Abstract
Background
Critically ill patients with acute kidney injury (AKI) can be divided into two subphenotypes, resolving or nonresolving, on the basis of the trajectory of serum creatinine. It is unknown if the biology underlying these two AKI recovery patterns is different.
Methods
We measured eight circulating biomarkers in plasma obtained from a cohort of patients admitted to an intensive care unit (ICU) (n = 1241) with systemic inflammatory response syndrome. The biomarkers were representative of several biologic processes: apoptosis (soluble Fas), inflammation (soluble tumor necrosis factor receptor 1, interleukin 6, interleukin 8) and endothelial dysfunction, (angiopoietin 1, angiopoietin 2, and soluble vascular cell adhesion molecule 1). We tested for associations between biomarker levels and AKI subphenotypes using relative risk regression accounting for multiple hypotheses with the Bonferroni correction.
Results
During the first 3 days of ICU admission, 868 (70%) subjects developed AKI; 502 (40%) had a resolving subphenotype, and 366 (29%) had a nonresolving subphenotype. Hospital mortality was 12% in the resolving subphenotype and 21% in the nonresolving subphenotype. Soluble Fas was the only biomarker associated with a nonresolving subphenotype after adjustment for age, body mass index, diabetes, and Acute Physiology and Chronic Health Evaluation III score (p = 0.005).
Conclusions
Identifying modifiable targets in the Fas-mediated pathway may lead to strategies for prevention and treatment of a clinically important form of AKI.
Electronic supplementary material
The online version of this article (doi:10.1186/s13054-017-1807-x) contains supplementary material, which is available to authorized users.
Keywords: Apoptosis, Acute kidney injury, Biomarkers
Background
Acute kidney injury (AKI) is common in the intensive care unit (ICU) and is associated with substantial morbidity and mortality [1–5]. Development of AKI occurs in response to a variety of toxic, inflammatory, and ischemic events, with the most common predisposing risk being sepsis. Biomarkers of functional (serum creatinine [SCr], blood urea nitrogen) or structural (neutrophil gelatinase-associated lipocalin, kidney injury molecule 1) kidney injury have been shown to have some utility in the early identification of AKI [6–8]. However, further exploration of the biologic pathways linked to the development of more severe forms of AKI is needed.
AKI is a heterogeneous entity, and increasing evidence shows that the Kidney Disease: Improving Global Outcomes (KDIGO) criteria for AKI severity may not adequately capture this heterogeneity [9–11]. In clinical trials, this heterogeneity may obscure treatment effects that are present only in subgroups of patients, potentially contributing to the increasing number of negative interventional trials in AKI [12]. We recently identified two AKI subphenotypes (resolving and nonresolving) on the basis of the trajectory of SCr in the first 3 days after hospital presentation [13]. A resolving trajectory, defined as a decrease in SCr of 0.3 mg/dl or 25% from the maximum, was associated with the same risk of hospital mortality as that of subjects with no AKI. A nonresolving trajectory, defined as AKI that did not meet the resolving criteria, was associated with a 60% higher risk of hospital mortality relative to patients with no AKI, even after adjusting for KDIGO stage of AKI and other potential confounders. Traditional prognostic risk factors, such as Acute Physiology and Chronic Health Evaluation III (APACHE III) score, vasopressor use, or sepsis status, did not differ across AKI subphenotypes. Prior work, primarily in animal models, has implicated different biologic processes, such as apoptosis, inflammation, and endothelial dysfunction, in the pathogenesis of AKI [14–17]. It is not yet known whether these different pathophysiologic processes might contribute to the development of a nonresolving as opposed to a resolving AKI subphenotype.
In this report, we present results of analyses based on testing of whether circulating levels of biomarkers representative of two main biologic pathways, endothelial dysfunction (angiopoietin 1 [Ang-1], angiopoietin-2 [Ang-2], angiopoietin ratio [Ang-2/Ang-1], soluble vascular cell adhesion molecule [sVCAM]) and inflammation/apoptosis (soluble Fas [sFas], soluble tumor necrosis factor receptor 1 [sTNFR-1], interleukin-6 [IL-6], and IL-8), are differentially associated with AKI subphenotypes in a cohort of ICU patients with the systemic inflammatory response syndrome (SIRS). These analyses seek to shed light on the biology of these two distinct AKI subphenotypes, resolving versus nonresolving.
Methods
Study design
We performed this study using previously collected data from the Harborview Medical Center cohort with systemic inflammatory response syndrome (HMC-SIRS) [18]. The HMC-SIRS cohort comprised consecutively enrolled subjects meeting criteria for SIRS [19], excluding patients with major trauma, intracranial hemorrhage, HIV infection or immunosuppression, or a current diagnosis of cancer, as previously described [20–22]. For this study, we excluded subjects with end-stage renal disease prior to study enrollment (n = 43) or if they were missing SCr values on day 1 or 2 of enrollment (n = 42). AKI was defined as an increase in SCr of 0.3 mg/dl from a “baseline” SCr value or a decrease in urine output < 0.5 ml/kg/h over 24 h within the first 3 days of enrollment. We derived an approximation of the baseline SCr from the nadir measured over the 3-day period. This approach to defining AKI has been described previously [23–25]. AKI severity was determined using modified KDIGO criteria based on the maximal difference between the nadir creatinine and the maximal creatinine or the minimal urine output over the 3-day period.
AKI subphenotypes were defined as previously described [13]. The resolving subphenotype was defined by a decrease of 0.3 mg/dl or 25% in SCr from its maximum during the first 3 days of study enrollment. All subjects with AKI who did not meet this criterion were classified as having a nonresolving subphenotype [13]. Sepsis-2 was defined by the presence of a suspected infection in addition to SIRS. Sepsis-3 was defined by the presence of a suspected infection and a Sequential Organ Failure Assessment score of 2 points or more [26]. Septic shock was defined by the need for vasopressor therapy [26] in subjects with sepsis.
Biomarker values
Blood for plasma biomarker measurements was collected during the first 24 h of study enrollment. The blood was collected in ethylenediaminetetraacetic acid (EDTA)-treated sterile tubes and centrifuged. Plasma was then aliquoted and frozen at −80 °C. The samples were stored for a variable number of years, but the plasma samples were freeze-thawed a maximum of one time prior to running the biomarker measurements. All biomarkers were measured on the same day using electrochemiluminescence immunoassays (Meso Scale Discovery, Rockville, MD, USA). The biomarkers were measured for research purposes. The biomarkers were run in singlets, and we used an EDTA plasma control sample on each plate to assess assay performance. Samples were diluted to fit within the dynamic range of each assay: IL-6, IL-8, and sTNFR-1 were diluted 0.08–2500 pg/ml; Ang-1 was diluted 3–100,000 pg/ml; Ang-2 was diluted 0.5–10,000 pg/ml; sVCAM-1 was diluted 0.05–1000 pg/ml; and sFas was diluted 40–5000 pg/ml. The samples that fell below the lower limit of detection or above the upper limit of detection were assigned those values. The number of samples below or above the limit of detection is provided in Additional file 1: Table S1. The intra-assay coefficients of variation ranged from 12 to 15 for the biomarkers. Additionally, we remeasured a random subset of these samples and analyzed the replicates using Pearson’s correlation. The averaged Pearson’s correlation was 0.95 with an SD of 0.06.
Statistical analysis
For baseline characteristics, we report continuous variables as mean ± SD and categorical variables as number and percent. Approximately 6% or less of the study participants were missing data on APACHE III (<1%), body mass index (1.7%), and race (6%). For the regression analyses, data for participants with missing values for these covariates were imputed using chained equations and combined using Rubin’s rules [27]. No imputations were completed for exposure or outcome measures. Associations between AKI subphenotype and hospital mortality were identified using relative risk (RR) regression [28], given that hospital mortality in subjects with AKI was relatively common (i.e., > 15%). The final model was adjusted for age, sex, race, body mass index, diabetes mellitus, APACHE III score, vasopressor use, mechanical ventilation, and KDIGO stage of AKI [13]. The covariates were chosen a priori on the basis of biologic plausibility that they could confound the associations of biomarkers with AKI subphenotypes.
Plasma biomarker concentrations were tabulated by AKI status (no AKI, resolving and nonresolving) and reported as median and IQR. Biomarker levels were log2-transformed because they are known to be heavily right-skewed with a very wide range. A two-tailed t test was performed to evaluate the association of biomarkers with resolving versus nonresolving AKI subphenotypes.
Univariate and multivariate associations between biomarker concentrations and AKI subphenotype are presented as RRs per doubling of the biomarker concentration. We performed RR regression using a multivariate generalized linear model to test for associations between biomarker levels (independent variable) and AKI subphenotype (dependent variable). Gaussian model and robust SE estimates were used if the binomial function did not allow for model convergence. Variables to include in the model were decided a priori on the basis of biologic plausibility and prior literature [1, 2, 29, 30]. The first adjusted model included baseline age, diabetes mellitus, and body mass index. The second model added APACHE III scores, which were based on the maximum values during the first hospital day. Data are presented as RR and 95% CI. All analyses were performed using Stata release 13.1 software (StataCorp, College Station, TX, USA).
Results
Detailed characteristics of the HMC-SIRS cohort have been published previously [22, 31]. The average age in the HMC-SIRS cohort was 54 years (SD ±16); 65% were men; 77% were Caucasian; 28% had a history of diabetes mellitus; and 9% had chronic kidney disease. Sepsis-3 was the admission diagnosis of 58% of subjects. During the first 3 days of ICU admission, 64% of subjects required mechanical ventilation for any period, and 23% required vasopressors (Table 1). Of 1241 eligible subjects, 868 subjects (70%) developed AKI within 3 days of study enrollment. The incidence of AKI is consistent with prior reports identifying the rate of AKI in ICU populations [32, 33]. Of the subjects with AKI, 502 subjects had a resolving subphenotype, and 366 had a nonresolving subphenotype. The proportion of subjects with a nonresolving subphenotype was greater than in our prior work identifying AKI subphenotypes in post-trauma and mixed medical-surgical ICU populations [13]. Subjects with the resolving and nonresolving subphenotypes had similar characteristics in multiple categories, including age, sex, body mass index, APACHE III score, cirrhosis, chronic kidney disease, need for vasopressors, and maximum SCr during the first 72 h of ICU care. Subjects with the nonresolving subphenotype had a higher rate of Sepsis-3 (66% versus 57%).
Table 1.
Patient characteristics in Harborview Medical Center cohort with systemic inflammatory response syndrome
| Clinical variables | No AKI | AKI | Total | |
|---|---|---|---|---|
| Resolving AKI | Nonresolving AKI | |||
| Total | 373 | 502 | 366 | 1241 |
| Baseline demographics | ||||
| Age, years | 53 ± 17 | 55 ± 15 | 55 ± 17 | 54 ± 16 |
| Male sex, n (%) | 233 (63) | 323 (64) | 250 (68) | 806 (65) |
| Body mass index, kg/m2 | 29.4 ± 10.2 | 31.2 ± 16.5 | 32.0 ± 19.4 | 30.8 ± 15.6 |
| Race, n (%) | ||||
| Caucasian | 267 (77) | 360 (76) | 272 (79) | 899 (77) |
| African American | 41 (12) | 66 (14) | 29 (8) | 136 (12) |
| Asian | 26 (7) | 35 (7) | 29 (8) | 90 (8) |
| Native American | 15 (4) | 13 (3) | 14 (4) | 42 (4) |
| Unknown | 24 (6) | 28 (6) | 22 (6) | 74 (6) |
| APACHE III (24 h) | 38 ± 18 | 57 ± 27 | 55 ± 27 | 50 ± 26 |
| Comorbidities, n (%) | ||||
| Diabetes mellitus | 78 (21) | 167 (33) | 98 (27) | 343 (28) |
| Cirrhosis | 37 (10) | 39 (8) | 38 (10) | 114 (9) |
| Chronic kidney disease | 16 (4) | 55 (11) | 43 (12) | 114 (9) |
| Congestive heart failure | 17 (5) | 58 (12) | 24 (7) | 99 (8) |
| Chronic obstructive lung disease | 64 (17) | 82 (16) | 59 (16) | 205 (17) |
| ICU events | ||||
| Mechanical ventilation (72 h) | 178 (48) | 354 (71) | 261 (71) | 793 (64) |
| Vasopressors (72 h) | 39 (10) | 140 (28) | 108 (30) | 287 (23) |
| Sepsis-2 | 227 (61) | 348 (69) | 268 (73) | 843 (68) |
| Sepsis-3 | 190 (51) | 287 (57) | 242 (66) | 719 (58) |
| Septic shock | 34 (14) | 122 (24) | 90 (25) | 246 (20) |
| Admission Scr | 0.8 ± 0.4 | 1.9 ± 1.7 | 1.6 ± 1.9 | 1.5 ± 1.6 |
| Maximum SCr (72 h) | 0.8 ± 0.4 | 2.0 ± 1.8 | 2.2 ± 2.4 | 1.7 ± 1.8 |
| KDIGO stage of AKI | ||||
| Stage 0 | 373 (100) | N/A | N/A | 373 (30) |
| Stage 1 | N/A | 219 (44) | 158 (43) | 377 (30) |
| Stage 2 | N/A | 155 (31) | 91 (25) | 250 (20) |
| Stage 3 | N/A | 128 (26) | 117 (32) | 245 (20) |
Abbreviations: AKI Acute kidney injury, APACHE III Acute Physiology and Chronic Health Evaluation III, ICU Intensive care unit, KDIGO Kidney Disease: Improving Global Outcomes, SCr Serum creatinine
Data are shown as mean ± SD, number of subjects (%), or median (IQR), as appropriate
Because we tested eight different associations between biomarker concentrations and a nonresolving AKI subphenotype, we completed a correlation matrix using a Spearman’s correlation test to evaluate collinearity between biomarkers. Other than correlations of 0.66 between sFas and sTNFR-1 and 0.64 between sTNFR-1 and Ang-2, the rest of the correlations between biomarkers were < 0.6 (Additional file 1: Table S2). Thus, we chose the most conservative estimate of a Bonferroni-corrected p value cutoff of 0.05/8 = 0.00625 to test for significance.
AKI subphenotypes, risk of death, and need for renal replacement therapy
Consistent with our prior report, the nonresolving AKI subphenotype was associated with a greater risk of hospital mortality than in patients with no AKI after adjustment for multiple factors, including APACHE III score, mechanical ventilation, vasopressor use, and KDIGO stage of AKI (Table 2) (adjusted RR [model C] 2.9, 95% CI 1.3, 6.4), whereas the resolving AKI subphenotype showed no increased risk of hospital mortality after adjustment for the same variables (Table 2) (adjusted RR [model C] 1.4, 95% CI 0.6, 3.7). There was an increased risk of death with a nonresolving AKI subphenotype within each KDIGO stage of AKI (Additional file 1: Table S3). To account for potential misclassification of AKI, we completed sensitivity analyses to evaluate the risk of a nonresolving subphenotype in a population of patients with KDIGO stage 2 or 3 AKI. In this population with more severe AKI, the risk of death in the nonresolving subphenotype persisted (Additional file 1: Table S4). Of all subjects in the cohort, 91 (7%) required new initiation of renal replacement therapy (RRT) during their hospitalization. Of the subjects with a resolving subphenotype, 23 (5%) required RRT compared with 65 (21%) with a nonresolving subphenotype. The need for RRT in the resolving subphenotype group was subsequent to a new AKI event. The adjusted risk of requiring inpatient initiation of RRT was greater in a nonresolving AKI subphenotype than among patients with a resolving AKI subphenotype (RR 9.7, 95% CI 2.1, 44.4) (Additional file 1: Table S5).
Table 2.
Risk for hospital mortality by Kidney Disease: Improving Global Outcomes stage and acute kidney injury subphenotype
| Relative risk (95% CI) | ||||||
|---|---|---|---|---|---|---|
| No. of patients | Deaths, n (%) | Unadjusted model | Adjusted model A | Adjusted model B | Adjusted model C | |
| No AKI | 373 | 11 (3) | 1.00 (reference) | |||
| KDIGO AKI stage | ||||||
| Stage 1 | 377 | 51 (14) | 4.5 (2.4, 8.6) | 3.9 (1.9, 7.7) | 2.2 (1.1, 4.6) | – |
| Stage 2 | 250 | 30 (12) | 4.0 (2.1, 7.9) | 3.3 (1.5, 7.0) | 1.6 (0.7, 3.9) | – |
| Stage 3 | 245 | 51 (21) | 7.0 (3.7, 13.1) | 5.9 (3.0, 11.6) | 1.9 (0.8, 4.4) | – |
| AKI subphenotype | ||||||
| Resolving | 502 | 57 (11) | 3.9 (2.1, 7.2) | 3.2 (1.5, 6.6) | 1.3 (0.6, 3.1) | 1.4 (0.6, 3.7) |
| Nonresolving | 366 | 75 (21) | 7.0 (3.8, 12.9) | 5.7 (3.0, 11.2) | 2.7 (1.3, 5.6) | 2.9 (1.3, 6.4) |
AKI Acute kidney injury, KDIGO Kidney Disease: Improving Global Outcomes
Adjustment variables were as follows:
Model A: age, sex, race
Model B: Model A + body mass index, diabetes mellitus, Acute Physiology and Chronic Health Evaluation III, vasopressor use, mechanical ventilation
Model C: Model B + KDIGO stage of AKI
Biomarker levels and risk for AKI subphenotypes
Among patients with AKI, univariate analysis showed that only sFas levels were significantly different between the resolving and nonresolving AKI subphenotypes after Bonferroni correction (Table 3). In multivariate analyses adjusting for potential confounders known to be associated with circulating biomarker levels and risk for AKI, including age, diabetes mellitus, body mass index, and APACHE III scores [1, 2, 34] (Table 4), we found that only sFas levels were associated with a nonresolving, as opposed to a resolving, AKI subphenotype (adjusted RR 1.16 per doubling of sFas levels, 95% CI 1.05, 1.28) after Bonferroni correction. Figure 1 shows the stepwise increase in sFas biomarker concentrations in those with no AKI, a resolving AKI subphenotype, and a nonresolving AKI subphenotype.
Table 3.
Plasma biomarker concentrations by acute kidney injury subphenotype
| Biomarker | No. of patients | Biomarker concentration, median (IQR) | |||
|---|---|---|---|---|---|
| No AKI | Resolving AKI | Nonresolving AKI | Resolving versus nonresolving (p value) | ||
| Endothelial dysfunction | |||||
| Ang-1, pg/ml | 1212 | 6382 (3114, 10,409) | 4393 (1957, 8856) | 4033 (1638, 8048) | 0.315 |
| Ang-2, pg/ml | 1221 | 7985 (4636, 14,996) | 14,924 (8367, 29,425) | 15,126 (7047, 35,138) | 0.287 |
| Ang-2/Ang-1 | 1212 | 1.3 (0.6, 3.5) | 3.6 (1.1, 12.4) | 3.6 (1.1, 18.1) | 0.039 |
| sVCAM-1, ng/ml | 1222 | 481 (382, 687) | 530 (388, 783) | 571 (446, 842) | 0.023 |
| Apoptosis and inflammation | |||||
| sTNFR-1, pg/ml | 1161 | 5380 (3961, 8000) | 10,063 (6147, 15,566) | 9838 (5765, 18,358) | 0.010 |
| sFas, pg/ml | 1223 | 8810 (6880, 11,926) | 11,586 (8095, 15,700) | 12,879 (8938, 17,682) | 0.001a |
| IL-6, pg/ml | 1149 | 75 (31, 178) | 137 (59, 351) | 147 (58, 375) | 0.536 |
| IL-8, pg/ml | 1160 | 11 (5, 20) | 13 (7, 35) | 14 (7, 33) | 0.420 |
Abbreviations: AKI Acute kidney injury, Ang-1 Angiopoietin 1, Ang-2 Angiopoietin 2, IL Interleukin, sFas Soluble Fas, sTNFR-1 Soluble tumor necrosis factor receptor 1, sVCAM-1 Soluble vascular cell adhesion molecule 1
a p < 0.00625 based on Bonferroni correction for multiple hypotheses
Table 4.
Associations between biomarker levels and risk of nonresolving acute kidney injury subphenotype
| Biomarkers | Unadjusted RRa (95% CI) | p Value | Adjustedb model A, RR (95% CI) | p Value | Adjusted model B, RR (95% CI) | p Value |
|---|---|---|---|---|---|---|
| Endothelial dysfunction | ||||||
| Ang-1 | 0.96 (0.91, 1.00) | 0.068 | 0.96 (0.91, 1.00) | 0.073 | 0.95 (0.91, 1.00) | 0.049 |
| Ang-2 | 1.00 (0.95, 1.07) | 0.850 | 0.99 (0.94, 1.06) | 0.851 | 1.00 (0.94, 1.07) | 0.923 |
| Ang-2/Ang-1 | 1.04 (1.00, 1.08) | 0.029 | 1.02 (0.98, 1.05) | 0.291 | 1.03 (1.00, 1.06) | 0.160 |
| sVCAM-1 | 1.12 (1.03, 1.22) | 0.007 | 1.11 (1.02, 1.21) | 0.017 | 1.11 (1.02, 1.21) | 0.016 |
| Apoptosis and inflammation | ||||||
| IL-6 | 1.00 (0.97, 1.05) | 0.604 | 1.00 (0.96, 1.04) | 0.977 | 1.00 (0.97, 1.04) | 0.830 |
| IL-8 | 1.01 (0.97, 1.05) | 0.718 | 1.00 (0.97, 1.04) | 0.781 | 1.00 (0.97, 1,05) | 0.676 |
| sFas | 1.21 (1.16, 1.28) | 0.001c | 1.14 (1.12, 1.26) | 0.001c | 1.16 (1.05, 1.28) | 0.005c |
| sTNFR-1 | 1.06 (0.98, 1.15) | 0.144 | 1.04 (0.96, 1.13) | 0.301 | 1.05 (0.97, 1.14) | 0.235 |
Abbreviations: Ang-1 Angiopoietin 1, Ang-2 Angiopoietin 2, IL Interleukin, RR Relative risk, sFas Soluble Fas, sTNFR-1 Soluble tumor necrosis factor receptor 1, sVCAM-1 Soluble vascular cell adhesion molecule 1
aRelative risks presented per doubling of each biomarker
bAdjustment variables were as follows:
Model A: age, diabetes mellitus, body mass index
Model B: model A + Acute Physiology and Chronic Health Evaluation III
c p < 0.00625 based on Bonferroni correction for multiple hypotheses
Fig. 1.
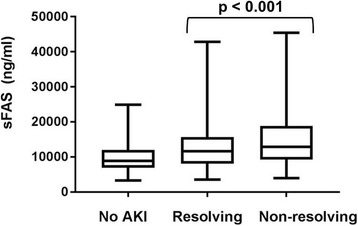
Soluble Fas (sFas) biomarker levels in the study cohort. Box plots showing median, interquartile range (box), and upper and lower adjacent values (bars) for biomarker levels, stratified by no acute kidney injury (AKI), resolving acute kidney injury, and nonresolving acute kidney injury. p Value is for comparison of resolving with nonresolving AKI
Subgroup analysis in septic shock
A greater percentage of patients in the nonresolving AKI subphenotype had septic shock (sepsis and requirement for vasopressor therapy during the first 72 h of ICU admission), potentially confounding our analyses. To minimize this possibility, we examined the subgroup of patients with septic shock (n = 205). In this subgroup, 34 (17%) had no AKI, 122 (60%) had a resolving subphenotype, and 90 (44%) had a nonresolving subphenotype. sFas continued to be strongly associated with a nonresolving subphenotype (RR 1.41, 95% CI 1.12, 1.80, p = 0.004). Of note, when we assessed for associations between biomarker levels and AKI subphenotype in the subgroup with septic shock, we found that, in addition to sFas, biomarkers of endothelial dysfunction were associated with AKI subphenotypes. Higher soluble VCAM (RR 1.29, 95% CI 1.08, 1.54, p = 0.005) and lower Ang-1 (RR 0.84, 95% CI 0.78, 0.89, p < 0.001) were associated with the nonresolving AKI subphenotype (Additional file 1: Table S6).
Discussion
In our analysis of a large cohort of critically ill subjects, we confirmed the presence of two AKI subphenotypes based on the trajectory of SCr in the first 3 days of ICU admission. As we previously demonstrated, subjects with a resolving AKI subphenotype have a similar risk of mortality and RRT as that of subjects with no AKI, but subjects with a nonresolving SCr trajectory have a twofold higher risk of death [13]. In contrast to a recently published work in which researchers excluded subjects with KDIGO stage 1 AKI to identify trajectories of AKI, we included all subjects with AKI in our analyses [11]. Minor changes in SCr are important [35], and KDIGO stage 1 AKI includes a large, heterogeneous population of all subjects with AKI (approximately 43% of subjects with AKI in our study were in KDIGO stage 1). To evaluate the pathophysiology of these distinct AKI subphenotypes, we measured plasma biomarkers associated with the development of AKI in key biologic pathways: inflammation, apoptosis, and endothelial dysfunction. We found that higher levels of sFas were associated with an elevated risk of developing a nonresolving AKI subphenotype.
Fas is a type 1 membrane protein that belongs to the tumor necrosis factor receptor 4 superfamily, which activates intracellular signaling after binding of Fas ligand (FasL) [36]. Fas ligation leads to a series of intracellular signaling events, culminating in activation of the death-inducing signaling complexes, which promote the activation of caspase-8-mediated apoptosis. Additionally, Fas ligation is believed to have an inflammatory role through cytokine production and then recruitment of proinflammatory cells [37]. sFas is a truncated form of Fas believed to result from proteolytic cleavage of membrane-bound receptors or alternative splicing of messenger RNA transcripts [38]. We have previously shown that genetic polymorphisms in FAS-related genes are associated with the development of AKI in subjects with acute respiratory distress syndrome (ARDS) [39]. Other studies have implicated the Fas pathway in the development of AKI in non-ARDS populations, such as patients with infection and chronic kidney disease [40–42]. Moreover, Ko et al. [14] showed in a murine model that a genetic deficiency of functional FasL protects mice from bilateral renal ischemia-reperfusion injury as measured by decreased apoptosis based on caspase 3 immunohistochemical staining, as well as decreases in SCr. Further, these authors also showed that pharmacologic blockade of FasL with an anti-FasL monoclonal immunoglobulin G antibody protected the kidneys of wild-type mice from ischemia-reperfusion injury.
It is well known that septic shock is a strong risk factor for AKI in the critically ill. We found a higher prevalence of sepsis and vasopressor use in the nonresolving AKI subphenotype than in the resolving AKI subphenotype. To account for the confounding of septic shock on the development of a nonresolving AKI subphenotype, we completed a sensitivity analysis limited to subjects with septic shock. We found that the association of sFas with increased risk of a nonresolving AKI subphenotype was unchanged. Of note, biomarker levels of endothelial dysfunction (Ang-1 and sVCAM) were also associated with risk for nonresolving AKI in the septic shock subset. These findings suggest that sFas is linked to the nonresolving AKI subphenotype, not just to the severity of sepsis, and that endothelial dysfunction may play a larger role in the development of AKI in patients with septic shock than in the overall AKI population.
The identification of distinct molecular profiles for different AKI subphenotypes has several potential implications. First, our findings suggest that inclusion of subjects with both resolving and nonresolving AKI subphenotypes in clinical trials of treatments for AKI adds a degree of disease heterogeneity that could reduce the efficacy of specific therapeutic interventions based on molecular mechanisms [43]. Second, our findings suggest that the Fas/FasL system may be differentially involved in the development of a nonresolving AKI subphenotype. We do not yet know whether the increased circulating levels of sFas are simply associated with or causal in the development of AKI. However, we do know from animal models that deficiency or blockade of the Fas/FasL system can protect from renal injury [14]. Thus, we favor a model in which increased sFas represents a byproduct of increased Fas pathway activity. Future studies will address the mechanistic role of sFas in the development of AKI. Third, our findings of associations between markers of endothelial dysfunction and the nonresolving AKI subphenotype in patients with septic shock support prior work demonstrating the protective effect of Ang1/Tie2 agonists in animal models of organ dysfunction in sepsis [44], and they suggest that targeting this pathway may have more of an impact in subjects with septic shock at risk for the nonresolving AKI subphenotype. Fourth, the identification of molecular subphenotypes in alternative heterogeneous diseases, such as asthma and lung cancer, has informed novel treatment strategies [45, 46]. Similarly, continued molecular characterization of AKI subphenotypes may identify therapeutic pathways for further investigation.
This study has several important strengths that support its novelty and robustness. First, to the best of our knowledge, this is the first study to link sFas concentrations and the development of AKI. Second, our use of a large, prospective ICU cohort allowed for more precise assessments of the associations between biomarkers and risk for AKI. Indeed, this is one of the largest published studies of biomarkers and risk for AKI. Third, we minimized the potential for type I error by using the Bonferroni correction to account for multiple hypothesis testing. Fourth, the simultaneous evaluation of two distinct pathways implicated in the pathogenesis of AKI allowed for a comparison of the relative strengths of association. Fifth, the association of sFas concentrations with a nonresolving subphenotype persisted in the subgroup of patients with severe AKI (KDIGO stage 2 or 3).
The study has several important limitations. First, our definition of “resolving” AKI required an SCr decrease of only 0.3 mg/dl or 25% from the maximum value. This relatively minimal decrease raises the possibility that a significant number of patients who actually had a nonresolving AKI subphenotype were misclassified as having a resolving AKI subphenotype. However, we have previously shown that this definition is superior to alternative definitions of the subphenotypes with regard to the separation by outcome (mortality) [13]. Even when stratified by KDIGO AKI stage, the patients with the nonresolving AKI subphenotype had a higher mortality and greater need for RRT than those with the resolving subphenotype. Also, misclassification of this type would be expected to add experimental noise and to have biased our results toward the null. Second, our study does not provide insight into the functional significance of elevated sFas in AKI. Future studies are needed to evaluate the relationship of sFas to sFasL and to address mechanistic questions of the role of sFas in AKI. Third, it is unknown if sFas is filtered from the glomerular capillary. In one prior study, researchers reported that sFas concentrations increased with worsening kidney function [47], but it is unknown if this increase was due to activation of the Fas/FasL system, entirely a function of decreased filtration of circulating sFas, or a combination of both. Fourth, clinical factors besides AKI, such as sepsis [48], major trauma [49], or active malignancy [50–52], have been associated with increased circulating levels of sFas. To account for these additional clinical factors, we excluded from our study patients with major trauma or active malignancy. Additionally, we completed a sensitivity analysis of patients with septic shock to determine if AKI influences sFas levels independently of sepsis.
Conclusions
We have shown that a biomarker of Fas pathway activity, sFas, is associated with the risk of developing a nonresolving AKI subphenotype in critically ill patients without major trauma, severe immune suppression, or active cancer. In contrast, biomarkers of endothelial dysfunction demonstrated an association with this subphenotype only in the subgroup of subjects with septic shock. These findings extend experimental data from animal models, suggesting that activation of the Fas pathway and, to a lesser extent, suppression of the Ang-1 axis play an important role in the pathogenesis of AKI. The continued molecular identification of AKI subphenotypes may allow recognition of subjects at high risk for poor outcomes, might facilitate the identification of novel therapeutic targets, and might allow for targeted enrollment in clinical trials.
Additional files
Supplemental data file that includes supplementary tables referenced in the text. (DOCX 32 kb)
Acknowledgements
The authors acknowledge the role of all support staff and participating patients in the study.
Funding
This work was supported by grants from the National Heart, Lung, and Blood Institute (R01 HL060710); the National Institute of Diabetes and Digestive and Kidney Diseases (F32DK112532); and the University of Washington Department of Medicine. The funding sources had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Availability of data and materials
The datasets generated during and/or analyzed during the present study are not publicly available, owing to currently ongoing research studies, but the data are available from the corresponding author on reasonable request.
Abbreviations
- AKI
Acute kidney injury
- Ang-1
Angiopoietin 1
- Ang-2
Angiopoietin 2
- APACHE III
Acute Physiology and Chronic Health Evaluation III
- ARDS
Acute respiratory distress syndrome
- EDTA
Ethylenediaminetetraacetic acid
- FasL
Fas ligand
- HMC-SIRS
Harborview Medical Center cohort with systemic inflammatory response syndrome
- ICU
Intensive care unit
- IL
Interleukin
- KDIGO
Kidney Disease: Improving Global Outcomes
- RR
Relative risk
- RRT
Renal replacement therapy
- SCr
Serum creatinine
- sFas
Soluble Fas
- SIRS
Systemic inflammatory response syndrome
- sTNFR-1
Soluble tumor necrosis factor receptor 1
- sVCAM
Soluble vascular cell adhesion molecule
Authors’ contributions
PKB, CM, CRC, WCL, JH, SRH, and MMW conceived of and designed the study. All authors acquired, analyzed, or interpreted data. PKB drafted the manuscript. All authors critically revised the manuscript for important intellectual content. PKB and CRC performed statistical analysis. JH, WCL, SRH, and MMW supervised the study. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The University of Washington Institutional Review Board approved this study. All patients provided necessary consent to participate in this study.
Consent for publication
No individual personal data are included in the study. All patients provided necessary consent to participate in this study.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s13054-017-1807-x) contains supplementary material, which is available to authorized users.
Contributor Information
Pavan K. Bhatraju, Phone: 206-897-5855, Email: bhatraju@uw.edu
Cassianne Robinson-Cohen, Email: cassyrc@uw.edu.
Carmen Mikacenic, Email: cmikacen@uw.edu.
Susanna Harju-Baker, Email: sharju-baker@medicine.washington.edu.
Victoria Dmyterko, Email: dmyterko@medicine.washington.edu.
Natalie S. J. Slivinski, Email: jonesn911@gmail.com
W. Conrad Liles, Email: wcliles@medicine.washington.edu.
Jonathan Himmelfarb, Email: himmej@u.washington.edu.
Susan R. Heckbert, Email: heckbert@uw.edu
Mark M. Wurfel, Email: mwurfel@uw.edu
References
- 1.Joannidis M, Metnitz PGH. Epidemiology and natural history of acute renal failure in the ICU. Crit Care Clin. 2005;21:239–49. doi: 10.1016/j.ccc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Hoste EAJ, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–23. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 3.Clermont G, Acker CG, Angus DC, Sirio CA, Pinsky MR, Johnson JP. Renal failure in the ICU: comparison of the impact of acute renal failure and end-stage renal disease on ICU outcomes. Kidney Int. 2002;62:986–96. doi: 10.1046/j.1523-1755.2002.00509.x. [DOI] [PubMed] [Google Scholar]
- 4.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–8. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 6.de Geus HRH, Bakker J, Lesaffre EMEH, le Noble JLML. Neutrophil gelatinase-associated lipocalin at ICU admission predicts for acute kidney injury in adult patients. Am J Respir Crit Care Med. 2011;183:907–14. doi: 10.1164/rccm.200908-1214OC. [DOI] [PubMed] [Google Scholar]
- 7.Siew ED, Ware LB, Bian A, Shintani A, Eden SK, Wickersham N, et al. Distinct injury markers for the early detection and prognosis of incident acute kidney injury in critically ill adults with preserved kidney function. Kidney Int. 2013;84:786–94. doi: 10.1038/ki.2013.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nejat M, Pickering JW, Devarajan P, Bonventre JV, Edelstein CL, Walker RJ, et al. Some biomarkers of acute kidney injury are increased in pre-renal acute injury. Kidney Int. 2012;81:1254–62. doi: 10.1038/ki.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coca SG, King JT, Rosenthal RA, Perkal MF, Parikh CR. The duration of postoperative acute kidney injury is an additional parameter predicting long-term survival in diabetic veterans. Kidney Int. 2010;78:926–33. doi: 10.1038/ki.2010.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perinel S, Vincent F, Lautrette A, Dellamonica J, Mariat C, Zeni F, et al. Transient and persistent acute kidney injury and the risk of hospital mortality in critically ill patients: results of a multicenter cohort study. Crit Care Med. 2015;43:e269–75. doi: 10.1097/CCM.0000000000001077. [DOI] [PubMed] [Google Scholar]
- 11.Kellum JA, Sileanu FE, Bihorac A, Hoste EAJ, Chawla LS. Recovery after acute kidney injury. Am J Respir Crit Care Med. 2017;195:784–91. doi: 10.1164/rccm.201604-0799OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ricci Z, Polito A, Polito A, Ronco C. The implications and management of septic acute kidney injury. Nat Rev Nephrol. 2011;7:218–25. doi: 10.1038/nrneph.2011.15. [DOI] [PubMed] [Google Scholar]
- 13.Bhatraju PK, Mukherjee P, Robinson-Cohen C, O’Keefe GE, Frank AJ, Christie JD, et al. Acute kidney injury subphenotypes based on creatinine trajectory identifies patients at increased risk of death. Crit Care. 2016;20:372. doi: 10.1186/s13054-016-1546-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ko GJ, Jang HR, Huang Y, Womer KL, Liu M, Higbee E, et al. Blocking Fas ligand on leukocytes attenuates kidney ischemia-reperfusion injury. J Am Soc Nephrol. 2011;22:732–42. doi: 10.1681/ASN.2010010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan HT, Tipping PG, Li XZ, Long DA, Woolf AS. Angiopoietin correlates with glomerular capillary loss in anti-glomerular basement membrane glomerulonephritis. Kidney Int. 2002;61:2078–89. doi: 10.1046/j.1523-1755.2002.00381.x. [DOI] [PubMed] [Google Scholar]
- 16.Jongman RM, van Klarenbosch J, Molema G, Zijlstra JG, de Vries AJ, van Meurs M. Angiopoietin/Tie2 dysbalance is associated with acute kidney injury after cardiac surgery assisted by cardiopulmonary bypass. PLoS One. 2015;10:e0136205. doi: 10.1371/journal.pone.0136205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simmons EM, Himmelfarb J, Tugrul S, Chertow GM, Mehta RL, Paganini EP, et al. Plasma cytokine levels predict mortality in patients with acute renal failure. Kidney Int. 2004;65:1357–65. doi: 10.1111/j.1523-1755.2004.00512.x. [DOI] [PubMed] [Google Scholar]
- 18.Glavan BJ, Holden TD, Goss CH, Black RA, Neff MJ, Nathens AB, et al. Genetic variation in the FAS gene and associations with acute lung injury. Am J Respir Crit Care Med. 2011;183:356–63. doi: 10.1164/rccm.201003-0351OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29:530–8. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- 20.Ko DC, Shukla KP, Fong C, Wasnick M, Brittnacher MJ, Wurfel MM, et al. A genome-wide in vitro bacterial-infection screen reveals human variation in the host response associated with inflammatory disease. Am J Hum Genet. 2009;85:214–27. doi: 10.1016/j.ajhg.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mikacenic C, Hahn WO, Price BL, Harju-Baker S, Katz R, Kain KC, et al. Biomarkers of endothelial activation are associated with poor outcome in critical illness. PLoS One. 2015;10:e0141251. doi: 10.1371/journal.pone.0141251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson-Cohen C, Katz R, Price BL, Harju-Baker S, Mikacenic C, Himmelfarb J, et al. Association of markers of endothelial dysregulation Ang1 and Ang2 with acute kidney injury in critically ill patients. Crit Care. 2016;20:207. doi: 10.1186/s13054-016-1385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu KD, Glidden DV, Eisner MD, Parsons PE, Ware LB, Wheeler A, et al. Predictive and pathogenetic value of plasma biomarkers for acute kidney injury in patients with acute lung injury. Crit Care Med. 2007;35:2755–61. doi: 10.1097/01.CCM.0000291649.72238.6D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu KL, Lee KT, Chang CH, Chen YC, Lin SM, Chu PH. Elevated plasma thrombomodulin and angiopoietin-2 predict the development of acute kidney injury in patients with acute myocardial infarction. Crit Care. 2014;18:R100. doi: 10.1186/cc13876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siew ED, Matheny ME, Ikizler TA, Lewis JB, Miller RA, Waitman LR, et al. Commonly used surrogates for baseline renal function affect the classification and prognosis of acute kidney injury. Kidney Int. 2010;77:536–42. doi: 10.1038/ki.2009.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubin DB. Multiple imputation for nonresponse in surveys. Hoboken, NJ: John Wiley & Sons; 2004. [Google Scholar]
- 28.Lumley T, Kronmal R, Ma S. Relative risk regression in medical research: models, contrasts, estimators, and algorithms. UW Biostatistics Working Paper 293. July 2006. http://biostats.bepress.com/uwbiostat/paper293. Date Accessed 8.10.17.
- 29.Siew ED, Davenport A. The growth of acute kidney injury: a rising tide or just closer attention to detail? Kidney Int. 2015;87:46–61. doi: 10.1038/ki.2014.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bagshaw SM, Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, et al. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol. 2007;2:431–9. doi: 10.2215/CJN.03681106. [DOI] [PubMed] [Google Scholar]
- 31.Hahn WO, Mikacenic C, Price BL, Harju-Baker S, Katz R, Himmelfarb J, et al. Host derived biomarkers of inflammation, apoptosis, and endothelial activation are associated with clinical outcomes in patients with bacteremia and sepsis regardless of microbial etiology. Virulence. 2016;7:387–94. doi: 10.1080/21505594.2016.1144003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu KD, Thompson BT, Ancukiewicz M, Steingrub JS, Douglas IS, Matthay MA, et al. Acute kidney injury in patients with acute lung injury: impact of fluid accumulation on classification of acute kidney injury and associated outcomes. Crit Care Med. 2011;39:2665–71. doi: 10.1097/CCM.0b013e318228234b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alobaidi R, Basu RK, Goldstein SL, Bagshaw SM. Sepsis-associated acute kidney injury. Semin Nephrol. 2015;35:2–11. doi: 10.1016/j.semnephrol.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bagshaw SM, George C, Bellomo R, ANZICS Database Management Committee Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care. 2008;12:R47. doi: 10.1186/cc6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–70. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka M, Suda T, Takahashi T, Nagata S. Expression of the functional soluble form of human Fas ligand in activated lymphocytes. EMBO J. 1995;14:1129–35. doi: 10.1002/j.1460-2075.1995.tb07096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park DR, Thomsen AR, Frevert CW, Pham U, Skerrett SJ, Kiener PA, et al. Fas (CD95) induces proinflammatory cytokine responses by human monocytes and monocyte-derived macrophages. J Immunol. 2003;170:6209–16. doi: 10.4049/jimmunol.170.12.6209. [DOI] [PubMed] [Google Scholar]
- 38.Cascino I, Fiucci G, Papoff G, Ruberti G. Three functional soluble forms of the human apoptosis-inducing Fas molecule are produced by alternative splicing. J Immunol. 1995;154:2706–13. [PubMed] [Google Scholar]
- 39.Bhatraju P, Hsu C, Mukherjee P, Glavan BJ, Burt A, Mikacenic C, et al. Associations between single nucleotide polymorphisms in the FAS pathway and acute kidney injury. Crit Care. 2015;19:368. doi: 10.1186/s13054-015-1084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schelling JR, Nkemere N, Kopp JB, Cleveland RP. Fas-dependent fratricidal apoptosis is a mechanism of tubular epithelial cell deletion in chronic renal failure. Lab Invest. 1998;78:813–24. [PubMed] [Google Scholar]
- 41.Ortiz-Arduan A, Danoff TM, Kalluri R, González-Cuadrado S, Karp SL, Elkon K, et al. Regulation of Fas and Fas ligand expression in cultured murine renal cells and in the kidney during endotoxemia. Am J Physiol. 1996;271:F1193–201. doi: 10.1152/ajprenal.1996.271.6.F1193. [DOI] [PubMed] [Google Scholar]
- 42.Góes MA, Iizuka IJ, Quinto BM, Dalboni MA, Monte JC, Santos BC, et al. Serum soluble-Fas, inflammation, and anemia in acute kidney injury. Artif Organs. 2013. doi:10.1111/aor.12019. [DOI] [PubMed]
- 43.Iwashyna TJ, Burke JF, Sussman JB, Prescott HC, Hayward RA, Angus DC. Implications of heterogeneity of treatment effect for reporting and analysis of randomized trials in critical care. Am J Respir Crit Care Med. 2015;192:1045–51. doi: 10.1164/rccm.201411-2125CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han S, Lee SJ, Kim KE, Lee HS, Oh N, Park I, et al. Amelioration of sepsis by TIE2 activation-induced vascular protection. Sci Transl Med. 2016;8:335ra55. doi: 10.1126/scitranslmed.aad9260. [DOI] [PubMed] [Google Scholar]
- 45.Fahy JV. Type 2 inflammation in asthma — present in most, absent in many. Nat Rev Immunol. 2015;15:57–65. doi: 10.1038/nri3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swanton C, Govindan R. Clinical implications of genomic discoveries in lung cancer. N Engl J Med. 2016;374:1864–73. doi: 10.1056/NEJMra1504688. [DOI] [PubMed] [Google Scholar]
- 47.Perianayagam MC, Murray SL, Balakrishnan VS, Guo D, King AJ, Pereira BJ, et al. Serum soluble Fas (CD95) and Fas ligand profiles in chronic kidney failure. J Lab Clin Med. 2000;136:320–7. doi: 10.1067/mlc.2000.109318. [DOI] [PubMed] [Google Scholar]
- 48.Doughty L, Clark RSB, Kaplan SS, Sasser H, Carcillo J. sFas and sFas ligand and pediatric sepsis-induced multiple organ failure syndrome. Pediatr Res. 2002;52:922–7. doi: 10.1203/00006450-200212000-00018. [DOI] [PubMed] [Google Scholar]
- 49.Paunel-Görgülü A, Flohé S, Scholz M, Windolf J, Lögters T. Increased serum soluble Fas after major trauma is associated with delayed neutrophil apoptosis and development of sepsis. Crit Care. 2011;15:R20. doi: 10.1186/cc9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zekri ARN, El-Din HMA, Bahnassy AA, Zayed NA, Mohamed WS, El-Masry SH, et al. Serum levels of soluble Fas, soluble tumor necrosis factor-receptor II, interleukin-2 receptor and interleukin-8 as early predictors of hepatocellular carcinoma in Egyptian patients with hepatitis C virus genotype-4. Comp Hepatol. 2010;9:1. doi: 10.1186/1476-5926-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jodo S, Kobayashi S, Nakajima Y, Matsunaga T, Nakayama N, Ogura N, et al. Elevated serum levels of soluble Fas/APO-1 (CD95) in patients with hepatocellular carcinoma. Clin Exp Immunol. 1998;112:166–71. doi: 10.1046/j.1365-2249.1998.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akhmedkhanov A, Lundin E, Guller S, Lukanova A, Micheli A, Ma Y, et al. Circulating soluble Fas levels and risk of ovarian cancer. BMC Cancer. 2003;3:33. doi: 10.1186/1471-2407-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental data file that includes supplementary tables referenced in the text. (DOCX 32 kb)
Data Availability Statement
The datasets generated during and/or analyzed during the present study are not publicly available, owing to currently ongoing research studies, but the data are available from the corresponding author on reasonable request.


