Bacterial pathogens use the type III secretion system to deliver dozens of effector proteins into host cells; for example, the plant pathogen Pseudomonas syringae can deliver more than 30 effectors. These effectors have multiple, nefarious functions that help the pathogen thrive in its host (reviewed in Toruño et al., 2016). Effectors target conserved host processes including pathways for entry into the host (for example, regulation of stomatal aperture in plants, perception of pathogens, regulation of cell death, transcription, RNA interference, defensive proteases, and other enzymes such as chitinases). Moreover, work in filamentous pathogens showed that pathogens do not indiscriminately unload all of their effectors at once—rather, the expression of different effectors peaks at different stages of infection, and in different organs. Depending on their target cellular process, effectors can localize to different subcellular compartments; for example, transcriptional activator-like effectors might localize to the nucleus and other effectors localize to the plasma membrane, cytoplasm, or other compartments.
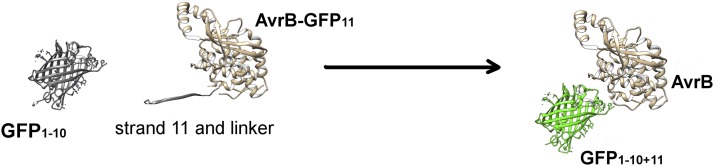
Based on this diversity of localization and expression, being able to track effector delivery and examine their localization will provide important information on effector function. However, effectors generally are low in abundance and large fluorescent tags can impede effector secretion. Work in mammalian systems used a split fluorescent protein system to visualize delivery of an effector into animal cells in culture. The β-barrel structure of GFP has 11 β-strands. For the split-GFP system, a nonfluorescent GFP protein with strands 1 to 10 (GFP1–10) is expressed in host cells and the effector is linked to the eleventh β-strand (GFP11). GFP maturation requires a conserved amino acid in strand 11; when the two proteins meet, reconstitution of the complete structure gives a fluorescent GFP (see figure). Work in mammalian systems has improved the solubility, folding, and assembly of the split-GFP system, and incorporated color variants.
The β-barrel structure of GFP contains 11 β-strands. The split-GFP approach uses GFP1–10, which contains the first 10 β-strands and is expressed in the host cell (and possibly localized to a subcellular compartment). The eleventh strand is attached to the effector molecule of interest, in this case AvrB (AvrB-GFP11). In the cell, the two proteins can reconstitute a fluorescent protein. (Reprinted from Henry et al. [2017], Figure 2A.)
To examine effectors in plant cells, previous studies have used transient overexpression from constitutive promoters, but such assays are prone to artifacts and plant cells have high autofluorescence. To remedy this, two Breakthrough Reports in the July issue of The Plant Cell used a split-GFP system to examine bacterial effector delivery in plant cells of intact plants, not in culture. Both studies used a super-folder version of GFP (sfGFP) and tested that the effectors retained the ability to induce cell death and host defenses (monitored as a reduction in bacterial growth) when each effector was fused to GFP11. The authors also tested whether the effector-GFP11 fusion could reconstitute a fluorescent GFP when delivered via Agrobacterium tumefaciens into plants expressing GFP1–10.
In one Breakthrough Report, Henry et al. (2017) examined the effectors in different plant tissues and organs. They first used dip inoculation and monitored cell death to show that Arabidopsis thaliana cells in the epidermis, mesophyll, and guard cells of the leaf could respond to the P. syringae pv tomato (Pst) effector AvrB. The authors examined AvrB, AvrPto, and AvrPtoB fused to GFP11 and showed delivery of these effectors into different cell types of Arabidopsis cells expressing GFP1–10; to avoid inducing cell death and defenses, they used Arabidopsis mutants for the host components require to perceive these effectors. After syringe inoculation, which directly delivers bacteria into the apoplast, they observed effector delivery in mesophyll and epidermal pavement cells. By contrast, after surface inoculation, they observed increased delivery in guard cells and epidermal pavement cells. Given the difference in size between the bacteria (∼1 μm) and the plant cells (∼10–200 μm), the authors also looked for fluorescent foci to examine whether multiple bacteria could inject effectors into the same cell. Indeed, they found 1 to 25 foci per cell in epidermal cells.
In addition to Pst, which infects leaves, the authors also used the split-GFP system in Ralstonia solanacearum, which infects the vascular system and causes bacterial wilt disease in hundreds of different plant species. The R. solanacearum PopP2 effector localizes to the nucleus and remained functional when fused to GFP11. Root inoculation of Arabidopsis expressing cytoplasmic GFP1–10 showed signal in nuclei in cells around the sites where lateral roots emerge and in vascular cells in the shoot. Therefore, the authors showed that the split-GFP system can be used in multiple cell types and with different bacterial pathogens.
In a complementary approach, a Breakthrough Report by Park et al. (2017) examined effectors in various subcellular compartments. The authors generated a set of transgenic Arabidopsis plants expressing GFP1–10 in the nucleus, Golgi, endoplasmic reticulum, plasma membrane, mitochondria, plastids, peroxisome, and cytoplasm. To examine whether the system could detect effectors, they used cytoplasmic GFP1–10 in Nicotiana benthamiana and effector-GFP11 in a Pst strain engineered to lack most effectors. Reasoning that pathogen-triggered immunity might kill the bacteria before they could deliver the tagged effector, the authors used a Pst strain that retained a few effectors that inhibit host immunity.
AvrB, a plasma membrane-localized effector from P. syringae pv glycinea, gave a fluorescent signal at the plasma membrane when AvrB-GFP11 was tested with transgenic Arabidopsis expressing cytoplasmic or plasma membrane-localized GFP1–10. Intriguingly, repeating these experiments in N. benthamiana showed transient vesicular localization, indicating that AvrB might undergo trafficking in plant cells. The authors observed localization of AvrRps4 (whole and different domains) to the nucleus, cytoplasm, and chloroplasts; although most of their results were consistent with studies using transient, constitutive expression, some observations did differ (specifically regarding the plastid localization of an N-terminal fragment of AvrRps4), indicating the importance of confirming results from overexpression studies.
These studies establish the split fluorescent protein system as a useful tool to examine the temporal and spatial dynamics of effector biology in plants. Future research will focus on improving the relatively weak signal, possibly by using the stronger yellow fluorescent protein, or multimers of the GFP11 tag, although it remains to be seen whether that affects delivery of effectors from bacteria. Moreover, advances in single-molecule imaging may enable detailed studies of the dynamics of plasma membrane-localized effectors. As Park et al. point out, secretion using a bacterial system might help in examination of fungal effectors, where split-GFP studies have been hampered, likely by aggregation and high autofluorescence at the fungal penetration site. Additional experiments will be required to test if the reconstituted GFP affects effector localization. Perhaps cytoplasmic GFP can be used to examine localization to multiple compartments, or reconstitution of the fluorescent protein may capture the effector in a specific compartment.
Footnotes
Articles can be viewed without a subscription.
References
- Henry E., Toruño T.Y., Jauneau A., Deslandes L., Coaker G.L. (2017). Direct and indirect visualization of bacterial effector delivery into diverse plant cell types during infection. Plant Cell 29: 1555–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E., Lee H.-Y., Woo J., Choi D., Dinesh-Kumar S.P. (2017). Spatiotemporal monitoring of Pseudomonas syringae effectors via type III secretion using split fluorescent protein fragments. Plant Cell 29: 1571–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toruño T.Y., Stergiopoulos I., Coaker G. (2016). Plant-pathogen effectors: cellular probes interfering with plant defenses in spatial and temporal manners. Annu. Rev. Phytopathol. 54: 419–441. [DOI] [PMC free article] [PubMed] [Google Scholar]