Abstract
Background
In 2014 a national campaign was launched to increase colorectal cancer (CRC) screening rates in the U.S. to 80% by 2018; it is unknown if there is sufficient colonoscopy capacity to reach this goal. We estimate the number of colonoscopies needed to screen 80% of the eligible population with fecal immunochemical testing (FIT) or colonoscopy, and if there is sufficient colonoscopy capacity to meet the need.
Methods
The Microsimulation Screening Analysis-colon (MISCAN-colon) model was used to simulate CRC screening test use in the U.S. (2014–2040), assuming the implementation of a national screening program in 2014 with FIT or colonoscopy with 80% participation. The 2012 Survey of Endoscopic Capacity (SECAP) estimated the number of colonoscopies that were performed and the number that could be performed.
Results
If a national screening program started in 2014, by 2024, approximately 47 million FITs and 5.1 million colonoscopies would be needed annually to screen the eligible population with a program using FIT as the primary screening test; approximately 11 to 13 million colonoscopies would be needed annually to screen the eligible population with a colonoscopy only screening program. Based on the SECAP survey, an estimated 15 million colonoscopies were performed in 2012 and an additional 10.5 million colonoscopies could be performed.
Conclusions
The estimated colonoscopy capacity is sufficient to screen 80% of the eligible U.S. population with FIT, colonoscopy, or a mix of tests. Future analyses should take into account the geographic distribution of colonoscopy capacity.
Keywords: colorectal cancer screening, colonoscopy, capacity
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer death among cancers that affect both men and women.1 Although screening for colorectal cancer has been shown to effectively reduce the incidence of and mortality from the disease, only 58% of adults aged 50–75 years were up-to-date with CRC screening in 2013.2 A recent initiative from the National Colorectal Cancer Roundtable (NCCRT), a coalition of public, private, and voluntary organizations, aims to increase CRC screening prevalence to 80% in the eligible population by 2018. Recent analyses estimated that reaching this goal would avert 280,000 new cases of and 200,000 deaths from CRC by 2030 and that 24.4 million people would need to be screened.3,4 No studies have estimated the number of CRC screening tests that would need to be performed each year if 80% prevalence is achieved, and whether current colonoscopy capacity would meet increased demand. Over the past decade, colonoscopy use has increased rapidly and has become the most commonly used test to screen for CRC, while relative use of fecal occult blood testing has declined.
We used microsimulation modeling to estimate the expected number of colonoscopies to screen 80% of the eligible population with either fecal immunochemical tests (FIT) or colonoscopy over 10 years. We also conducted a national Survey of Endoscopic Capacity (SECAP) to estimate the number of colonoscopies performed in a year in the U.S., and the number of additional colonoscopies that could be performed (capacity). Resources, or capacity, are defined as non-monetary resources, such as number of staff, facility space, equipment and time needed to perform colonoscopies, and does not include the actual cost of the procedures paid for by individuals or insurers.
Methods
Estimation of Screening Test Need
The Microsimulation Screening Analysis-Colon (MISCAN-colon) model was used to simulate CRC screening test use in the U.S. (from 2014 to 2040), assuming the implementation of a nationwide screening program in 2014. The main outcome of the model was the number of colonoscopies required per year to screen 80% of the population. Screening was implemented over 10 years using FIT or colonoscopy as the primary screening test.
The model has been used previously to inform U.S. Preventive Services Task Force guideline recommendations and has been described in detail elsewhere.5,6 In brief, the model simulates a large population of individuals from birth to death. CRC is assumed to arise in this population according to the adenoma-carcinoma sequence, in which every cancer is preceded by an adenoma.7 The risk for developing one or more adenomas depends on age, sex, and baseline individual risk. Adenomas can progress from small (1 to 5 mm) to medium (6 to 9 mm) to large (≥10 mm). Most adenomas are assumed to be non-progressive and will never develop into cancer. The progressive adenomas have the ability to become malignant and transform into stage I cancers. These cancers may successively progress to stage II, III, and IV until they are diagnosed in one of these stages. After diagnosis, the individual may or may not die from CRC depending on the stage-specific survival and competing causes of death. With screening, underlying lesions (adenomas and pre-clinical cancers) may be detected, depending on the sensitivity of the test for that lesion and, for endoscopic tests, whether the lesion is within reach of the endoscope.
The current and future age distribution and size of the population were based on the 2008 population estimates of the U.S. Census Bureau.8 The simulated population size was equal to one tenth of the actual United States population size. This size was chosen to limit computation time while also avoiding significant random outcome variation. The lifetime risk of CRC in the unscreened population was estimated to be approximately 6.5%. Natural history of the adenoma-carcinoma sequence was calibrated to adenoma prevalence data9–13 and CRC incidence data from the Surveillance, Epidemiology and End Results (SEER) program from 1975 to 1979 when incidence rates and adenoma prevalence had not yet been affected by screening.14 To align the modeled CRC incidence in 2012 (given observed screening patterns from 1978 forward) with 2012 SEER estimates, the adenoma onset rate was increased by 10% in isolation from other natural history parameters. Stage-specific CRC survival was based on 2000–2010 SEER data. Mortality rates from other causes were imputed from U.S. life tables.15 The inputs for sensitivity and specificity of CRC screening tests were based on previous reviews of the literature and have been published previously.5
Simulated Scenarios
Age-specific use rates of colonoscopy, flexible sigmoidoscopy, and guaiac fecal occult blood test (FOBT) until the start of a hypothetical national screening program in 2014 were based on National Health Interview Survey (NHIS) data from 1987 through 2010.2 Based on these data, it was estimated that in 2013, 67% of U.S. adults aged ≥50 years had ever been screened with any test, 8.8% had a home FOBT within the last year, 4% had a sigmoidoscopy within the last 5 years, and 55% had a colonoscopy within the past 10 years. We assumed that there was no further increases in overall screening uptake in the period from the last NHIS to the start of the hypothetical screening program.
The model enrolled all U.S. adults aged 50 to 75 years into a national screening program over 10 years, starting with the first cohort in 2014, consisting of 1/10 of the age-eligible population. The model assumed that the remaining eligible population would continue to be screened at a projected estimate based on 2010 NHIS data until enrollment into the hypothetical national program. People were not invited for screening in the program until 1 year after their last FOBT, 5 years after their most recent sigmoidoscopy, or 10 years after their most recent colonoscopy. In the first scenario, we evaluated a program of annual FIT in which 80% of eligible adults participated; in the second scenario, we evaluated a program colonoscopy every 10 years with 80% participation. People with a positive FIT were referred for follow-up colonoscopy and people with an adenoma detected were followed with colonoscopy surveillance, with the interval (3 to 5 years) dependent on the number and size of adenomas detected on the most recent colonoscopy.16,17
Sensitivity analysis
In a sensitivity analysis we evaluated various alternative modeling scenarios, to inform readers on implications of other possible screening tests and adherence rates. Alternative modeling scenarios included: alternative primary screening tests, including annual guaiac fecal occult blood testing (FOBT), 10-yearly computed tomographic colonography (CTC), and 5-yearly flexible sigmoidoscopy (FSIG); higher assumed participation rates (100%) for FIT and colonoscopy screening; and a scenario of currently observed test use patterns in NHIS (with both under- and over-use), with an assumed linear increase in overall screening participation rates from 58% in 2013 to 80% by 2018. Test performance characteristics used in the primary and sensitivity analysis are provided in Supporting Information, Table 1.
Estimation of Endoscopic Capacity
The Survey of Endoscopic Capacity II (SECAP II) was conducted in 2012. The survey methodology was unchanged from the original SECAP study; a detailed description of the survey methodology has been published previously.18 In brief, a list of all U.S. medical facilities known to have purchased or leased lower endoscopic equipment between January 1, 2006 and December 31, 2010 was obtained from three major endoscopic equipment manufacturers: Olympus America, Inc., Fujinon, Inc., and Pentax Precision Instrument Corporation. The lists were merged and duplicates removed to create a sampling frame. A random sample of 2100 facilities (31% of all facilities), stratified by region and location (urban or rural), was selected to participate in the survey. A telephone screening questionnaire was administered to confirm study eligibility and to identify the person in charge of endoscopy. Of the 2100 facilities, 258 (12%) were found to be ineligible (did not currently perform screening sigmoidoscopy or colonoscopy on adults or could not be located). A self-administered questionnaire, personalized cover letter, postage-paid return envelope, and $40.00 incentive were sent by Federal Express to a person identified by each eligible facility. Respondents were asked to provide an estimate of the total number of sigmoidoscopies and colonoscopies performed by all endoscopists at the practice site per week, the percentage of procedures performed by endoscopist specialty, and the additional number of sigmoidoscopies and colonoscopies that could be performed with no other investment of resources.
Of the 1842 eligible facilities, 1269 returned valid surveys (overall response rate 68.9%). To provide national capacity estimates, the universe of facilities was adjusted based on the ineligibility rate, and survey data were weighted to adjust for the sampling weight and non-response. Annual estimates of capacity were obtained by multiplying the weighted weekly estimates of current and potential capacity by the number of workweeks per year (50 weeks). Survey data were analyzed with Stata 12.1.
For the estimation of endoscopic capacity, two questions were critical to the analysis: 1) the number of procedures currently performed and 2) the additional number of procedures that could be performed. If answers to both of these questions were missing, the survey was excluded from analysis. If the survey was missing data for one of the two key question, then these values were imputed using a variation of the hot-deck method, as described previously.18
RESULTS
Simulation Results
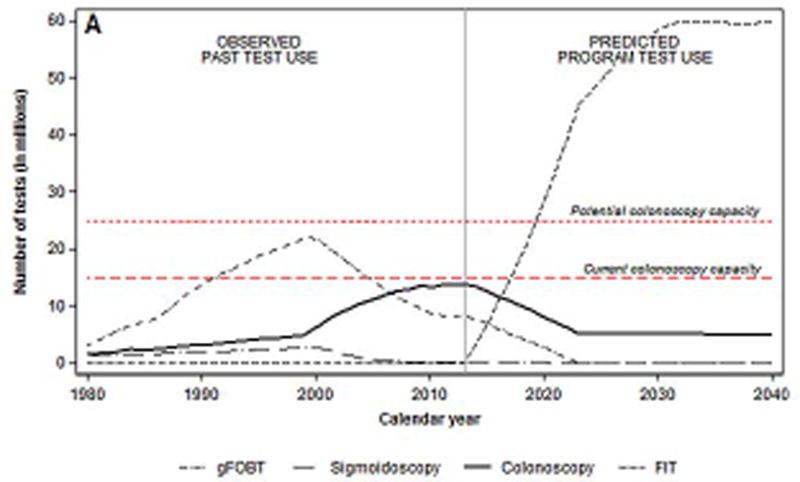
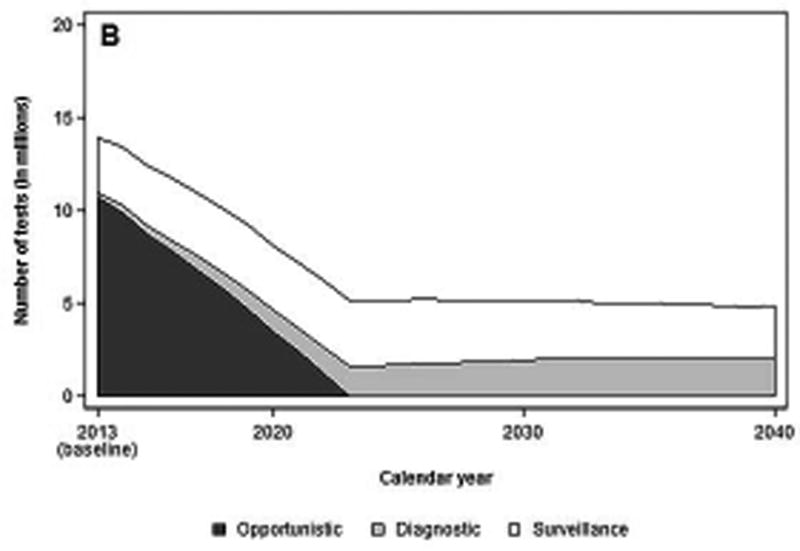
Based on recent CRC screening patterns, an estimated 8.4 million FOBTs and 14 million colonoscopies were performed in 2013. Of these, approximately 3.3 million colonoscopies were estimated to have been performed for diagnostic and surveillance purposes (Figure 1a).
Figure 1.
a. Number of colorectal cancer tests per year before and after start of hypothetical national screening program with FIT* in 2014, by test type
*Fecal immunochemical test
† Fecal occult blood test
b. Number of colonoscopies per year, before and after start of a hypothetical national screening program with FIT, by colonoscopy indication
FIT Scenario
Assuming the introduction of a FIT screening program in 2014, a total of 3.3 million FITs would need to be performed to screen 80% of eligible adults aged 50 to 75 invited to the first round of screening (1/10 of the eligible population). The total number of colonoscopies needed in 2014 would be 13.4 million: 3.5 million for diagnostic or surveillance purposes, and 9.9 million for screening performed outside the program (Figure 1b). By 2024, approximately 47 million FITs and 5.1 million diagnostic (32%) and surveillance (68%) colonoscopies would have to be performed. The number of FITs would gradually increase to approximately 60 million tests annually by 2030, but the number of colonoscopies would remain steady.
Colonoscopy Scenario
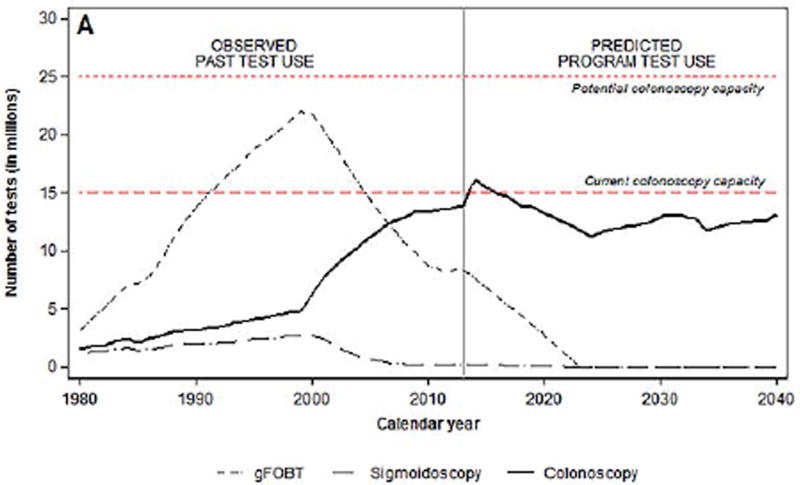
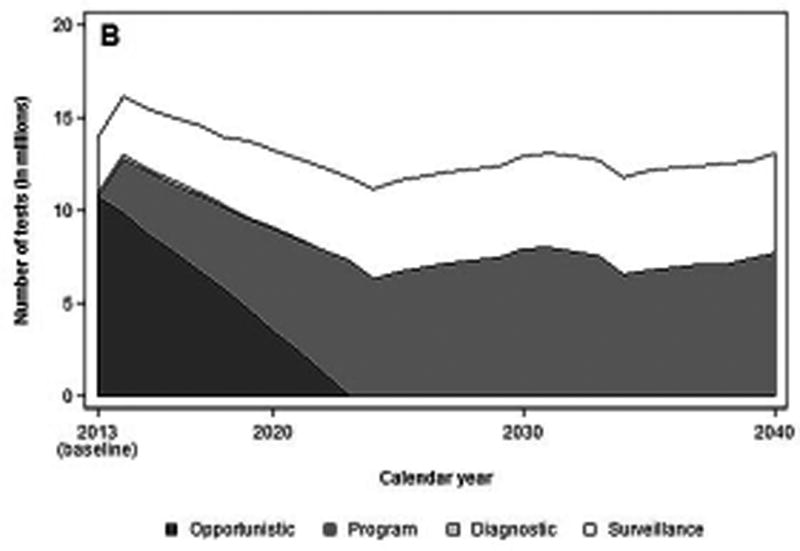
The introduction of a colonoscopy screening program in 2014 would require 12.8 million screening colonoscopies and 3.4 million diagnostic and surveillance colonoscopies (Figures 2a and 2b). By 2024, 11 to 13 million colonoscopies would have to be performed annually, with ~57% being performed for screening and~43% for surveillance, and remain level through 2030.
Figure 2.
a. Number of colorectal cancer tests per year before and after start of a hypothetical national screening program with colonoscopy, by test type
*Fecal immunochemical test
† Fecal occult blood test
b. Number of colonoscopies per year, before and after start of a hypothetical national screening program with colonoscopy, by colonoscopy indication
Sensitivity analysis
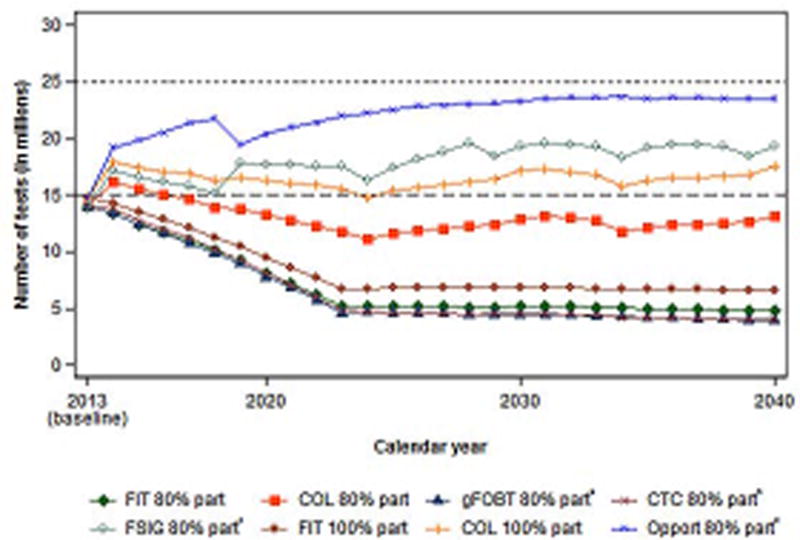
Estimated colonoscopy requirements assuming 80% participation of all eligible adults were similar for annual FIT, annual gFOBT, and 5-yearly CTC testing (Figure 3). FSIG every 5 years would require 16.3 colonoscopies in 2014, and 18–19 million sigmoidoscopies and colonoscopies annually by 2030. Continuation of currently observed test use patterns with 80% participation would require 23 million colonoscopies annually by 2030.
Figure 3.
Predicted colonoscopy use under various modeling scenarios (in millions).
FIT = Fecal Immunochemical Test; COL = Colonoscopy; gFOBT = guaiac Fecal Occult Blood Test; FSIG = Flexible Sigmoidoscopy; Opport. = Opportunistic; part = participation.
* Annual testing with Hemoccult II.
†10-yearly testing with CT colonography.
‡ 5-yearly testing with flexible sigmoidoscopy. Numbers represent sigmoidoscopy and colonoscopy use.
§ In the scenario with opportunistic screening, we assumed future screening patterns according to age and type of test were similar to those observed in 2013 National Health Interview survey data. The screen rate was increased linearly from approximately 60% to 80% from 2013 to late 2018.
The required colonoscopy capacity with either FIT or colonoscopy screening with 100% participation was approximately one-third higher than the capacity needed for the base-case of 80% assumed participation. The 100% FIT scenario would require 14.3 million colonoscopies in 2014, and would require 68 million FITs and 6.9 million colonoscopies annually by 2030. The 100% colonoscopy scenario would require 14.1 million colonoscopies in 2014 and 17 million colonoscopies annually by 2030.
Survey
Of the 1269 facilities included in the final analysis, 767 (60.9%) were hospital departments, 403 (31.8%) were ambulatory endoscopy or surgery centers, 98 (7.7%) were physician practices and 1 was unknown (data not shown). The majority of survey respondents identified themselves as nurse administrators/managers (60.2%). The majority of sites were classified as urban (68.2%). After weighting, there were an estimated 5988 (95% confidence intervals [CI] 5832 to 6144) facilities in the U.S. that performed any lower endoscopy in 2012. Of these, 5858 (97.8%) facilities reported performing colonoscopy and 1831 (30.6%) reported performing sigmoidoscopy.
Survey respondents estimated that 51.1 (95% CI 46.1 to 56.1) colonoscopies were performed per week (Table 1). Respondents estimated that 43.2% of colonoscopies were performed for screening. The total mean potential maximum number of colonoscopies that could be performed per week was 87.
Table 1.
Current and potential number of colonoscopies, Survey of Endoscopic Capacity II - 2012
| Total (SE)* |
|
|---|---|
| Number of facilities† | 5858 |
| (202.6) | |
| Current weekly number | 51.1 |
| (mean) | (2.5) |
| Percent performed for screening | 43.2 |
| (0.6) | |
| Potential Maximum weekly number | 87.0 |
| (mean) | (5.4) |
| Current annual‡ volume | 15.0 |
| (1.2) | |
| Potential annual‡ volume | 25.5 |
| (2.4) | |
| Available annual‡ capacity | 10.5 |
| (2.6) |
SE = Standard error
Facilities included hospitals, ambulatory surgery centers, and physician offices where colonoscopies were performed for the purpose of colorectal cancer screening of adults.
Assuming 50 work weeks per year
Survey responses were weighted to determine national estimates for current and potential capacity to provide colonoscopies in the U.S. In 2012, approximately 15 million total colonoscopies were performed (Table 1). Respondents reported they could increase their colonoscopy volume to 25.5 million annually for an available capacity of 10.5 million colonoscopies annually.
DISCUSSION
This report estimates the number of colonoscopies that would be needed to screen 80% of the eligible population and compares this need to estimates of colonoscopy capacity. The MISCAN microsimulation model estimated that 13.4 million colonoscopies would be needed in the first year of a population CRC screening program with FIT, gradually declining to 5.2 million colonoscopies with full implementation of the program after 10 years. A colonoscopy program implemented over 10 years would require 16.2 million colonoscopies in the first year, and 12 to 13 million colonoscopies annually with full implementation. According to the survey, in 2012, 15 million colonoscopies were performed, of which respondents estimated that 42.3% (6.3 million) were performed for screening. Respondents indicated that an additional 10.5 million colonoscopies could be performed per year, suggesting that the increased demand for screening colonoscopy could be absorbed. The FIT screening program would require no screening colonoscopies, and the demand for diagnostic and surveillance colonoscopies could presumably be met by shifting currently available resources. The colonoscopy screening program would require approximately 7 million screening colonoscopies and 5 million surveillance colonoscopies annually. Assuming no change in available capacity, the increased demand for screening and surveillance colonoscopies is matched by currently available colonoscopy capacity as reported. Given that a colonoscopy screening program is, for a given participation level, the strategy with the highest colonoscopy demand, there would also be sufficient capacity to meet colonoscopy demand for most of the scenarios modeled in the sensitivity analysis (FIT only or colonoscopy only with 100% participation, and annual gFOBT, 5-yearly CTC or FSIG with 80% participation). If recently observed CRC test use patterns continued with 80% participation, estimated capacity could meet colonoscopy need within the estimated standard error (22–24 million needed annually vs. 23.1–27.9 estimated annual capacity).
The percentage of the adult population that is up-to-date with CRC screening has steadily increased over the past decade, primarily through increased use of colonoscopy.19–21 Our data do not show a concomitant increase in the number of colonoscopies performed annually. Although the SECAP survey is cross-sectional, and may not have captured a true rise and subsequent decline in the number of colonoscopies performed, at least one other study found that the use of screening colonoscopy increased prior to the recent economic recession, then subsequently declined.22 After rapid growth from 2000 to 2006, a decline in the number of colonoscopies performed per Medicare beneficiary has also been noted.23
The MISCAN microsimulation model estimated that approximately 13.7 million colonoscopies were performed in 2012 for screening and follow-up; analysis of the 2012 Behavioral Risk Factor Surveillance System (BRFSS)24 and of the 2010 NHIS2 estimated that 14.9 million people and 11.4 million people respectively had a colonoscopy within the past year. The 2012 SECAP estimate of 15 million colonoscopies performed closely matches these estimates. Of note, the number of adults aged 50 years or older that reported sigmoidoscopy or colonoscopy within the previous year remained largely unchanged from the 2003 (11.3 million) to the 2010 (11.8 million) NHIS, despite a substantial increase in the proportion of adults in this age group that reported being up-to-date with CRC screening by colonoscopy within 10 years.2
In the base-case analysis, future test use for a national CRC screening program with either FIT or colonoscopy estimated that 5 to 16 million colonoscopies would be needed annually, assuming 80% of the eligible population would participate. These model projections included only colonoscopies performed for screening, diagnostic, or surveillance purposes and assumed no under- or overuse of screening and therefore may have under-estimated actual test need in these scenarios. The 2012 SECAP survey estimated that 43.2% of colonoscopies were performed for screening purposes consistent with previous estimates of 38% to 49.7%.22,25 Surveillance colonoscopies have accounted for up to one-quarter of colonoscopies performed, suggesting that a substantial proportion of colonoscopies performed are for reasons other than screening or surveillance (i.e., diagnostic).25,26 Several studies of the Medicare population have found over- and under-use of both screening and surveillance colonoscopies.27–33 In our sensitivity analysis, continuation of current CRC test use patterns, reflecting current patterns of under- and overuse, required substantially more colonoscopies than even the colonoscopy only scenario. The modeled colonoscopy need may also have been overestimated as we assumed 80% adherence to screening and surveillance. Despite concerted efforts to increase CRC screening rates in the population, rates remain well below 80% due to a variety of patient, provider, and system level factors.20,21,35 Among those who are screened for CRC with FIT or other tests that require follow-up with colonoscopy, adherence to follow-up is well below 100%.35,36
Full implementation of a national CRC screening program with FIT would require approximately 5 million diagnostic and surveillance colonoscopies annually. While the number of colonoscopies required is practically achievable, a national FIT program would also require nearly 60 million FITs annually by 2040. FIT does not require many resources on the part of the patient (can be done at home, does not require bowel preparation or dietary changes), but a complete FIT screening program can require substantial additional resources to ensure that test kits are distributed to the eligible population, remind people to complete and return the kits, ensure complete follow-up for those with positive results, process all returned kits in the provider’s office or in the lab, and to ensure that all eligible adults repeat the test annually.37 It is unknown if adequate resources exist to implement a FIT screening program on such a large scale. Our model estimates that a national colonoscopy screening program would require substantially more colonoscopies annually than a FIT program. It is unknown if it is feasible to shift resources towards more screening and surveillance and if sufficient capacity would remain to perform colonoscopies needed for other reasons.
This study is subject to some other limitations. First, our model estimates of future test need for a national CRC screening program were based on recent population projections, currently available screening methods, and current screening guidelines which may not apply to the entire time horizon of the study. Second, we could not validate directly the number of colonoscopies that survey respondents indicated they were performing or could perform. Respondents were asked to estimate the number of additional colonoscopies they could do without additional resources, but it is unknown if the estimate truly reflects what could be done without changes to current practice or if it reflects shifting resources away from other procedures. Analysis of additional SECAP questions indicated that there were limiting factors to increasing capacity and, if needed, facilities would invest in additional resources (physicians, nurses, equipment, etc.) to increase capacity (Supporting Information, Tables 2 and 3). As described earlier, our estimate of annual colonoscopy volume was consistent with estimates from other sources.2,24 Third, the survey sampling frame included facilities that purchased or leased equipment between 2006 and 2010. This excludes facilities that use equipment purchased or leased outside of this time frame and may underestimate the number of colonoscopies currently performed and available capacity. Fourth, the study was not designed to model market forces as it relates to the supply of colonoscopy in response to increasing demand (e.g., the market could respond by increasing the supply of endoscopists). Fifth, this study could not account for the geographic distribution of CRC screening need or of colonoscopy capacity. The survey was not designed to estimate colonoscopy capacity at the local level, and simulating future screening need at this level would require an impractical number of models (to account for population size and past screening behavior for each geographical unit).
CRC screening is conducted with a variety of tests, most commonly colonoscopy and less frequently with FOBT or FIT. While it is unlikely that all eligible adults will be screened with a single test type, this analysis shows that the estimated colonoscopy capacity would be sufficient to screen with a mix of tests. Future analyses should take into account the geographic distribution of colonoscopy capacity and screening need, to determine if there is a surplus of capacity in some areas of the country and insufficient capacity in others.
Supplementary Material
Acknowledgments
Funding Source: This research was supported by the CDC. The funding source had a role in the design, conduct, and reporting of the study. This study was approved by the CDC Institutional Review Board for Protection of Human Subjects and the U.S. Office of Management and Budget under the Paperwork Reduction Act. MISCAN-Colon is part of the Cancer Intervention and Surveillance Modeling Network (CISNET; http://cisnet.cancer.gov) sponsored by the U.S. National Cancer Institute.
Grant info : P30 CA008748 - Cancer Center Support Grant; Behavioral Research; Bioinfomatics; Biostatistic; CTRP Supplement; DEVELOPMENTAL FUNDS; Developmental Funds; Genomics; Microchemistry & Proteomics; PLANNING and EVALUATION; Planning & Evaluation; Web Survey Core.
Footnotes
Conflict of Interest: There are no conflict of interest disclosures from any author.
Author Contributions: Joseph DA – writing original draft/investigation; Meester RGS – writing original draft/investigation/data curation; Zauber AG – writing original draft/investigation; Manninen DL – writing original draft/investigation; Winges L – writing original draft/investigation; Dong FB – data curation/review and editing; Peaker B – review and editing/investigation; van Ballegooijen M – investigation/data curation/review and editing
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.U.S. Preventive Service Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–37. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 2.National Center for Health Statistics. Data file documentation, National Health Interview Survey, 2010 (machine readable data file and documentation) Hyattsville, MD: National Center for Health Statistics, Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 3.Meester RGS, Doubeni CA, Zauber AG, et al. Public health impact of achieving 80% colorectal cancer screening rates in the United States by 2018. Cancer. 2015;121:2281–5. doi: 10.1002/cncr.29336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fedewa SA, Ma J, Sauer AG, et al. How many individuals will need to be screened to increase colorectal cancer screening prevalence to 80% by 2018? Cancer. 2015;121:4258–65. doi: 10.1002/cncr.29659. [DOI] [PubMed] [Google Scholar]
- 5.Lansdorp-Vogelaar I, Kuntz KM, Knudsen AB, Wilschut JA, Zauber AG, van Ballegooijen Stool DNA testing to screen for colorectal cancer in the Medicare population. Ann Intern Med. 2010;153:368–77. doi: 10.1059/0003-4819-153-6-201009210-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cancer intervention and surveillance modeling network, colorectal cancer model profiles. [March 31, 2014];Sloan-Kettering Institute for Cancer Research (MISCAN-colon) 2008 Accessed at http://cisnet.cancer.gov/colorectal/profiles.html.on.
- 7.Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer. 1975;36:2251–70. doi: 10.1002/cncr.2820360944. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Census Bureau. US Population projections 2008. Accessed at http://www.census.gov/population/projections/ on [February xx 2014]
- 9.Disario JA, Foutch PG, Mai HD, Pardy K, Manne RK. Prevalence and malignant potential of colorectal polyps in asymptomatic, average-risk men. Am J Gastroenterol. 1991;86:941–45. [PubMed] [Google Scholar]
- 10.Johnson DA, Gurney MS, Volpe RJ, et al. A prospective study of the prevalence of colonic neoplasms in asymptomatic patients with and age-related risk. Am J Gastroenterol. 1990;85:969–74. [PubMed] [Google Scholar]
- 11.Koretz RL. Malignant polyps: are they sheep in wolves’ clothing? Ann Intern Med. 1993;118:63–68. doi: 10.7326/0003-4819-118-1-199301010-00011. [DOI] [PubMed] [Google Scholar]
- 12.Lieberman DA, Smith FW. Screening for colon malignancy with colonoscopy. Am J Gastroenterol. 1991;86:946–51. [PubMed] [Google Scholar]
- 13.Rex DK, Lehman GA, Hawes RH, Ulbright TM, Smith JJ. Screening colonoscopy in asymptomatic average-risk persons with negative fecal occult bclood tests. Gastroenterol. 1991;100:64–7. doi: 10.1016/0016-5085(91)90583-7. [DOI] [PubMed] [Google Scholar]
- 14.Surveillance, epidemiology and End Results (SEER) Program. Bethesda, MD: National Cancer Institute; Apr, 2004. SEER*Stat Database: Incidence-SEER 9 Regs Public Use. Nov 2003 Sub (1973–2001), DCCPS Surveillance Research Program, Cancer Statistics Branch. Based on the November 2003 submission. Accessed at http://seer.cancer.gov on [date] [Google Scholar]
- 15.The Berkeley Mortality Database: Data for the U.S. Accessed at http://www.demog.berkeley.edu/~bmd/states.html on February 012014.
- 16.Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the U.S. Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. CA Cancer J Clin. 2006;56:143–59. doi: 10.3322/canjclin.56.3.143. [DOI] [PubMed] [Google Scholar]
- 17.Lieberman DA, Rex DK, Winawer SJ, Giadiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterol. 2012;143:844–57. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Seeff LC, Richards TB, Shapiro JA, et al. How many endoscopies are performed for colorectal cancer screening? Results from CDC’s survey of endoscopic capacity. Gastroenterol. 2004;127:1670–77. doi: 10.1053/j.gastro.2004.09.051. [DOI] [PubMed] [Google Scholar]
- 19.Shapiro JA, Klabunde CN, Thompson TD, Nadel MR, Seeff LC, White A. Patterns of colorectal cancer test use, including CT colonography, in the 2010 National Health Interview Survey. Cancer Epidemiol Biomark Prev. 2012;21:895–904. doi: 10.1158/1055-9965.EPI-12-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klabunde CN, Cronin KA, Breen N, Waldron WR, Ambs AH, Nadel MR. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol Biomark Prev. 2011;20:1611–21. doi: 10.1158/1055-9965.EPI-11-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CDC. Colorectal cancer screening – United States, 2002, 2004, 2006, and 2008. MMWR. 2011;60:42–46. [PubMed] [Google Scholar]
- 22.Dorn SD, Wei D, Farley JF, et al. Impact of the 2008–2009 economic recession on screening colonoscopy utilization among the insured. Clin Gastroenterol Hepatol. 2012;10:278–84. doi: 10.1016/j.cgh.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koenig L, Gu Q. Growth of ambulatory surgery centers, surgery volume, and savings to medicare. Am J Gastroenterol. 2013;1–8:10–15. doi: 10.1038/ajg.2012.183. [DOI] [PubMed] [Google Scholar]
- 24.CDC. Behavioral Risk Factor Surveillance System Survey Data. Atlanta, Georgia: U.S. Department of Health and Human Services, CDC; 2012. [Google Scholar]
- 25.Lieberman DA, Holub J, Eisen G, Kraemer D, Morris CD. Utilization of colonoscopy in the United States: results from a national consortium. Gastrointest Endosc. 2005;62:875–83. doi: 10.1016/j.gie.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 26.Leiberman DA, de Garmo PL, Fleischer DE, Eisen GM, Helfand M. Patterns of endoscopy use in the United States. Gastroenterol. 2000;118:619–24. doi: 10.1016/s0016-5085(00)70269-1. [DOI] [PubMed] [Google Scholar]
- 27.Goodwin JS, Singh A, Reddy N, Riall TS, Kuo YF. Overuse of screening colonoscopy in the Medicare population. Arch Intern Med. 2011;171:1335–43. doi: 10.1001/archinternmed.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheffield KM, Han Y, Kuo YF, Riall TS, Goodwin JS. Potentially inappropriate screening colonoscopy in medicare patients. JAMA Intern Med. 2013;173:542–50. doi: 10.1001/jamainternmed.2013.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper GS, Kuo TD, Barnholtz-Slaon JS, Koroukian SM, Schluchter MD. Use of colonoscopy for polyp surveillance in Medicare beneficiaries. Cancer. 2013;119:1800–7. doi: 10.1002/cncr.27990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laiyemo AO, Pinsky PF, Marcus PM, et al. Utilization and yield of surveillance colonoscopy in the continued follow-up of the Polyp Prevention Trial. Clin Gastroenterol Hepatol. 2009;7:562–7. doi: 10.1016/j.cgh.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boolchand V, Olds G, Singh J, Singh P, Chak A, Cooper GS. Colorectal cancer screening after polypectomy: a national survey study of primary care physicians. Ann Intern Med. 2006;145:654–59. doi: 10.7326/0003-4819-145-9-200611070-00007. [DOI] [PubMed] [Google Scholar]
- 32.Schoen RE, Pinsky PF, Weissfeld JL, et al. Utilization of surveillance colonoscopy in community practice. Gastroenterol. 2010;138:71–81. doi: 10.1053/j.gastro.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mysliwiec PA, Brown ML, Klabunde CN, Ransohoff DF. Are physicians doing too much colonoscopy? A national survey of colorectal surveillance after polypectomy. Ann Intern Med. 2004;141:264–71. doi: 10.7326/0003-4819-141-4-200408170-00006. [DOI] [PubMed] [Google Scholar]
- 34.Holden D, Jonas DE, Porterfield DS, Reuland D, Harris R. Systematic review: enhancing the use and quality of colorectal cancer screening. Ann Intern Med. 2010;152:666–68. doi: 10.7326/0003-4819-152-10-201005180-00239. [DOI] [PubMed] [Google Scholar]
- 35.Seeff LC, Royalty J, Helsel WE, et al. Clinical outcomes from the CDC’s colorectal cancer screening demonstration program. Cancer. 2013;119(15 suppl):2820–33. doi: 10.1002/cncr.28163. [DOI] [PubMed] [Google Scholar]
- 36.Nadel MR, Royalty J, Shapiro JA, et al. Assessing screening quality in the CDC’s colorectal cancer screening demonstration program. Cancer. 2013;119(15 suppl):2834–41. doi: 10.1002/cncr.28164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Humphrey LL, Shannon J, Partin MR, O’Malley J, Chen Z, Helfand M. Improving the follow-up of positive hemoccult screening tests: an electronic intervention. J Gen Intern Med. 2011;26:691–7. doi: 10.1007/s11606-011-1639-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levin TR, Jamieson L, Burley DA, Reyes J, Oehrli M, Caldwell C. Organized colorectal cancer screening in integrated health care systems. Epidemiol Rev. 2011;33:101–10. doi: 10.1093/epirev/mxr007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.