The Alpha Thalassemia-mental Retardation X-linked homolog functions as a H3.3 chaperone and modulates gene expression in Arabidopsis.
Abstract
Histones are essential components of the nucleosome, the major chromatin subunit that structures linear DNA molecules and regulates access of other proteins to DNA. Specific histone chaperone complexes control the correct deposition of canonical histones and their variants to modulate nucleosome structure and stability. In this study, we characterize the Arabidopsis thaliana Alpha Thalassemia-mental Retardation X-linked (ATRX) ortholog and show that ATRX is involved in histone H3 deposition. Arabidopsis ATRX mutant alleles are viable, but show developmental defects and reduced fertility. Their combination with mutants of the histone H3.3 chaperone HIRA (Histone Regulator A) results in impaired plant survival, suggesting that HIRA and ATRX function in complementary histone deposition pathways. Indeed, ATRX loss of function alters cellular histone H3.3 pools and in consequence modulates the H3.1/H3.3 balance in the cell. H3.3 levels are affected especially at genes characterized by elevated H3.3 occupancy, including the 45S ribosomal DNA (45S rDNA) loci, where loss of ATRX results in altered expression of specific 45S rDNA sequence variants. At the genome-wide scale, our data indicate that ATRX modifies gene expression concomitantly to H3.3 deposition at a set of genes characterized both by elevated H3.3 occupancy and high expression. Together, our results show that ATRX is involved in H3.3 deposition and emphasize the role of histone chaperones in adjusting genome expression.
INTRODUCTION
Gene regulation in the eukaryotic genome requires a controlled balance between packaging the large linear DNA molecules and permitting regulated access to protein complexes involved in DNA transcription, replication, and repair. Within nucleosomes, the basic building blocks of chromatin, the double-stranded DNA helix wraps around octamers of histone proteins. Canonical histones are deposited on newly synthesized DNA to maintain nucleosomal density following passage of the replication fork. These canonical histones can then be replaced with specific histone variants to modify nucleosome composition, stability, higher-order chromatin organization, and DNA accessibility in a site-specific manner (Talbert and Henikoff, 2010). Most eukaryotes express variants of the canonical histone H3.1, such as the replacement variant H3.3, as well as tissue-specific H3 variants (Talbert et al., 2012). In mammals, H3.3 deposition is associated with dynamic chromatin regions such as transcriptionally active genes and regulatory regions, with high nucleosome turnover, and DNA accessibility. The plant model Arabidopsis thaliana also encodes H3.1 and H3.3 proteins that differ by only four amino acids, as well as H3.3-like variants expressed in specific reproductive tissues (Ingouff et al., 2010; Okada et al., 2005). Genome-wide studies revealed preferential enrichment of H3.1 at heterochromatic regions and of H3.3 at active genes, promoters, and telomeric repeats (Stroud et al., 2012; Wollmann et al., 2012; Vaquero-Sedas and Vega-Palas, 2013; Shu et al., 2014).
To ensure the incorporation of the adequate histone type at the right time and genomic location, specialized proteins called histone chaperones associate with histones during their shuttling from cytoplasm to nucleoplasm and deposit histones on DNA. These histone chaperones operate in a coordinated network. For the assembly of H3-H4 dimers into nucleosomes, several chaperone complexes have been characterized in eukaryotes. The CAF-1 (Chromatin Assembly Factor 1) complex deposits H3 in a DNA-synthesis-coupled manner during DNA replication and repair (Tagami et al., 2004; Smith and Stillman, 1989) and is composed of the subunits FASCIATA1 (FAS1), FAS2, and MULTICOPY SUPPRESSOR OF IRA1 in Arabidopsis (Kaya et al., 2001). The HIR complex incorporates H3 histones in a DNA synthesis-independent manner throughout the cell cycle or in resting cells. In Arabidopsis, this complex is conserved and composed of HISTONE REGULATOR A (HIRA), UBINUCLEIN 1/2 (UBN1/UBN2), and CALCINEURIN BINDING PROTEIN1 (CABIN1) (Nie et al., 2014; Duc et al., 2015). In mammals, these chaperone complexes show clear variant specificity, with CAF-1 depositing the canonical histone H3.1, while the HIR complex assembles H3.3 (Ricketts et al., 2015; Tagami et al., 2004). Little is known so far concerning such a specificity of the different complexes for H3 histone variants in plants, except that the Arabidopsis HIR complex binds H3.3 (Nie et al., 2014). In mammals, additional proteins that function as histone chaperones have been described, such as the DEK (Sawatsubashi et al., 2010) and the ATRX/DAXX (Alpha Thalassemia-mental Retardation X-linked syndrome/Death-domain Associated protein) heterocomplex, which shows specificity for H3.3 (Drané et al., 2010; Goldberg et al., 2010; Lewis et al., 2010; Wong et al., 2010).
ATRX/DAXX and HIR show differential chromatin binding patterns in mammals (He et al., 2015; Pchelintsev et al., 2013) and are known to deposit histone H3.3 at distinct genomic regions. Indeed, while HIRA deposits H3.3 at genic regions (Ray-Gallet et al., 2011; Pchelintsev et al., 2013; Goldberg et al., 2010), ATRX/DAXX incorporates this histone variant at pericentromeric repeats, telomeres, endogenous retroviral elements, and silenced imprinted alleles (Elsässer et al., 2015; Voon et al., 2015; Filipescu et al., 2013; Gokhman et al., 2013; Xue et al., 2003; McDowell et al., 1999; Goldberg et al., 2010; Lewis et al., 2010). ATRX has the capacity to bind lysine 9 (K9) methylated histone tails via its ATRX-DNMT3-DNMT3L (ADD) N-terminal domain (Iwase et al., 2011; Eustermann et al., 2011). This function is thought to contribute to the specific deposition pattern of H3.3 histones. Besides the ADD domain, ATRX contains a C-terminal SWI/SNF2-like ATPase motif found in chromatin remodeling proteins. These proteins use the energy of ATP to modulate histone-DNA interactions within nucleosomes and contribute to a wide range of cellular processes, including recombination (Alexeev et al., 2003), DNA replication (Collins et al., 2002), and histone exchange (Mizuguchi et al., 2004; Konev et al., 2007). The human ATRX was described about two decades ago due to various disorders associated with mutations in the ATRX gene, such as the X-linked-thalassemia mental retardation syndrome, characterized by several developmental abnormalities (Gibbons et al., 1995a, 1995b). Besides its role as a component of a histone chaperone complex, several studies analyzed the effects of ATRX loss of function in mammals and defined additional roles for this protein, including telomere maintenance (notably by protecting them from replication fork stalling), DNA replication (defects in ATRX leading to a prolongation of the S-phase), and heterochromatin silencing (reviewed in Clynes et al., 2013). Indeed, several recent studies connect ATRX/DAXX with silencing of retrotransposons and satellite sequences via the incorporation of H3.3 and the recruitment of histone methyltransferase activity (Sadic et al., 2015; Elsässer et al., 2012; Voon et al., 2015; He et al., 2015).
The ATRX gene has been conserved through evolution, but intriguingly, in invertebrates such as Drosophila melanogaster, ATRX is split into two proteins, dADD1 (CG8290), which harbors a homolog of the human ATRX ADD domain (López-Falcón et al., 2014; Alekseyenko et al., 2014), and dATRX/XNP (X-linked Nuclear Protein), which contains the SWI/SNF-like ATPase domain (Bassett et al., 2008; Emelyanov et al., 2010). The dATRX/XNP protein shares similar functions with its mammalian counterpart; however, XNP isoforms localize to chromosome arms and heterochromatin regions (Bassett et al., 2008). ATRX null alleles are embryo-lethal in human and mouse (Garrick et al., 2006), and semilethal in fly (Lee et al., 2007). In plants, despite the identification of a putative ATRX-encoding gene in Arabidopsis (Shaked et al., 2006; Otero et al., 2014), the involvement of this protein in histone H3 deposition and chromatin function has not been analyzed yet.
In this study, we investigated the role of ATRX in histone H3 deposition and chromatin function in Arabidopsis. We showed that Arabidopsis plants harboring atrx mutant alleles are viable but show reduced vigor and fertility. Genetic analyses using a combination of mutants in the different histone H3 incorporation systems suggest that ATRX functions as a histone H3.3 chaperone. ATRX controls histone H3.3 cellular pools and chromatin incorporation and thereby fine-tunes gene expression. Genomic sites with medium to high H3.3 occupancy, including the 45S rDNA loci, show decreased H3.3 upon loss of ATRX. Moreover, at 45S rDNA, ATRX controls histone H3 occupancy and H3.3 levels, rRNA gene transcription, and variant dosage. This study characterized ATRX as a player in the plant chaperone network.
RESULTS
Identification and Characterization of the Arabidopsis ATRX Ortholog
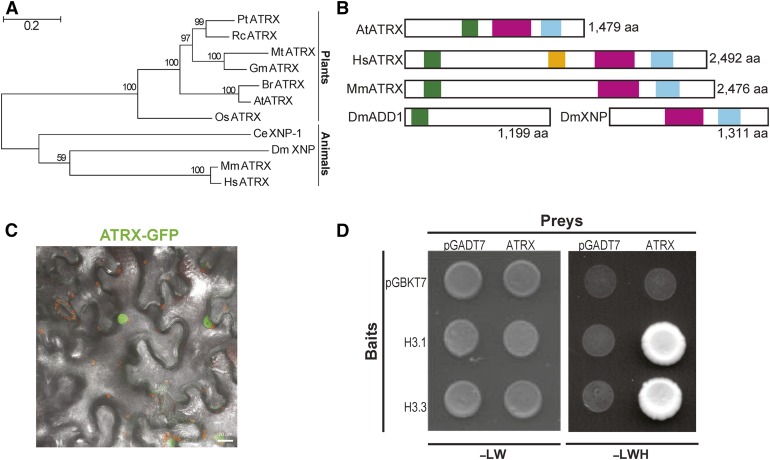
Using the conserved domains of the human ATRX, we confirmed the identification of At1g08600, also called CHROMATIN REMODELLING20 (Shaked et al., 2006; Otero et al., 2014), as the unique Arabidopsis ATRX ortholog. Plant ATRX proteins shared similarities with mammalian and invertebrate ATRX proteins, although they formed a distinct monophyletic group (Figure 1A). As in mammals, and contrary to Drosophila that expresses the two distinct proteins dADD1 (López-Falcón et al., 2014; Alekseyenko et al., 2014) and dATRX/XNP, the Arabidopsis ATRX harbors simultaneously the N-terminal ADD domain, which contains a pocket for recognition of histone H3 tails (Iwase et al., 2011), and the C-terminal helicase domain (Figure 1B). The plant protein was shorter than its mammalian counterpart, devoided of the large central region involved in DAXX interaction (Tang et al., 2004) (Figure 1B), and localized to the nucleus when transiently expressed in tobacco (Nicotiana benthamiana) leaves (Figure 1C). Both the plant and the human ADD domains contained the GATA and PHD zinc finger helices and shared 36.8% similarity for this domain as can be seen in the 3D model (Supplemental Figures 1A and 1D). The ADD domain is known to interact with histone H3 tails and preferentially with methylated H3K9 (Iwase et al., 2011). The C-terminal region of the Arabidopsis ATRX contained the DExDc and the HELICc subdomains constituting the ATPase domain required for chromatin remodeling and characteristic of the SNF2 family proteins (46.2% and 54.8% similarity, respectively, for DExDc and HELICc domains with HsATRX; Supplemental Figures 1B and 1C). In a yeast two-hybrid assay, Arabidopsis ATRX interacts with the canonical histone H3.1 and the variant H3.3 (Figure 1D) and ATRX can immunoprecipitate Arabidopsis H3.3 when both proteins are transiently coexpressed in tobacco leaves (Supplemental Figures 1E to 1G). Taken together, the Arabidopsis ATRX protein shares major features with its human counterpart and interacts with H3, leading us to explore its potential role in histone dynamics and assembly.
Figure 1.
Characterization of the ATRX Ortholog in Arabidopsis.
(A) Phylogenetic tree of ATRX proteins. Pt, Populus trichocarpa; Rc, Ricinus communis; Mt, Medicago truncatula; Gm, Glycine max; At, Arabidopsis thaliana; Br, Brassica rapa; Os, Oryza sativa; Mm, Mus musculus; Hs, Homo sapiens; Dm, Drosophila melanogaster; Ce, Caenorhabditis elegans. Bar = 0.2 substitutions/per site. The percentage of trees in which the associated taxa clustered together is shown next to the branches.
(B) Functional domains of ATRX proteins. The ADD (ATRX-DNMT3-DNMT3L) domain is displayed as a green box, the DAXX-I (DAXX-interacting) domain as an orange box, the DEXDc (DEAD-like helicase superfamily) domain as a pink box, and the HELICc (HELICase superfamily C-terminal) domain as a blue box.
(C) Merged maximum intensity projection of confocal fluorescence and bright-field images of N. benthamiana leaves transiently expressing ATRX-GFP fusion (green) proteins. Chlorophyll fluorescence appears in red. Bar = 20 μm.
(D) Interaction of ATRX with histones H3.1 and H3.3 in a yeast two-hybrid assay. Photographs were taken after 3 d of yeast cell growth on leucine-tryptophan-/yeast nitrogen base medium (-LW) or on the selective leucine-tryptophan-histidine-yeast nitrogen base (-LWH) medium. The pGADT7 (prey) and pGBKT7 (bait) empty vectors were used as negative controls.
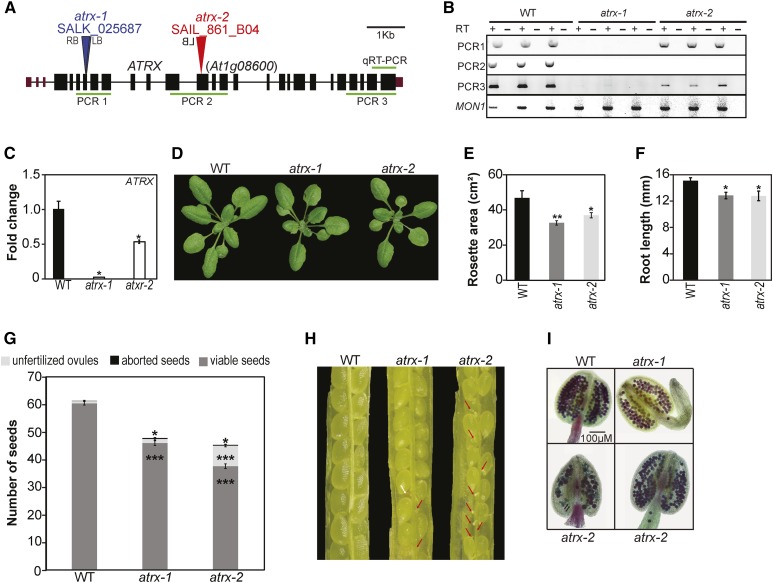
We first quantified transcript levels of ATRX by qRT-PCR, which showed expression in all tested plant tissues (Supplemental Figure 1H). Two different mutant alleles in which T-DNAs are inserted in an exon (Figure 2A) were obtained and homozygous mutant plants established. RT-PCR and qRT-PCR analyses revealed that the atrx-1 allele is a knockout mutant (Figures 2B and 2C; Supplemental Figures 2A and 2B) as the remaining transcript would encode a short truncated protein devoid of the ADD and helicase domains (Supplemental Figure 2C). The atrx-2 allele produced transcripts comprising part of the ATRX 5′ and 3′ regions (Figure 2B; Supplemental Figures 2B and 2C), suggesting that truncated versions of the ATRX protein containing the majority of the ADD and/or part of the HELICc domain could be produced.
Figure 2.
Characterization of the Arabidopsis atrx Mutant Alleles.
(A) Gene structure of Arabidopsis ATRX. Exons, black rectangles; untranslated regions, purple rectangles; introns, lines; T-DNA insertion, triangle; LB, left border; RB, right border.
(B) Analysis of ATRX transcripts produced in mutants with the atrx-1 or atrx-2 alleles by RT-PCR on three biological replicates consisting of independent pools of about twenty 2.5-week-old in vitro-grown whole plantlets sampled at the same time. The amplified regions are displayed by green lines in (A). MON1 (At2g28390) was used as a control.
(C) Mean expression of ATRX in atrx mutants analyzed by qRT-PCR in the same samples than in (B). Transcript levels in the wild type were set to 1. The analyzed region is displayed in (A).
(D) Representative 3-week-old wild-type and atrx mutant plantlets grown on soil.
(E) Quantification of rosette surface area (in cm2) of atrx mutant plants. Mean rosette area is shown for at least six 2-week-old plants for each genotype. Student’s t test; **P < 0.01.
(F) Root length quantification (in mm) of atrx mutant plants. Mean root length is calculated from at least four 5-d-old in vitro-grown plants for each genotype. A representative experiment out of three independent ones is displayed. Student’s t test; *P < 0.05.
(G) Quantification of seed content in atrx mutant siliques compared with the wild type. Mean seed number was calculated from at least 30 siliques pooled from four plants per genotype grown at the same time. Student’s t test compared with the wild type; *P < 0.05 and ***P < 0.001.
(H) Representative dissected siliques from atrx mutants. Red arrows indicate unfertilized ovules and the white arrow an aborted seed.
(I) Representative anthers out of four independent 4-week-old plants grown at the same time, for which pollen viability was assessed by Alexander staining. Both atrx-1 and atrx-2 anthers show reduced pollen content, and atrx-2 anthers contain nonviable pollen (green color) indicated by black arrows.
Error bars of all panels represent se of the mean.
Plants carrying either mutant allele were viable and showed no obvious vegetative abnormalities compared with wild-type plants (Figure 2D), except slightly reduced rosette surface and root growth (Figures 2E and 2F). In addition, atrx mutant siliques showed fewer viable seeds and an increased number of aborted seeds, and more unfertilized ovules were scored in atrx-2 mutant plants (Figures 2G and 2H; Supplemental Figure 2H). The atrx anthers develop normally but Alexander staining revealed reduced pollen content in both mutants (Figure 2I). Given that in mammals, ATRX mutations are associated with replication defects (Leung et al., 2013; Huh et al., 2012), we performed flow cytometry analysis of nuclei from dissected whole cotyledons. For the atrx-1 knockout allele, we noticed broader peaks in the flow cytometry profile (Supplemental Figure 2D). The latter might be caused by delayed S-phase progression or replication defects. While we found no hypersensitivity to the DNA replication inhibitor hydroxyurea (HU) (Supplemental Figure 2E), we noted an increased number of nuclei in early compared with late S-phase after 5-ethynyl-2′-deoxyuridine staining of root apexes in the mutant (Supplemental Figure 2F). This might be indicative of a slower progression through early S-phase. Furthermore, a previous study has implicated ATRX in the DNA damage response using an RNAi line (Shaked et al., 2006). We confirmed moderate sensitivity to γ-irradiation in our atrx mutant alleles using a DNA-damage sensitivity assay, which monitors emergence of true leaves after seed irradiation (Supplemental Figure 2G).
Taken together, atrx mutant alleles are viable but display moderate growth, reproductive, and DNA replication defects.
Epistatic Relationships between Players in the H3 Incorporation Pathways
Since ATRX is an essential component of a histone chaperone complex in several organisms and is highly conserved through evolution, we hypothesized that ATRX may play a similar role in Arabidopsis. Hence, we investigated the genetic interaction between known Arabidopsis histone H3 chaperone complexes and ATRX, by crossing mutants of the HIR (Nie et al., 2014; Duc et al., 2015) and the CAF-1 complex (Kaya et al., 2001) to each atrx mutant allele.
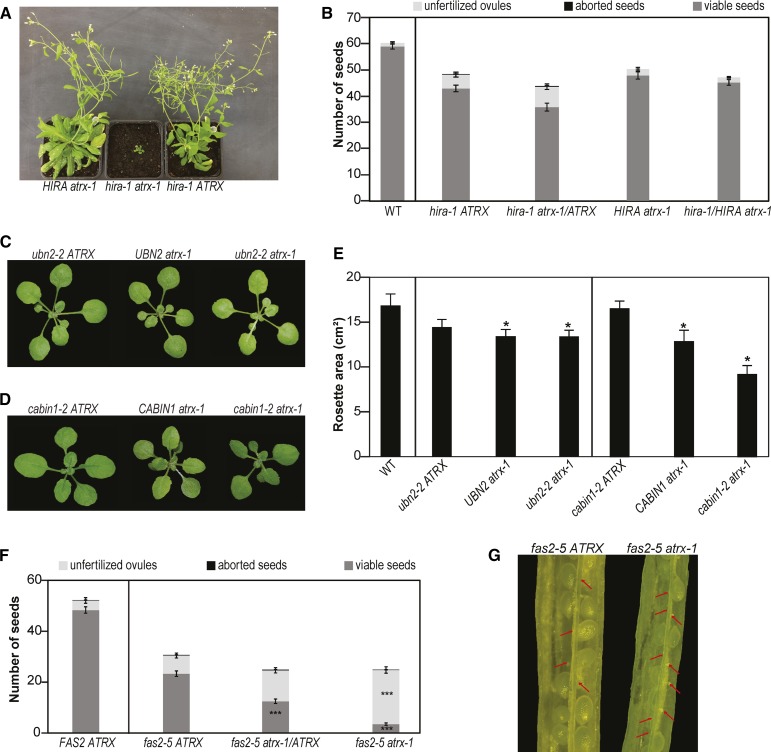
For the hira-1 mutants that lack the central subunit of the HIR complex (Duc et al., 2015), we obtained in the F2 generation only one double mutant plant from the cross with atrx-1 (n = 111) and none with atrx-2 (n = 115; Supplemental Tables 1A and 1B), although the respective loci are genetically unlinked. We confirmed the distorted segregation by analyzing the F3 progeny (Supplemental Tables 2A and 2B). The few hira-1 atrx-1 double mutants recovered were sterile and severely affected in growth in contrast to the hira-1 and atrx-1 single mutant sister plants (Figure 3A). Furthermore, different combinations of F2 plants heterozygous for one mutation and homozygous for the other showed normal flowers (Supplemental Figures 3A and 3B), but reduced number of pollen grains (Supplemental Figures 3C and 3D) and, for certain combinations, reduced seed set compared with single mutants (Figure 3B; Supplemental Figure 3E). Finally, to confirm that the observed lethality of hira-1 atrx double mutations is indeed caused by loss of ATRX and not by an unlinked mutation, we analyzed the F1 progeny of a cross between an atrx-1/atrx-1 hira-1/HIRA plant and an atrx-2/atrx-2 hira-1/HIRA plant. Out of the 43 genotyped plants transheterozygous for atrx (i.e., atrx-1/atrx-2), none was homozygous for hira-1 (Supplemental Table 1C). Jointly, this shows that the simultaneous loss of HIRA and ATRX impairs plant viability or causes severe developmental defects in the surviving plants. For the crosses with mutants for two other subunits of the HIR complex, UBN2 and CABIN1, we obtained viable double mutants with expected or reduced frequency, respectively (Supplemental Tables 1D to 1G, 2C, and 2D), and that displayed a reduced leaf surface compared with the corresponding single mutant for a member of the HIR complex (Figures 3C to 3E; Supplemental Figures 3F to 3H).
Figure 3.
Epistatic Relationship between ATRX and CAF-1 or HIR Histone Chaperone Complexes.
(A) Representative 7-week-old F3 sister plants grown on soil.
(B) Quantification of seed content in hira-1, hira-1 atrx-1/ATRX, atrx-1, and atrx-1 hira-1/HIRA mutant siliques. Mean seed number was calculated from at least 30 siliques pooled from four plants grown at the same time. A Student’s t test revealed no statistically significant difference between the genotypes.
(C) and (D) Representative 3-week-old F3 plants derived from crosses of atrx-1 alleles with ubn2-2 (C) or cabin1-2 (D) grown on soil.
(E) Quantification of total rosette surface area of the F3 sister plants described in (C) and (D). Mean rosette area was calculated from at least six 2-week-old plants for each genotype. Student’s t test in comparison to ubn2-2 ATRX for UBN2 atrx-1 and ubn2-2 atrx-1 mutants and in comparison to cabin1-2 ATRX for CABIN1 atrx-1 and cabin1-2 atrx-1 mutants; *P < 0.05.
(F) Quantification of seed content in siliques of FAS2 ATRX, fas2-5 ATRX, fas2-5 atrx-1/ATRX, and fas2-5 atrx-1 F2 sister plants. Mean for seed number was calculated from at least 30 siliques pooled from four plants grown at the same time. Student’s t test in comparison to fas2-5 ATRX; ***P < 0.001.
(G) Representative dissected siliques from fas2-5 ATRX and fas2-5 atrx-1 F2 sister plants. Red arrows indicate unfertilized ovules.
Error bars of all panels represent se of the mean.
To explore the relationship with the CAF-1 complex, which is thought to be involved in replication-coupled histone deposition, we crossed both atrx alleles with the fas2-5 mutant, a knockout mutant for the second largest subunit of the CAF-1 complex (Duc et al., 2015). Double mutants were obtained with the expected frequencies (Supplemental Table 3). When we looked closer at the development of the double mutant plants, we noticed flowers similar to fas2-5 (Supplemental Figures 3I and 3J), but with aggravated anther shapes (Supplemental Figures 3K and 3L), and further reduction of the already low seed set (Figures 3F and 3G; Supplemental Figures 3M and 3N).
Taken together, this analysis reveals aggravated developmental defects and severe growth deficiencies or lethality when combining atrx mutations with plants deficient in CAF-1 and HIR complexes, respectively. In particular, the lethality for mutant combinations within the replication-independent system of H3 incorporation suggests that Arabidopsis ATRX could play a complementary role in histone H3.3 variant deposition in plants.
Loss of ATRX Affects Histone Pools and H3 Occupancy
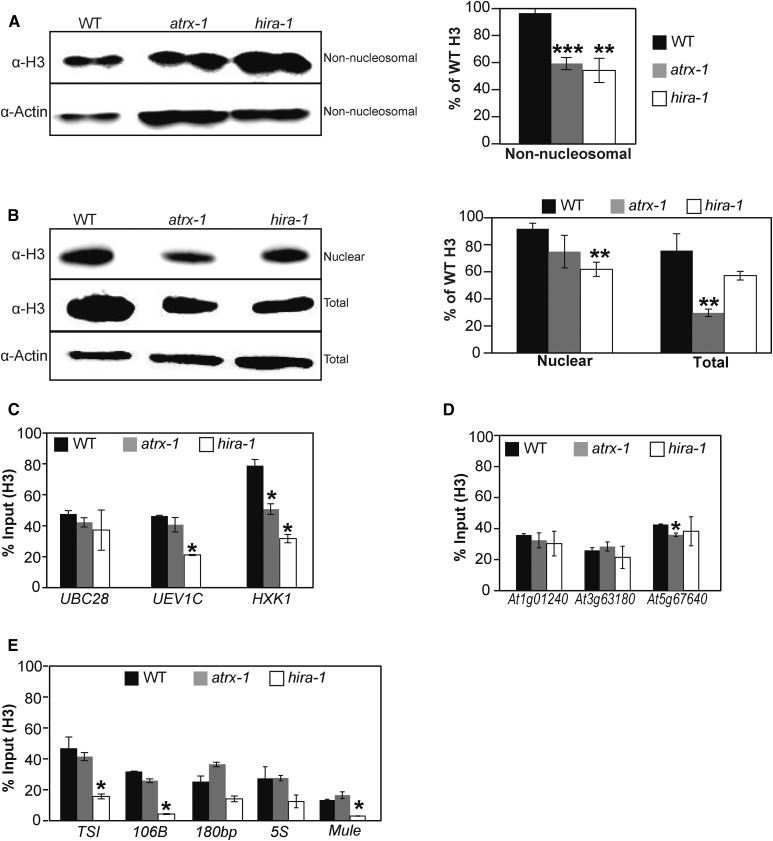
Based on the above results, we reasoned that loss of Arabidopsis ATRX function might alter histone H3 pools. We used different protein extraction protocols (Durut et al., 2014; Honda et al., 1966) to recover histones from distinct cellular fractions (see Methods for details) of the wild type, hira-1, and the knockout atrx-1 mutant. In comparison to wild-type plants, non-nucleosomal and total H3 amounts were significantly reduced in atrx-1, and non-nucleosomal and nuclear fractions were affected in hira-1 (Figures 4A and 4B). The reduction observed in atrx-1 occurred without changes in expression levels of the H3.1- and H3.3-encoding genes (Supplemental Figure 4A). Consistent with the association of H3 and H4 proteins as heterodimers and heterotetramers (Banks and Gloss, 2004), non-nucleosomal and total H4 levels were concomitantly reduced in atrx-1 (Supplemental Figures 4B and 4C). These results show that loss of both HIRA and ATRX affects H3 and H4 histone pools.
Figure 4.
Effects of ATRX Loss on Histone Pools and Nucleosome Occupancy.
(A) and (B) Left: Histone H3 protein levels quantified by immunoblot in non-nucleosomal fractions (A) and nuclear and total extracts (B). Twenty (A) or six (B) micrograms of proteins were loaded per lane. Right: Quantification of H3 band intensities normalized to Actin from two independent experiments, each comprising two biological replicates for each genotype, consisting of independent pools of 1 g of 2.5-week-old in vitro-grown plantlets collected at the same time, and several blots. Student’s t test compared with the wild type; **P < 0.01 and ***P < 0.001.
(C) to (E) Histone H3 occupancy at three active genes (UBC28, UEV1C, and HXK1) (C), at three genes situated in subtelomeric regions (At1g01240, At3g63180, and At5g67640) (D), and at centromeric and pericentromeric repeats (TSI, 106B, 180bp, and ribosomal 5S rDNA loci) and at a transposon on a chromosome arm (At2g15810, Mule) (E) was assessed by H3-ChIP qPCR in three biological replicates consisting of pools of 1 g of 2.5-week-old in vitro-grown wild-type, atrx-1, and hira-1 mutant plants. Student’s t test compared with the wild type; *P < 0.05.
Error bars of all panels represent se of the mean.
We further investigated the possible contribution of ATRX to histone deposition by analyzing histone H3 occupancy at specific genomic sites. For this purpose, we combined H3-ChIP (chromatin immunoprecipitation) with quantitative PCR on wild-type, atrx-1, and hira-1 plants. Since the combination of atrx and hira-1 mutations was lethal, we hypothesized that ATRX affects incorporation of the replacement variant H3.3. Therefore, we first measured H3 occupancy in the 3′ regions of three transcriptionally active genes (UBC28, UEV1C, and HXK1) that have different expression levels (Duc et al., 2015) and were previously shown to be enriched in H3.3 (Stroud et al., 2012). While H3 occupancy was lower in hira-1 for two of the three genes (Duc et al., 2015), it was reduced only at HXK1 in atrx-1 (Figure 4C). Given that ATRX binds to telomeres and subtelomeric regions of human chromosomes (Law et al., 2010), we analyzed three genes in subtelomeric regions. Only a minor reduction of H3 occupancy was found at At5g67640 (Figure 4D). In mammals, ATRX is involved in H3.3 deposition at pericentromeric regions (Voon et al., 2015). We therefore determined H3 occupancy at two centromeric regions: the 180-bp repeats and the 106B long terminal (LTR)-like repeats (Thompson et al., 1996; Fransz et al., 1998); and at two pericentromeric regions: TSI (Transcriptionally Silent Information) (Steimer et al., 2000) and at the 5S ribosomal DNA loci; as well as at Mule (Mutator-like, At2g15810), a DNA transposon located on a chromosome arm. While histone H3 occupancy was reduced in hira-1 at TSI, 106B, and Mule (Duc et al., 2015), it was not affected in atrx-1 (Figure 4E). We further checked whether the H3K9me2 mark, the major plant heterochromatin signature present in pericentromeric regions and in patches of heterochromatin on chromosome arms (Bernatavichute et al., 2008), was maintained in atrx-1. Indeed, once normalized to H3, H3K9me2 levels were unchanged at the heterochromatic repeats TSI, 106B, and 180bp as well as at the Mule transposon or at At5g67640, a gene situated in the sub-telomeric region (Supplemental Figure 4D). This finding suggests that ATRX does not affect the setting or maintenance of this H3K9me2 repressive histone mark at several heterochromatic sequences. In agreement with this observation, silencing of the centromeric and pericentromeric 180bp, 106B, and TSI repeat sequences (Supplemental Figure 4E) and of several transposable elements (list of tested loci available in Methods) was unchanged in atrx-1, except for the Mule transposon (Supplemental Figure 4F) and three Gypsy LTR transposons GP2NLTR, Atlantys2_I, and Athila2_I that were reactivated in atrx-1 in our transcriptome analysis presented below (Supplemental Data Set 1).
Therefore, loss of ATRX affects histone pools and has a moderate influence on histone H3 occupancy at certain active genes. In contrast to mammals, ATRX in Arabidopsis is required neither for H3 incorporation at the tested pericentromeric regions nor to maintain transcriptional silencing at repeats and transposable elements.
Arabidopsis ATRX Influences Histone H3.3 Deposition
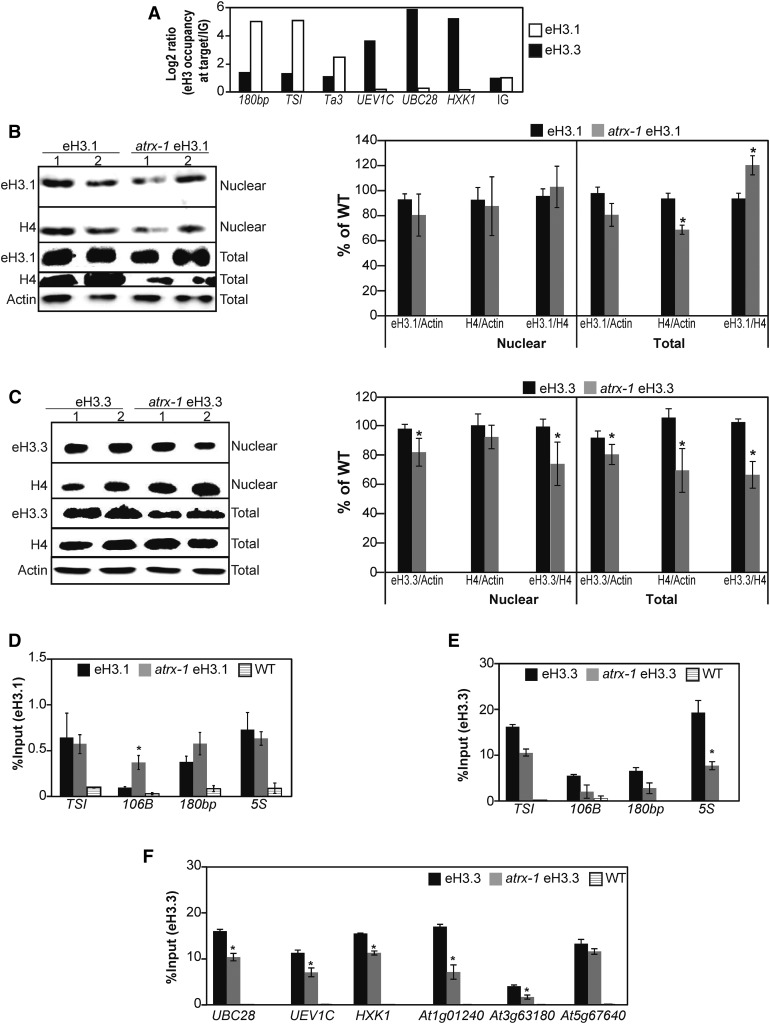
Arabidopsis canonical histones H3.1 and variants H3.3 differ by only four amino acids, and no variant-specific antibodies are available so far in plants. Hence, to follow specifically each H3 histone type, we used plants expressing FLAG-HA-tagged versions of H3.1 (epitope-tagged H3.1, eH3.1) or H3.3 (eH3.3) under the control of their endogenous promoters. We first validated chromatin incorporation of eH3.1 and eH3.3 proteins by ChIP using a FLAG antibody. As expected, eH3.1 was preferentially enriched at heterochromatic loci (180bp, TSI, and the Ta3 retrotransposon localized in the pericentromeric region of chromosome 1), while transcriptionally active genes were enriched in eH3.3 (UEV1C, UBC28, and HXK1) (Figure 5A). We then crossed the eH3.1 and eH3.3 transgenic lines with plants carrying the atrx-1 allele. From the progeny, we selected sister plants with and without the atrx-1 mutation that expressed sufficient eH3.1 or eH3.3 for further analysis. We chose two lines for each genotype with similar transgene expression levels (Supplemental Figures 5A and 5B) to ensure that changes in eH3.1 and eH3.3 abundance between wild-type and atrx-1 mutant plants were caused by ATRX loss and not by differential expression of the transgene.
Figure 5.
Effect of ATRX Loss on Incorporation and Balance of the Canonical Histone H3.1 and the H3.3 Variant.
(A) Canonical eH3.1 and variant eH3.3 occupancy at heterochromatic repeats (180bp, TSI, and Ta3) and at three active genes (UBC28, UEV1C, and HXK1) assessed by ChIP-qPCR in one biological replicate sampled at the same time for each genotype and consisting of a pool of 0.5 g of 2.5-week-old in vitro-grown plants. The eH3.1 and eH3.3 occupancy is normalized to the occupancy at an intergenic region (IG; set to 1).
(B) and (C) Left: Histone eH3.1 (B) and eH3.3 (C) protein levels quantified by immunoblot in nuclear fractions and total extracts prepared with Honda buffer (Honda et al., 1966). Six micrograms of proteins were loaded per lane. Right: Quantification of H4 and eH3.1 (B) or eH3.3 (C) band intensities relative to Actin in total extracts from two independent experiments comprising in total four biological replicates of pools of 1 g of 2.5-week-old in vitro-grown plants and several blots. Student’s t test compared with the wild type; *P < 0.05.
(D) and (E) Histone eH3.1 (D) and eH3.3 (E) occupancy at heterochromatic repeats (TSI, 106B, and 180bp) and at the 5S ribosomal DNA loci assessed by ChIP-qPCR in three biological replicates sampled at the same time and consisting of pools of 1 g of 2.5-week-old in vitro-grown eH3.1, atrx-1 eH3.1, and wild-type plants. Student’s t test compared with the wild type; *P < 0.05.
(F) Histone eH3.3 occupancy at three active genes (UBC28, UEV1C, and HXK1) and at three genes situated in subtelomeric regions (At1g01240, At3g63180, and At5g67640) assessed by ChIP-qPCR in the same plant material as in (E). Student’s t test compared with the wild type; *P < 0.05.
Error bars of all panels represent se of the mean.
We first determined the amount of eH3.1 and eH3.3 in total and nuclear histone pools by immunoblot. Since the α-H3 antibody recognized endogenous and epitope-tagged H3.1 and H3.3, blots were hybridized with a α-H4 antibody for normalization. The quantification of H4 levels confirmed the moderate reduction of the H3-H4 pool in the total protein extract in both atrx-1 eH3.1 and atrx-1 eH3.3 lines (Figures 5B and 5C). The eH3.1/H4 ratio was increased in the total extract of atrx-1 eH3.1 mutants compared with the wild type (Figure 5B), while the eH3.3/H4 ratio was reduced in both total and nuclear extracts of atrx-1 eH3.3 compared with the wild type (Figure 5C). Based on these results, we conclude that storage and deposition of H3.3 are affected by the absence of ATRX.
To analyze the chromatin distribution of H3.1 or H3.3, a ChIP coupled to quantitative PCR was performed on plants expressing eH3.1 or eH3.3. A ChIP performed with the H3 antibody revealed that atrx-1 eH3.1 and atrx-1 eH3.3 display similar H3 enrichment patterns (Supplemental Figures 5C and 5D) as previously observed for atrx-1 (Figures 4C to 4E). Then, to determine potential changes in the incorporation of eH3.1 and eH3.3, we performed a ChIP with a FLAG antibody using wild-type plants as a control for background noise (Figures 5D and 5E). We evaluated eH3.1 and eH3.3 levels at heterochromatic regions (TSI, 106B, and 180bp) and at 5S rDNA. In atrx-1 mutants, the eH3.1 abundance was maintained at TSI, 180bp, and 5S rDNA regions; only 106B repeats displayed a higher abundance of eH3.1 (Figure 5D). In contrast to eH3.1, eH3.3 was significantly less enriched at TSI and 5S rDNA regions in atrx-1 mutants (Figure 5E). Furthermore, analysis of three transcriptionally active genes (UBC28, UEV1C, and HXK1) and three genes in subtelomeric regions revealed a significant reduction of eH3.3 occupancy at these targets except for At5g67640 (Figure 5F).
Taken together, our results from immunoblot and ChIP-qPCR analyses were consistent with the hypothesis that Arabidopsis ATRX is involved in H3.3 deposition.
ATRX Controls Gene Expression and H3.3 Distribution
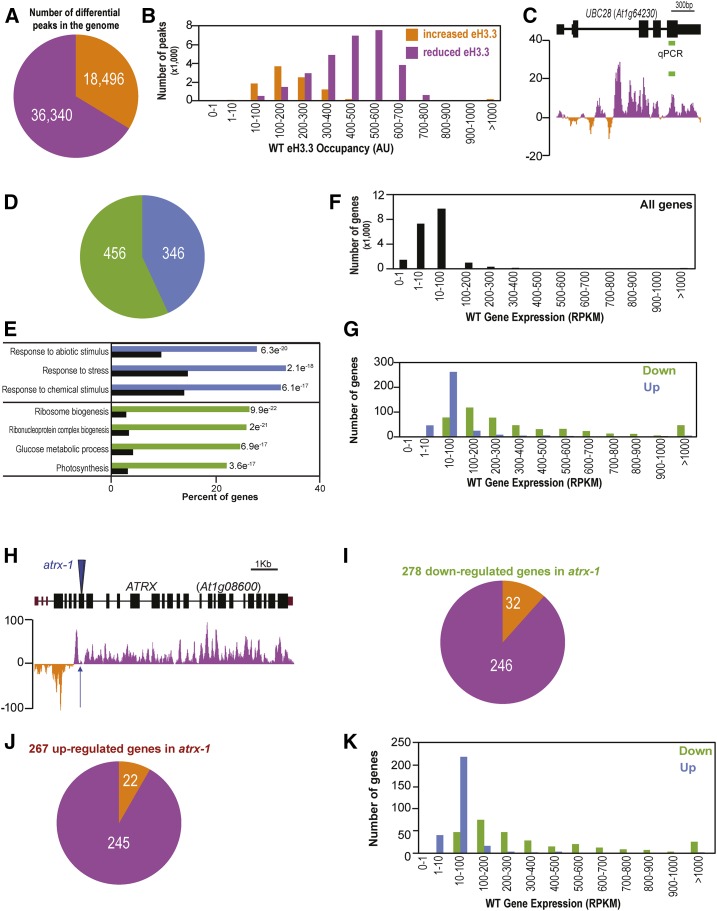
To determine how the distribution of H3.3 is affected by ATRX loss of function at a genome-wide scale, we performed ChIP-seq on chromatin prepared from whole seedlings expressing the eH3.3 transgene. The genome-wide distribution of eH3.3 along the chromosomes (Supplemental Figure 6A, left) showed the expected profile in the wild type (Stroud et al., 2012; Wollmann et al., 2012; Vaquero-Sedas and Vega-Palas, 2013; Shu et al., 2014), the profile being similar in atrx-1 mutants (Supplemental Figure 6A, right). We therefore assessed changes in eH3.3 peak summit occupancy between the wild type and atrx-1 using DANPOS2 (Chen et al., 2013). We identified 54,836 genomic peaks with significantly different eH3.3 occupancy in atrx-1 mutants compared with the wild type (Supplemental Data Set 2, smt_diff_FDR < 0.05), out of which 66% correspond to a reduced occupancy in atrx-1 (Figure 6A), as expected for a H3.3 chaperone. Upon ATRX loss, reduced eH3.3 occupancy was observed at regions of medium to high eH3.3 occupancy, while increased eH3.3 occupancy was found at regions of low eH3.3 occupancy (Figure 6B). This suggests that ATRX affects regions with different levels of H3.3 occupancy in a distinct manner. Given that in mammals, ATRX/DAXX was shown to control silencing of retrotransposons notably through the incorporation of H3.3 (Elsässer et al., 2015), we analyzed changes in eH3.3 incorporation at transposable elements (TEs). In Arabidopsis, TEs are primarily localized in the pericentromeric regions, which are H3.3 poor (Supplemental Figure 6A, left). Only 29% of TEs showed differential H3.3 occupancy, and 43% of these TEs presented reduced H3.3 occupancy in atrx-1 (Supplemental Figure 6B and Supplemental Data Set 3). Since 70% of the genomic peaks with eH3.3 occupancy changes localized to gene bodies, we further focused on the effect of ATRX loss on H3.3 incorporation at genes. Among the 38,645 peaks located in 19,396 gene bodies, 75% corresponded to a reduced H3.3 occupancy in atrx-1, confirming the variations in eH3.3 occupancy observed by ChIP-qPCR (Figure 6C; Supplemental Figures 6C and 6D). The distribution was similar for the few (0.07%) intergenic regions (Supplemental Figure 6E and Supplemental Data Set 4) that showed differential eH3.3 occupancy. Therefore, ATRX appears to preferentially control H3.3 occupancy at genic sites.
Figure 6.
Effects of ATRX Loss on Gene Expression and Histone Variant H3.3 Occupancy.
(A) Pie chart showing the number of peaks situated in the Arabidopsis genome with increased (orange) or reduced (violet) eH3.3 occupancy in atrx-1 relative to the wild type. Numbers of peaks are indicated in the pie chart. ChIP-seq was performed in two biological replicates for each genotype sampled at the same time consisting of pools of 2 g of 2.5-week-old in vitro-grown eH3.3 and atrx-1 eH3.3 plants. Peak occupancy was determined with DANPOS2.
(B) Distribution of gene-localized peaks according to their increased (orange) or reduced (violet) eH3.3 occupancy in the wild-type ChIP-seq data set. Peak occupancy was determined with DANPOS2 (AU, arbitrary units).
(C) Differential eH3.3 summit occupancy calculated at single nucleotide resolution in the ChIP-seq data sets in comparison to ChIP-qPCR data from Figure 5F. The active gene UBC28 is presented. Peaks with higher eH3.3 occupancy in atrx-1 compared with the wild type are shown in orange (negative values), while those with lower eH3.3 occupancy are in violet (positive values). The region amplified in ChIP-qPCR is displayed by a green line. Reduced eH3.3 occupancy was monitored by ChIP-qPCR at this locus (Figure 5F).
(D) Pie chart showing the number of differentially expressed genes. Upregulated and downregulated genes in atrx-1 compared with the wild type are displayed in blue and green, respectively, and numbers of differentially expressed genes are indicated in the pie chart. RNA-seq was conducted on total RNAs extracted from three biological replicates sampled at the same time consisting of pools of around twenty 2.5-week-old in vitro-grown wild-type and atrx-1 plants.
(E) Top GO terms for upregulated and downregulated genes in atrx-1 compared with the wild type. The P values are indicated. Terms for upregulated genes are in blue, while those for downregulated genes are in green. Background levels are shown in black. e, exponent (scientific format, in which “e” multiplies the preceding number by 10 to the -nth power).
(F) and (G) Distribution of all genes (F) and of differentially expressed genes in atrx-1 ([G], in blue for upregulated genes and in green for downregulated genes) according to their expression levels in the wild type RNA-seq data set (RPKM).
(H) Differential eH3.3 summit occupancy calculated at single nucleotide resolution at the ATRX locus from the ChIP-seq data set. Peaks with higher eH3.3 occupancy in atrx-1 compared with the wild type are shown in orange, while those with lower eH3.3 occupancy are in violet. Peak occupancy was determined with DANPOS2. The blue arrow indicates the T-DNA insertion location for the atrx-1 mutant.
(I) Pie chart showing distribution of genes with differential summit eH3.3 occupancy determined with DANPOS2 in atrx-1 eH3.3 in the ChIP-seq data, for the 278 downregulated genes identified by DeSeq2 in atrx-1 in the RNA-seq data. The 246 genes with reduced eH3.3 occupancy in atrx-1 relative to the wild type are in violet. The 32 genes with increased eH3.3 occupancy in atrx-1 relative to the wild type are in orange.
(J) Pie chart showing distribution of genes with differential summit eH3.3 occupancy determined with DANPOS2 in atrx-1 eH3.3 in the ChIP-seq data, for the 267 upregulated genes identified by DeSeq2 in atrx-1 in the RNA-seq data. The 245 genes with reduced eH3.3 occupancy in atrx-1 relative to the wild type are in violet. The 22 genes with increased eH3.3 occupancy in atrx-1 relative to the wild type are in orange.
(K) Distribution of genes downregulated (green) and upregulated (blue) in atrx-1 according to their expression levels in the wild-type RNA-seq data set. Differential expression was determined with DeSeq2.
H3.3 occupancy is known to be correlated with gene expression (Stroud et al., 2012; Wollmann et al., 2012; Shu et al., 2014). Hence, we analyzed how the defective H3.3 incorporation upon ATRX loss influences gene expression by performing RNA-seq on rRNA-depleted total RNAs prepared in triplicates from whole 2.5-week-old wild-type and atrx-1 seedlings. We identified 456 down- and 346 upregulated genes in atrx-1 compared with wild-type plants (|log2fold change|>0 and FDR < 0.1; Figure 6D; Supplemental Data Set 5). Enriched Gene Ontology (GO) terms were determined to uncover modulated cellular components (Figure 6E). While terms such as response to stress as well as abiotic and chemical stimuli were enriched among upregulated genes, the term “ribosome” emerged as strongly enriched in downregulated genes (71 genes out of 456 corresponding to the GO term “ribosome”). Compared with all the genes expressed in the RNA-seq data set (Figure 6F), downregulated genes in atrx-1 belonged to medium and highly expressed genes (84% with transcript levels >100 reads per kilobase per million mapped reads [RPKM]), while 88% of upregulated genes showed transcript levels between 1 and 100 RPKM (Figure 6G). This suggests a role for ATRX in the positive regulation of highly expressed genes.
We then asked whether eH3.3 occupancy changes induced by ATRX loss are associated with gene expression changes. For this purpose, we first looked at the ATRX locus itself, as no ATRX transcripts were observed downstream of the T-DNA insertion site in atrx-1 mutants (Supplemental Figure 2B). Indeed, eH3.3 occupancy is significantly reduced in atrx-1 compared with the wild type in this region (Figure 6H). This prompted us to analyze in detail genes that showed significantly increased or decreased eH3.3 occupancy in atrx-1 and which were differentially expressed in the RNA-seq data set. It appears that 278 out of 456 downregulated genes (Figure 6I) and 267 out of 346 upregulated genes (Figure 6J) presented significantly altered eH3.3 occupancy in our ChIP-seq data, and both sets of genes showed similar H3.3 occupancy distribution in wild-type plants. The vast majority of the downregulated genes (88%) showed reduced eH3.3 occupancy in atrx-1 (Figure 6I) and were characterized by a medium to high gene expression level (Figure 6K), suggesting that modulation of the expression of these genes may be associated with an ATRX-dependent deposition of H3.3. Unexpectedly, most upregulated genes also showed reduced H3.3 occupancy (Figure 6J). These genes differed from the downregulated ones by their low expression level (Figure 6K), implying that, at this set of genes, H3.3 occupancy does not positively regulate gene expression and that other mechanisms dominate.
To conclude, ATRX controls H3.3 occupancy and fine-tunes gene expression of moderately to highly expressed genes.
Arabidopsis ATRX Maintains H3 Occupancy and Expression at 45S rDNA Genes
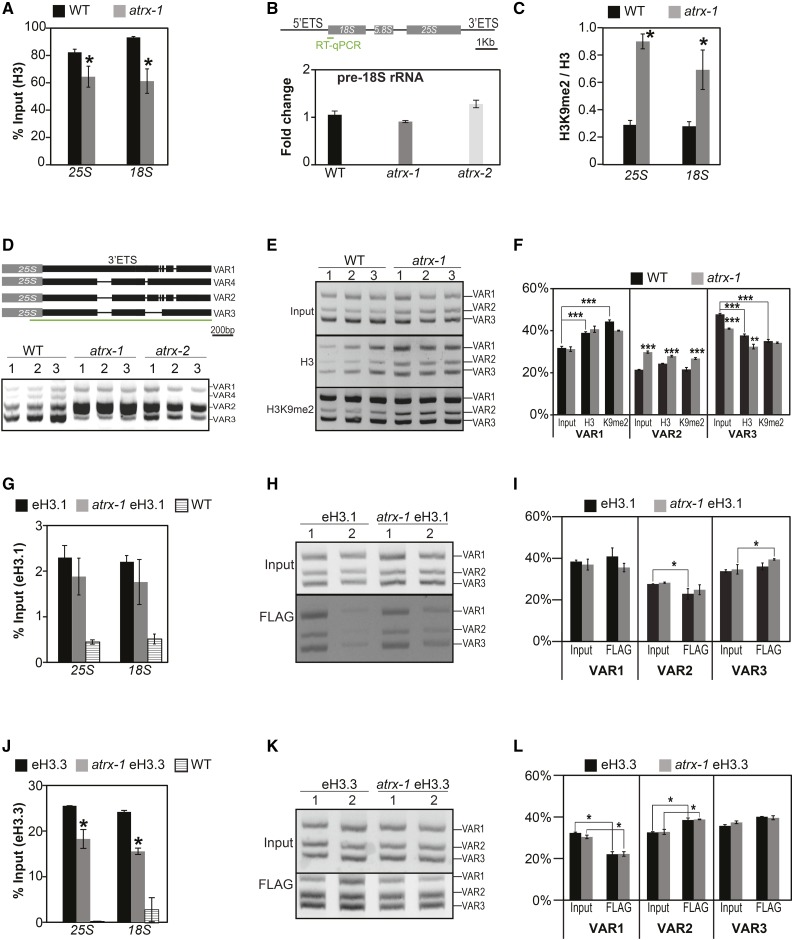
Many riboprotein-coding genes were downregulated in our transcriptome analysis in atrx-1 mutants, suggesting altered ribosome biogenesis in the mutant. Besides riboproteins, major components of the ribosome are the structural 18S, 5.8S, and 25S rRNAs encoded by repetitive arrays of 45S ribosomal genes, which are organized in nucleolus organizer regions (NORs) that are highly enriched in H3.3 (Shi et al., 2011; Shu et al., 2014). We therefore analyzed histone occupancy by H3-ChIP in atrx-1 mutants and found that histone occupancy was reduced at the 45S rDNA loci (Figure 7A). Given that the formation of a repressive chromatin environment is required to silence 45S rRNA genes since only 10 to 27% of 45S rDNA genes are transcribed (Pontes et al., 2003; Lawrence et al., 2004), we asked whether reduced histone occupancy leads to altered 45S rRNA expression and nuclear organization. We found that despite reduced histone H3 occupancy, 45S pre-rRNA levels (Figure 7B) were not significantly altered in atrx potentially due to compensation by increased H3K9me2 enrichment relative to H3 at the 45S rDNA loci (Figure 7C). Furthermore, in atrx-1, the repetitive 45S rDNA arrays remained clustered into highly condensed chromatin regions termed chromocenters (Supplemental Figure 7A). The atrx-1 nuclei showed an increased number of NORs (Supplemental Figure 7B), which were associated with the nucleolus, in agreement with increased endoreduplication and chromocenter numbers observed in atrx mutant cotyledons (Supplemental Figures 7C and 7D).
Figure 7.
Effects of ATRX Loss on Nucleosome Occupancy and Balance between Histone Variants at 45S rDNA Loci.
(A) Mean histone H3 occupancy at 25S and 18S rDNA loci assessed by H3-ChIP qPCR from three biological replicates consisting of independent pools of 1 g of 2.5-week-old in vitro-grown wild-type and atrx-1 mutant plants collected at the same time. Student’s t test; *P < 0.05.
(B) Quantification of pre-rRNA transcripts by qRT-PCR. Top: 45 rDNA unit with the 18S, 5.8S, and 25S rRNA genes (gray boxes) with positions of the external transcribed spacers (5′ETS and 3′ETS). The region amplified in qRT-PCR is displayed by a green line. Bottom: Quantification of pre-rRNA transcripts by qRT-PCR on cDNA prepared from three biological replicates consisting of independent pools of about twenty 2.5-week-old in vitro-grown wild-type, atrx-1, and atrx-2 plantlets collected at the same time. Wild type was set to 1.
(C) Mean ratio of histone H3K9me2/H3 occupancy at 25S and 18S rDNA loci assessed by ChIP-qPCR from three biological replicates consisting of independent pools of 1 g of 2.5-week-old in vitro-grown wild-type and atrx-1 mutant plants collected at the same time. Student’s t test; *P < 0.05.
(D) Analysis of 45S rRNA variants. Top: Schema of 3′ETS rRNA gene variants. Black lines joining rectangles indicate deletions in sequences of each rRNA gene variant. The green line represents the amplified region presented in the bottom panel. Bottom: RT-PCR on cDNA, prepared from three biological replicates consisting of independent pools of about twenty 2.5-week-old in vitro-grown wild-type, atrx-1, and atrx-2 plants grown and collected at the same time. Detection of pre-rRNA transcripts was performed with primers enclosing the external transcribed 45S spacer and detecting all variants. The amplified region is displayed by a green line in the top panel.
(E) Analysis of 45S variant abundance in the input, H3-ChIP, and H3K9me2-ChIP (K9me2) fractions presented in (A) and (C).
(F) Quantification of relative band intensities presented in (E). Student’s t test; **P < 0.01 and ***P < 0.001.
(G) and (J) Mean histone eH3.1 (G) and eH3.3 (J) occupancy at 25S and 18S rDNA loci from three biological replicates consisting of independent pools of about 1 g of 2.5-week-old in vitro-grown eH3, atrx-1 eH3, and wild-type plants assessed by ChIP-qPCR.
(H) and (K) Analysis of the relative 45S variant abundance in the input and Flag-ChIP samples in (G) and (J).
(I) and (L) Quantification of relative band intensities presented in (H) and (K). Student’s t test; *P < 0.05.
Error bars of all panels represent se of the mean.
While the quantity of 45S pre-rRNA transcripts was not affected, altered chromatin structure could influence the regulation of the different 45S sequence variants. These variants (VAR1 to 4) are defined by small sequence variations in the 3′ external transcribed spacer region and can be separated by size after amplification with a primer pair common to all variant types (Pontvianne et al., 2010) (Figure 7D, top panel). In our conditions, mainly VAR2 and VAR3 were transcribed in wild-type plants (Abou-Ellail et al., 2011; Pontvianne et al., 2010), while VAR2 was predominantly expressed in both atrx-1 and atrx-2 mutant alleles (Figure 7D, bottom panel; Supplemental Figure 7E). To explain this observation, we determined the relative abundance of the rDNA variants, as well as their enrichment in H3 or H3K9me2, by submitting ChIP samples to PCR (Figures 7E and 7F). In the wild type, VAR1 is enriched in H3 and H3K9me2 when compared with the ChIP input DNA, while VAR3 is depleted (Figure 7F). This finding is in agreement with previous observations showing that VAR1 is transcriptionally silent in wild-type plants (Pontvianne et al., 2012). When comparing wild-type and atrx-1 mutants, we noticed a similar pattern for VAR1, but found that VAR2 was more abundant in the atrx-1 genome than in wild-type plants, while VAR3 was underrepresented (Figure 7F). This illustrates a change in the relative variant abundance in atrx-1 mutants, while 45S rDNA copy numbers remained unchanged (Supplemental Figure 7H). Furthermore, the H3/input and the H3K9me2/input ratios revealed reduced H3 and H3K9me2 enrichment at VAR2 in atrx-1 (Supplemental Figures 7F and 7G). Together, this is consistent with the increased VAR2 transcript levels observed in the atrx-1 mutant.
We then used the atrx-1 eH3.1 and atrx-1 eH3.3 lines to analyze whether the reduced H3 occupancy is caused by loss of H3.1 or H3.3. We first confirmed the reduced H3 occupancy at 45S rDNA in atrx-1 compared with the respective wild-type lines (Supplemental Figures 7I and 7L). While no significant change in eH3.1 occupancy was observed at 45S rDNA loci (Figure 7G), eH3.3 occupancy was decreased (Figure 7J), suggesting that deficient H3.3 deposition caused the reduced nucleosomal occupancy at 45S rDNA loci in atrx-1 mutants. When we looked at eH3.1 and eH3.3 enrichment at specific 45S variants, we noticed, compared with input, less eH3.3 enrichment at VAR1 as well as more eH3.3 and less eH3.1, respectively, at VAR2 in wild-type and atrx-1 plants (Figures 7H, 7I, 7K, and 7L), in agreement with their relative expression patterns in these lines (Supplemental Figures 7J, 7K, 7M, and 7N). In the atrx-1 eH3.1 and atrx-1 eH3.3 lines, VAR2 abundance was similar in atrx-1 DNA compared with the wild type (Figures 7H, 7I, 7K, and 7L), likely because these two lines are issued from a cross between plants expressing the epitope-tagged histones and an atrx-1 mutant plant that led to homogenization. Nevertheless, VAR2 remained predominantly expressed in atrx-1 eH3.1 and atrx-1 eH3.3, respectively, compared with eH3.1 and eH3.3 plants (Supplemental Figures 7J, 7K, 7M, and 7N). This suggests that increased VAR2 abundance at the RNA level in atrx-1 mutants results not only from increased VAR2 abundance at the DNA level, but may also be the consequence of ATRX loss of function.
To summarize, ATRX loss affects H3.3 occupancy and H3K9me2 enrichment at 45S rDNA repeats and leads to a modified variant dosage and altered 45S rRNA variant expression.
DISCUSSION
We investigated the role of ATRX in the model plant Arabidopsis using two independent mutant alleles in the single ATRX gene generated by T-DNA insertions. For both mutant alleles, and in contrast to loss of the mammalian ATRX that is embryo lethal, we found that Arabidopsis atrx mutants are viable. The atrx plants show reduced vigor, reduced vegetative growth, and reduced fertility, features that correlate well with the reduced riboprotein-coding gene expression.
Both atrx alleles, as well as transheterozygotes, result in lethality in combination with HIR complex mutations and affect rDNA expression, but dissimilarities concerning the seed set have been observed between the two atrx alleles. This might be explained by differences in the number of subsequent generations homozygous for the atrx mutation, by the presence of potential partial proteins still produced in the atrx-2 allele, or by a second mutation. Normal seed set in F1 ATRX/atrx-2 (Supplemental Figure 2H) ruled out the hypothesis of a partial ATRX protein acting as a dominant negative. The analysis of F1 atrx-1/atrx-2 plants revealed a seed set similar to atrx-1 but different from the atrx-2 mutant (Supplemental Figure 2H). This rather supports the hypothesis of a second mutation in the atrx-2 mutant allele, which seems to enhance the fertility defects, such as those observed in combination with hira-1 (Supplemental Figure 3E). Hence, the knockout allele atrx-1 was chosen for in-depth molecular analysis.
ATRX is a multifunctional protein carrying both an ADD domain involved in histone tail binding and a SWI/SNF helicase domain implicated in chromatin remodeling. Therefore, ATRX is likely to play a broader role in chromatin structure not necessarily linked to its potential histone chaperone function. Indeed, the moderate sensitivity to γ-irradiation observed in the mutant plants might be attributed to reduced nucleosomal occupancy as well as to the loss of chromatin remodeling activity (Shaked et al., 2006). One of the phenotypes we noted in atrx-1 mutants is an aberrant cell cycle profile with enlarged peaks in the 2C and 4C populations, which could be explained by an S-phase defect. While we found no hypersensitivity to HU, which inhibits replication by diminishing the available nucleotide pool, we noticed more early S-phase nuclei relative to late ones in root tips, which could suggest a delayed progression through early S-phase. The binding of mammalian ATRX to genic and intergenic sites rich in variable number tandem repeats that can form G-quadruplexes (Law et al., 2010) may imply that ATRX is also needed for the resolution of such structures interfering with proper DNA replication in Arabidopsis. Further studies are needed to explore fully the role of ATRX in the replication process in plants.
ATRX as a Player in the Arabidopsis H3.3 Chaperone Network
Lethality and developmental defects observed in combination with mutations for HIR or CAF-1 histone chaperone complexes, along with the reduced amounts of H3.3 histones in different cellular pools and in chromatin, strongly suggest that ATRX functions as a histone H3.3 chaperone in Arabidopsis. The most severe phenotypes were observed in crosses with mutants for the HIR complex that is involved in the replication-independent histone deposition in mammals (Tagami et al., 2004) and that interacts with H3.3 in Arabidopsis (Nie et al., 2014). Therefore, in the absence of a functional HIR complex, ATRX may be required as an alternative pathway of H3.3 deposition, recalling observations in Drosophila: hira and xnp single mutants are viable and reveal only moderate defects in H3.3 deposition, while double mutants die during larval development and are unable to assemble H3.3 into chromatin (Schneiderman et al., 2012). In hira mutants, H3 occupancy is affected at many genomic locations including active genes as well as transcriptionally repressed centromeric and pericentromeric repeats or transposons (this study; Duc et al., 2015). By contrast, in atrx mutants, H3 occupancy changes are moderate compared with hira and the majority of loci with differential H3.3 occupancy identified in the ChIP-seq data set (70%) situate in genes. Therefore, ATRX is rather implicated in deposition of the histone variant H3.3 in genes and may function as a fail-safe mechanism for impaired HIR-mediated H3.3 deposition, which is consistent with the recent finding that a complete loss of H3.3 through the disruption of the three H3.3-encoding genes is lethal in Arabidopsis (Wollmann et al., 2017). Within the mammalian ATRX/DAXX complex, DAXX binds histone H3.3 and determines the variant specificity of the heterocomplex (Liu et al., 2012; Elsässer et al., 2012), while ATRX guides the heterocomplex to specific genomic sites through interaction with methylated histone tails. Arabidopsis ATRX contains a conserved ADD domain and interacts with H3 histones in Y2H assays and in planta. To date, no DAXX homolog could be identified in the Arabidopsis genome based on protein sequence homology (this study; Otero et al., 2014), consistent with the absence of the mammalian DAXX binding domain in Arabidopsis ATRX. The Arabidopsis ATRX therefore might affect H3.3 deposition by interacting with a functional homolog of DAXX or with yet unknown partners. The identification of these binding partners will be an interesting avenue to better understand ATRX function in Arabidopsis. An alternative hypothesis that cannot be completely excluded is that ATRX affects H3.3 occupancy in an indirect manner, e.g., by modulating chromatin organization due to its remodeling activity or via changes in the enrichment in other core histones. Indeed, in mammals, ATRX coimmunoprecipitates with macroH2A, and ATRX loss leads to increased macroH2A enrichment at specific genomic sites (Ratnakumar et al., 2012). Furthermore, genome-wide analysis of the distribution of ATRX and DAXX proteins in embryonic stem cells revealed only a relatively small percentage of common binding sites (He et al., 2015), suggesting that ATRX and DAXX may have independent functions at certain targets.
ATRX and Heterochromatin
In mammals, ATRX binds to repetitive satellite sequences and is involved in heterochromatin formation and silencing of certain transposable elements (He et al., 2015; Sadic et al., 2015; Voon et al., 2015). This is in agreement with the observation of H3.3 enrichment at pericentromeric domains in mouse embryonic stem cells (Drané et al., 2010; Goldberg et al., 2010; Wong et al., 2010) and at specific classes of transposable elements (Elsässer et al., 2015; He et al., 2015; Voon et al., 2015). In atrx mutant plants, however, transcriptional silencing of repetitive elements or transposons is not impaired, as monitored by qRT-PCR and RNA-seq. This discrepancy might be resolved by the fact that H3.3 marks active chromatin and telomeres in Arabidopsis, while repetitive elements and transposons are globally enriched in H3.1 and not in H3.3 (Shu et al., 2014; Vaquero-Sedas and Vega-Palas, 2013; Stroud et al., 2012; Wollmann et al., 2012; this study). Furthermore, in mammals, the localization of ATRX to heterochromatin requires binding to methylated H3K9 via the ADD domain (Dhayalan et al., 2011; Iwase et al., 2011; Eustermann et al., 2011). While we found that the overall structure of the ADD domain is conserved in Arabidopsis ATRX and that ATRX can bind histones, the amino acids Y203, Y204, and Q219, critical for binding to H3K9me3 (Iwase et al., 2011), are not conserved (Supplemental Figure 1A). Therefore, it can be envisaged that, in plants, ATRX may not be targeted specifically to heterochromatin but may rather localize to regions with ongoing nucleosome displacement (Schneiderman et al., 2009) or bind similarly to the HIR complex directly to nucleosome-free gaps in DNA to direct histone deposition (Schneiderman et al., 2012; Ray-Gallet et al., 2011).
ATRX and Chromatin Organization at 45S rDNA Loci
Chromatin modifications are known to modulate rRNA expression in various species (Sandmeier et al., 2002; McStay and Grummt, 2008; Grummt and Längst, 2013). Indeed, in most eukaryotes, 45S rRNA genes are present in excess, resulting in selective silencing of a subset of genes by epigenetic mechanisms involving histone modifications (Preuss and Pikaard, 2007; Pontvianne et al., 2012, 2013). In Arabidopsis, the 45S rDNA loci are highly enriched in H3.3, and its presence correlates with transcriptional activity of the loci (Shi et al., 2011; Shu et al., 2014). Loss of ATRX affects H3.3 occupancy at 45S rDNA loci, thereby leading to reduced nucleosomal occupancy. This is in agreement with genome-wide studies in mammals, which identified ATRX at ribosomal genes (Law et al., 2010; He et al., 2015). Notably, H3K9me2 levels are globally increased at 45S rDNA in atrx-1, and we could speculate that the increased amount of this mark compensates for reduced nucleosomal occupancy. The existence of 45S rDNA sequence variants in the Arabidopsis genome that differ by small indels (Pontvianne et al., 2010) allows to evidence changes in rRNA gene choice. Indeed, it has been previously reported that VAR1, which is the most abundant at the DNA level, is silenced in Col-0 wild-type plants. This is in agreement with higher histone occupancy and H3K9 methylation, along with reduced H3.3 occupancy when normalized to input, the opposite being observed for VAR3, corroborating their expression profiles. While the relative abundance of VAR1 rDNA as well as its enrichment in H3K9me2/H3 is unchanged, expression shifts to increased VAR2 and reduced VAR3 rRNA in the atrx mutant. This might be partly explained by increased VAR2 and decreased VAR3 abundance in the atrx-1 genome, as well as reduced histone occupancy and a lower H3K9me2 enrichment at VAR2. Changes in variant abundance have previously been observed in different mutants, such as nucleolin2 (Durut et al., 2014), atxr5 atxr6 (Mohannath et al., 2016; Pontvianne et al., 2012), or CAF-1 mutants (Mozgová et al., 2010; Pavlištová et al., 2016), and have been suggested to occur through gene conversion or selective rereplication coupled to intra-NOR recombination. Since 45S rRNA genes may form complex DNA structures prone to replication defects (Muchová et al., 2015), we could speculate that replication defects in atrx contribute to changes in relative 45S rDNA variant abundance. Given the downregulation of many ribosomal proteins in atrx-1 concomitant with different 45S rDNA variant expression, it would be of interest to explore further whether this observation hints toward communication between the different transcriptional machineries involved in ribosome synthesis (Laferté et al., 2006).
Role of ATRX in Genomic H3.3 Distribution and Gene Expression
The amount of the variant histone H3.3 relative to H4 is reduced in all analyzed cellular pools, likely due to the combination of a reduced chaperone-bound histone fraction, which is shuttled for degradation, and deficient H3.3 deposition. At the nucleosomal level, H3.3 occupancy is reduced in atrx at a genome-wide level in particular within genes, pointing out a role for ATRX in histone deposition. Most of these genes with reduced H3.3 occupancy belong to classes with medium to elevated H3.3 levels, suggesting that ATRX contributes to H3.3 deposition at regions globally rich in H3.3 and corresponding to key metabolic players such as genes coding for ribosomal proteins. Differential H3.3 enrichments were globally modest in comparison to the H3.3 differences observed at the ATRX locus, which is equally affected in all cell types. This was expected since differences in H3.3 incorporation are likely to occur in a stochastic manner in agreement with our genetic analysis showing that ATRX is not the only pathway involved in H3.3 deposition. Alternatively, H3.3 incorporation may be mediated by ATRX only in specific cell types.
H3.3 occupancy has been globally correlated with gene expression even though it might not be indispensable for transcription (Wollmann et al., 2012, 2017; Stroud et al., 2012). For genes belonging to classes with medium to high expression, we observed that downregulation of gene expression is associated with reduced H3.3 occupancy in atrx-1, suggesting a role for ATRX in favoring gene expression at these loci. By contrast, the genes that are upregulated in atrx-1 also predominantly display reduced H3.3 occupancy. Several explanations for this can be put forward, such as that H3.3 loss rather reflects reduced nucleosomal occupancy resulting from increased transcription and polymerase II abundance at these genes. However, a remarkable feature of these upregulated genes is their low expression while being enriched in H3.3, a characteristic recently described for certain subsets of genes (Liu et al., 2016). Hence, these genes are likely controlled by mechanisms different from those regulating constitutively expressed genes. Interestingly, upregulated genes notably correspond to genes associated with stress.
In conclusion, ATRX plays a major role in the maintenance of H3.3 variant occupancy at a genome-wide level at genes and at the 45S rRNA gene loci, which are H3.3-rich regions. Loss of ATRX affects H3.3 histone pools and H3.3 deposition and therefore modulates the H3.1/H3.3 balance in the cell. At 45S rRNA gene loci, ATRX loss results in altered 45S rRNA gene choice and at a genome-wide level in gene expression changes. Hence, the Arabidopsis ATRX protein shares some properties with its mammalian and invertebrate counterparts and functions in H3.3 deposition as part of a complementary pathway to HIR-mediated nucleosome assembly.
METHODS
Plant Material
Mutant Arabidopsis thaliana lines were obtained from the Nottingham Arabidopsis Stock Center and/or were gifts from other laboratories. We used the following mutant Arabidopsis lines atrx-1 (SALK_025687), atrx-2 (SAIL-861-B04), fas2-5 (SALK_147693) (Duc et al., 2015), hira-1 (WiscDsLox362H05) (Ingouff et al., 2010), ubn2-2 (GABI_018D02), cabin1-2 (SALK_099927), (Duc et al., 2015), the double mutant atxr5 (SALK_130607) atxr6 (SAIL_181_D09) (Jacob et al., 2009), atr-2 (SALK_032841) (Rounds and Larsen, 2008), and the triple mutant ku80 (FLAG_DMT5) xrcc1 (SALK_125373) xpf (N3819) (Charbonnel et al., 2011) kindly provided by S. Amiard (GReD). Except xrcc1 ku80 xpf, all mutants are in the Columbia background. Plants were grown on soil in a growth chamber under 16-h-light/8-h-dark cycles at 22°C (white fluorescent tubes, 100 to 130 µmol m−2 s−1). The eH3.1 and eH3.3 lines were generated by transcriptional fusion of the genomic fragment containing the promoter and the genomic coding region of either HTR9 or HTR5 (stop codon excluded) with the FLAG-HA tag and the OCS (octopine synthase) terminator using classic cloning with restriction enzymes and the Gateway technology. Monolocus homozygous lines were selected based on segregation of hygromycin resistance also encoded in the transgene, and further crosses to atrx-1 were performed. For in vitro culture, seeds were surface-sterilized and sown on germination medium containing 0.8% (w/v) agar, 1% (w/v) sucrose, and Murashige and Skoog salts (M0255; Duchefa Biochemie). After 2 d of stratification at 4°C in the dark, plants were grown under 16-h-light/8-h-dark cycles at 23°C (white fluorescent tubes, 120 µmol m−2 s−1).
Phylogenetic Analysis
To identify the Arabidopsis ATRX ortholog, we performed interspecies BLAST searches with the mammalian protein sequence. Conserved domains were aligned using the program Muscle with the ESPript (Easy Sequencing in PostScript) program (Robert and Gouet, 2014) with default settings. Trees were constructed from protein sequences (Supplemental Data Set 6). The evolutionary history was inferred by using the maximum likelihood method based on the JTT matrix-based model (Jones et al., 1992). The tree with the highest log likelihood (−15825.7621) was used. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Distance bootstrap analyses consisted of 1000 replicates. Initial trees for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using a JTT model and then selecting the topology with a superior log likelihood value. The tree is drawn to scale, with branch lengths measured in number of substitutions per site. The analysis involved 11 amino acid sequences. All positions containing gaps and missing data were eliminated. There were a total of 1002 positions in the final data set. Evolutionary analyses were conducted in MEGA6 (Tamura et al., 2013).
Yeast Two-Hybrid Assay
The full-length cDNAs encoding histones H3.1 and H3.3 were cloned into the pGBKT7 vector as baits and the sequence encoding full-length Arabidopsis ATRX was cloned into the pGADT7 vector as prey. Vectors pGADT7 and pGBKT7 were respectively transformed into Saccharomyces cerevisiae Y187 and AH109 strains based on the manufacturer’s instructions of the MatchMaker III GAL4 two-hybrid system (Clontech). Screening and interaction studies between preys and baits were performed by mating compatible yeast strains following Clontech’s instructions. Interactions between ATRX and histones H3.1 and H3.3 were determined by growing transformants on media depleted of leucine, tryptophan, and histidine following the manufacturer’s recommendations.
Confocal Images of ATRX-GFP in Nicotiana benthamiana
The full-length cDNA of ATRX was cloned behind the cauliflower mosaic virus 35S promoter in translational fusion with GFP at the C terminus into a plasmid using the Gateway technology (see Supplemental Table 4 for primer sequences). This plasmid was transiently expressed by agroinfiltration in N. benthamiana leaves with the viral silencing suppressor p19. Infected leaves were sectioned 3 to 5 d after infiltration. Infiltrated N. benthamiana leaves were imaged with Zeiss LSM 880 microscope (Carl Zeiss Vision) with a 40× objective.
Documentation of Phenotypes
Images of dissected siliques and flowers were taken using a Leica binocular and the LAS 3.6 software (Leica Microsystems), with 0.63× and 2× magnification, respectively. Viability of mature pollen grains was assayed as described (Alexander, 1969). Anthers from stained flowers were isolated and photographed using a Zeiss Axioplan microscope and Axiovision 4.2 software (Carl Zeiss Vision). For seed counting, we distinguished viable green seeds from unfertilized ovules as described (Duc et al., 2015). For rosette area measurement, digital photographs of double mutants and their respective single mutant sister plants were processed with the ImageJ (Fiji) software to measure rosette area (total area of rosette leaves).
RNA Extraction and RT-PCR
RNAs were extracted with Tri-Reagent (Euromedex) according to the manufacturer’s instructions, then treated with RQ1 DNase I (Promega) and purified with phenol-chloroform extraction. Reverse transcription was done either with oligo(dT)15 or with random hexamers using M-MLV reverse transcriptase (Promega). For analysis of ATRX expression in atrx alleles, reverse transcription was done with the ATRX_RT_Rev primer (5′-GGGACCCGTTGAACTTCCTCCC-3′) combined with random hexamers. Obtained cDNAs were diluted 1:3 and used in PCR (Promega Flexi) or in quantitative PCR with the LightCycler 480 SYBR Green I Master kit on the Roche LightCycler 480. Transcript levels of interest were normalized to MON1 (At2g28390) (Czechowski et al., 2005) or to UBC28 (At1g64230) for Supplemental Figure 2, using the comparative threshold cycle method. RT-qPCR histograms show means of transcript levels ±se obtained for two independent PCR amplifications of three biological replicates. The y axis shows the fold change relative to the wild type (wild type set to 1) after normalization to MON1 (At2g28390) expression. For analysis of silencing release, the following loci were tested: Ta3 and Athila LTR pericentromeric retrotransposons, Mule transposons At1g40097 and At1g43280, the Mu1 (At4g08680) transposons and SINE retrotransposon AtSN1 (At3TE63860) (Vaillant et al., 2006), the CACTA1 transposon and the T5L23.26 CACTA-like transposon (located at the heterochromatin knob of chromosome 4) (Ono et al., 2006), and the COPIA78 LTR transposon (Pecinka et al., 2010).
Analysis of 45S rDNA and rRNA Variants
For analysis of 45S rDNA expression, cDNA synthesis was performed on 2.5 µg of DNaseI-treated RNA with the 3allrRNAvar primer using M-MLV reverse transcriptase (Promega). Obtained cDNAs were diluted 1:2 and used in PCR (Promega Flexi) with 25 to 40 cycles. For the relative abundance of each class of rDNA and rRNA variants, PCR was performed with 5allrRNAvar/3allrRNAvar primers. Quantification of variants was performed on non-saturated signals using Multi Gauge software (Fujfilm).
ChIP Analysis
The 2.5-week-old plantlets were formaldehyde cross-linked, and ChIP was performed as previously described (Bowler et al., 2004) with minor modifications: Chromatin was sheared with the Diagenode Bioruptor (10 cycles of 30 s on and 1.5 min off). Protein A-coupled Dynabeads (Invitrogen) or Anti-FLAG M2 Magnetic beads (Sigma-Aldrich; M8823) were used, and the sonicated chromatin was precleared in presence of Invitrogen magnetic beads for 3 h, before immunoprecipitation with the anti-H3 antibody (Abcam; ab1791, lots GR242835-1, GR265016-1, and GR172700-1), with the anti-H3K9me2 antibody (Abcam; ab1220, lot GR166768-3), or with the Anti-FLAG M2 Magnetic beads (Sigma-Aldrich; M8823, lot SBL1128V). DNA was quantified using qPCR and normalized relatively to input.
Protein Extraction and Immunoblot Analysis
To recover the non-nucleosomal fraction of histones present in the cytoplasm and in the nucleoplasm, proteins were extracted from 100 mg of plantlets according to Durut et al. (2014). For the total extract corresponding to the histones present in the cytoplasm, nucleoplasm, chromatin, and the nucleus, proteins were recovered from 1 g of plantlets with Honda buffer (2.5% Ficoll 400, 5% Dextran T40, 0.4 M sucrose, 25 mM Tris-HCl, pH 7.4, 10 mM MgCl2, 10 mM β-mercaptoethanol, 0.5 mM PMSF, and complete Protease Inhibitor Cocktail Tablets [Roche]) (Honda et al., 1966). Briefly, ground tissues were homogenized in 15 mL of Honda buffer and filtered through a double layer of Miracloth (Millipore). After removal of an aliquot that constitutes the “total” extract, Triton X-100 was added at a 0.5% concentration and samples were submitted to rotation on a wheel at cold for 15 min. After 5 min centrifugation at 1500g, pellets were washed with Honda buffer containing 0.1% Triton X-100. These nucleus-rich preparations were centrifuged at 1500g for 5 min, and recovered pellets were washed with Honda buffer without Triton X-100. Nuclei were recovered after 5 min centrifugation at 1500g to constitute the “nuclear” fraction. Immunoblots were probed with the anti-H3 antibody (Abcam, ab1791, lots GR242835-1, GR265016-1, and GR172700-1; 1/3000), anti-HA antibody (Abcam; ab9110, lot GR235874-6; 1/1000), or with the anti-H4 antibody (Abcam; ab10158, lots GR264160-1 and GR273051-1; 1/1000). Equal loading was confirmed with an anti-Actin antibody (Sigma-Aldrich; clone 10-B2, A0480, lot 054M4805V; 1/3000). Primary antibodies were revealed by incubation with anti-rabbit (Abliance; BI2407, lot 14052; 1/3000) or anti-mouse (Abliance; BI2413C, lot 13101; 1/3000) secondary antibodies. Immunoblot chemiluminescence was revealed using ECL protein gel blotting detection reagents (GE Healthcare Biosciences). Densitometric analysis of immunoreactive protein bands was performed on nonsaturated signals using Multi Gauge software (Fujfilm) and H3, H4, and HA levels normalized to Actin with the wild type set to 100% and Actin being used as a loading control.
RNA-Seq Library Construction
RNAs were isolated from 2.5-week-old in vitro-grown plantlets using Tri-Reagent (Euromedex) and then treated with RQ1 DNase I (Promega) and purified with phenol-chloroform extraction. RNA-seq libraries were prepared using the TruSeq Stranded Total RNA sample preparation kit with Ribo-Zero Plant (Illumina) following the manufacturer’s protocol. All six samples, along with their inputs, were pooled together and run on one lane, each on a separate flow cell of an Illumina HiSeq 4000 for paired-end sequencing (GEO accession number GSE87918).
ChIP-Seq Assay
ChIP-seq was performed on chromatin from 2.5-week-old in vitro-grown plantlets sheared to mononucleosomes using Anti-FLAG M2 Magnetic beads (Sigma-Aldrich). Preparation of ChIP-seq samples and library construction were performed as described (Veluchamy et al., 2016). Single-end sequencing using Illumina GAIIx with a read length of 75 bp was performed on two eH3.3 and two atrx-1 eH3.3 ChIP samples, along with their inputs, at the IPS2 Transcriptomic platform (Paris-Saclay; GEO accession number GSE87918).
Analysis of ChIP-Seq Data
The quality of reads was checked using FASTQC (www.bioinformatics.babraham.ac.uk/projects/fastqc/) with a cutoff Phred score of 20. Reads with minimum length of 36 bp were kept after trimming using Trimmomatic. Parameters for Trimmomatic were set as follows: minimum length of 36 bp, mean Phred quality score greater than 30, leading and trailing base removal with base quality below 3, and sliding window of 4:15. Reads were then mapped onto TAIR10 using Bowtie with the unique read mapping parameter (Langmead and Salzberg, 2012). Peak detection was performed using MACS2 (Zhang et al., 2008). To analyze changes in location and occupancy of eH3.3 binding position, we used DANPOS2 (Chen et al., 2013). DANPOS2 settings were used as follows: minimum read depth, 5; window size, 50 bp; minimal distance between peaks, 100 bp. To analyze occupancy, we used unique reads mapping to a position and masked transposable element and low complexity regions. Analysis and visualization of the data were performed using the IGB browser (Integrated Genome Browser; Nicol et al., 2009). Mean and sd of the coverage depth were calculated and plotted using Qualimap.
Gene Ontology
Analysis for GO term enrichment was performed using the Agrigo tool (http://bioinfo.cau.edu.cn/agriGO/) (Du et al., 2010) with TAIR10 annotations. Down- and upregulated genes were analyzed separately.
Primers
All primer sequences are listed in Supplemental Table 4.
Accession Numbers
The sequencing data corresponding to RNA-seq and ChIP-seq libraries have been deposited in the Gene Expression Omnibus under accession number GSE87918. Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: At1g65470 (FAS1), At5g64630 (FAS2), At3g44530 (HIRA), At1g21610 (UBN1), At1g77310 (UBN2), At1g64230 (UBC28), At4g29130 (HXK1), At2g36060 (UEV1C), At2g15810 (Mule), At1g08600 (ATRX), and At2g28390 (MON1); and for the ATRX homologs: Populus trichocarpa (XP_002319663), Ricinus communis (XP_015576552), Medicago truncatula (XP_003590986), Glycine max (XP_003555577), Arabidopsis thaliana (AtATRX, NP_001184937), Brassica rapa (XP_018515512), Oryza sativa (XP_015614509), Mus musculus (MmATRX, AAC08741), Homo sapiens (HsATRX, NP_000480), Drosophila melanogaster (DmXNP, NP_651398 et DmADD1, NP_725094), and Caenorhabditis elegans (Q9U7E0).
Supplemental Data
Supplemental Figure 1. Evolutionary conservation of the ATRX chaperones and expression of Arabidopsis ATRX.
Supplemental Figure 2. Analysis of atrx mutant alleles.
Supplemental Figure 3. Genetic interaction between CAF-1 and HIR histone chaperones and ATRX.
Supplemental Figure 4. Effects of ATRX loss on H3K9me2 enrichment and expression of several silent loci.
Supplemental Figure 5. Effects of ATRX loss on H3.1 and H3.3 levels.
Supplemental Figure 6. Analysis of effects of ATRX loss on transcription by RNA-seq and H3.3 occupancy by ChIP-seq.
Supplemental Figure 7. Effects of ATRX loss at 45S rDNA loci.
Supplemental Table 1. Segregation analysis in F2 populations from crosses between atrx alleles and mutants in the HIR complex.
Supplemental Table 2. Segregation analysis in F3 populations from crosses between atrx alleles and mutants in the HIR complex.
Supplemental Table 3. Segregation analysis in F2 populations from crosses between fas2-5 and atrx alleles.
Supplemental Table 4. Primer list.
Supplemental Data Set 1. List of transposons differentially expressed in atrx-1.
Supplemental Data Set 2. List of the differential peaks identified in atrx-1 eH3.3.
Supplemental Data Set 3. List of the differential peaks identified in atrx-1 eH3.3 located in transposons.
Supplemental Data Set 4. List of the differential peaks identified in atrx-1 eH3.3 located in intergenic regions.
Supplemental Data Set 5. List of genes differentially expressed in atrx-1.
Supplemental Data Set 6. Alignments used to generate the phylogeny presented in Figure 1A.
Acknowledgments
We thank S. Amiard for seeds and help with the 5-ethynyl-2′-deoxyuridine detection, HU, and γ-irradiation experiments; P. Pouchin and N. Goué for help with bioinformatics analyses; A. Lhomme for technical assistance; E. Vanrobays for the NLS-GFP construct; O. Mittelsten Scheid for critical reading of the manuscript; and J. Sáez-Vásquez and F. Pontvianne for valuable advice with 45S rDNA variant analysis and for critical reading of the manuscript. The RNA-seq was performed by the IGBMC Microarray and Sequencing platform, a member of the “France Génomique” consortium (ANR grant ANR-10-INBS-0009). This work was supported by doctoral stipends from the Region Auvergne, ANR grants ‘Dynam’Het’ ANR-11 JSV2 009 01 and ‘SINUDYN’ ANR-12 ISV6 0001. This work was further supported by the CNRS, INSERM, and Université Clermont Auvergne.
AUTHOR CONTRIBUTIONS
C.D. and A.V.P. designed the research. C.D., M.B., G.D., L.S., D.L., S.L.G., A.P., S.C., and A.V.P. performed experiments. C.D., A.P., and M.J. gathered and analyzed RNA-seq data, and C.D., D.L., A.V., and M.B. gathered and analyzed ChIP-seq data. C.D. and A.V.P. analyzed the data. A.P. contributed analysis tools. C.D., C.T., and A.V.P. wrote the article.
Glossary
- HU
hydroxyurea
- ChIP
chromatin immunoprecipitation
- TEs
transposable elements
- GO
Gene Ontology
- RPKM
reads per kilobase per million mapped reads
- NOR
nucleolus organizer region
References
- Abou-Ellail M., Cooke R., Sáez-Vásquez J. (2011). Variations in a team: major and minor variants of Arabidopsis thaliana rDNA genes. Nucleus 2: 294–299. [DOI] [PubMed] [Google Scholar]
- Alekseyenko A.A., Gorchakov A.A., Zee B.M., Fuchs S.M., Kharchenko P.V., Kuroda M.I. (2014). Heterochromatin-associated interactions of Drosophila HP1a with dADD1, HIPP1, and repetitive RNAs. Genes Dev. 28: 1445–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander M.P. (1969). Differential staining of aborted and nonaborted pollen. Stain Technol. 44: 117–122. [DOI] [PubMed] [Google Scholar]
- Alexeev A., Mazin A., Kowalczykowski S.C. (2003). Rad54 protein possesses chromatin-remodeling activity stimulated by the Rad51-ssDNA nucleoprotein filament. Nat. Struct. Biol. 10: 182–186. [DOI] [PubMed] [Google Scholar]
- Banks D.D., Gloss L.M. (2004). Folding mechanism of the (H3-H4)2 histone tetramer of the core nucleosome. Protein Sci. 13: 1304–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett A.R., Cooper S.E., Ragab A., Travers A.A. (2008). The chromatin remodelling factor dATRX is involved in heterochromatin formation. PLoS One 3: e2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernatavichute Y.V., Zhang X., Cokus S., Pellegrini M., Jacobsen S.E. (2008). Genome-wide association of histone H3 lysine nine methylation with CHG DNA methylation in Arabidopsis thaliana. PLoS One 3: e3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler C., Benvenuto G., Laflamme P., Molino D., Probst A.V., Tariq M., Paszkowski J. (2004). Chromatin techniques for plant cells. Plant J. 39: 776–789. [DOI] [PubMed] [Google Scholar]
- Charbonnel C., Allain E., Gallego M.E., White C.I. (2011). Kinetic analysis of DNA double-strand break repair pathways in Arabidopsis. DNA Repair (Amst.) 10: 611–619. [DOI] [PubMed] [Google Scholar]
- Chen K., Xi Y., Pan X., Li Z., Kaestner K., Tyler J., Dent S., He X., Li W. (2013). DANPOS: dynamic analysis of nucleosome position and occupancy by sequencing. Genome Res. 23: 341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clynes D., Higgs D.R., Gibbons R.J. (2013). The chromatin remodeller ATRX: a repeat offender in human disease. Trends Biochem. Sci. 38: 461–466. [DOI] [PubMed] [Google Scholar]
- Collins N., Poot R.A., Kukimoto I., García-Jiménez C., Dellaire G., Varga-Weisz P.D. (2002). An ACF1-ISWI chromatin-remodeling complex is required for DNA replication through heterochromatin. Nat. Genet. 32: 627–632. [DOI] [PubMed] [Google Scholar]
- Czechowski T., Stitt M., Altmann T., Udvardi M.K., Scheible W.R. (2005). Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 139: 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhayalan A., Tamas R., Bock I., Tattermusch A., Dimitrova E., Kudithipudi S., Ragozin S., Jeltsch A. (2011). The ATRX-ADD domain binds to H3 tail peptides and reads the combined methylation state of K4 and K9. Hum. Mol. Genet. 20: 2195–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drané P., Ouararhni K., Depaux A., Shuaib M., Hamiche A. (2010). The death-associated protein DAXX is a novel histone chaperone involved in the replication-independent deposition of H3.3. Genes Dev. 24: 1253–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z., Zhou X., Ling Y., Zhang Z., Su Z. (2010). agriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Res. 38: W64–W70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duc C., Benoit M., Le Goff S., Simon L., Poulet A., Cotterell S., Tatout C., Probst A.V. (2015). The histone chaperone complex HIR maintains nucleosome occupancy and counterbalances impaired histone deposition in CAF-1 complex mutants. Plant J. 81: 707–722. [DOI] [PubMed] [Google Scholar]
- Durut N., et al. (2014). A duplicated NUCLEOLIN gene with antagonistic activity is required for chromatin organization of silent 45S rDNA in Arabidopsis. Plant Cell 26: 1330–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsässer S.J., Huang H., Lewis P.W., Chin J.W., Allis C.D., Patel D.J. (2012). DAXX envelops a histone H3.3-H4 dimer for H3.3-specific recognition. Nature 491: 560–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsässer S.J., Noh K.-M., Diaz N., Allis C.D., Banaszynski L.A. (2015). Histone H3.3 is required for endogenous retroviral element silencing in embryonic stem cells. Nature 522: 240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emelyanov A.V., Konev A.Y., Vershilova E., Fyodorov D.V. (2010). Protein complex of Drosophila ATRX/XNP and HP1a is required for the formation of pericentric beta-heterochromatin in vivo. J. Biol. Chem. 285: 15027–15037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eustermann S., Yang J.-C., Law M.J., Amos R., Chapman L.M., Jelinska C., Garrick D., Clynes D., Gibbons R.J., Rhodes D., Higgs D.R., Neuhaus D. (2011). Combinatorial readout of histone H3 modifications specifies localization of ATRX to heterochromatin. Nat. Struct. Mol. Biol. 18: 777–782. [DOI] [PubMed] [Google Scholar]
- Filipescu D., Szenker E., Almouzni G. (2013). Developmental roles of histone H3 variants and their chaperones. Trends Genet. 29: 630–640. [DOI] [PubMed] [Google Scholar]
- Fransz P., Armstrong S., Alonso-Blanco C., Fischer T.C., Torres-Ruiz R.A., Jones G. (1998). Cytogenetics for the model system Arabidopsis thaliana. Plant J. 13: 867–876. [DOI] [PubMed] [Google Scholar]
- Garrick D., Sharpe J.A., Arkell R., Dobbie L., Smith A.J.H., Wood W.G., Higgs D.R., Gibbons R.J. (2006). Loss of Atrx affects trophoblast development and the pattern of X-inactivation in extraembryonic tissues. PLoS Genet. 2: e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R.J., Picketts D.J., Higgs D.R. (1995a). Syndromal mental retardation due to mutations in a regulator of gene expression. Hum. Mol. Genet. 4: 1705–1709. [DOI] [PubMed] [Google Scholar]
- Gibbons R.J., Picketts D.J., Villard L., Higgs D.R. (1995b). Mutations in a putative global transcriptional regulator cause X-linked mental retardation with α-thalassemia (ATR-X syndrome). Cell 80: 837–845. [DOI] [PubMed] [Google Scholar]
- Gokhman D., Livyatan I., Sailaja B.S., Melcer S., Meshorer E. (2013). Multilayered chromatin analysis reveals E2f, Smad and Zfx as transcriptional regulators of histones. Nat. Struct. Mol. Biol. 20: 119–126. [DOI] [PubMed] [Google Scholar]
- Goldberg A.D., et al. (2010). Distinct factors control histone variant H3.3 localization at specific genomic regions. Cell 140: 678–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grummt I., Längst G. (2013). Epigenetic control of RNA polymerase I transcription in mammalian cells. Biochim. Biophys. Acta 1829: 393–404. [DOI] [PubMed] [Google Scholar]
- He Q., Kim H., Huang R., Lu W., Tang M., Shi F., Yang D., Zhang X., Huang J., Liu D., Songyang Z. (2015). The Daxx/Atrx complex protects tandem repetitive elements during DNA hypomethylation by promoting H3K9 trimethylation. Cell Stem Cell 17: 273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda S.I., Hongladarom T., Laties G.G. (1966). A new isolation medium for plant organelles. J. Exp. Bot. 17: 460–472. [Google Scholar]
- Huh M.S., Price O’Dea T., Ouazia D., McKay B.C., Parise G., Parks R.J., Rudnicki M.A., Picketts D.J. (2012). Compromised genomic integrity impedes muscle growth after Atrx inactivation. J. Clin. Invest. 122: 4412–4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingouff M., Rademacher S., Holec S., Soljić L., Xin N., Readshaw A., Foo S.H., Lahouze B., Sprunck S., Berger F. (2010). Zygotic resetting of the HISTONE 3 variant repertoire participates in epigenetic reprogramming in Arabidopsis. Curr. Biol. 20: 2137–2143. [DOI] [PubMed] [Google Scholar]
- Iwase S., Xiang B., Ghosh S., Ren T., Lewis P.W., Cochrane J.C., Allis C.D., Picketts D.J., Patel D.J., Li H., Shi Y. (2011). ATRX ADD domain links an atypical histone methylation recognition mechanism to human mental-retardation syndrome. Nat. Struct. Mol. Biol. 18: 769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob Y., Feng S., LeBlanc C.A., Bernatavichute Y.V., Stroud H., Cokus S., Johnson L.M., Pellegrini M., Jacobsen S.E., Michaels S.D. (2009). ATXR5 and ATXR6 are H3K27 monomethyltransferases required for chromatin structure and gene silencing. Nat. Struct. Mol. Biol. 16: 763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.T., Taylor W.R., Thornton J.M. (1992). The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 8: 275–282. [DOI] [PubMed] [Google Scholar]
- Kaya H., Shibahara K.I., Taoka K.I., Iwabuchi M., Stillman B., Araki T. (2001). FASCIATA genes for chromatin assembly factor-1 in Arabidopsis maintain the cellular organization of apical meristems. Cell 104: 131–142. [DOI] [PubMed] [Google Scholar]
- Konev A.Y., Tribus M., Park S.Y., Podhraski V., Lim C.Y., Emelyanov A.V., Vershilova E., Pirrotta V., Kadonaga J.T., Lusser A., Fyodorov D.V. (2007). CHD1 motor protein is required for deposition of histone variant H3.3 into chromatin in vivo. Science 317: 1087–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laferté A., Favry E., Sentenac A., Riva M., Carles C., Chédin S. (2006). The transcriptional activity of RNA polymerase I is a key determinant for the level of all ribosome components. Genes Dev. 20: 2030–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B., Salzberg S.L. (2012). Fast gapped-read alignment with Bowtie 2. Nat. Methods 9: 357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M.J., et al. (2010). ATR-X syndrome protein targets tandem repeats and influences allele-specific expression in a size-dependent manner. Cell 143: 367–378. [DOI] [PubMed] [Google Scholar]
- Lawrence R.J., Earley K., Pontes O., Silva M., Chen Z.J., Neves N., Viegas W., Pikaard C.S. (2004). A concerted DNA methylation/histone methylation switch regulates rRNA gene dosage control and nucleolar dominance. Mol. Cell 13: 599–609. [DOI] [PubMed] [Google Scholar]
- Lee N.G., Hong Y.K., Yu S.Y., Han S.Y., Geum D., Cho K.S. (2007). dXNP, a Drosophila homolog of XNP/ATRX, induces apoptosis via Jun-N-terminal kinase activation. FEBS Lett. 581: 2625–2632. [DOI] [PubMed] [Google Scholar]
- Leung J.W.-C., Ghosal G., Wang W., Shen X., Wang J., Li L., Chen J. (2013). Alpha thalassemia/mental retardation syndrome X-linked gene product ATRX is required for proper replication restart and cellular resistance to replication stress. J. Biol. Chem. 288: 6342–6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P.W., Elsaesser S.J., Noh K.M., Stadler S.C., Allis C.D. (2010). Daxx is an H3.3-specific histone chaperone and cooperates with ATRX in replication-independent chromatin assembly at telomeres. Proc. Natl. Acad. Sci. USA 107: 14075–14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Wang C., Wang G., Becker C., Zaidem M., Weigel D. (2016). Genome-wide analysis of chromatin packing in Arabidopsis thaliana at single-gene resolution. Genome Res. 26: 1057–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.-P., Xiong C., Wang M., Yu Z., Yang N., Chen P., Zhang Z., Li G., Xu R.-M. (2012). Structure of the variant histone H3.3-H4 heterodimer in complex with its chaperone DAXX. Nat. Struct. Mol. Biol. 19: 1287–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Falcón B., Meyer-Nava S., Hernández-Rodríguez B., Campos A., Montero D., Rudiño E., Vázquez M., Zurita M., Valadez-Graham V. (2014). Characterization of the Drosophila group ortholog to the amino-terminus of the alpha-thalassemia and mental retardation X-Linked (ATRX) vertebrate protein. PLoS One 9: e113182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell T.L., et al. (1999). Localization of a putative transcriptional regulator (ATRX) at pericentromeric heterochromatin and the short arms of acrocentric chromosomes. Proc. Natl. Acad. Sci. USA 96: 13983–13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McStay B., Grummt I. (2008). The epigenetics of rRNA genes: from molecular to chromosome biology. Annu. Rev. Cell Dev. Biol. 24: 131–157. [DOI] [PubMed] [Google Scholar]
- Mizuguchi G., Shen X., Landry J., Wu W.-H., Sen S., Wu C. (2004). ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science 303: 343–348. [DOI] [PubMed] [Google Scholar]
- Mohannath G., Pontvianne F., Pikaard C.S. (2016). Selective nucleolus organizer inactivation in Arabidopsis is a chromosome position-effect phenomenon. Proc. Natl. Acad. Sci. USA 113: 13426–13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozgová I., Mokros P., Fajkus J. (2010). Dysfunction of chromatin assembly factor 1 induces shortening of telomeres and loss of 45S rDNA in Arabidopsis thaliana. Plant Cell 22: 2768–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchová V., Amiard S., Mozgová I., Dvořáčková M., Gallego M.E., White C., Fajkus J. (2015). Homology-dependent repair is involved in 45S rDNA loss in plant CAF-1 mutants. Plant J. 81: 198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol J.W., Helt G.A., Blanchard S.G. Jr., Raja A., Loraine A.E. (2009). The Integrated Genome Browser: free software for distribution and exploration of genome-scale datasets. Bioinformatics 25: 2730–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie X., Wang H., Li J., Holec S., Berger F. (2014). The HIRA complex that deposits the histone H3.3 is conserved in Arabidopsis and facilitates transcriptional dynamics. Biol. Open 3: 794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T., Endo M., Singh M.B., Bhalla P.L. (2005). Analysis of the histone H3 gene family in Arabidopsis and identification of the male-gamete-specific variant AtMGH3. Plant J. 44: 557–568. [DOI] [PubMed] [Google Scholar]
- Ono T., Kaya H., Takeda S., Abe M., Ogawa Y., Kato M., Kakutani T., Mittelsten Scheid O., Araki T., Shibahara K. (2006). Chromatin assembly factor 1 ensures the stable maintenance of silent chromatin states in Arabidopsis. Genes Cells 11: 153–162. [DOI] [PubMed] [Google Scholar]
- Otero S., Desvoyes B., Gutierrez C. (2014). Histone H3 dynamics in plant cell cycle and development. Cytogenet. Genome Res. 143: 114–124. [DOI] [PubMed] [Google Scholar]
- Pavlištová V., Dvořáčková M., Jež M., Mozgová I., Mokroš P., Fajkus J. (2016). Phenotypic reversion in fas mutants of Arabidopsis thaliana by reintroduction of FAS genes: variable recovery of telomeres with major spatial rearrangements and transcriptional reprogramming of 45S rDNA genes. Plant J. 88: 411–424. [DOI] [PubMed] [Google Scholar]
- Pchelintsev N.A., McBryan T., Rai T.S., van Tuyn J., Ray-Gallet D., Almouzni G., Adams P.D. (2013). Placing the HIRA histone chaperone complex in the chromatin landscape. Cell Reports 3: 1012–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecinka A., Dinh H.Q., Baubec T., Rosa M., Lettner N., Mittelsten Scheid O. (2010). Epigenetic regulation of repetitive elements is attenuated by prolonged heat stress in Arabidopsis. Plant Cell 22: 3118–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontes O., Lawrence R.J., Neves N., Silva M., Lee J.-H., Chen Z.J., Viegas W., Pikaard C.S. (2003). Natural variation in nucleolar dominance reveals the relationship between nucleolus organizer chromatin topology and rRNA gene transcription in Arabidopsis. Proc. Natl. Acad. Sci. USA 100: 11418–11423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontvianne F., et al. (2010). Nucleolin is required for DNA methylation state and the expression of rRNA gene variants in Arabidopsis thaliana. PLoS Genet. 6: e1001225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontvianne F., Blevins T., Chandrasekhara C., Feng W., Stroud H., Jacobsen S.E., Michaels S.D., Pikaard C.S. (2012). Histone methyltransferases regulating rRNA gene dose and dosage control in Arabidopsis. Genes Dev. 26: 945–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontvianne F., Blevins T., Chandrasekhara C., Mozgová I., Hassel C., Pontes O.M., Tucker S., Mokros P., Muchová V., Fajkus J., Pikaard C.S. (2013). Subnuclear partitioning of rRNA genes between the nucleolus and nucleoplasm reflects alternative epiallelic states. Genes Dev. 27: 1545–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss S., Pikaard C.S. (2007). rRNA gene silencing and nucleolar dominance: insights into a chromosome-scale epigenetic on/off switch. Biochim. Biophys. Acta 1769: 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnakumar K., et al. (2012). ATRX-mediated chromatin association of histone variant macroH2A1 regulates α-globin expression. Genes Dev. 26: 433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray-Gallet D., Woolfe A., Vassias I., Pellentz C., Lacoste N., Puri A., Schultz D.C., Pchelintsev N.A., Adams P.D., Jansen L.E., Almouzni G. (2011). Dynamics of histone H3 deposition in vivo reveal a nucleosome gap-filling mechanism for H3.3 to maintain chromatin integrity. Mol. Cell 44: 928–941. [DOI] [PubMed] [Google Scholar]
- Ricketts M.D., Frederick B., Hoff H., Tang Y., Schultz D.C., Singh Rai T., Grazia Vizioli M., Adams P.D., Marmorstein R. (2015). Ubinuclein-1 confers histone H3.3-specific-binding by the HIRA histone chaperone complex. Nat. Commun. 6: 7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert X., Gouet P. (2014). Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 42: W320–W324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounds M.A., Larsen P.B. (2008). Aluminum-dependent root-growth inhibition in Arabidopsis results from AtATR-regulated cell-cycle arrest. Curr. Biol. 18: 1495–1500. [DOI] [PubMed] [Google Scholar]
- Sadic D., Schmidt K., Groh S., Kondofersky I., Ellwart J., Fuchs C., Theis F.J., Schotta G. (2015). Atrx promotes heterochromatin formation at retrotransposons. EMBO Rep. 16: 836–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmeier J.J., French S., Osheim Y., Cheung W.L., Gallo C.M., Beyer A.L., Smith J.S. (2002). RPD3 is required for the inactivation of yeast ribosomal DNA genes in stationary phase. EMBO J. 21: 4959–4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawatsubashi S., et al. (2010). A histone chaperone, DEK, transcriptionally coactivates a nuclear receptor. Genes Dev. 24: 159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman J.I., Orsi G.A., Hughes K.T., Loppin B., Ahmad K. (2012). Nucleosome-depleted chromatin gaps recruit assembly factors for the H3.3 histone variant. Proc. Natl. Acad. Sci. USA 109: 19721–19726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman J.I., Sakai A., Goldstein S., Ahmad K. (2009). The XNP remodeler targets dynamic chromatin in Drosophila. Proc. Natl. Acad. Sci. USA 106: 14472–14477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaked H., Avivi-Ragolsky N., Levy A.A. (2006). Involvement of the Arabidopsis SWI2/SNF2 chromatin remodeling gene family in DNA damage response and recombination. Genetics 173: 985–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L., Wang J., Hong F., Spector D.L., Fang Y. (2011). Four amino acids guide the assembly or disassembly of Arabidopsis histone H3.3-containing nucleosomes. Proc. Natl. Acad. Sci. USA 108: 10574–10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu H., Nakamura M., Siretskiy A., Borghi L., Moraes I., Wildhaber T., Gruissem W., Hennig L. (2014). Arabidopsis replacement histone variant H3.3 occupies promoters of regulated genes. Genome Biol. 15: R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S., Stillman B. (1989). Purification and characterization of CAF-I, a human cell factor required for chromatin assembly during DNA replication in vitro. Cell 58: 15–25. [DOI] [PubMed] [Google Scholar]
- Steimer A., Amedeo P., Afsar K., Fransz P., Mittelsten Scheid O., Paszkowski J. (2000). Endogenous targets of transcriptional gene silencing in Arabidopsis. Plant Cell 12: 1165–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud H., Greenberg M.V., Feng S., Bernatavichute Y.V., Jacobsen S.E. (2013). Comprehensive analysis of silencing mutants reveals complex regulation of the Arabidopsis methylome. Cell 152: 352–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud H., Otero S., Desvoyes B., Ramírez-Parra E., Jacobsen S.E., Gutierrez C. (2012). Genome-wide analysis of histone H3.1 and H3.3 variants in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 109: 5370–5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagami H., Ray-Gallet D., Almouzni G., Nakatani Y. (2004). Histone H3.1 and H3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell 116: 51–61. [DOI] [PubMed] [Google Scholar]
- Talbert P.B., et al. (2012). A unified phylogeny-based nomenclature for histone variants. Epigenetics Chromatin 5: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbert P.B., Henikoff S. (2010). Histone variants--ancient wrap artists of the epigenome. Nat. Rev. Mol. Cell Biol. 11: 264–275. [DOI] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. (2013). MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J., Wu S., Liu H., Stratt R., Barak O.G., Shiekhattar R., Picketts D.J., Yang X. (2004). A novel transcription regulatory complex containing death domain-associated protein and the ATR-X syndrome protein. J. Biol. Chem. 279: 20369–20377. [DOI] [PubMed] [Google Scholar]
- Thompson H.L., Schmidt R., Dean C. (1996). Identification and distribution of seven classes of middle-repetitive DNA in the Arabidopsis thaliana genome. Nucleic Acids Res. 24: 3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillant I., Schubert I., Tourmente S., Mathieu O. (2006). MOM1 mediates DNA-methylation-independent silencing of repetitive sequences in Arabidopsis. EMBO Rep. 7: 1273–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquero-Sedas M.I., Vega-Palas M.A. (2013). Differential association of Arabidopsis telomeres and centromeres with histone H3 variants. Sci. Rep. 3: 1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veluchamy A., Jégu T., Ariel F., Latrasse D., Mariappan K.G., Kim S.K., Crespi M., Hirt H., Bergounioux C., Raynaud C., Benhamed M. (2016). LHP1 regulates H3K27me3 spreading and shapes the three-dimensional conformation of the Arabidopsis genome. PLoS One 11: e0158936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon H.P.J., Hughes J.R., Rode C., De La Rosa-Velázquez I.A., Jenuwein T., Feil R., Higgs D.R., Gibbons R.J. (2015). ATRX plays a key role in maintaining silencing at interstitial heterochromatic loci and imprinted genes. Cell Reports 11: 405–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmann H., et al. (2017). The histone H3 variant H3.3 regulates gene body DNA methylation in Arabidopsis thaliana. Genome Biol. 18: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmann H., Holec S., Alden K., Clarke N.D., Jacques P.É., Berger F. (2012). Dynamic deposition of histone variant H3.3 accompanies developmental remodeling of the Arabidopsis transcriptome. PLoS Genet. 8: e1002658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong L.H., McGhie J.D., Sim M., Anderson M.A., Ahn S., Hannan R.D., George A.J., Morgan K.A., Mann J.R., Choo K.H. (2010). ATRX interacts with H3.3 in maintaining telomere structural integrity in pluripotent embryonic stem cells. Genome Res. 20: 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y., Gibbons R., Yan Z., Yang D., McDowell T.L., Sechi S., Qin J., Zhou S., Higgs D., Wang W. (2003). The ATRX syndrome protein forms a chromatin-remodeling complex with Daxx and localizes in promyelocytic leukemia nuclear bodies. Proc. Natl. Acad. Sci. USA 100: 10635–10640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Liu T., Meyer C.A., Eeckhoute J., Johnson D.S., Bernstein B.E., Nusbaum C., Myers R.M., Brown M., Li W., Liu X.S. (2008). Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9: R137. [DOI] [PMC free article] [PubMed] [Google Scholar]