Abstract
Objective
To test the hypothesis that intrauterine hyperoxemia is associated with an increased risk of neonatal morbidity.
Methods
This was a secondary analysis of a prospective study of singleton, non-anomalous deliveries at or beyond 37° weeks at an institution with universal umbilical cord gas policy from 2010 to 2014. The primary outcome was a composite of neonatal morbidity including neonatal death, meconium aspiration syndrome, intubation, mechanical ventilation, hypoxic-ischemic encephalopathy, and hypothermic therapy. Intrauterine hyperoxemia was defined as cord venous PO2 ≥ 90th percentile of the cohort. Adjusted relative risks (aRR) were estimated for neonatal morbidity controlling for confounders. Analysis was performed for the entire cohort and stratified by the presence of acidemia defined as umbilical artery pH<7.1.
Results
Of 7,789 patients with validated paired cord gases, 106 (1.4%) had the composite neonatal morbidity. There was no difference in composite neonatal morbidity in patients with and without intrauterine hyperoxemia in the entire cohort (1.5% vs 1.3%, aRR 1.5, 95% CI 0.9–2.7). The rate of acidemia was not significantly different in the two groups (1.9% vs 1.8%, aRR 1.5, 95% CI 0.9–2.5). In stratified analysis, there was evidence of effect modification (p for interaction <0.001), with a significant association between intrauterine hyperoxemia and neonatal morbidity in the presence of acidemia (41.2% vs 21.4%, aRR 2.3, 95% CI 1.1–3.5), but not in its absence (0.8% vs 1.0%, aRR 1.0, 95% CI 0.5–2.2).
Conclusion
Intrauterine hyperoxemia, compared with normoxemia, is associated with a small, but significantly increased risk of neonatal morbidity in acidemic neonates.
Introduction
Maternal oxygen administration is a ubiquitously used intrapartum intervention to address concerning fetal heart rate tracing (FHT) patterns. This form of intrauterine resuscitation is often performed for FHT patterns that are thought to be associated with fetal hypoxemia or acidemia(1). The theoretical benefit of maternal hyperoxygenation is to increase oxygen delivery to the fetus via the umbilical cord vein, thereby reducing adverse outcomes related to fetal asphyxia.
Although animal and human studies have shown increased umbilical venous oxygen content and alleviation of fetal heart rate decelerations with maternal hyperoxygenation(2–5), there is a paucity of data linking hyperoxygenation to improved neonatal outcomes(6). Moreover, there are potential harmful effects of hyperoxygenation via free radical production (4). Hyperoxygenation in the setting of neonatal resuscitation has been shown to increase the risk of neonatal morbidity (6–11). Due to these findings, the American Academy of Pediatrics now recommend initial neonatal resuscitation with room air rather than supplemental oxygen(12, 13). The detrimental outcomes associated with hyperoxygenation at time of neonatal resuscitation demand a closer look at the safety of intrauterine fetal oxygen exposure.
We tested the hypothesis that intrauterine hyperoxemia is associated with an increased risk of neonatal morbidity by investigating the relationship between high fetal umbilical cord venous oxygen content at time of birth and neonatal outcomes. We were particularly interested in the relationship between neonatal morbidity and hyperoxemia in the setting of acidemia.
Materials and Methods
This was a secondary analysis of a large prospective cohort study at a tertiary care center with a universal cord gas policy from 2010 to 2014. This study was approved by the Washington University School of Medicine Human Research Protection Office.
Per institutional protocol, umbilical cord gases were collected for every patient by trained technicians. Both arterial and venous blood samples are obtained from a clamped segment of cord immediately after delivery and analyzed in the hospital laboratory using the ABL800 benchtop gas analyzer (Radiometer). The coefficient of variation of the partial pressure of oxygen (PO2) using this analyzer ranges from 1.9–2.9%. All singleton, term, non-anomalous deliveries with validated and paired venous and arterial cord gases were eligible. Patient with unpaired and unvalidated cord gases were excluded. Paired cord gases were validated by confirming that the arterial cord pH was at least 0.02 lower in the artery than the vein(14, 15).
Information regarding maternal demographics, antenatal care, labor outcomes, cord gas values, and neonatal diagnoses were collected from medical records by trained research staff. The primary outcome was a composite of neonatal morbidity that included neonatal death, hypoxic ischemic encephalopathy, need for hypothermia treatment, intubation, mechanical ventilation, and meconium aspiration syndrome.(14, 16) When neonates had more than one morbidity only one was counted per patient. Given the wide and skewed range of oxygen content in the umbilical cord(17) we defined hyperoxemia as cord venous PO2 ≥ 90th percentile (39 mmHg) of the cohort. Coincidentally this cut-point is consistent with the upper range of published normal values of umbilical venous PO2.(18)
Baseline characteristics of patients with and without fetal umbilical cord hyperoxemia were compared using χ2 or Fisher exact test, Mann-Whitney U test, or Student t test as appropriate. Multivariable logistic regression was used to adjust for confounders. Potential confounders were selected on the basis of biologic plausibility, risk factors that have been identified in the literature for the various outcomes, and results of univariable and stratified analyses. Backwards elimination was used to reduce the number of variables in each model. Variables were included in the final model if they had at least a 10% effect on the magnitude of the odds ratio associated with the primary exposure. Model fit for the final model was assessed with the Hosmer-Lemeshow goodness-of-fit test (19). Because the frequency of the primary outcome was>10% in some subgroups and odds ratios would overestimate the relative risks, we estimated adjusted relative risks using the method proposed by Zhang et al.(20)
Analysis was performed for the entire cohort and was then stratified by the presence of acidemia defined as umbilical artery pH<7.1. We included an interaction term in the logistic regression model to test for effect modification by acidemia on the relationship between hyperoxemia and neonatal morbidity. This test assessed whether the magnitude of the association between hyperoxemia and neonatal morbidity significantly differed in acidemic and non-acidemic neonates. The original prospective cohort study from which our sample was obtained did not contain information on intrapartum oxygen supplementation. We conducted additional chart review to abstract data on intrapartum oxygen supplementation for the acidemic cohort.
We included all consecutive patients meeting inclusion criteria; no a priori sample size estimation was performed. All tests were 2-tailed with P<0.05 considered significant. STATA Version 12.1 (STATA Corp., College Station, TX) was used to perform all analyses.
Results
A total of 8,580 deliveries occurred during the study period. Of these, we excluded 791 for missing validated or paired cord gases, leaving 7,789 for analysis. In all, 907 (11.6%) had cord venous PO2 ≥ 90th percentile (Figure 1). Composite neonatal morbidity was diagnosed in 106 (1.4%) neonates. Intubation (68.9%), mechanical ventilation (45.3%), and hypothermic therapy (37.7%) were the most common outcomes (Table 1).
Figure 1.
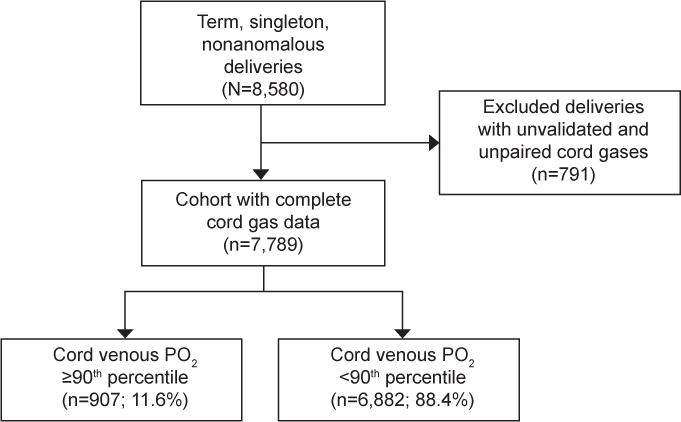
Flow chart of study population. PO2, partial pressure of oxygen.
Table 1.
Composite neonatal morbidity (n=106/7789 = 1.4%)
| Component | n | % |
|---|---|---|
| Intubation | 73 | 68.9 |
| Mechanical ventilation | 48 | 45.3 |
| Hypothermia treatment | 40 | 37.7 |
| Hypoxic encephalopathy | 33 | 31.1 |
| Meconium aspiration syndrome | 22 | 20.8 |
| Neonatal death | 4 | 3.8 |
Baseline characteristics of patients with fetal umbilical cord hyperoxemia were compared to those without hyperoxemia. Patients with fetal hyperoxemia were more likely to be younger, African American, smoke cigarettes, and have hypertension. Cesarean delivery, cesarean for the specific indication of non-reassuring fetal status, and intrapartum maternal fever were less common in the hyperoxemic group. There was no difference in BMI or diabetes between groups. Although patients with fetal hyperoxemia had slightly higher mean umbilical cord arterial pH compared to those without hyperoxemia (7.28±0.07 versus 7.27±0.06, p<0.01), there was no significant difference in the proportion with acidemia (umbilical arterial pH <7.1) between the groups (Table 2).
Table 2.
Baseline demographics of study cohort
| Demographic | Entire Cohort (n=7789) |
Venous PO2≥90th%ile* (n=907) |
Venous PO2< 90th%ile (n= 6882) |
P-value |
|---|---|---|---|---|
|
| ||||
| Maternal Age (years) | 26 ± 5.9 | 25 ± 5.6 | 26 ± 6.0 | <0.001 |
|
| ||||
| Race | <0.001 | |||
| African American | 5085 (65.8) | 717 (79.2) | 4368 (64.0) | |
| Caucasian | 1744 (22.6) | 122 (13.5) | 1622 (23.8) | |
| Hispanic | 552 (7.1) | 38 (4.2) | 514 (7.5) | |
| Other | 350 (4.5) | 28 (3.1) | 322 (4.7) | |
|
| ||||
| BMI (kg/m2) | 32.3 ± 7.4 | 32.5 ±7.6 | 32.3 ± 7.3 | 0.35 |
|
| ||||
| Obese | 4424 (56.8) | 525 (57.9) | 3899 (56.7) | 0.48 |
|
| ||||
| Gestational age, weeks | 39 ± 1.21 | 39 ± 1.24 | 39 ± 1.21 | 0.04 |
|
| ||||
| Nulliparous | 3320 (42.6) | 288 (31.8) | 3032 (44.1) | <0.001 |
|
| ||||
| Maternal fever >38.1 C | 358 (4.6) | 21 (2.3) | 337 (4.9) | <0.001 |
|
| ||||
| Mode of delivery | <0.001 | |||
| Spontaneous vaginal | 6033 (77.5) | 787 (86.8) | 5246 (76.2) | |
| Operative vaginal | 373 (4.8) | 37 (4.1) | 336 (4.9) | |
| Cesarean | 1383 (17.8) | 83 (9.2) | 1300 (18.9) | |
|
| ||||
| Cesarean indication of non-reassuring fetal status | 1118 (14.4) | 86 (9.5) | 1032 (15.0) | <0.001 |
|
| ||||
| Smoking | 1085 (13.9) | 149 (16.4) | 936 (13.6) | 0.02 |
|
| ||||
| Illicit drug use | 901 (11.6) | 124 (13.7) | 777 (11.3) | 0.04 |
|
| ||||
| Alcohol | 86 (1.1) | 11 (1.2) | 75 (1.1) | 0.75 |
|
| ||||
| Diabetes | 120 (1.5) | 7 (0.8) | 113 (1.6) | 0.05 |
|
| ||||
| Hypertension | 372 (4.8) | 58 (6.4) | 314 (4.6) | 0.02 |
|
| ||||
| Birthweight (grams) | 3249 ± 463 | 3229 ± 433 | 3252 ± 467 | 0.16 |
|
| ||||
| Umbilical arterial pH | 7.3 ± 0.1 | 7.28 ± 0.07 | 7.27 ± 0.06 | <0.01 |
|
| ||||
| Umbilical arterial pH <7.1 | 147 (1.9) | 17 (1.9) | 130 (1.9) | 0.98 |
|
| ||||
| NICU admission | 123 (1.6) | 19 (2.1) | 104 (1.5) | 0.19 |
|
| ||||
| Oxytocin | 5227 (67.1) | 598 (65.9) | 4629 (67.2) | 0.42 |
|
| ||||
| Induction of labor | 3438 (44.1) | 407 (44.9) | 3031 (44.0) | 0.64 |
|
| ||||
| Prolonged 1st stage† | 336 (5.1) | 48 (5.8) | 288 (5.0) | 0.33 |
|
| ||||
| Prolonged 2nd stage‡ | 443 (6.6) | 43 (5.2) | 400 (6.8) | 0.07 |
90th%ile Venous PO2 is 39 mmHg
Data represents n(%) or mean ± standard deviation
>95th percentile of first stage of labor: 15.3 hours for nulliparas and >12.8 hours for multiparas
>3 hours for nulliparous patients without epidurals, >4 hours for nulliparous patients with epidurals. >2 hours for multiparous patients without epidurals, >3 hours for multiparous patients with epidurals
For the entire cohort, there was no difference in composite neonatal morbidity between those with and without fetal hyperoxemia (1.9% versus 1.8%, aRR 1.5, 95% CI 0.9–2.5). However, in the stratified analysis among those with acidemia, hyperoxemia was associated with a significantly higher risk of the composite neonatal morbidity among acidemic neonates (41.2% versus 21.4%, aRR 2.3, 95% CI 1.1–3.5). There was no difference in neonatal morbidity between those with and without hyperoxemia among non-acidemic neonates (0.8% versus 1.0%, aRR 1.0, 95% CI 0.5–2.2). The test of interaction confirmed that the magnitude of the association between hyperoxemia and neonatal morbidity was significantly different in acidemic compared with non-acidemic neonates (P for interaction <0.001) (Table 3)
Table 3.
Neonatal outcomes by cord venous O2 content and acidemia
| Outcome | Venous PO2 ≥90th%ile |
Venous PO2 < 90th%ile |
RR (95% CI) |
Adjusted RR† (95%CI) |
P for interaction |
|---|---|---|---|---|---|
| Entire cohort | N=907 | N=6882 | |||
| Cord arterial pH <7.1 | 17 (1.9) | 126 (1.8) | 1.0 (0.7–1.6) | 1.5 (0.9–2.5) | |
| Composite neonatal morbidity |
14 (1.5) | 92 (1.3) | 1.1 (0.7–1.9) | 1.5 (0.9–2.7) | |
| Acidemic cohort* | N=17 | N=126 | |||
| Composite neonatal morbidity | 7 (41.2) | 27 (21.4) | 2.2 (0.9–5.4) | 2.3 (1.1–3.5) | <0.001 |
| Non-acidemic cohort | N=890 | N= 6756 | |||
| Composite neonatal morbidity | 7 (0.8) | 65 (1.0) | 0.8 (0.4–1.7) | 1.0 (0.5–2.2) |
RR relative risk
Acidemic defined as umbilical artery pH<7.1
Adjusted for nulliparity, hypertension, and delivery mode
Bolded data represent statistically significant results
On further analysis of the acidemic cohort, there were no significant differences in clinical characteristics including mode of delivery, prolonged labor, and cesarean for non-reassuring fetal status between acidemic fetuses with and without hyperoxemia. (Table 4) There was also no difference in median arterial pH [7.07 (IQR 7.01, 7.09) versus 7.04 (IQR 7.01,7.09), P= 0.71], base excess [−11.1 mEq/L (−12.8, −9.3) versus −10.8 mEweq/L (−13, −9.4), P= 0.92], or PCO2 [83 mmHg (78, 90) versus 88 mmHg (76, 93) P=0.96] between those with and without hyperoxemia. Frequency of oxygen supplementation in the acidemic cohort was high, and not different between those with and without hyperoxemia (80.0% versus 90.1%, P=0.22). The median duration of oxygen supplementation was not different between acidemic patients with and without hyperoxemia (230 versus 175 minutes, P=0.19). There was no statistically significant difference between groups when duration of oxygen supplementation was assessed categorically (Table 5). Finally, in sensitivity analysis where we included only patients who had oxygen supplementation, there was a significantly higher risk of neonatal morbidity in the setting of acidemia and hyperoxemia compared with acidemia without hyperoxemia (41.7% versus 21.1%, aRR 2.6; 95% CI 1.1 – 3.9).
Table 4.
Baseline demographics of acidemic cohort
| Demographic | Acidemia* + Venous PO2≥ 90th%ile (n=17) |
Acidemia* + Venous PO2<90th%ile (n=126) |
P-value |
|---|---|---|---|
|
| |||
| Maternal age | 28 (19, 31) | 28 (22, 33) | 0.29 |
|
| |||
| Race | |||
| African American | 12 (70.6) | 78 (62.4) | 0.75 |
| Caucasian | 4 (23.5) | 34 (27.2) | |
| Hispanic | 1 (5.9) | 6 (4.8) | |
| Other | 0 (0) | 7 (5.6) | |
|
| |||
| BMI (kg/m2) | 33 (30, 41) | 32 (27, 39) | 0.56 |
|
| |||
| Obese | 12 (70.6) | 77 (61.1) | 0.45 |
|
| |||
| Gestational age, weeks | 39 (39,41) | 39 (38,40) | 0.13 |
|
| |||
| Nulliparous | 11 (64.7) | 68 (54.0) | 0.40 |
|
| |||
| Maternal fever>38.1 C | 1 (5.9) | 9 (7.4) | 0.85 |
|
| |||
| Mode of delivery | |||
| Spontaneous vaginal | 8 (47.1) | 32 (25.4) | 0.17 |
| Operative vaginal | 1 (5.9) | 15 (11.9) | |
| Cesarean | 8 (47.1) | 79 (62.7) | |
|
| |||
| Operative delivery (operative vaginal or cesarean) | 9 (52.9) | 94 (74.6) | 0.06 |
|
| |||
| Cesarean indication of non-reassuring fetal status | 8 (47.1) | 80 (63.5) | 0.19 |
|
| |||
| Smoking | 2 (11.8) | 12 (9.5) | 0.77 |
|
| |||
| Illicit drug use | 2 (11.8) | 9 (7.1) | 0.50 |
|
| |||
| Alcohol | 0 (0) | 0 (0) | – |
|
| |||
| Diabetes | 0 (0) | 10 (10.8) | 0.28 |
|
| |||
| Hypertension | 2 (11.8) | 22 (17.5) | 0.56 |
|
| |||
| Birthweight (grams) | 3501 ± 118 | 3232 ± 43.9 | 0.04 |
|
| |||
| NICU admission | 6 (35.3) | 25 (19.8) | 0.15 |
|
| |||
| Oxytocin | 10 (58.8) | 84 (66.7) | 0.52 |
|
| |||
| Induction of labor | 7 (41.2) | 62 (49.2) | 0.53 |
|
| |||
| Prolonged 1st stage† | 0 (0) | 8 (11.1) | 0.29 |
|
| |||
| Prolonged 2nd stage‡ | 0 (0) | 9 (12.5) | 0.26 |
Umbilical artery pH<7.1
>95th percentile of first stage of labor: 15.3 hours for nulliparas and >12.8 hours for multiparas
>3 hours for nulliparas without epidurals, >4 hours for nulliparas with epidurals. >2 hours for multiparas without epidurals, >3 hours for multiparas with epidurals
Data represents mean ± standard deviation, n(%), or median (interquartile range)
Table 5.
Intrapartum oxygen supplementation in acidemic cohort
| Intrapartum oxygen supplementation |
Acidemia* + Venous PO2 ≥90th%ile n=15† |
Acidemia*+ Venous PO2<90th%ile n=121† |
P value |
|---|---|---|---|
|
| |||
| Any oxygen supplementation | 12 (80.0) | 109 (90.1) | 0.22 |
|
| |||
| Duration of oxygen supplementation (mins) |
230 (119,381) | 175 (68,290) | 0.19 |
| Oxygen ≥2 hours | 14 (82.4) | 86 (68.3) | 0.28 |
| Oxygen ≥3 hours | 12 (70.6) | 69 (54.8) | 0.22 |
| Oxygen ≥4 hours | 11 (64.7) | 55 (43.7) | 0.10 |
| Oxygen ≥5 hours | 9 (52.9) | 43 (34.1) | 0.13 |
| Oxygen ≥6 hours | 8 (47.1) | 31 (24.6) | 0.05 |
Umbilical artery pH<7.1
Missing data for 2/17 in acidemia + venous PO2≥90th percentile group and 5/126 in acidemia + venous PO2<90th percentile group
Data represent n (%) or median (interquartile range)
Discussion
In this large prospective cohort, we found no difference in morbidity between neonates with and without hyperoxemia. However, the risk of neonatal morbidity varied depending on the presence of acidemia with a small, but significantly increased risk of adverse neonatal outcomes with hyperoxemia in the setting of acidemia, but not in its absence.
An increase in adverse outcomes has also been observed in clinical trials evaluating intrauterine oxygen exposure. In a trial of 56 laboring women randomized to oxygen or room air for at least 30 minutes before delivery, Nesterenko et al found that more infants in the oxygen group required delivery room resuscitation.(21)A similar trial by Thorp et al comparing oxygen to room air in the second stage of labor revealed that babies in the oxygen group were four times more likely to have umbilical artery pH values <7.20 and that duration of oxygen exposure had an inverse relationship with cord arterial pH.(22) In a double-blinded trial of room air versus oxygen during elective cesarean delivery, Khaw et al demonstrated that oxygen was associated with increased maternal and umbilical cord levels of malondialdehyde, a marker of free radical activity and oxidative stress.(4) However, none of these studies directly related fetal hyperoxemia to clinical neonatal outcomes and did not stratify on the basis of acidemia.
The observed interaction between acidemia and hyperoxemia on neonatal morbidity is biologically plausible and may be attributable to hypoxia-reoxygenation injury. Fetal acidemia occurs when hypoxia causes a shift from aerobic to anaerobic metabolism. The hydrogen ions produced by anaerobic metabolism in this setting overwhelm the buffer capacity of the fetus resulting in a fall in pH.(23) Subsequent reoxygenation results in calcium influx at a cellular level that increases substrate availability for the enzyme xanthine oxidase, a generator of free radicals.(6, 24, 25) The buildup of free radicals then triggers cellular damage and subsequent injury to fetal organs including the brain and lungs.(10, 26) In addition, the decreased cerebral blood flow associated with hyperoxia can further amplify the severity of reperfusion injury.(27) This is supported by studies of neonatal resuscitation which showed an increased risk of neonatal mortality, retinopathy of prematurity, and bronchopulmonary dysplasia when resuscitation was performed with 100% oxygen rather than room air.(8, 10, 28) Furthermore, hyperoxemia at the time of resuscitation is associated with impaired neonatal inspiratory responses attributed to hyperoxemia-related oversaturation of peripheral chemoreceptors.(9) Although neonatal hyperoxemic injury may differ in pathophysiology from that of intrauterine hyperoxemia, the potential harm of excess fetal oxygen exposure must be carefully evaluated. We found that patients with hyperoxemia were less likely to have cesareans for non-reassuring fetal tracings. This is consistent with prior studies linking increased umbilical venous oxygen content with alleviation of fetal decelerations. However, although fetal heart tracings may improve as a result of hyperoxygenation, it may not improve neonatal outcomes.(3, 29) Although we did not assess the diagnosis of chorioamnionitis, we observed a lower proportion of patients with intrapartum maternal fever in the hyperoxemia group. We suspect that the relationship between chorioamnionitis, fetal heart tracings, and oxygen administration is complex and cannot account for changes in placental regulation that may occur secondary to inflammation and infection.
The large prospective cohort design is a strength of this study. The large sample size enabled us to stratify our analysis by acidemia, which revealed evidence of interaction. The universal cord gas collection and objective validation of cord gas values allowed for accurate, generalizable estimates of cord venous content and reduced the potential for selection bias.
There are limitations that should be considered. We defined hyperoxemia by umbilical venous PO2 ≥ 90th percentile without reference to whether the patients received supplemental oxygen. The lack of oxygen supplementation data for the entire cohort limits our ability to directly link hyperoxemia with oxygen administration. However, prior studies have demonstrated an association between maternal hyperoxygenation and increased umbilical vein PO2. (5, 29). Among the acidemic cohort for which we had data on oxygen supplementation, we found high frequency of oxygen supplementation. Although the overall frequency of oxygen supplementation was not significantly different between the acidemic subgroups with and without hyperoxemia, the median duration of oxygen supplementation was nominally higher in the subgroup with hyerpoxemia (230 versus 175 min., P=0.19) and there was a tendency towards a higher proportion of patients in the hyperoxemia group receiving oxygen for greater than 6 hours compared to the normoxic group. These differences may not be statistically significant due to the limited sample size of the acidemic cohort. It is possible that fetal acidemia triggers changes in villous oxygen absorption, independent of maternal oxygen administration, that account for the observed changes in neonatal morbidity. However, in sensitivity analysis limited only to patients who received oxygen, and thus hyperoxemia associated with oxygen administration, we observed a similarly increased risk of neonatal morbidity in the setting of acidemia and hyperoxemia compared with acidemia without hyperoxemia.
The use of a composite neonatal outcome measure may also be seen as a weakness. This was necessary for adequate statistical power to perform detailed analysis, which would not be possible if the relatively rare individual outcomes were used. Moreover, we used an established composite with carefully selected components of neonatal morbidities that are directly or indirectly linked to intrauterine hypoxia.(14, 16) Finally, although we used appropriate statistical methods to control for confounders, there is the possibility of residual confounding by unmeasured factors.
In conclusion, this study demonstrates a small, but significantly increased risk of neonatal morbidity with intrauterine hyperoxemia in the presence of acidemia. Since intrauterine resuscitation is commonly used for suspected fetal acidemia, this finding calls for a closer look at the safety of liberal and prolonged oxygen administration for intrapartum management of suspected fetal acidemia. While these findings are insufficient to recommend practice change, they support the need for further studies including an ongoing randomized trial comparing outcomes associated with intrauterine resuscitation using oxygen or room air.
Précis.
Intrauterine hyperoxemia, compared with normoxemia, is associated with a small, but significantly increased risk of neonatal morbidity in acidemic neonates.
Acknowledgments
Dr. Cahill is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD061619-01, PI Cahill) and was a Robert Wood Johnson Foundation Faculty Physician Scholar, which partially supported this work. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official view of the NIH or Robert Wood Johnson Foundation. Dr Temming is supported by a National Institutes of Health (NIH) T32 training grant (5T32HD055172-07). This publication was also made possible by grant number UL1 TR000448 from the NIH National Center for Advancing Translational Sciences (NCATS), components of the NIH, and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCATS or NIH.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
Each author has indicated that he or she has met the journal’s requirements for authorship.
Presented at the Annual Meeting for the Society for Maternal-Fetal Medicine, January 23–28, 2017, Las Vegas, Nevada.
References
- 1.Cahill AG, Roehl KA, Odibo AO, Macones GA. Association and prediction of neonatal acidemia. American journal of obstetrics and gynecology. 2012 Sep;207(3):206.e1–8. doi: 10.1016/j.ajog.2012.06.046. [DOI] [PubMed] [Google Scholar]
- 2.Young DC, Popat R, Luther ER, Scott KE, Writer WD. Influence of maternal oxygen administration on the term fetus before labor. American journal of obstetrics and gynecology. 1980 Feb;136(3):1. 321–4. doi: 10.1016/0002-9378(80)90856-x. [DOI] [PubMed] [Google Scholar]
- 3.Althabe O, Jr, Schwarcz RL, Pose SV, Escarcena L, Caldeyro-Barcia R. Effects on fetal heart rate and fetal pO2 of oxygen administration to the mother. American journal of obstetrics and gynecology. 1967 Jul;98(6):15. 858–70. doi: 10.1016/0002-9378(67)90205-0. [DOI] [PubMed] [Google Scholar]
- 4.Khaw KS, Wang CC, Ngan Kee WD, Pang CP, Rogers MS. Effects of high inspired oxygen fraction during elective caesarean section under spinal anaesthesia on maternal and fetal oxygenation and lipid peroxidation. Br J Anaesth. 2002 Jan;88(1):18–23. doi: 10.1093/bja/88.1.18. [DOI] [PubMed] [Google Scholar]
- 5.Ramanathan S, Gandhi S, Arismendy J, Chalon J, Turndorf H. Oxygen transfer from mother to fetus during cesarean section under epidural anesthesia. Anesthesia and analgesia. 1982 Jul;61(7):576–81. [PubMed] [Google Scholar]
- 6.Hamel MS, Anderson BL, Rouse DJ. Oxygen for intrauterine resuscitation: of unproved benefit and potentially harmful. American journal of obstetrics and gynecology. 2014 Aug;211(2):124–7. doi: 10.1016/j.ajog.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Davis PG, Tan A, O’Donnell CP, Schulze A. Resuscitation of newborn infants with 100% oxygen or air: a systematic review and meta-analysis. Lancet (London, England) 2004 Oct;364(9442):9–15. 1329–33. doi: 10.1016/S0140-6736(04)17189-4. [DOI] [PubMed] [Google Scholar]
- 8.Tan A, Schulze A, O’Donnell CP, Davis PG. Air versus oxygen for resuscitation of infants at birth. The Cochrane database of systematic reviews. 2005;(2):Cd002273. doi: 10.1002/14651858.CD002273.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vento M, Asensi M, Sastre J, Garcia-Sala F, Pallardo FV, Vina J. Resuscitation with room air instead of 100% oxygen prevents oxidative stress in moderately asphyxiated term neonates. Pediatrics. 2001 Apr;107(4):642–7. doi: 10.1542/peds.107.4.642. [DOI] [PubMed] [Google Scholar]
- 10.Vento M, Asensi M, Sastre J, Lloret A, Garcia-Sala F, Vina J. Oxidative stress in asphyxiated term infants resuscitated with 100% oxygen. The Journal of pediatrics. 2003 Mar;142(3):240–6. doi: 10.1067/mpd.2003.91. [DOI] [PubMed] [Google Scholar]
- 11.Sola A, Saldeno YP, Favareto V. Clinical practices in neonatal oxygenation: where have we failed? What can we do? Journal of perinatology : official journal of the California Perinatal Association. 2008 May;28(Suppl 1):S28–34. doi: 10.1038/jp.2008.47. [DOI] [PubMed] [Google Scholar]
- 12.NRP Neonatal Resuscitation Textbook. 6th. American Academy of Pediatrics; 2011. (English version) [Google Scholar]
- 13.Perlman JM, Wyllie J, Kattwinkel J, Wyckoff MH, Aziz K, Guinsburg R, et al. Part 7: Neonatal Resuscitation: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2015 Oct 20;132(16 Suppl 1):S204–41. doi: 10.1161/CIR.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 14.Tuuli MG, Stout MJ, Shanks A, Odibo AO, Macones GA, Cahill AG. Umbilical cord arterial lactate compared with pH for predicting neonatal morbidity at term. Obstet Gynecol. 2014 Oct;124(4):756–61. doi: 10.1097/AOG.0000000000000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Westgate J, Garibaldi JM, Greene KR. Umbilical cord blood gas analysis at delivery: a time for quality data. British journal of obstetrics and gynaecology. 1994 Dec;101(12):1054–63. doi: 10.1111/j.1471-0528.1994.tb13581.x. [DOI] [PubMed] [Google Scholar]
- 16.Tuuli MG, Stout MJ, Macones GA, Cahill AG. Umbilical Cord Venous Lactate for Predicting Arterial Lactic Acidemia and Neonatal Morbidity at Term. Obstet Gynecol. 2016 Apr;127(4):674–80. doi: 10.1097/AOG.0000000000001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arikan GM, Scholz HS, Petru E, Haeusler MC, Haas J, Weiss PA. Cord blood oxygen saturation in vigorous infants at birth: what is normal? BJOG: an international journal of obstetrics and gynaecology. 2000 Aug;107(8):987–94. doi: 10.1111/j.1471-0528.2000.tb10401.x. [DOI] [PubMed] [Google Scholar]
- 18.Creasy RKRR, Iams J, Lockwood CJ, Moore T, Greene M. Creasy and Resnik’s Maternal-Fetal Medicine: Principles and practice. 7th. Philadelphia, PA: Elsevier/Saunders; 2014. [Google Scholar]
- 19.Hosmer D, Lemeshow S. Applied Logistic Regression, 2001. New York, NY: John Wiley & Sons; 2001. pp. 147–156. [Google Scholar]
- 20.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. Jama. 1998 Nov;280(19):18. 1690–1. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 21.Nesterenko TH, Acun C, Mohamed MA, Mohamed AN, Karcher D, Larsen J, Jr, et al. Is it a safe practice to administer oxygen during uncomplicated delivery: a randomized controlled trial? Early human development. 2012 Aug;88(8):677–81. doi: 10.1016/j.earlhumdev.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Thorp JA, Trobough T, Evans R, Hedrick J, Yeast JD. The effect of maternal oxygen administration during the second stage of labor on umbilical cord blood gas values: a randomized controlled prospective trial. American journal of obstetrics and gynecology. 1995 Feb;172(2 Pt 1):465–74. doi: 10.1016/0002-9378(95)90558-8. [DOI] [PubMed] [Google Scholar]
- 23.Uzan S, Berkane N, Verstraete L, Mathieu E, Breart G. Acid base balance in the fetus during labor: pathophysiology and exploration methods. Journal de gynecologie, obstetrique et biologie de la reproduction. 2003 Feb;321(1 Suppl):S68–78. [PubMed] [Google Scholar]
- 24.McCord JM. Oxygen-derived free radicals in postischemic tissue injury. The New England journal of medicine. 1985 Jan;312(3):17. 159–63. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 25.Zweier JL, Talukder MA. The role of oxidants and free radicals in reperfusion injury. Cardiovascular research. 2006 May;70(2):1. 181–90. doi: 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 26.Fellman V, Raivio KO. Reperfusion injury as the mechanism of brain damage after perinatal asphyxia. Pediatric research. 1997 May;41(5):599–606. doi: 10.1203/00006450-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Lundstrom KE, Pryds O, Greisen G. Oxygen at birth and prolonged cerebral vasoconstriction in preterm infants. Archives of disease in childhood Fetal and neonatal edition. 1995 Sep;73(2):F81–6. doi: 10.1136/fn.73.2.f81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saugstad OD, Ramji S, Vento M. Resuscitation of depressed newborn infants with ambient air or pure oxygen: a meta-analysis. Biology of the neonate. 2005;87(1):27–34. doi: 10.1159/000080950. [DOI] [PubMed] [Google Scholar]
- 29.Khazin AF, Hon EH, Hehre FW. Effects of maternal hyperoxia on the fetus. I. Oxygen tension. American journal of obstetrics and gynecology. 1971 Feb;109(4):15. 628–37. doi: 10.1016/0002-9378(71)90639-9. [DOI] [PubMed] [Google Scholar]


