) | 0 | 0.0 | 1 | 0.6 | 6 | 0.33 |
| Reporting bias (dishonesty, drug/money seeking behavior) | 7 | 0.43 | 3 | 0.17 | 0 | 0.0 |
| Biomedical Risk (addiction, drug interaction, disease transmission) | 4 | 0.23 | 5 | 0.29 | 2 | 0.01 |
| Confidentiality | 2 | 0.1 | 2 | 0.15 | 3 | 0.15 |
| Funding | 1 | .07 | 2 | 0.08 | 0 | 0.0 |
| IRB approval | 0 | 0.0 | 0 | 0.0 | 3 | 0.15 |
| Post-trial issues | 0 | 0.0 | 0 | 0.0 | 3 | 0.07 |
| Investigator/institutional reluctance | 0 | 0.0 | 1 | 0.8 | 3 | 0.13 |
| Ignorance | 2 | 0.09 | 0 | 0.0 | 2 | 0.08 |
Smith’s Salience Index
Reported responses with >3 respondents
Focus Group Discussions and In-depth Interviews
The interactive and dynamic conversations in each interview and discussion group suggested that there was a comfortable rapport between the discussion leader and the participants in each group. Our analysis of the data revealed concordant and discordant perspectives among stakeholder groups and some unanticipated perspectives. The most prominent themes were the need for research, barriers associated with physical risk and stigma, personal biases, and predicted study complications. We also examined the pattern of how views evolved during the FGDs.
We found that each stakeholder group agreed on the need for research, but expressed distinct concerns associated with undertaking, approving, or participating in an actual study. Employing the Social Ecological Model to analyze the noted barriers, we identified dilemmas and a fundamental problem in the structure of drug policy.
Need for research
All stakeholder groups ultimately indicated that there is a need for research because of the clinical implications of developing additional treatment for pain. Clinicians and PLWHA agreed that neuropathy lacks adequate treatment. IRB members compared research on controlled drugs with studies on other potentially harmful drugs that provide valuable evidence of their safety and efficacy, suggesting that studies of Schedule I drugs could similarly prove informative.
In one discussion, clinicians described refractory pain and medications with undesirable side effects as indicators of the need for improved treatment. One clinician remarked,
What I really want is a better toolbox […] What I’m looking for, certainly, is something with a longer half-life, less sedation, less side effects. (Clinician)
Another declared,
I’m not saying I agree or not with marijuana use, but if it’s going to help people that are ill and it’s going --to help them with the pain and suffering, right, I think that is something that definitely needs to be studied and measured and managed. (Clinician)
Clinicians agreed that the current pain management medications are insufficient and that substances like marijuana could manage pain that other medications do not ameliorate. They also mentioned the absence of evidence to support prescribing decisions. In addition, they wanted research into non-narcotic pain management options without burdensome side-effects.
During one FGD, individuals living with HIV expressed the need for more effective neuropathy treatment. One remarked,
I get angry because I say ‘You mean to tell me you got all this medicine, technology… and you can’t give me anything to alleviate this so that I can function as a regular human being? (PLWHA)
Another expressed,
I had neuropathy too. I don’t know what my doctors give me, but it wasn’t working. I used to have to get up and have to put my feet on the cold floor. And it’d go away for about ten minutes and then come back. (PLWHA)
Others who had AIDS neuropathy were dissatisfied with their current treatments, but resigned to tolerate it. One explained,
My T-cell was completely zero. And it was like that for a year [sic]. [My doctor] right now, is more concerned about building my T-cell count up. … sometimes I just have to do deal with the side effects. There’s nothing I can do about it. (PLWHA)
Most IRB members had little to no experience with neuropathy or its treatment, and their responses indicated an entirely different perspective on the need for research than clinicians and patients. When asked why research on Schedule I drugs should be pursued, they focused on intellectual justifications, such as to simply better understand the drug itself. Nevertheless, they acknowledged that information is required in order to make and implement informed decisions about prescribing strictly controlled drugs.
Physical Risk
All stakeholders initially responded that the risk of physical harm would be a barrier to human subject research using Schedule I drugs. When queried about our three comparator-drugs, most did not know what Gabapentin was. Participants accepted research on its therapeutic uses. The absence of reservations suggested that there was no stigma associated with it. The clinicians and PLWHA who were familiar with Gabapentin remarked that it had little street value and did not allow users to get high.
Across groups, stakeholders perceived marijuana to be associated with less risk than heroin. Participants from all groups also mentioned their concern about the risks of addiction, misuse, and diversion. As the discussions progressed, however, participants began to question their original presumptions about risks and to see the risk of physical harm as less of a barrier to research than they had originally assumed.
PLWHA almost unanimously agreed that the risks associated with heroin are barriers that would make them reluctant to participate in any trials. Many of the PLWHA described their firsthand experience with addiction. In the FGDs they offered stories from their own lives and the lives of family members and friends as reason for resisting exposure to heroin. Explaining his reluctance to participate in a study with heroin as the study drug, one person explained,
Especially if it’s a substance I know I can get addicted to, because I’ve done a lot of drugs, and I’ve been addicted to a lot of drugs. And right now, at this point in my life, I’m too old to get another habit. So…no. (PLWHA)
Another worried,
It’s such an addictive drug. I know too many people that that drug has taken out. (PLWHA)
At the same time, most participants living with HIV expressed openness or positive opinions about participating marijuana trials.
Several clinicians and IRB members also expressed rather strong concerns about addictive potential. As two IRB members opined,
The enslaving effect, of course, is a side effect, which we cannot control. (IRB)
I think particularly if you’re talking about an illegal substance, there’s that automatic presumption that addiction is a problem, period. (IRB)
A clinician expressed a similar fear with these words,
My worry with these particular drugs, heroin, is the addiction and the CNS [central nervous system] related HIV problems. (Clinician)
Others, however, viewed the risk of addiction as something to control, rather than a reason to prevent such research. As one IRB member explained,
I honestly wouldn’t be terribly concerned that in my trial that they were going to become addicted. … I assume that we would be looking for risk protections the same way we would for any drug. (IRB)
All stakeholder groups also mentioned the potential for harm to non-trial participants. Clinicians and PLWHA focused on the risk of diversion and concern that participants might sell their medications. Some PLWHA confessed that they would participate in a trial so that they could profit by selling the drug. One offered that,
She [My doctor] can prescribe it [heroin] to me twenty-five times and I’m going to be on the next corner selling it to the next dope fiend. So she ain’t going to give me that. I don’t want it. That’s going to cause me to go to jail, because I’m going to find a way to hustle with it. (PLWHA)
PLWHA also predicted that allowing people to consume heroin legally would have dire effects on their communities. One predicted that,
“They [are] going to become addicted. Then they’re going to be bootlegging and stealing out the stores. And 125th street is going to be on fire. (PLWHA)
Stigma
IRB members and clinicians frequently mentioned stigma associated with illicit substances and drug users. One described it saying,
There’s this presumption on IRBs … of people who are known active illicit substance users, you always have the worst image of them and the assumption that they are all in the trial for the money so they can get their next fix. But I think it speaks to what is an inherent bias among a lot of IRB members as well as clinicians--that addiction is the worst possible thing. (IRB)
Other IRB members elaborated and expressed similar concerns.
If somebody sees your prescription for heroin, or if your boss finds out you’re on heroin versus … Oxycodone I don’t know how an employer would feel about having an employee that they know is receiving marijuana or heroin or something like that on the job. (IRB)
We already know in the states where medical marijuana has been legal, people have been fired -- even though they have a legitimate prescription for it. (IRB)
IRB members and clinicians also expressed the view that stigma associated with drugs and drug users would create barriers to research by making investigators reluctant to conduct trials involving these substances. IRB members remarked about the institutional risks. One declared,
I think of the stigma around the use of an illicit substance and what it implies both for the participants of the study but, more importantly, the institution. [Institutions are] afraid of the public backlash and how it would affect them as an institution and their fundraising activities and PR and everything else. (IRB)
Another noted that,
There’s the politics involved. [The] last thing you need is The New York Times saying how the department of health is giving people heroin. (IRB)
IRB members and clinicians were also aware that conducting this type of research could tarnish the reputation of a clinician, an IRB member, or an investigator. One clinician remarked,
I would totally, about five or ten minutes ago, [have] said that I would definitely help accomplish some cannabis research. But now that I’m thinking about it, if your name gets tarnished … the price is powerful. (Clinician)
Another offered,
Maybe it’s not such a good idea for me to be pioneering just because you wouldn’t--I wouldn’t want to set anything back by being considered less reputable. I certainly wouldn’t stake my next academic degree on that being my thesis. (Clinician)
An IRB member added,
I think that there is always the concern, particularly following some of the trials that have gone bad. IRB members think about the potential risk for them should it go wrong because IRBs are under increasing scrutiny particularly when you have a severe adverse event. (IRB)
IRB and clinician participants identified specific systems and institutional arrangements that would obstruct research with controlled substances. IRB members mentioned possible intervention by institutional risk managers. Clinicians mentioned their worries about IRB review committees. None of the individuals living with HIV mentioned reputational risks as relevant barriers to research.
Personal Perspectives
The FGDs initiated a deliberative process that allowed clinicians and IRB members to notice how their own perspectives skewed their opinions. One IRB member even suggested using a fictitious name for the study drug while accurately ascribing its relevant risks in proposals for studying heroin. This strategy was proposed as a way to avoid the name-associated bias.
In each FGD with PLWHA, some participants voiced negative opinions about human subject research and researchers. The widely shared attitude toward research participation was expressed by a person who declared,
I would do them as long as you’re talking to me, I’m on the computer. But when it comes to clinical trials, I’m not too enthused about doing anything—me being a guinea pig for anybody (PLWHA)
Several participants shared the view that they were part of a targeted population. As one explained,
A lot of people of color, black people, don’t enter studies like that. Look at Tuskegee. [nods of agreement] You look back at places like that and see what they did to people in the study. They’re people of minorities; you know, they needed money … Years after, people didn’t even know about it. I did some research on it. Most black people scared of studies. (PLWHA)
Whether or not the story was true, one participant shared this anecdote, which expressed the group’s trepidations about research participation.
Listen, we know someone who did this study … She went in. She did the study. She sat there. They gave her all the crack she wanted … But when she came out—[…] she was so messed up that she immediately went to a long-term rehab. Long-term. (PLWHA)
Study Complications
FGD participants raised concerns about possible deception by study participants, that could undermine the validity of a clinical trial. For example, one clinician offered this prediction,
If you give too much [incentive], you’re looking at a bunch of folks who are looking for that. They call and say they have terrible pain and they want $200. (Clinician)
An overwhelming majority of PLWHA participants shared this view. One expressed the concern with particular clarity.
One of the biggest barriers in this entire problem is how honest the client is going to be. Because, most of us, if you tell us you’re going to pay us two-thousand dollars to do something, we’ll lie, steal, and cheat to get that money. (PLWHA)
Clinicians and PLWHA also predicted that study participants would drop out of studies once they received the drugs or money. For example, one clinician remarked,
They might not finish the research because they’ll get high or use the money to get [high] (Clinician)
And one PLWHA said,
Most of the time, the barriers to a study is that most people only do it in the beginning, just to get the initial incentive and the money. And then once they get it, they’re all getting high again and they’re not going to complete the study. (PLWHA)
Because of their disease, PLWHA are vulnerable to a number of harms related to their immune-compromised physical condition and the social stigma associated with the illness. Participants from all groups expressed these concerns:
When you’re on enough drugs already, and you’re already consuming enough, to try something, not knowing the outlook or the outcome—I think it’s enough. You take enough drugs already. (PLWHA)
Because we got a whole lot of things going on, …issues with us. My liver fucked up already. I don’t know what’s going—I mess around with some research and be done. (PLWHA)
I don’t know if we are specifically talking about studying this in the HIV+ population, but I think that’s like a very interesting subject to consider and makes it a lot more complicated and maybe not the place to start. (Clinician)
What I’m trying to say is start with the least controversial population. […]I mean, there’s a lot of people with neuropathy out there. (Clinician)
What would have to change though is that you’d have to have some studies that weren’t done on some of these diseases […] that already have that stigma. (IRB)
Evolution of Views
As the discussions progressed, clinicians and IRB stakeholders identified inconsistencies in their own opinions about issues related to research on controlled substances and began to challenge each other’s statements. As their comments demonstrate, the participants displayed reflection, insights, and willingness to revise unfounded beliefs. Late in each FGD, comments such as these were made:
Am I having a hard time with this because it is a drug that I think of as illicit? … Maybe the answer is just give people informed consent and you let them know. We do invasive surgeries. We do chemo. Am I sitting here, swimming through stigma in my own head right now? Maybe you just give them consent. (Clinician)
I sort of almost feel neutral about it. But the more I think about it, why not study it? […] Because there’s so much politics and maybe some of this, like, really old school sort of sheltered […] stigma that research--I mean, smoke it, eat it, you know, extract the different--So let’s research it. (Clinician)
Upon reflection, the groups noticed that their initial views on the risks of heroin were based on presumptions rather than evidence. To that affect, one clinician asked,
If we can get rid of those problems with the heroin and treat pain with heroin, what I would be questioning [is] What’s the difference between heroin and morphine, or heroin and Oxycontin? (Clinician)
An IRB member showed a similar recognition, stating that,
I honestly wouldn’t be terribly concerned that in my trial that they were going to become addicted. I mean heroin is addictive but it’s not like one dose I mean that you’re suddenly addicted in any physiological sense. I assume that we would be looking for risk protections the same way we would for any drug in terms of phase in and phase out. (IRB)
Another IRB member remarked,
We looked at a lot of nicotine drugs in schizophrenics—and you know you give nicotine, because they do better on it, but you know it is addictive. (IRB)
Another IRB member responded,
Now what? I feel like I’m talking out of both sides of my mouth because really we didn’t have any trouble with the nicotine studies (IRB)
And another IRB member expressed that insight saying,
We kind of walked through it while we sat here and there’s not a real good reason to say no to this kind of research [Laughing]. (IRB)
Within the span of a 90-minute discussion, both IRB and clinician stakeholders came to recognize that their unsupported beliefs had impacted their previous attitudes towards the risk of providing heroin in research. Seeing that transformation in group after group was unanticipated, startling, and encouraging.
Participant Insights
Across stakeholder groups, participants began to identify gaps in their knowledge about the therapeutic effects of Schedule I drugs such as heroin. Their ignorance appeared to make participants feel uncomfortable. The discomfort seemed to foster critical reflection, and that, in turn, led the participants to identify their ignorance as a barrier to research. A few even mentioned that their ignorance was a reason to pursue research because knowledge gained from studies would be the grounds for evidence-based practice in the future. Ultimately, nearly all clinicians and IRB members reached the conclusion that with appropriate study design and considerable caution, the risks of physical harm involved in studying heroin or marijuana as a possible treatment for HIV symptoms were surmountable. Some granted that symptom relief, even with addictive consequences, could be a significant benefit for people living with extreme, refractory pain. They referenced the “benefit ratio” (IRB), implying that addiction may be an easier ailment than living with unremittant pain, thereby benefiting the patient. An IRB member expressed,
So what if a person becomes addicted to a prescription painkiller? If they’re in unremitting pain, so…? (IRB)
Other group members added that offering heroin at HIV clinics could reduce harms associated with illicit drug self-medication, spreading infection with shared needles, and risking legal and social penalties. It could also provide the benefits that come with accessing the health care system. In sum, one participant remarked,
I’m sure we’ll have a lot more patients coming into the clinic [if we offer heroin]. And it would be more like the safe injecting rooms in Vancouver and Switzerland and New Zealand where people could be observed using in a safer setting, where overdoses could be identified more quickly and prevented. I mean I think there are tangible health benefits to the user or the participant. (Clinician)
Discussion
The findings from this qualitative pilot study reveal the impact of unexamined beliefs and attitudes on studying the therapeutic potential of Schedule I drugs for HIV symptom management. The FGDs, in-depth interviews, and free-listing exercise data demonstrate similarities and differences in the initial views of each stakeholder group.
The participants’ initial opinions as recorded in the free-listing exercise suggest that stakeholders in each group have distinctive concerns. These groups also have little awareness of the perspectives of other interested parties. Clinicians and IRB members express concerns with their own and their institution’s reputations, and patients express concern with dishonesty. This finding makes it is easy to appreciate just how different their perspectives are. The FGDs and interviews further confirmed these divergent perspectives.
The Evolution of Views in Focus Group Discussions
During the FGDs, participants expressed their views on the various topics. In all discussions, it was clear that most participants had not previously considered the medicinal potential of these Schedule I drugs. Yet, each conversation led participants to become skeptical of their initial qualms, challenge their initial views, and recognize them as reflections of unsubstantiated bias. In every group, and in instance after instance, the open discussion led participants to confirm the need for research on controlled drugs in order to explore their potential for alleviating serious medical problems associated with HIV.
Although we did not begin with a commitment to any particular philosophic method, the sui generous result that we observed in the FGDs is compatible with deliberative democracy theory.26 Deliberative democracy is an approach in contemporary moral philosophy embraced by theorists known as “contractarian constructivists.” Amy Guttmann, Dennis Thompson, Leonard Fleck, and other proponents of this approach to resolving challenging ethical issues, maintain that democratic deliberations allow people to discuss their differences in understanding and opinion. According to these theorists, the agreements that result from such a process represent conclusions about what justice requires.
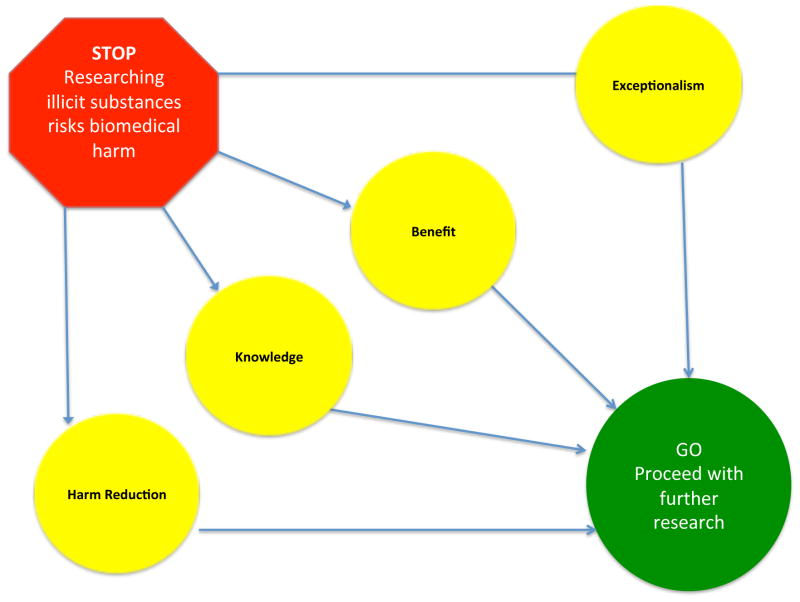
It has, however, been noted that the focus group process can produce a convergence of views. Our finding is remarkable because all eight of our FGDs reached the same conclusions on a set of related issues. This consistent, overlapping consensus, across all stakeholder groups and expressed concerns, could not have been produced by a single dominant voice.27 Figure 1 illustrates the evolution of views within all eight of the FGDs on the core question about the risk of physical harm as a barrier to research on controlled drugs. Participants provided similar reasons for concluding that the risk of physical harm should not be a barrier to research during the discussion of each of the potential barriers that they had identified. We labeled these reasons for rejecting the potential barriers (1) exceptionalism, (2) benefit, (3) knowledge, and (4) harm reduction.
Figure 1.
Evolution of participants' positions on risks versus benefits of therapeutic research on controlled substances for PLWHA.
(1) Exceptionalism refers to the participant realization that the risks of research with heroin and marijuana are not greater than the risks of other studies with interventions such as chemotherapy, oxycodone and nicotine, which could also involve a risk of harm to subjects. (2) Benefit refers to the appreciation of the potential for marijuana and/or heroin to provide effective, affordable, and accessible treatment for refractory pain and other symptoms. (3) Knowledge indicates the information that would result from studies of the safety and efficacy of the drugs. That information would allow physicians to consider whether or not to administer them in future treatment decisions. (4) Harm reduction refers to the potential benefit that the legal provision of a controlled substance can provide to mitigate other risks, such as improper self-medication, avoidable illness, or crime.
Through their deliberation, the clinicians, IRB members, and PLWHA systematically refuted many of their own previous opinions of barriers to research on controlled substances. They concluded that further research should be considered, and in some cases that it was even ethically required. Participants living with HIV, however, persisted in expressing strong reservations about studies that would provide heroin. They continued to assert that participation in heroin trials was inconceivable for them or their loved ones. Personal experiences with addiction and intimates who had overdosed were pervasive, and those experiences had a powerful effect on their views. The deliberation that produced these four arguments led the FGD participants from all three stakeholder-groups to conclude that the risks of physical harm from research should not constitute a barrier to research. Our FGDs modeled how a deliberative democratic process can be effective in disabusing participants of their biases and helping them to revise their attitudes toward research on controlled substances. With discussion, participants in each FGD reached agreement on the most pressing issues. Where they disagreed, their differences were explicitly revealed. Further deliberations may help us to refine our understanding of genuine barriers to research with controlled substances and to identify the legitimate limits for conducting such research according to the highest ethical standards.
Existing Literature
We have only identified a few studies that investigated potential barriers to research with illicit substances in the U.S.,28 and a paper by Bell and Salmon was the only study that explored ethical review committee bias against illicit drug users. It found that assumptions about users’ incapacity to participate in trials were unwarranted.29 Our study findings are consistent with theirs in that we have also noted that IRB members were skeptical about individuals who use substances being unable to provide informed consent and adhere to a study protocol. At the same time, PLWHA reported that they would refuse to participate in clinical trials with heroin out of concern for the risks. Their responses demonstrate their ability to distinguish between greater and lesser risks, and therefore suggest that they do have the capacity to make responsible decisions about study participation.
Strength and Limitations
The uniqueness of this study lies in its three stakeholder groups. We gained invaluable insights about the differences in their perspectives, as well as the similarities in the conclusions that they reached. Comparing and contrasting the perspectives that they revealed in their responses to the same questions illuminated the similarities and differences in the experiences of each stakeholder group and how those differences lead them to perceive research with controlled substances. The inclusion of individuals who live with HIV and have experience with addiction was an especially innovative and valuable feature of our study because the PLWHA contributed voices from the population of potential controlled substance study participants.
Because of the small sample size, the purposive sample, and the limited range of participant demographics, our pilot study was not representative of the stakeholder groups at large. The IRB members were all recruited from a bioethics conference, and most described themselves as liberals. The clinician group was drawn from the New York area and a significant majority of them were women. Some clinicians voiced strong antipathies toward narcotics and western medicine, which limited the scope of the conversation about research with controlled substances.
The group discussions involving PLWHA were conducted in two clinics that include only patients with limited education and low socioeconomic status as demonstrated in the demographics survey responses. In some cases, the educational limitations of these participants may have prevented them from completing the survey. Several either expressed or demonstrated their inability to read or write. This factor may have accounted for why only 40% of them completed the free-listing exercise. All of these limitations make it difficult to draw strong generalizable conclusions.
Before conducting group discussions we had anticipated that in-depth interviews would be necessary in order for respondents to be willing to share personal, sensitive, and potentially compromising information and views on controversial topics. From our experience, however, we learned, to the contrary, that people were at least as forthcoming in groups as there were in private conversations. The data collected from in-depth interviews were consistent with FGDs. Furthermore, the group discussions allowed participants to challenge each other and gain insight into the validity of their previously unexamined views on research with illicit drugs. Although we found that FGDs are a suitable tool, in our future work we plan to incorporate more varied study vignettes based on actual clinical trials to allow us to delve more deeply into the thorny issues of research ethics that could present barriers to controlled drug research. We will need to sample more geographically and demographically diverse populations.
Conclusions
By including the voices of clinicians, IRB members, and potential research participants, our work adds significantly to the small body of prior research on barriers to controversial drug research. The inclusion of all three stakeholder-groups is especially valuable because it allowed us to compare their perspectives and identify their limited awareness of the perspectives of the other interested stakeholders. We were also able to identify the strong influence of their personal experiences and the impact of bias and stigma on their opinions. These factors constitute considerable barriers to research on the therapeutic benefits of controversial controlled drugs.
We also witnessed how our FGDs eventually led participants to refute the exceptionalism of controlled substances research and to acknowledge the potential for benefit, knowledge, and harm reduction that could result from research with controlled substances. This transformation in attitudes provides some reason to anticipate that with effort focused on understanding and addressing people’s concerns, the existing policies that inhibit research on tightly controlled drugs can be reformed so that needed research may proceed. Additionally, diminishing the social stigma associated with schedule I drugs will reduce the risks for IRB members and providers. Achieving such regulatory and social reform will enable investigators to undertake studies and IRB members to approve them, and thereby allow clinicians to fulfill their duty to their patients by providing drugs that are effective in meeting their patients’ medical needs.
While our work highlights specific areas of stigma and bias impacting science, it also has revealed that there is more that we need to study. We need to inform our understanding of the nature of the risk of physical harm by eliciting the views of experts on addiction and the known effects of marijuana and heroin. Furthermore, it is important, specifically, to explore the research ethics issues of informed consent, vulnerability, undue inducement, and therapeutic misconception that will be especially complex in this context. Developing a detailed understanding of how these ethical concepts bear on the ethical conduct of research with controlled substances will be critical in formulating guidance on how such studies may be conducted, particularly in studies that propose to involve participants from vulnerable groups or people who may be current or past drug users. An informed perspective on these issues should be developed before leaping into clinical trials with controlled substances, which are clearly not without risks or adverse effects. For example, we are aware that cannabis use during adolescence may increase the subsequent risk to develop schizophrenia.30 Further research with a greater diversity of stakeholders is necessary in order to develop a broader consensus on these ethical issues. Evidence-based recommendations, including input from relevant experts on controlled substances, needs to be created so that investigators can employ that guidance on how clinical trials of controlled drugs may be conducted in accordance with the highest ethical standards. During the period when this manuscript was under review, the momentum for medical cannabis use has grown,31 new indications for marijuana been identified,32 and off label treatment with intravenous ketamine for depression has become widespread.33 These developments emphasize the urgent need to address barriers to research, so that treatment choices can be informed by sound clinical evidence.
References
- 1.Gossop Michael, Keaney Francis, Sharma Pankaj, Jackson Mark. The Unique Role of Diamorphine in British Medical Practice: A Survey of General Practitioners and Hospital Doctors. European Addiction Research. 2005;11(2):76–82. doi: 10.1159/000083036. [DOI] [PubMed] [Google Scholar]
- 2.Kavalali Ege T, Monteggia Lisa M. Synaptic Mechanisms Underlying Rapid Antidepressant Action of Ketamine. The American Journal of Psychiatry. 2012 Nov;169(11):1150–1156. doi: 10.1176/appi.ajp.2012.12040531. [DOI] [PubMed] [Google Scholar]; Li Jih-Heng, Vicknasingam Balasingam, Cheung Yuet-Wah, Zhou Wang, Nurhidayat Adhi Wibowo, Des Jarlais Don C, Schottenfeld Richard. To Use or Not to Use: An Update on Licit and Illicit Ketamine Use. Substance Abuse and Rehabilitation. 2011;2:11–20. doi: 10.2147/SAR.S15458. [DOI] [PMC free article] [PubMed] [Google Scholar]; Roback Mark G, Wathen Joe E, MacKenzie Todd, Bajaj Lalit. A Randomized, Controlled Trial of I.v. versus I.m. Ketamine for Sedation of Pediatric Patients Receiving Emergency Department Orthopedic Procedures. Annals of Emergency Medicine. 2006 Nov;48(5):605–612. doi: 10.1016/j.annemergmed.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Sindicich N, Degenhardt L, Hall W. The Use of MDMA for Therapeutic Purposes. The Health and Psychological Effects of “Ecstasy” (MDMA) Use. 2010;12(62) [Google Scholar]
- 4.Conlin Anne Elizabeth, McLean Laurie. Systematic Review and Meta-Analysis Assessing the Effectiveness of Local Anesthetic, Vasoconstrictive, and Lubricating Agents in Flexible Fibre-Optic Nasolaryngoscopy. Journal of Otolaryngology - Head & Neck Surgery. 2008 Apr;37(2):240–249.Elliott R, Fischer CT, Rennie DL. Evolving Guidelines for Publication of Qualitative Research Studies in Psychology and Related Fields. The British Journal of Clinical Psychology / the British Psychological Society. 1999 Sep;38(3):215–229. doi: 10.1348/014466599162782.Iskedjian Michael, Bereza Basil, Gordon Allan, Piwko Charles, Einarson Thomas R. Meta-Analysis of Cannabis Based Treatments for Neuropathic and Multiple Sclerosis-Related Pain. Current Medical Research and Opinion. 2007 Jan;23(1):17–24. doi: 10.1185/030079906x158066.Kendall JM, Reeves BC, Latter VS. Multicentre Randomised Controlled Trial of Nasal Diamorphine for Analgesia in Children and Teenagers with Clinical Fractures. BMJ (Clinical Research Ed) 2001 Feb;322(7281):261–265. doi: 10.1136/bmj.322.7281.261.Lynch Mary E, Campbell Fiona. Cannabinoids for Treatment of Chronic Non-Cancer Pain; a Systematic Review of Randomized Trials. British Journal of Clinical Pharmacology. 2011 Nov;72(5):735–744. doi: 10.1111/j.1365-2125.2011.03970.x.McInnes Rhona J, Hillan Edith, Clark Diana, Gilmour Harper. Diamorphine for Pain Relief in Labour: A Randomised Controlled Trial Comparing Intramuscular Injection and Patient-Controlled Analgesia. BJOG: An International Journal of Obstetrics and Gynaecology. 2004 Oct;111(10):1081–1089. doi: 10.1111/j.1471-0528.2004.00131.x.Roback, et al. A Randomized, Controlled Trial of I.v. versus I.m. Ketamine for Sedation of Pediatric Patients Receiving Emergency Department Orthopedic Procedures. doi: 10.1016/j.annemergmed.2006.06.001. see note 2 above.
- 5.Silva Edinete M, Cirne-Santos Claudio C, Frugulhetti Izabel C, Galvao-Castro Bernardo, Saraiva Elvira M, Kuehne Martin E, Bou-Habib Dumith Chequer. Anti-HIV-1 Activity of the Iboga Alkaloid Congener 18-Methoxycoronaridine. Planta Medica. 2004 Sep;70(9):808–812. doi: 10.1055/s-2004-827227. [DOI] [PubMed] [Google Scholar]; Costantino Cristina Maria, Gupta Achla, Yewdall Alice W, Dale Benjamin M, Devi Lakshmi A, Chen Benjamin K. Cannabinoid Receptor 2-Mediated Attenuation of CXCR4-Tropic HIV Infection in Primary CD4+ T Cells. In: Wu Yuntao., editor. PLoS ONE. 3. Vol. 7. Mar 20, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.French L, Gray C, Leonard G, Perron M, Pike G, Richer L, Séguin J, Veillette S, Evans C, Artiges E, Banaschewski T, Bokde A, Bromberg U, Bruehl R, Buchel C, Cattrell A, Pangelinan M, Poustka L, Rietschel M, Smolka M, Walter H, Whelan R, Timpson N, Schumann G, Smith G, Pausova Z, Paus T. Early cannabis use, polygenic risk score for schizophrenia and brain maturation in adolescence. JAMA Psychology. 2015;72(10):1002–11. doi: 10.1001/jamapsychiatry.2015.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dines A, Wood D, Yates C, Heyerdahl F, Hovda K, Giraudon I, Sedefov R, Dargan P Euro-DEN Research Group. Acute recreational drug and new psychoactive substance toxicity in Europe: 12 months data collection from the European Drug Emergencies Netowrk (Euro-DEN) Clinical Toxicology. 2015;53(9):893–900. doi: 10.3109/15563650.2015.1088157. [DOI] [PubMed] [Google Scholar]; Gibbons S. ‘Leval highs’--novel and emergin psychoactive drugs: a chemiical overview for the toxicologist. Clinical Toxicology. 2012;50(1):15–24. doi: 10.3109/15563650.2011.645952. [DOI] [PubMed] [Google Scholar]
- 7.Abrams DI. Medical Marijuana: Tribulations and Trials. Journal of Psychoactive Drugs. 1998;30(2):163–169. doi: 10.1080/02791072.1998.10399686. [DOI] [PubMed] [Google Scholar]; Cohen Peter J. Medical Marijuana: The Conflict between Scientific Evidence and Political Ideology. Part Two of Two. Journal of Pain & Palliative Care Pharmacotherapy. 2009a;23(2):120–140. doi: 10.1080/15360280902900620. [DOI] [PubMed] [Google Scholar]
- 8.DEA. United States Code (USC) Controlled Substances Act: Section 812. Schedules of Controlled Substances. United Office of Diversion Control, Drug Enforcement Administration. 2007 www.gpoaccess.gov.
- 9.Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB APA Council of Research Task Force on Novel Biomarkers and Treatments. Ketamine and Other NMDA Antagonists: Early Clinical Trials and Possible Mechanisms in Depression. Am J Psychiatry. 2015;172(10):950–66. doi: 10.1176/appi.ajp.2015.15040465. [DOI] [PubMed] [Google Scholar]
- 10.Andreae Michael H, Carter George M, Shaparin Naum, Suslov Kathryn, Ellis Ronald J, Ware Mark A, Abrams Donald I, et al. Inhaled Cannabis for Chronic Neuropathic Pain: An Individual Patient Data Meta-Analysis. The Journal of Pain: Official Journal of the American Pain Society. 2007 Sep; doi: 10.1016/j.jpain.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen Medical Marijuana: The Conflict between Scientific Evidence and Political Ideology. Part Two of Two. doi: 10.1080/15360280902900620., see note 7 above; Abrams Medical Marijuana: Tribulations and Trials. doi: 10.1080/02791072.1998.10399686., see note 7 above Nahas GG, Greenwood A. The First Report of the National Commission on Marihuana (1972): Signal of Misunderstanding or Exercise in Ambiguity. Bulletin of the New York Academy of Medicine. 1974 Jan;50(1):55–75.
- 12.Ellis Ronald J, Toperoff Will, Vaida Florin, van den Brande Geoffrey, Gonzales James, Gouaux Ben, Bentley Heather, Hampton Atkinson J. Smoked Medicinal Cannabis for Neuropathic Pain in HIV: A Randomized, Crossover Clinical Trial. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2009 Feb 6;34(3):672–680. doi: 10.1038/npp.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh Sabyasachi, Chandran Arthi, Jansen Jeroen P. Epidemiology of HIV-Related Neuropathy: A Systematic Literature Review. AIDS Research and Human Retroviruses. 2012 Jan;28(1):36–48. doi: 10.1089/AID.2011.0116. [DOI] [PubMed] [Google Scholar]
- 14.Finnerup Nanna Brix, Sindrup Soren Hein, Jensen Troels Staehelin. The Evidence for Pharmacological Treatment of Neuropathic Pain. Pain. 2010 Sep;150(3):573–581. doi: 10.1016/j.pain.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 15.Giles M, Workman C. Clinical Manifestations and the Natural History of HIV. HIV Management in Australasia. 2009:125–32. [Google Scholar]
- 16.Elbrit D, Bayon C. Neurocognitive Impairment, Depression, and Anxiety in HIV-1-Infected Patients across Western Europe and Canada: The Cranium Study - Ethnicity Analysis. International AIDS Society. 2012;15(6):18276.Giles, Workman Clinical Manifestations and the Natural History of HIV , see note 15 above; Harrington RD, Woodward JA, Hooton TM, Horn JR. Life-Threatening Interactions between HIV-1 Protease Inhibitors and the Illicit Drugs MDMA and Gamma-Hydroxybutyrate. Archives of Internal Medicine. 1999 Oct;159(18):2221–2224. doi: 10.1001/archinte.159.18.2221.Klitzman Robert. From ‘Male Bonding Rituals’ to ‘Suicide Tuesday’: A Qualitative Study of Issues Faced by Gay Male Ecstasy (MDMA) Users. Journal of Homosexuality. 2006;51(3):7–32. doi: 10.1300/J082v51n03_02.Kousik Sharanya M, Celeste Napier T, Carvey Paul M. The Effects of Psychostimulant Drugs on Blood Brain Barrier Function and Neuroinflammation. Frontiers in Pharmacology. 2012;3:121. doi: 10.3389/fphar.2012.00121.
- 17.Nutt David J, King Leslie A, Phillips Lawrence D. Drug Harms in the UK: A Multicriteria Decision Analysis. Lancet (London, England) 2010 Nov;376(9752):1558–1565. doi: 10.1016/S0140-6736(10)61462-6. [DOI] [PubMed] [Google Scholar]
- 18.Bourgain Catherine, Falissard Bruno, Blecha Lisa, Benyamina Amine, Karila Laurent, Reynaud Michel. A Damage/benefit Evaluation of Addictive Product Use. Addiction (Abingdon, England) 2012 Feb;107(2):441–450. doi: 10.1111/j.1360-0443.2011.03675.x.Cohen Medical Marijuana: The Conflict between Scientific Evidence and Political Ideology. Part Two of Two. doi: 10.1080/15360280902900620., see note 7 above; Cohen Peter J. Medical Marijuana: The Conflict between Scientific Evidence and Political Ideology. Part One of Two. Journal of Pain and Palliative Care Pharmacotherapy. 2009b doi: 10.1080/15360280902727973.
- 19.Charmaz Kathy. Constructing Grounded Theory: A Practical Guide through Qualitative Analysis (Introducing Qualitative Methods Series) Sage Publications Ltd; 2006. [Google Scholar]
- 20.Ibid.
- 21.Rapley Timothy John. The Art(fulness) of Open-Ended Interviewing: Some Considerations on Analysing Interviews. Qualitative Research. 2001 Dec 1;1(3):303–323. [Google Scholar]
- 22.Hill Clara E, Knox Sarah, Thompson Barbara J, Williams Elizabeth Nutt, Hess Shirley A, Ladany Nicholas. Consensual Qualitative Research: An Update. Journal of Counseling Psychology. 2005;52(2):196–205. [Google Scholar]
- 23.Charmaz Constructing Grounded Theory: A Practical Guide through Qualitative Analysis (Introducing Qualitative Methods Series) , see note 19 above.
- 24.Bernard H Russell. Research Methods in Anthropology: Qualitative and Quantitative Approaches. 2011. [Google Scholar]; Altamira Rowman, Guest G, Bunce A, Johnson L. How Many Interviews Are Enough? An Experiment with Data Saturation and Variability. Field Methods. 2006;18:59–82. [Google Scholar]; Liamputtong Pranee. Focus Group Methodology: Principles and Practice. London: 2011. [Google Scholar]; Sofaer Shoshanna. Qualitative Research Methods. International Journal for Quality in Health Care. 2002 Aug 1;14(4):329–336. doi: 10.1093/intqhc/14.4.329. [DOI] [PubMed] [Google Scholar]; Willig Carla. Introducing Qualitative Research in Psychology. 3. Berkshire, England: Open University Press; 2013. [Google Scholar]
- 25.Quinlan Marsha. Considerations for Collecting Freelists in the Field: Examples from Ethobotany. Field Methods. 2005 Aug 1;17(3):219–234. [Google Scholar]
- 26.Gutmann Amy, Thompson Dennis. Why Deliberative Democracy? Princeton, NJ: Princeton University Press; 2004. [Google Scholar]; Fleck Leonard M. Just Caring: Health Care Rationing and Democratic Deliberation. Oxford: University Press; 2009. [Google Scholar]
- 27.Rawls J. Political Liberalism. New York, NY: Columbia University Press; 1993. [Google Scholar]
- 28.Anderson EE, Solomon S. Research Ethics Education for Community-Engaged Research: A Review and Research Agenda …. Research Ethics. 2012 doi: 10.1525/jer.2012.7.2.3.Bell Kirsten, Salmon Amy. What Women Who Use Drugs Have to Say about Ethical Research: Findings of an Exploratory Qualitative Study. Journal of Empirical Research on Human Research Ethics: JERHRE. 2011 Dec;6(4):84–98. doi: 10.1525/jer.2011.6.4.84.Gossop The Unique Role of Diamorphine in British Medical Practice: A Survey of General Practitioners and Hospital Doctors. doi: 10.1159/000083036., see note 1 above.
- 29.Bell Kirsten, Salmon Amy. Good Intentions and Dangerous Assumptions: Research Ethics Committees and Illicit Drug Use Research. Research Ethics. 2012;8:191–199. [Google Scholar]
- 30.French, et al. Early cannabis use, polygenic risk score for schizophrenia and brain maturation in adolescence. doi: 10.1001/jamapsychiatry.2015.1131. see note 6 above.
- 31.McCall C. Momentum grows for medical use of marijuana. Lancet. 2015;24(386 (10004)):1615–6. doi: 10.1016/S0140-6736(15)00674-1. [DOI] [PubMed] [Google Scholar]
- 32.Friedman D, Devinsky O. Cannabinoids in the Treatment of Epilepsy. N Engl J Med. 2016;7(374(1)):94–5. doi: 10.1056/NEJMc1512758. [DOI] [PubMed] [Google Scholar]
- 33.NPR.org. Club Drug Ketamine Gains Traction As A Treatment For Depression. 2015 Retrieved 1 February 2016, from http://www.npr.org/sections/health-shots/2015/09/28/443203592/club-drug-ketamine-gains-traction-as-a-treatment-for-depression.