Abstract
The aim of this study was to compare osseointegration and surface characteristics of zirconia implants made by the powder injection molding (PIM) technique and made by the conventional milling procedure in rabbit tibiae. Surface characteristics of 2 types of implant were evaluated. Sixteeen rabbits received 2 types of external hex implants with similar geometry, machined zirconia implants and PIM zirconia implants, in the tibiae. Removal torque tests and histomorphometric analyses were performed. The roughness of PIM zirconia implants was higher than that of machined zirconia implants. The PIM zirconia implants exhibited significantly higher bone-implant contact and removal torque values than the machined zirconia implants (P < 0.001). The osseointegration of the PIM zirconia implant is promising, and PIM, using the roughened mold etching technique, can produce substantially rough surfaces on zirconia implants.
Keywords: zirconia implant, powder injection molding, osseointegration, rabbit experiment
INTRODUCTION
Due to its proven long-term reliability, titanium is still the most dominantly used implant material1). However, there are some reported problematic disadvantages, such as sensitivity to the titanium2–4), electrical conductivity5), corrosive properties6), and accumulation of the metal ions in regional lymph nodes7), which have made researchers and clinicians explore alternatives8). Above all, the dark grayish color transmitted through thin gingival tissue or exposed after gingival shrinkage has been a major practical problem in the long-term maintenance of the implant restoration, especially in the anterior esthetic zone9–11).
While exploring tooth-colored implant materials, ceramics have been frequently proposed as alternatives to titanium12). Among them, yttria-stabilized zirconia ceramic (Y-TZP) has received considerable attention and has been studied most extensively13). In addition to its natural mimicking aesthetics, this material has a high bending strength and fracture toughness, resistance to corrosion and wear, biocompatibility, minimal ion release, and low affinity to bacterial colonization14–17).
For successful osseointegration, both not only the material itself but also the structure of the surface is one of the most important factors18). In order to improve osseointegration, efforts have been made to roughen the particularly hard zirconia surface textures, as with titanium and titanium alloy implants12). The successful osseointegration of zirconia implants with or without surface modification has been reported in several preclinical and clinical studies10,14,17,19–25). However, the influence of the surface characteristics of the zirconia implant on the complicated process of osseointegration is just beginning to be understood26).
Injection molding is a kind of plasticity shaping technique that utilizes binder, plasticizer, lubricant, and a coupling agent27). Injection molding is suitable for making fine ceramics, and the products shaped by this technique are highly precise and of good quality for clinical application, requiring little or no modification28). One additional advantage of this technique is that it offers the opportunity for mass production at a low cost29). In a previous in vivo rabbit experiment30), the novel roughened surface of the zirconia implant made by powder injection molding (PIM) was introduced and showed promising results in comparison with a machined titanium implant. The roughness incorporated into the PIM zirconia implant was not from additional surface modifications, but from the roughness of the mold’s inner surface.
Until now, there has been no comparison of the effect of roughness on peri-implant bone response between conventional milled zirconia implants and PIM zirconia implants. Therefore, the objective of this study is to compare osseointegration in rabbit tibiae, analyzing the effects of surface properties of the zirconia implants made by the PIM technique and made by the conventional milling procedure.
MATERIALS AND METHODS
Animals
Sixteen 3-month-old male New Zealand White rabbits, each weighing 2.5 to 3.0 kg, were used in the study. Animal selection, management, and surgical procedures followed protocols approved by the Institutional Animal Care and Use Committee of the Seoul National University, Seoul, Korea. The animals were provided access to water ad libitum and were fed a standard laboratory diet throughout the study.
Implant design
A total of 64 implants with a similar macro-design (external hex threaded type with a diameter 4.0 mm and a length of 7.0 mm) were used in this animal experiment. Implants were divided into 2 groups (Fig. 1). Group 1 (n = 32) consisted of machined-surface zirconia implants manufactured by Dentime (Seoul, Korea). Group 2 (n = 32) was the zirconia implant, which was created using the PIM technique with a roughened mold, manufactured according to a proprietary process of Cetatech (Seoul, Korea). Briefly, fine zirconia powder was mixed with binders in solvents. After injection molding of this feedstock, the binders were released and then the implant was sintered. The surface texture was bestowed upon the implant from the intaglio surface of the mold which was electrochemically modified. From each group, 20 implants were used for histomorphometric analysis and the remainders were used for the removal torque test.
Fig. 1.

Implants used in the experiment with the similar macro-design (external hex threaded type with a 4.0 mm diameter and a length of 7.0 mm).
(a) Group 1 (n = 32): machined-surface zirconia implant.
(b) Group 2 (n = 32): zirconia implant created using the PIM technique with a roughened inner surface mold.
Surface analysis
Surface topography was qualitatively examined using scanning electron microscopy (S-4700 Hitachi, Tokyo, Japan) and quantitatively measured by confocal 3-dimensional laser scanning microscopy (LMS 5-Pascal, Carl Zeiss, Oberhausen, Germany). A low pass Gaussian filter was used to calculate 3D roughness parameters such as Sa (arithmetic mean deviation peak-to-valley height of the surface), Sdr (increment of the interfacial surface area relative to a flat plane base line), Sds (density of summits, i.e. the number of peaks per area) and Sq (root mean square height of the surface) (Table 1).
Table 1.
Topographic analyses of the implant surface roughness, values obtained from confocal microscopy. Mean values (± standard deviation [SD]) from 5 implants, each tested at 4 different positions; Sa: arithmetic mean deviation of the surface roughness, Sdr: increment of the interfacial surface area relative to a flat plane base line, Sds: density of summits, i.e., the number of peaks per area, and Sq: root mean square height of the surface
| Group | Sa(㎛) | Sdr(%) | Sds(/㎛2) | Sq(㎛) |
|---|---|---|---|---|
| Group 1 (milled Zr) | 0.58 ± 0.05 | 12.68 ± 4.73 | 0.04 ± 0.02 | 0.74 ± 0.05 |
| Group 2 (PIM Zr) | 1.67 ± 0.11 | 31.82 ± 6.11 | 0.08 ± 0.03 | 2.14 ± 0.11 |
Assessment of surface chemistry was also performed on the implants produced for this animal experiment. Implants were placed in the vacuum chamber of an XPS device (EMAX, Horiba, Stanmore, England). A monochromatic X-ray source was used with an accelerating voltage of 15 kV and an emission current of 15 mA. The chemical purity of all surfaces was measured at different magnifications.
The surface profile of each sample was investigated using contact mode with a commercial atomic force microscope (AFM) (XE-100, Park Systems, Suwon, Korea). V-shaped silicon nitride cantilevers were used with a bending constant of 0.5 N/m as measured by the supplier. The images were analyzed with specific software (XEI, Park Systems, Suwon, Korea).
Surgical procedures
For the experiment, the hind legs of the animals were shaved before they were brought into the surgical area. The rabbits were anesthetized with an intramuscular injection of 10 mg/kg of zolazepam (Zoletil, Yuhan Corp., Seoul, Korea) and 10 mg/kg of xylazine hydrochloride (Rompun, Bayer, Frankfurt, Germany). Infiltration anesthesia (lidocaine 2% and epinephrine 1:100,000; 0.5 to1 mL/site) was used at the experimental sites. The animals received a slow, constant infusion of lactated Ringer’s solution (10 to 20 mL/kg/h intravenously) to maintain hydration during surgery. Surgeries were performed using aseptic routines by an experienced surgeon. The skin was incised, and each tibia was exposed after muscle dissection and periosteal elevation. The implant sites were prepared on the flat tibia surface using a dental drill with sterile and profuse saline irrigation.
Two implants were placed into each tibial metaphysis. Each group of implants was randomly allocated to the left and right hind limbs. The fascia and skin were closed in layers with interrupted single and mattress sutures as appropriate using resorbable (Vicryl 5.0, Ethicon, Somerville, NJ, USA) and non-resorbable materials (GORETEX Suture CV5, WL Gore & Associates, Flagstaff, AZ, USA).
Postsurgical procedures and animal sacrifice
A nonsteroidal analgesic agent with anti-inflammatory and antipyretic activity (flunixin meglumine, 0.8 mg/kg, intramuscularly twice daily for 3 days) was administered, and a broad-spectrum antibiotic (cefazolin, 40 mg/kg, intramuscularly two times daily for 3 days) was administered for infection control.
The animals were euthanized at 4 weeks with an overdose of sodium pentobarbital (100 mg/kg). Block biopsy specimens of the implant sites were collected, fixed in 10% buffered formalin, and processed for biomechanical and histomorphometric analysis.
Removal torque test
Immediately following sacrifice, the bone section containing the implant of interest was trimmed. The coupling mount was attached to the implants and the mount was connected to one arm of a torsional universal testing machine (55MT1-31, Instron, Norwood, MA, USA), which produces a linearly increasing torque. The bone section was coupled to the opposing arm and secured. A rotational unscrewing force was applied to the implant, and the peak torque value was recorded in Ncm from real time torque values, which were measured using computer software (Partner 7.1e, Instron, Norwood, MA, USA).
Histomorphometric analysis
The specimens were rinsed in water to wash out the paraformaldehyde, dehydrated in ascending alcohol fractions, and were then embedded in light-curing methacrylate (Technovit 7200 VCL, Kulzer, Hanau, Germany). The implants were cut in a mid-axial apico-coronal plane using a Macro cutting and grinding technique (EXAKT 310 CP series; EXACT Apparatebau, Norderstedt, Germany) and were subsequently ground and polished to a final thickness of 30 to 40 μm. The specimens were surface-stained with hematoxylin and eosin.
A single masked, calibrated examiner (Y. P.) performed the histomorphometric analysis. Digital images were captured, and a computer-based image analysis system with a custom macro (Image-Pro Plus, Media Cybernetic, Silver Spring, MD, USA) was used to quantify findings. The percentages of bone-implant contact (BIC) within the cortical resident bone of 20 implants per group were measured.
Statistical analysis
Examiner reliability for the histomorhometric evaluation was assessed using the concordance correlation coefficient, which ranged from 0.92 to 0.95, which demonstrates high reliability for all the parameters. Statistical analyses were performed using PASW statistics 18 (SPSS Inc, Chicago, IL, USA). Data were expressed as the mean plus or minus the standard deviation. Independent samples t-tests were used for between-group comparisons (α = 0.05).
RESULTS
Clinical observations
The healing period was uneventful. All animals survived the treatment and were available for evaluation. During the healing period, one of the machined implants was lost for an unidentified reason, and the remaining 63 implants were primarily stable and appeared to be clinically osseointegrated.
Surface characteristics
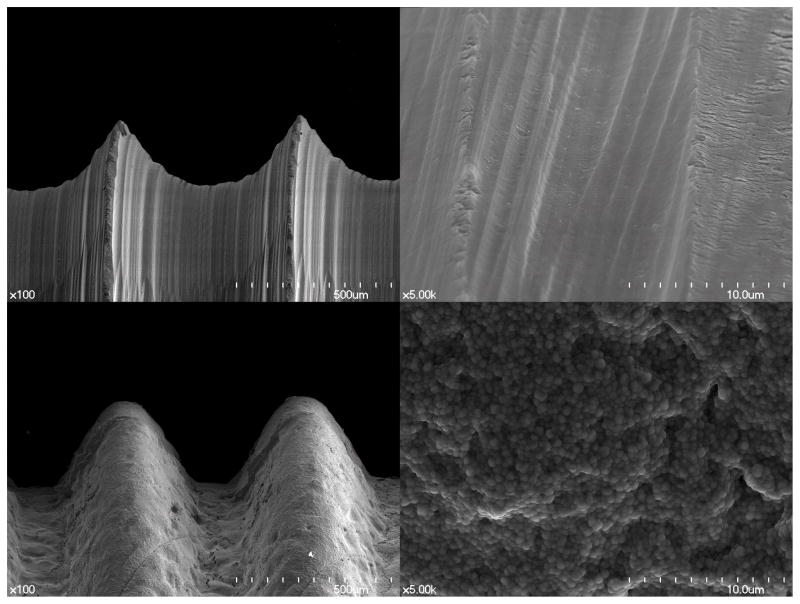
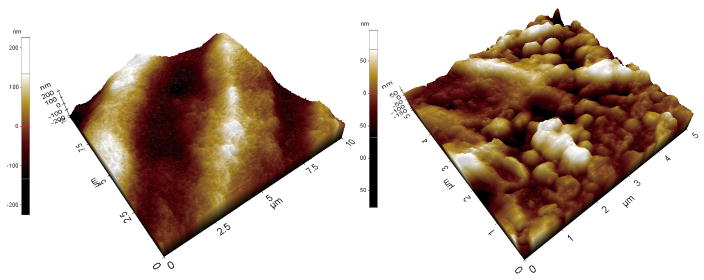
The SEM micrographs showed substantially different microstructures for the two zirconia implant surfaces (Fig. 2). The image of the PIM zirconia implant surface demonstrated elevations and depressions as well as the typical grain structure found in the as-sintered zirconia (Fig. 2). However, the machined zirconia implant surface exhibited parallel grooves in line with the direction of the milling bur movements, instead of the grain structures. When evaluating the surfaces using AFM, the grain structures of both zirconia surfaces could be easily identified, and the differences between them were obvious (Fig. 3). Sa, Sdr and Sq values in Group 2 (PIM zirconia implants) were greater than those of Group 1 (machined zirconia implants) (Table 1). However, the EDX results of Group 1 and Group 2 were quite similar but Group 2 showed higher O peak and lower Zr peak compared to Group 1 (Fig. 4).
Fig. 2.
SEM micrographs of tested zirconia implants. Group 1 implant with enlargement 100-fold (a) and with enlargement 5000-fold (b), Group 2 implant with enlargement 100-fold (c) and with enlargement 5000-fold (d)
Fig. 3.
AFM images of tested zirconia implants. Group 1 implant (a) and Group 2 implant (b)
Fig. 4.
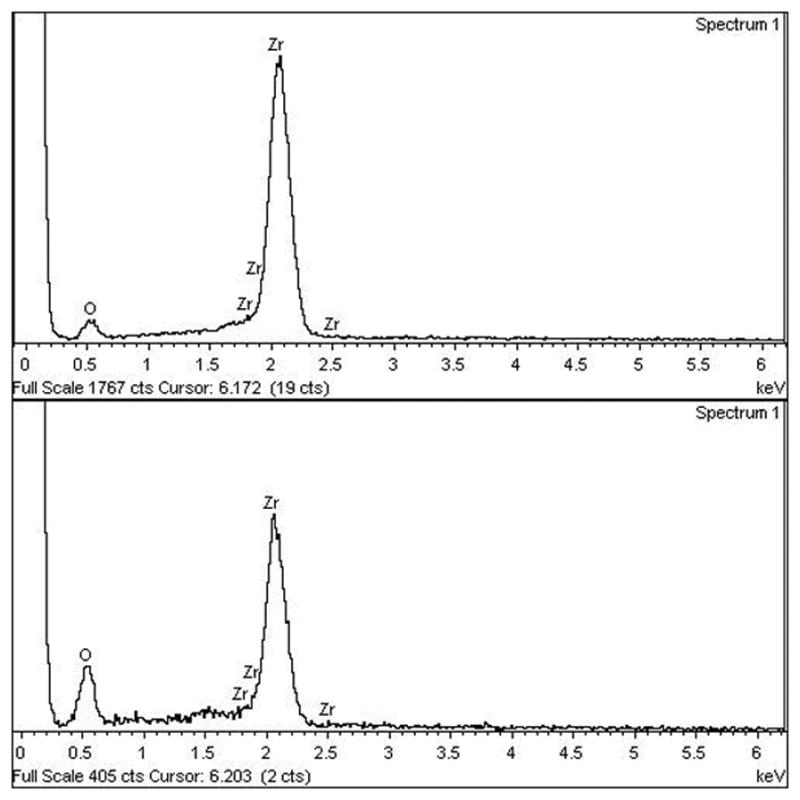
EDS images of tested zirconia implants. Group 1 implant (a) and Group 2 implant (b)
Removal torque test and histomorphometric analysis
The means and standard deviations of the tests are summarized in Table 2. Average BICs for Groups 1 and 2 were 32.15% and 58.38%, respectively (Fig. 6). Average removal torque values for Groups 1 and 2 were 19.44 Ncm and 57.63 Ncm, respectively. A Kolmogorov–Smirnov normality test showed that the samples were distributed normally for both the removal torque test and histomorphometric analysis. Levene’s test showed that the samples fulfilled the homogeneity of variance requirement. Independent samples t-tests indicated that the BIC and removal torque values were significantly different between groups.
Table 2.
Bone–implant contact (BIC) ratio values (percent, mean ± standard deviation [SD]) and removal torque (RT) values (percent, mean ± standard deviation [SD]) for tested implants
| Group | BIC (%)* | RT (Ncm)* |
|---|---|---|
|
|
|
|
| (n = 20 per each group) | (n = 11 for group 1 and n = 12 for group 2) | |
| Group 1 (milled Zr) | 32.15 ± 10.76 | 19.44 ± 7.42 |
| Group 2 (PIM Zr) | 58.38 ± 11.28 | 57.63 ± 11.64 |
denotes statistically significant difference between groups
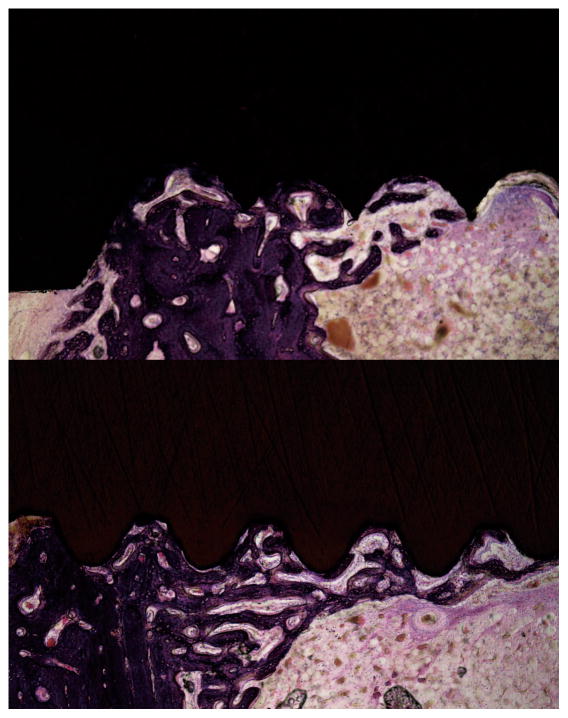
Fig. 6.
Undecalcified, ground and polished sections stained with Hematoxylin-Eosin. Group 1 implant (left) and Group 2 implant (right) with 12.5 times (top) and 40 times (bottom) magnification
DISCUSSION
Established in the past two decades, the powder injection molding technique is a manufacturing solution for producing intricate parts using metal or ceramic powders31). It is capable of transforming a complicated design into final product of high final properties with the advantages of dimensional precision and near-net-shape formation29). The process usually involves compounding fine ceramic powder with a blend of polymers or wax in a solvent such as toluene32). Prior to sintering, the organic binders embedded in the molded parts must be removed via thermal pyrolysis or solvent detracting33).
The fabrication or modification of a dense, hard zirconia surface is not so easy to achieve sufficient roughness34). A number of approaches are currently used to increase the surface roughness of machined zirconia to enhance the peri-implant bone response such as sandblasting35,36), coating24,37,38), acid or alkali etching26,39,40), laser treatment8), and UV irradiation41). For now, it is unclear whether or not these surface treatments can affect the integrity and mechanical properties of the implant. With regard to the preparation of a one-piece implant, it is known that the preparation significantly and negatively affects the fracture strength of the implant42). According to one phase transformation study observing zirconia implant crystalline structures, the sandblasted area showed trace amounts of monoclinic phase, while the untreated machined surface showed only tetragonal phase43). Therefore, the effects of surface modification on implant integrity should be further evaluated, especially when using subtractive modifications like blasting or etching.
On the other hand, surface coating, which is an additive method, has a common problem of delayed detachment1). Although the numerous coating materials have shown excellent results from the perspective of the early osseointegration by increasing bioactivity of the surface, delayed detachment is still a problem44). The detachment can result in defects at the interface between the bone and implant, provoking the eventual loss of the implant. Unfortunately, this type of catastrophe is not usually noticed until several months or several years after function. Furthermore, few studies have addressed the long-term integrity of the surface coating on the zirconia implant45).
One advantage of the PIM technique is that the roughness created by the roughened mold needs no additional surface treatment, which avoids potential damage to the integrity of zirconia surface30). To achieve the optimal range of roughness, the mold roughness can be adjusted in this technique. This innate roughness bestowed on the implant surface in the manufacturing process may not cause any weakness from further surface modification in contrast to the roughness from subtractive or additive treatment. However, for a few parameters, the surface properties cannot be well characterized because one surface may have the same parameters but a different spatial distribution or three-dimensional topography46). Also, it is not clearly understood which morphologic feature is the best at every step of peri-implant osteogenesis. Each step, which includes osteoblast adhesion, proliferation, differentiation involving the production of specific protein and bone matrix, could require unique morphological features.
Roughening titanium implant surfaces has been reported as an effective way to improve bone integration. The results of the present study show that the BIC of rough PIM zirconia implants was higher than that of machined zirconia implants, consistent with the results of the titanium implant studies1,46). These results are also in line with previous studies24,30,36 that compared the osseointegration of rough and smooth zirconia implants. In case of removal torque, the difference between the groups was more prominent, probably due to the friction difference. However, one limitation of this study is that the macrodesign of the implants were similar, but not identical, and slight differences in geometry might affect the final results. Furthermore, this study does not address whether roughness is always beneficial to osseointegration around the zirconia implants, since this question is beyond the scope of our aims. Since the existing biomechanical and histomorphometrical investigations of zirconia implant surfaces are few in number, further studies are necessary in order to elucidate the methods of enhancing osseointegration of zirconia implants.
CONCLUSION
Within the limit of the present study, the roughness of PIM zirconia implants was beneficial for osseointegration, showing higher BIC and removal torque value than that of machined zirconia implants. The roughness of PIM zirconia implants does not need any further subtractive surface treatment which might cause damage to the surface integrity. In this respect, the PIM technique for surface roughness seemed promising, which the roughness parameter can be adjusted according to the needs.
Fig. 5.
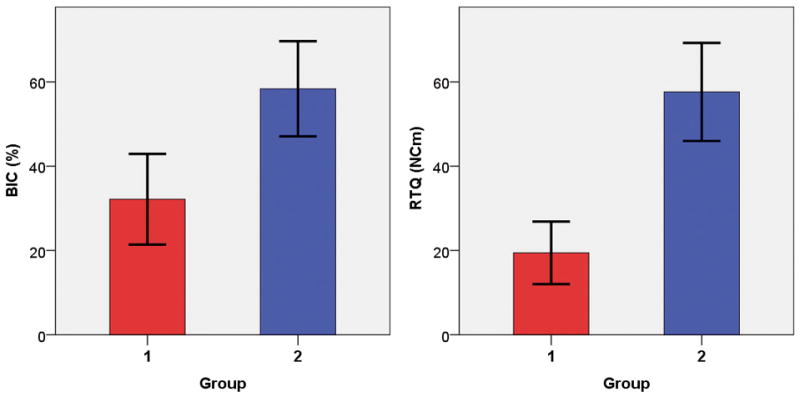
Comparisons of BIC and removal torque values of the 2 implant groups. *denotes a statistical significance.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the the Ministry of Science, ICT & Future Planning (NRF-2012R1A1A1001758).
References
- 1.Albrektsson T, Sennerby L, Wennerberg A. State of the art of oral implants. Periodontol 2000. 2008;47:15–26. doi: 10.1111/j.1600-0757.2007.00247.x. [DOI] [PubMed] [Google Scholar]
- 2.Flatebo RS, Johannessen AC, Gronningsaeter AG, Boe OE, Gjerdet NR, Grung B, et al. Host response to titanium dental implant placement evaluated in a human oral model. J Periodontol. 2006;77:1201–1210. doi: 10.1902/jop.2006.050406. [DOI] [PubMed] [Google Scholar]
- 3.Javed F, Al-Hezaimi K, Almas K, Romanos GE. Is titanium sensitivity associated with allergic reactions in patients with dental implants? A systematic review. Clin Implant Dent Relat Res. 2013;15:47–52. doi: 10.1111/j.1708-8208.2010.00330.x. [DOI] [PubMed] [Google Scholar]
- 4.Siddiqi A, Payne AG, De Silva RK, Duncan WJ. Titanium allergy: could it affect dental implant integration? Clin Oral Implants Res. 2011;22:673–680. doi: 10.1111/j.1600-0501.2010.02081.x. [DOI] [PubMed] [Google Scholar]
- 5.Tschernitschek H, Borchers L, Geurtsen W. Nonalloyed titanium as a bioinert metal--a review. Quintessence Int. 2005;36:523–530. [PubMed] [Google Scholar]
- 6.Gittens RA, Olivares-Navarrete R, Tannenbaum R, Boyan BD, Schwartz Z. Electrical implications of corrosion for osseointegration of titanium implants. J Dent Res. 2011;90:1389–1397. doi: 10.1177/0022034511408428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weingart D, Steinemann S, Schilli W, Strub JR, Hellerich U, Assenmacher J, et al. Titanium deposition in regional lymph nodes after insertion of titanium screw implants in maxillofacial region. Int J Oral Maxillofac Surg. 1994;23:450–452. doi: 10.1016/s0901-5027(05)80045-1. [DOI] [PubMed] [Google Scholar]
- 8.Delgado-Ruiz RA, Calvo-Guirado JL, Abboud M, Ramirez-Fernandez MP, Mate-Sanchez JE, Negri B, et al. Histologic and Histomorphometric Behavior of Microgrooved Zirconia Dental Implants with Immediate Loading. Clin Implant Dent Relat Res. 2013 doi: 10.1111/cid.12069. [DOI] [PubMed] [Google Scholar]
- 9.Shon WJ, Chung SH, Kim HK, Han GJ, Cho BH, Park YS. Peri-implant bone formation of non-thermal atmospheric pressure plasma-treated zirconia implants with different surface roughness in rabbit tibiae. Clin Oral Implants Res. 2014;25:573–579. doi: 10.1111/clr.12115. [DOI] [PubMed] [Google Scholar]
- 10.Oliva J, Oliva X, Oliva JD. One-year follow-up of first consecutive 100 zirconia dental implants in humans: a comparison of 2 different rough surfaces. Int J Oral Maxillofac Implants. 2007;22:430–435. [PubMed] [Google Scholar]
- 11.Kan JY, Rungcharassaeng K, Lozada JL, Zimmerman G. Facial gingival tissue stability following immediate placement and provisionalization of maxillary anterior single implants: a 2- to 8-year follow-up. Int J Oral Maxillofac Implants. 2011;26:179–187. [PubMed] [Google Scholar]
- 12.Schliephake H, Hefti T, Schlottig F, Gedet P, Staedt H. Mechanical anchorage and peri-implant bone formation of surface-modified zirconia in minipigs. J Clin Periodontol. 2010;37:818–828. doi: 10.1111/j.1600-051X.2010.01549.x. [DOI] [PubMed] [Google Scholar]
- 13.Shin D, Blanchard SB, Ito M, Chu TM. Peripheral quantitative computer tomographic, histomorphometric, and removal torque analyses of two different non-coated implants in a rabbit model. Clin Oral Implants Res. 2011;22:242–250. doi: 10.1111/j.1600-0501.2010.01980.x. [DOI] [PubMed] [Google Scholar]
- 14.Kohal RJ, Finke HC, Klaus G. Stability of prototype two-piece zirconia and titanium implants after artificial aging: an in vitro pilot study. Clin Implant Dent Relat Res. 2009;11:323–329. doi: 10.1111/j.1708-8208.2008.00116.x. [DOI] [PubMed] [Google Scholar]
- 15.Piconi C, Maccauro G. Zirconia as a ceramic biomaterial. Biomaterials. 1999;20:1–25. doi: 10.1016/s0142-9612(98)00010-6. [DOI] [PubMed] [Google Scholar]
- 16.Rimondini L, Cerroni L, Carrassi A, Torricelli P. Bacterial colonization of zirconia ceramic surfaces: an in vitro and in vivo study. Int J Oral Maxillofac Implants. 2002;17:793–798. [PubMed] [Google Scholar]
- 17.Kohal RJ, Knauf M, Larsson B, Sahlin H, Butz F. One-piece zirconia oral implants: one-year results from a prospective cohort study. 1. Single tooth replacement. J Clin Periodontol. 2012;39:590–597. doi: 10.1111/j.1600-051X.2012.01876.x. [DOI] [PubMed] [Google Scholar]
- 18.Mai R, Kunert-Keil C, Grafe A, Gedrange T, Lauer G, Dominiak M, et al. Histological behaviour of zirconia implants: an experiment in rats. Ann Anat. 2012;194:561–566. doi: 10.1016/j.aanat.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Kohal RJ, Patzelt SB, Butz F, Sahlin H. One-piece zirconia oral implants: one-year results from a prospective case series. 2. Three-unit fixed dental prosthesis (FDP) reconstruction. J Clin Periodontol. 2013;40:553–562. doi: 10.1111/jcpe.12093. [DOI] [PubMed] [Google Scholar]
- 20.Oliva J, Oliva X, Oliva JD. Zirconia implants and all-ceramic restorations for the esthetic replacement of the maxillary central incisors. Eur J Esthet Dent. 2008;3:174–185. [PubMed] [Google Scholar]
- 21.Oliva J, Oliva X, Oliva JD. Five-year success rate of 831 consecutively placed Zirconia dental implants in humans: a comparison of three different rough surfaces. Int J Oral Maxillofac Implants. 2010;25:336–344. [PubMed] [Google Scholar]
- 22.Kohal RJ, Klaus G. A zirconia implant-crown system: a case report. Int J Periodontics Restorative Dent. 2004;24:147–153. [PubMed] [Google Scholar]
- 23.Akagawa Y, Ichikawa Y, Nikai H, Tsuru H. Interface histology of unloaded and early loaded partially stabilized zirconia endosseous implant in initial bone healing. J Prosthet Dent. 1993;69:599–604. doi: 10.1016/0022-3913(93)90289-z. [DOI] [PubMed] [Google Scholar]
- 24.Sennerby L, Dasmah A, Larsson B, Iverhed M. Bone tissue responses to surface-modified zirconia implants: A histomorphometric and removal torque study in the rabbit. Clin Implant Dent Relat Res. 2005;7(Suppl1):S13–20. doi: 10.1111/j.1708-8208.2005.tb00070.x. [DOI] [PubMed] [Google Scholar]
- 25.Scarano A, Di Carlo F, Quaranta M, Piattelli A. Bone response to zirconia ceramic implants: an experimental study in rabbits. J Oral Implantol. 2003;29:8–12. doi: 10.1563/1548-1336(2003)029<0008:BRTZCI>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 26.Saulacic N, Erdosi R, Bosshardt DD, Gruber R, Buser D. Acid and alkaline etching of sandblasted zirconia implants: a histomorphometric study in miniature pigs. Clin Implant Dent Relat Res. 2014;16:313–322. doi: 10.1111/cid.12070. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Wang K, Liu G, Wang D. Fracture resistance of inter-joined zirconia abutment of dental implant system with injection molding technique. Clin Oral Implants Res. 2012 doi: 10.1111/j.1600-0501.2012.02539.x. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Xie ZP, Li JB, Huang Y. Progress of ceramic injection molding. Rare Metal Materials and Engineering. 2004;33:1121–1126. [Google Scholar]
- 29.Lin SIE. Near-net-shape forming of zirconia optical sleeves by ceramics injection molding. Ceramics International. 2001;27:205–214. [Google Scholar]
- 30.Park YS, Chung SH, Shon WJ. Peri-implant bone formation and surface characteristics of rough surface zirconia implants manufactured by powder injection molding technique in rabbit tibiae. Clin Oral Implants Res. 2013;24:586–591. doi: 10.1111/j.1600-0501.2012.02468.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee SY. Sintering behavior and mechanical properties of injection-molded zirconia powder. Ceramics International. 2004;30:579–584. [Google Scholar]
- 32.Song JH, Evans JRG. The Injection-Molding of Fine and Ultra-Fine Zirconia Powders. Ceramics International. 1995;21:325–333. [Google Scholar]
- 33.Liu DM, Tseng WJ. Influence of debinding rate, solid loading and binder formulation on the green microstructure and sintering behaviour of ceramic injection mouldings. Ceramics International. 1998;24:471–481. [Google Scholar]
- 34.Wang G, Luo G, Soo YL, Sabirianov RF, Lin HJ, Mei WN, et al. Phase stabilization in nitrogen-implanted nanocrystalline cubic zirconia. Phys Chem Chem Phys. 2011;13:19517–19525. doi: 10.1039/c1cp22132a. [DOI] [PubMed] [Google Scholar]
- 35.Kohal RJ, Weng D, Bachle M, Strub JR. Loaded custom-made zirconia and titanium implants show similar osseointegration: an animal experiment. J Periodontol. 2004;75:1262–1268. doi: 10.1902/jop.2004.75.9.1262. [DOI] [PubMed] [Google Scholar]
- 36.Gahlert M, Gudehus T, Eichhorn S, Steinhauser E, Kniha H, Erhardt W. Biomechanical and histomorphometric comparison between zirconia implants with varying surface textures and a titanium implant in the maxilla of miniature pigs. Clinical Oral Implants Research. 2007;18:662–668. doi: 10.1111/j.1600-0501.2007.01401.x. [DOI] [PubMed] [Google Scholar]
- 37.Lee J, Sieweke JH, Rodriguez NA, Schupbach P, Lindstrom H, Susin C, et al. Evaluation of nano-technology-modified zirconia oral implants: a study in rabbits. J Clin Periodontol. 2009;36:610–617. doi: 10.1111/j.1600-051X.2009.01423.x. [DOI] [PubMed] [Google Scholar]
- 38.Sollazzo V, Pezzetti F, Scarano A, Piattelli A, Bignozzi CA, Massari L, et al. Zirconium oxide coating improves implant osseointegration in vivo. Dent Mater. 2008;24:357–361. doi: 10.1016/j.dental.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Gahlert M, Roehling S, Sprecher CM, Kniha H, Milz S, Bormann K. In vivo performance of zirconia and titanium implants: a histomorphometric study in mini pig maxillae. Clin Oral Implants Res. 2012;23:281–286. doi: 10.1111/j.1600-0501.2011.02157.x. [DOI] [PubMed] [Google Scholar]
- 40.Aboushelib MN, Salem NA, Taleb AL, El Moniem NM. Influence of surface nano-roughness on osseointegration of zirconia implants in rabbit femur heads using selective infiltration etching technique. J Oral Implantol. 2013;39:583–590. doi: 10.1563/AAID-JOI-D-11-00075. [DOI] [PubMed] [Google Scholar]
- 41.Att W, Takeuchi M, Suzuki T, Kubo K, Anpo M, Ogawa T. Enhanced osteoblast function on ultraviolet light-treated zirconia. Biomaterials. 2009;30:1273–1280. doi: 10.1016/j.biomaterials.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 42.Kohal RJ, Klaus G, Strub JR. Zirconia-implant-supported all-ceramic crowns withstand long-term load: a pilot investigation. Clin Oral Implants Res. 2006;17:565–571. doi: 10.1111/j.1600-0501.2006.01252.x. [DOI] [PubMed] [Google Scholar]
- 43.Zinelis S, Thomas A, Syres K, Silikas N, Eliades G. Surface characterization of zirconia dental implants. Dent Mater. 2010;26:295–305. doi: 10.1016/j.dental.2009.11.079. [DOI] [PubMed] [Google Scholar]
- 44.Park YS, Yi KY, Lee IS, Han CH, Jung YC. The effects of ion beam-assisted deposition of hydroxyapatite on the grit-blasted surface of endosseous implants in rabbit tibiae. Int J Oral Maxillofac Implants. 2005;20:31–38. [PubMed] [Google Scholar]
- 45.Chung SH, Kim HK, Shon WJ, Park YS. Peri-implant bone formations around (Ti,Zr)O(2) -coated zirconia implants with different surface roughness. J Clin Periodontol. 2013;40:404–411. doi: 10.1111/jcpe.12073. [DOI] [PubMed] [Google Scholar]
- 46.Wennerberg A, Albrektsson T. Effects of titanium surface topography on bone integration: a systematic review. Clin Oral Implants Res. 2009;20(Suppl4):172–184. doi: 10.1111/j.1600-0501.2009.01775.x. [DOI] [PubMed] [Google Scholar]