Abstract
Purpose
Mucosal melanoma is an aggressive malignancy with a poor response to conventional therapies. The efficacy and safety of nivolumab (a programmed death-1 checkpoint inhibitor), alone or combined with ipilimumab (a cytotoxic T-lymphocyte antigen-4 checkpoint inhibitor), have not been reported in this rare melanoma subtype.
Patients and Methods
Data were pooled from 889 patients who received nivolumab monotherapy in clinical studies, including phase III trials; 86 (10%) had mucosal melanoma and 665 (75%) had cutaneous melanoma. Data were also pooled for patients who received nivolumab combined with ipilimumab (n = 35, mucosal melanoma; n = 326, cutaneous melanoma).
Results
Among patients who received nivolumab monotherapy, median progression-free survival was 3.0 months (95% CI, 2.2 to 5.4 months) and 6.2 months (95% CI, 5.1 to 7.5 months) for mucosal and cutaneous melanoma, with objective response rates of 23.3% (95% CI, 14.8% to 33.6%) and 40.9% (95% CI, 37.1% to 44.7%), respectively. Median progression-free survival in patients treated with nivolumab combined with ipilimumab was 5.9 months (95% CI, 2.8 months to not reached) and 11.7 months (95% CI, 8.9 to 16.7 months) for mucosal and cutaneous melanoma, with objective response rates of 37.1% (95% CI, 21.5% to 55.1%) and 60.4% (95% CI, 54.9% to 65.8%), respectively. For mucosal and cutaneous melanoma, respectively, the incidence of grade 3 or 4 treatment-related adverse events was 8.1% and 12.5% for nivolumab monotherapy and 40.0% and 54.9% for combination therapy.
Conclusion
To our knowledge, this is the largest analysis of data for anti–programmed death-1 therapy in mucosal melanoma to date. Nivolumab combined with ipilimumab seemed to have greater efficacy than either agent alone, and although the activity was lower in mucosal melanoma, the safety profile was similar between subtypes.
INTRODUCTION
Ipilimumab, which blocks cytotoxic T-lymphocyte antigen-4,1 has demonstrated long-term survival in approximately 20% of patients with advanced melanoma.2 Another immune checkpoint inhibitor, nivolumab, blocks the interaction of the programmed death-1 receptor (PD-1) with its ligands, PD-L1 and PD-L2.1 In phase III trials, nivolumab monotherapy showed improved overall survival (OS) and a greater objective response rate (ORR) versus dacarbazine in untreated patients with BRAF wild-type melanoma3 and a greater ORR versus chemotherapy in melanoma patients who experienced disease progression and were receiving ipilimumab or ipilimumab and a BRAF inhibitor.4 In phase II and III clinical trials, nivolumab in combination with ipilimumab improved progression-free survival (PFS) and ORR versus ipilimumab alone in treatment-naïve patients with advanced melanoma.5,6
Several new agents have been approved for the treatment of cutaneous melanoma since 2011, including the combination of nivolumab and ipilimumab, yet there is a paucity of published information regarding the efficacy and safety of these agents in other melanoma subtypes. In white populations, the primary sites of melanoma are cutaneous (82%), uveal (8%), acral (3%), and mucosal (2%), with approximately 5% being unknown.7 Mucosal melanomas primarily occur in the head and neck region (eg, nasal and oral cavities), followed by the GI tract (anorectum) and female genital tract (vulva and vagina).8,9 Accordingly, they occur at a higher incidence in females than in males.10 Although mucosal melanomas are rare in white populations, accounting for 2% or less of all melanomas,7,10 the incidence has been reported to be up to 23% in Chinese populations.11 Prognosis for these patients is poor, with a 5-year survival rate less than that reported for cutaneous or uveal melanoma.9
Mucosal melanoma is an aggressive subtype that is largely resistant to traditional therapies.11,12 A major challenge with mucosal melanoma is that well-established protocols for staging and treatment are lacking, and in the absence of discernable signs or symptoms recognizable by the patient, diagnosis often occurs at late stages.9 Anatomic location often precludes complete surgical resection because negative margins are difficult to achieve.9 Response rates with chemotherapy are poor and are generally similar to those observed in cutaneous melanoma.13 Patients with mucosal, acral, and chronically sun-damaged melanomas infrequently have BRAF mutations, but amplifications or activating mutations in the receptor tyrosine kinase, KIT, are common.14,15 Although typically of short duration, antitumor activity with KIT inhibitors such as imatinib has been observed in mucosal melanoma with certain KIT mutations.14,15
Although ipilimumab and anti–PD-1 agents have demonstrated activity in mucosal melanoma, the evidence is based on small study populations, retrospective analyses, and single case reports.16-20 In two retrospective analyses and data from an expanded access program, ipilimumab treatment resulted in an ORR of 7% to 12%, median PFS of 2.3 to 4.3 months, and median OS of 6.4 months in patients with metastatic mucosal melanoma.16-18 In a phase II study, 1-year OS rates of 38% and 14% were reported for ipilimumab-treated patients with cutaneous (n = 83) and mucosal (n = 7) melanoma, respectively.19 A patient with mucosal melanoma was reported to achieve a durable, near-complete response when treated with an anti–PD-1 agent after ipilimumab.20 To better understand the benefit of anti–PD-1–based therapy in this melanoma subtype, we conducted a pooled analysis of data from patients with mucosal melanoma who received nivolumab alone or combined with ipilimumab in clinical trials.
PATIENTS AND METHODS
Study Population
Patients included in the current analyses had a confirmed histologic diagnosis of unresectable stage III or stage IV (advanced) melanoma. Those with primary uveal melanoma were excluded from four of the six nivolumab clinical trials from which the data in these analyses were derived, but patients with primary mucosal melanoma were eligible to participate in all studies. In these studies, M staging of mucosal melanomas was based on cutaneous melanoma criteria. Information regarding the exact location of the primary site of mucosal melanomas was not collected during the trials.
Clinical Trials
Data were pooled from 889 patients with advanced melanoma who had received nivolumab monotherapy (3 mg/kg every 2 weeks until progression or unacceptable toxicity) in one of five ongoing clinical trials: (1) a phase I dose-ranging study in previously treated patients (CA209-003; n = 17)21; (2) a phase I biomarker study to evaluate the immunomodulatory effects of nivolumab (CA209-038; n = 85)22; (3) a phase III trial of nivolumab versus chemotherapy in treatment-naïve patients with wild-type BRAF (CheckMate 066; n = 206)3; (4) a phase III trial of nivolumab versus chemotherapy in patients who experienced disease progression after ipilimumab or ipilimumab and a BRAF inhibitor if positive for a BRAF V600 mutation (CheckMate 037; n = 268)4; and (5) a phase III trial of nivolumab monotherapy or nivolumab plus ipilimumab versus ipilimumab monotherapy in treatment-naïve patients (CheckMate 067; n = 313).6
To evaluate the efficacy and safety of nivolumab combined with ipilimumab in mucosal melanoma, data were pooled from CheckMate 067 and an ongoing phase II trial (CheckMate 069) of nivolumab plus ipilimumab versus ipilimumab alone in treatment-naïve patients.5 Across melanoma subtypes, 407 patients (313 from CheckMate 067; 94 from CheckMate 069) had received nivolumab (1 mg/kg) plus ipilimumab (3 mg/kg) every 3 weeks for up to four doses, and after combination therapy, patients could have received nivolumab monotherapy at 3 mg/kg every 2 weeks until progression or unacceptable toxicity; 357 patients had received ipilimumab monotherapy (3 mg/kg every 3 weeks for four doses).
Data Analyses
For comparisons of patient demographics between subtypes, P values were based on the χ2 test for categorical variables and two-sample t test for continuous variables. Median PFS was based on Kaplan-Meier estimates, with two-sided 95% CIs computed using the Brookmeyer and Crowley method. Hazard ratios and corresponding 95% CIs were estimated using an unstratified Cox proportional hazards model. In an exploratory analysis, P values for comparisons of PFS between treatment groups within each subtype were calculated using an unstratified log-rank test. Tumor response was assessed using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 in all studies except CA209-003, in which RECIST version 1.0 (with modification) was used.3-6,21,22 The proportion of patients with a confirmed complete or partial response (ORR) was calculated for each pooled data set, with 95% CIs on the basis of the Clopper-Pearson method. Kaplan-Meier methodology was used to calculate the duration of response, defined as the time between the date of the first documented objective response and the date of the first subsequent disease progression or death, whichever occurred first. OS was not included in the analyses because of the lack of mature data for most of the studies. No formal comparisons were made between subtypes for any efficacy end point.
ORR and PFS were also evaluated in the pooled data sets according to PD-L1 status, which was evaluated with a verified immunohistochemical assay using a rabbit monoclonal antihuman antibody (clone 28-8), described previously.23 Each biopsied tissue sample was scored with a cutoff of ≥ 5% or < 5% of tumor cells having cell-surface PD-L1 staining of any intensity in a section with at least 100 evaluable tumor cells. Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0). Patients were evaluated for safety if they had received at least one dose of nivolumab monotherapy or one dose each of nivolumab and ipilimumab as combination therapy.
RESULTS
Patient Characteristics and Treatment
Among 889 patients who received nivolumab monotherapy, 86 (10%) with mucosal melanoma and 665 (75%) with cutaneous melanoma were included in the analyses. For those who received nivolumab combined with ipilimumab (n = 407), 35 patients (9%) with mucosal melanoma and 326 (80%) with cutaneous melanoma were included; 36 of 357 patients (10%) with mucosal melanoma and 269 (75%) with cutaneous melanoma had received ipilimumab monotherapy. The remaining 11% to 15% of patients within each pooled group were diagnosed with acral melanoma, uveal melanoma, or unknown primaries.
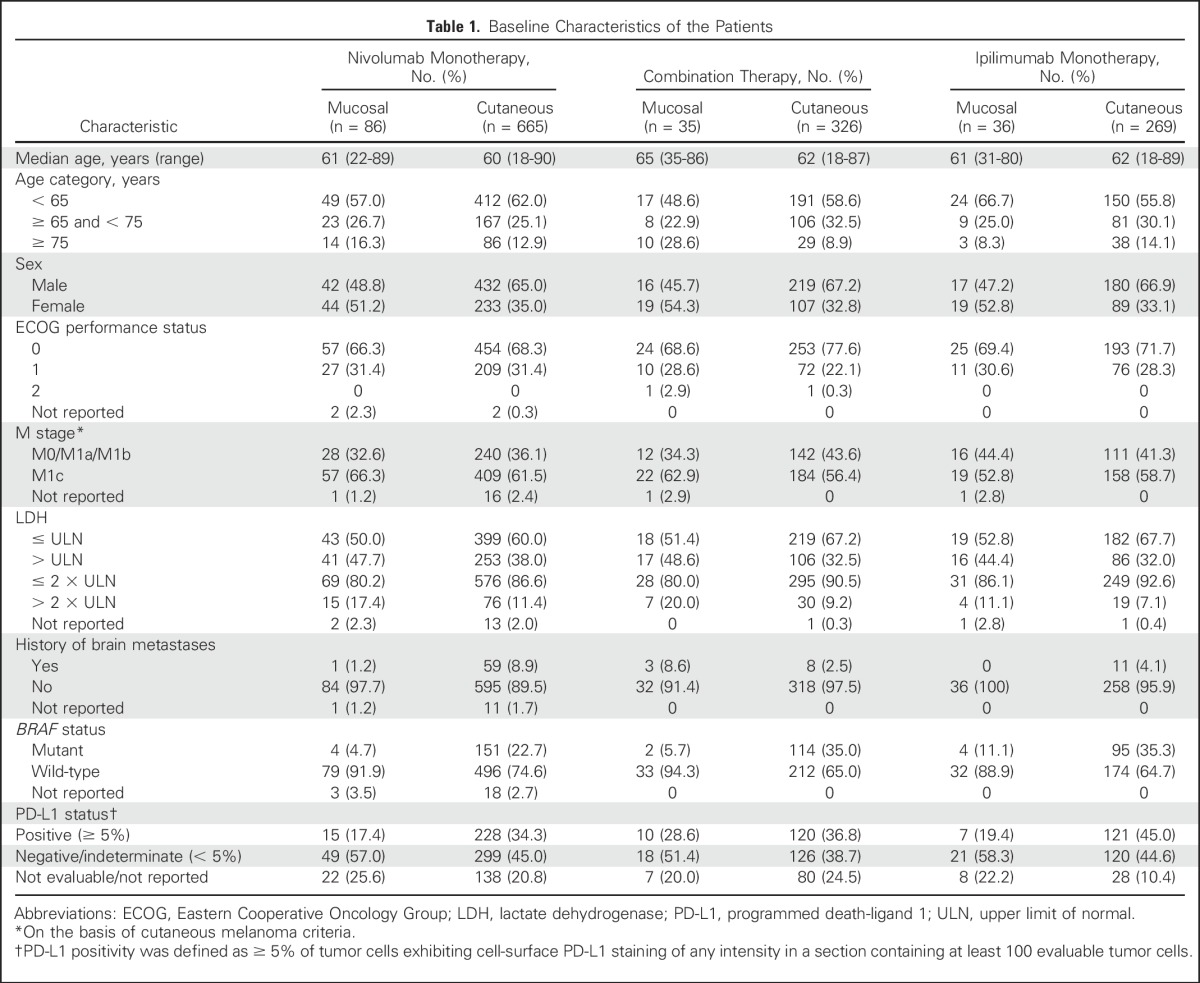
Baseline demographics were balanced between mucosal and cutaneous melanoma subtypes and across treatment groups, age, Eastern Cooperative Oncology Group performance status, and M stage (Table 1). However, relative to cutaneous melanoma, a higher percentage of patients with mucosal melanoma were female (P = .0035 for nivolumab monotherapy; P = .0114 for combination therapy), and a lower percentage had tumor PD-L1 expression ≥ 5% (P = .0071 for nivolumab monotherapy). Although the differences were not statistically significant, more patients with mucosal melanoma had elevated lactate dehydrogenase (LDH) levels. More patients with cutaneous melanoma had a BRAF mutation, consistent with the known molecular pathology of this subtype compared with mucosal melanoma. Other genetic abnormalities, such as mutations in KIT, were not tested in our study population.
Table 1.
Baseline Characteristics of the Patients
Patients with mucosal melanoma who were treated with nivolumab monotherapy had received a median of 7.0 doses (range, 1 to 34), and those with cutaneous melanoma had received a median of 11.0 doses (range, 1 to 61 doses). In the combination group, a median of 4.0 doses (range, 1 to 28 doses) of nivolumab and 4.0 doses (range, 1 to 4 doses) of ipilimumab were received by patients with mucosal melanoma; patients with cutaneous melanoma received similar dosing (nivolumab, median of 4.0 doses [range, 1 to 39 doses]; ipilimumab, median of 4.0 doses [range, 1 to 4 doses]). Patients treated with ipilimumab monotherapy, regardless of melanoma subtype, received a median of 4.0 doses (range, 1 to 4 doses). In the three treatment groups, median follow-up times ranged from 6.2 to 8.6 months for mucosal melanoma and 10.0 to 11.7 months across melanoma subtypes.
Efficacy
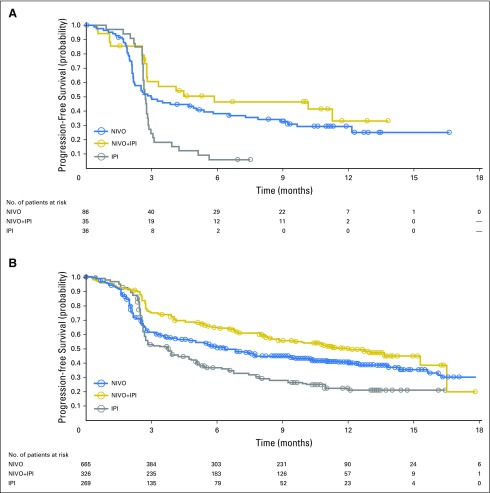
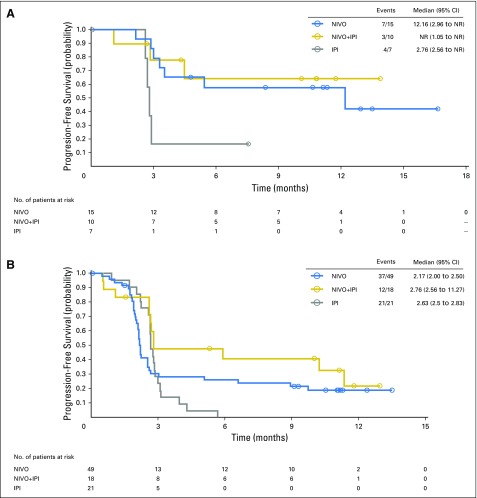
Median PFS was 3.0 months (95% CI, 2.2 to 5.4 months), 5.9 months (95% CI, 2.2 to not reached), and 2.7 months (95% CI, 2.6 to 2.8 months) for patients with mucosal melanoma who received nivolumab monotherapy, combination therapy, and ipilimumab monotherapy, respectively (Fig 1A). For patients with cutaneous melanoma, median PFS was 6.2 months (95% CI, 5.2 to 7.5 months), 11.7 months (95% CI, 8.9 to 16.7), and 3.9 months (95% CI, 2.9 to 4.4 months), respectively (Fig 1B). ORR was 23.3% (95% CI, 14.8% to 33.6%), 37.1% (95% CI, 21.5% to 55.1%), and 8.3% (95% CI, 1.8% to 22.5%) for mucosal melanoma, and 40.9% (95% CI, 37.1% to 44.7%), 60.4% (95% CI, 54.9% to 65.8%), and 21.2% (95% CI, 16.5% to 26.6%) for cutaneous melanoma, among those who received nivolumab, combination therapy, or ipilimumab, respectively (Table 2).
Fig 1.
Progression-free survival in patients with (A) mucosal melanoma and (B) cutaneous melanoma who received nivolumab (NIVO) alone, combination therapy of NIVO plus ipilimumab (NIVO+IPI), or ipilimumab alone (IPI). Symbols indicate censored observations. Hazard ratios in (A): 0.61 (95% CI, 0.39 to 0.96; NIVO v IPI; P = .116); 0.42 (95% CI, 0.23 to 0.75; combination therapy versus ipilimumab; P = .003). Hazard ratios in (B): 0.73 (95% CI, 0.61 to 0.87; NIVO v IPI; P = .04); 0.49 (95% CI, 0.40 to 0.61; NIVO+IPI; P < .0001).
Table 2.
Best Overall Response
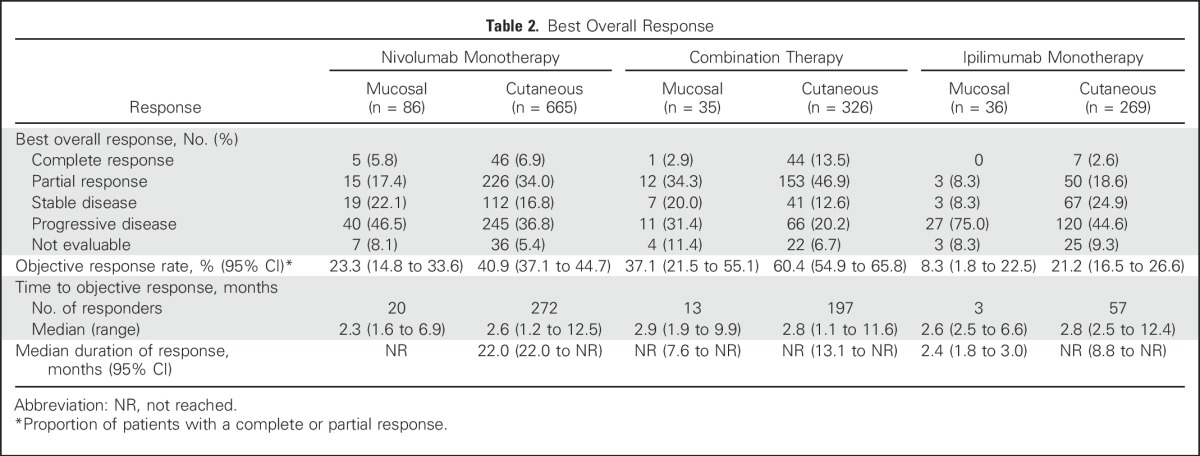
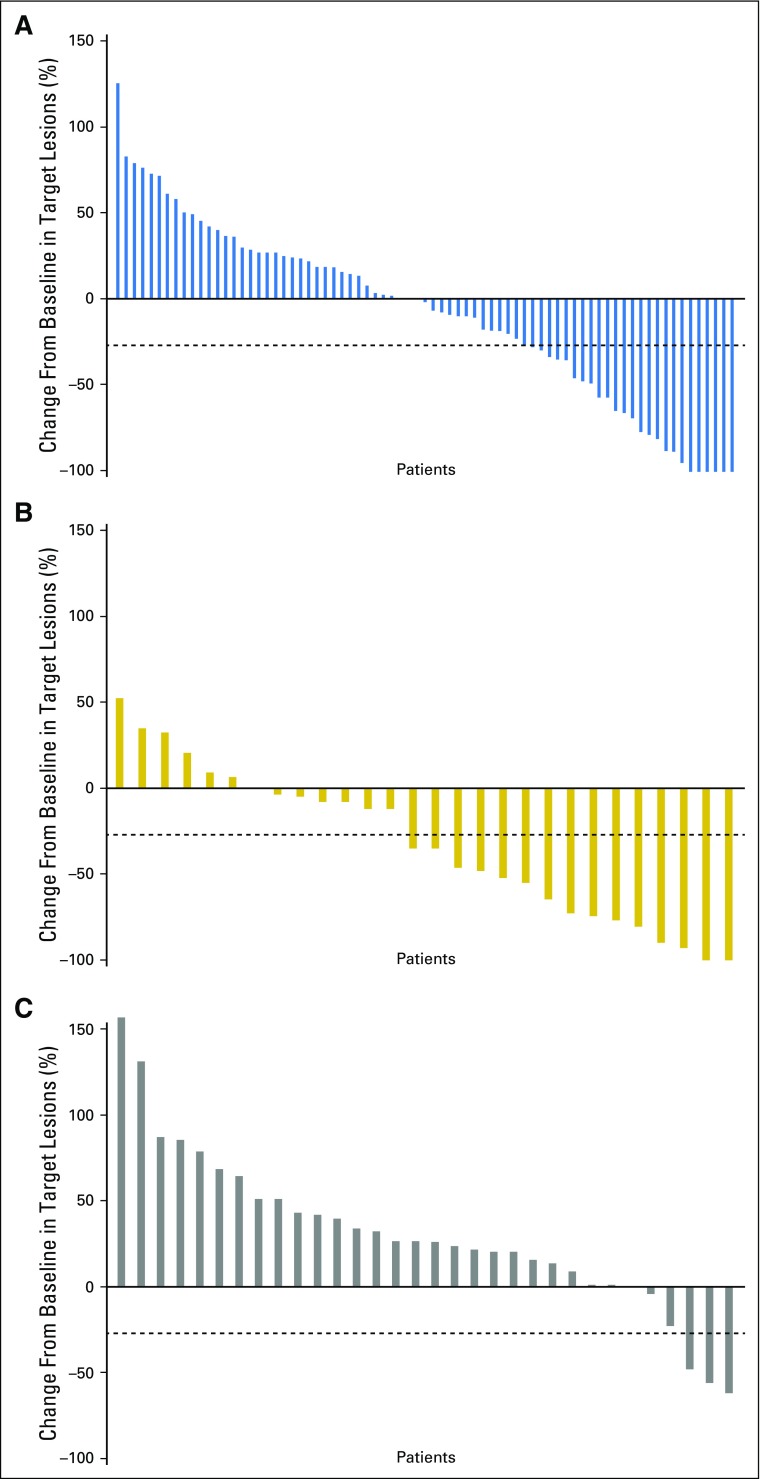
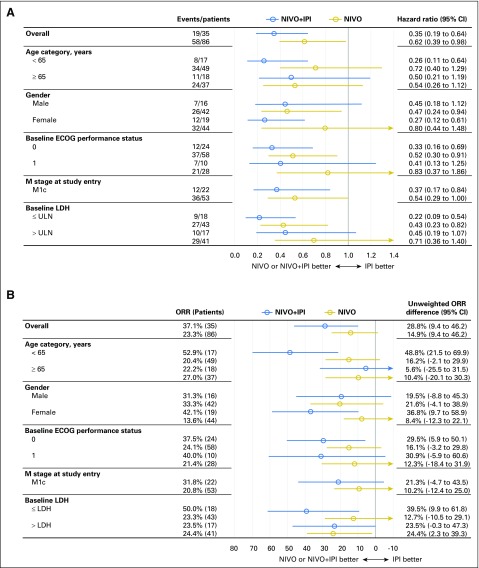
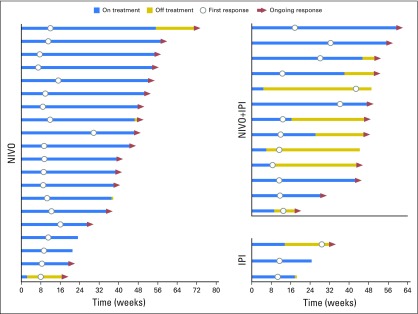
Median time to response was similar for both melanoma subtypes, regardless of treatment, and median duration of response was not reached in most groups (Table 2). There were ongoing responses in 85% of responders who received nivolumab alone or combination therapy (Appendix Fig A1, online only). In patients with mucosal melanoma, median reduction in tumor burden in the target lesions was −1.4% for nivolumab monotherapy, −34.2% for combination therapy, and +28.6% for ipilimumab monotherapy (Fig 2). Subgroup analyses in patients with mucosal melanoma suggested improved PFS and higher ORR with nivolumab monotherapy or combination therapy versus ipilimumab monotherapy across patient subgroups (Fig 3). Moreover, there seemed to be longer PFS and higher ORR across patient subgroups for combination therapy compared with nivolumab monotherapy.
Fig 2.
Waterfall plots showing tumor burden change from baseline in patients with mucosal melanoma who received (A) nivolumab alone (n = 75; median change, −1.4%); (B) combination therapy (n = 28; median change, −34.2%); and (C) ipilimumab alone (n = 32; median change, +28.6%). Dashed lines indicate a 30% reduction in tumor burden.
Fig 3.
Subgroup analyses of (A) progression-free survival and (B) objective response rate (ORR) for patients with mucosal melanoma. Horizontal bars indicate 95% CIs. ECOG, Eastern Cooperative Oncology Group; IPI, ipilimumab alone; LDH, lactate dehydrogenase; NIVO, nivolumab alone, NIVO+IPI, combination therapy.
Efficacy by PD-L1 status
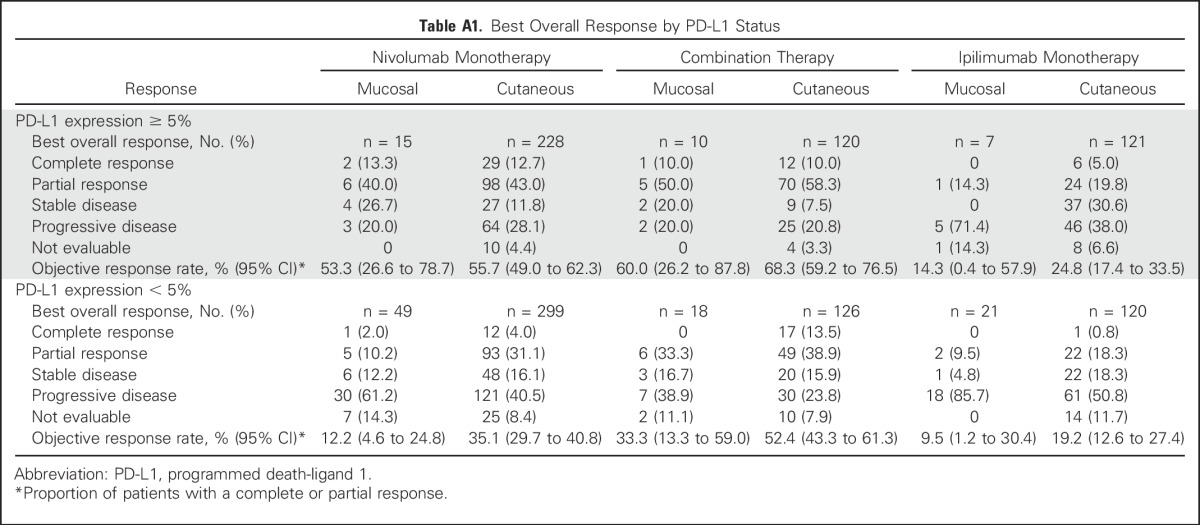
In patients with mucosal melanoma and tumor PD-L1 expression ≥ 5% (n = 32), ORR was 53.3% (95% CI, 26.6% to 78.7%), 60.0% (95% CI, 26.2% to 87.8%), and 14.3% (95% CI, 0.4% to 57.9%) for nivolumab monotherapy, combination therapy, and ipilimumab monotherapy, respectively (Appendix Table A1, online only); among patients with PD-L1 expression < 5% (n = 88), ORR was 12.2% (95% CI, 4.6% to 24.8%), 33.3% (95% CI, 13.3% to 59.0%), and 9.5% (95% CI, 1.2% to 30.4%), respectively. The magnitude of differences in ORR between patients with PD-L1 expression ≥ 5% and those with PD-L1 expression < 5% were greater for mucosal melanoma than for cutaneous melanoma (Appendix Table A1). Median PFS among patients with mucosal melanoma and tumor PD-L1 expression ≥ 5% was 12.2 months (95% CI, 3.0 months to not reached) for nivolumab monotherapy, not reached for combination therapy, and 2.8 months (95% CI, 2.6 months to not reached) for ipilimumab monotherapy (Appendix Fig A2). Among patients with mucosal melanoma and tumor PD-L1 expression < 5%, median PFS ranged from 2.2 to 2.8 months across treatment groups (Appendix Fig A2).
Safety
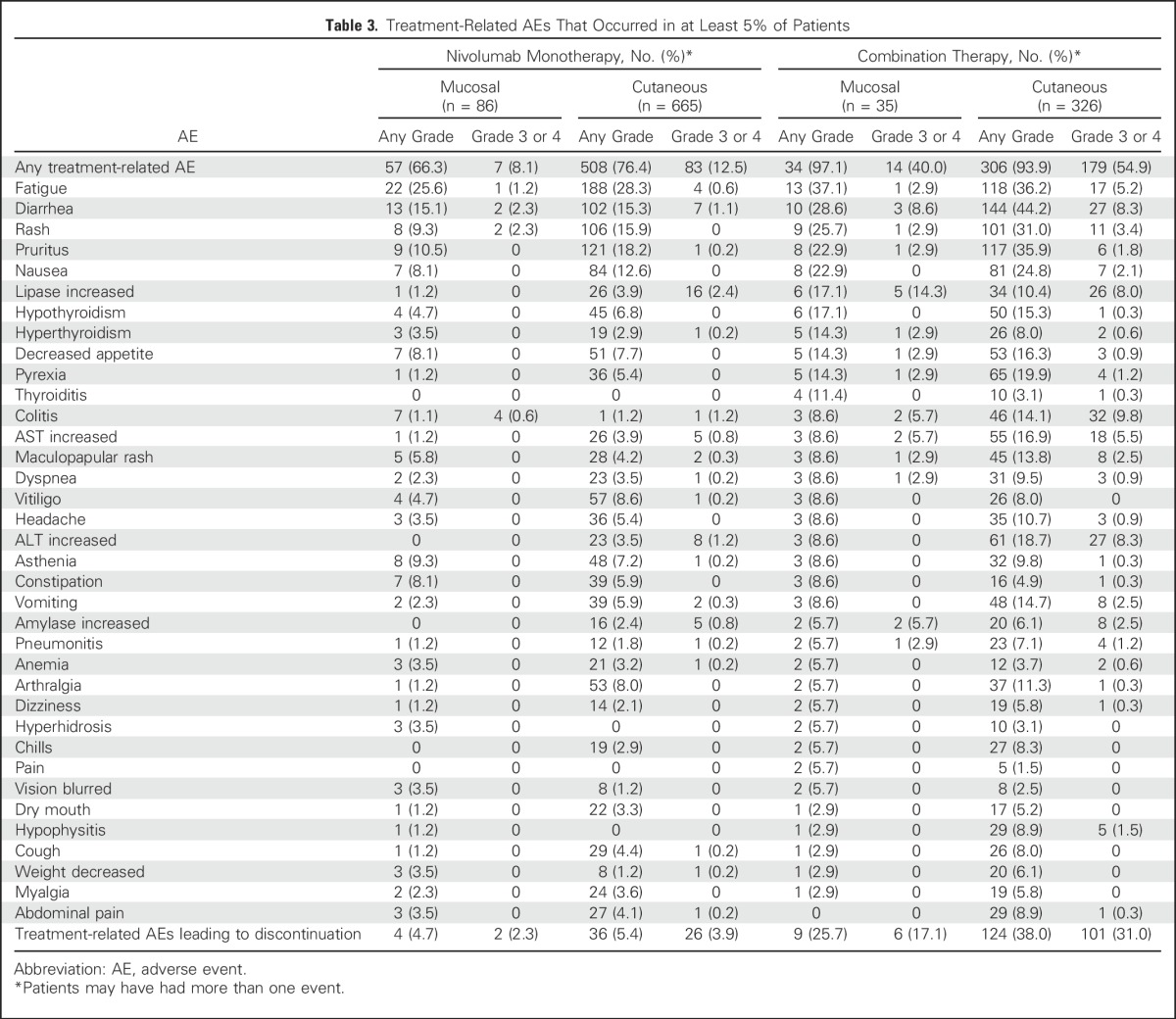
Table 3 summarizes the AEs that were considered to be related to study drug treatment in at least 5% of patients. The types and frequencies of treatment-related AEs were generally similar among patients with mucosal and cutaneous melanoma. However, the frequencies of treatment-related grade 3 or 4 AEs were higher for patients with cutaneous melanoma, particularly for those who received combination therapy (54.9% v 40.0%). In patients with mucosal melanoma, the most common treatment-related grade 3 or 4 AEs were diarrhea and rash in those who received nivolumab monotherapy and increased lipase and diarrhea for those who received combination therapy. In mucosal and cutaneous melanoma, respectively, the rates of discontinuation due to treatment-related AEs of grade 3 or 4 were 2.3% and 3.9% for nivolumab monotherapy and 17.1% and 31.0% for combination therapy. There were no drug-related deaths in patients with mucosal or cutaneous melanoma who received nivolumab monotherapy or in patients with cutaneous melanoma who received combination therapy. One drug-related death (2.9%) was reported in a patient with mucosal melanoma who received combination therapy. This patient had a history of cardiac disease and died of ventricular arrhythmia 29 days after the last dose of the study drug.
Table 3.
Treatment-Related AEs That Occurred in at Least 5% of Patients
DISCUSSION
To our knowledge, this pooled analysis represents the largest report to date of the efficacy and safety of an immune checkpoint inhibitor in mucosal melanoma. Although relatively small numbers of patients with mucosal melanoma were enrolled in individual nivolumab studies, this pooled analysis of data from six clinical studies has allowed for a more rigorous evaluation of anti–PD-1–based therapy in this subtype. The inclusion of these patients in the clinical trials and exclusion of other melanoma subtypes from most of the studies likely explains the higher incidence of mucosal melanoma in our analyses than is observed in the general population. Nivolumab combined with ipilimumab consistently showed a clinically meaningful improvement in PFS and ORR compared with either agent alone, with most tumor responses being durable. These results were observed across patient subgroups, including those with M1c disease and elevated LDH levels. Safety profiles were consistent with those observed in cutaneous melanoma.
Primary mucosal melanomas can arise from virtually any mucosal membrane, with the female genital tract being a common site of origin.8,9 In our study population, there was a higher percentage of females among patients with mucosal melanoma, versus a higher percentage of males in patients with cutaneous melanoma. Mucosal melanomas are considered to be the most aggressive of all melanoma subtypes.11 A higher percentage of patients with mucosal melanoma in our study had elevated LDH compared with patients with cutaneous melanoma. Although no formal comparisons were made between subtypes, efficacy outcomes seemed to be poorer in mucosal melanoma than in cutaneous melanoma. The exact reasons for the apparent differences in response to treatment between these subtypes remain unclear, yet studies have shown distinct biologic differences among noncutaneous melanomas and between cutaneous and noncutaneous melanomas.8,11,24 These differences include higher ratios of metastasis at diagnosis for mucosal and unknown primary melanomas,8 and a different pattern of metastasis for mucosal melanomas compared with other subtypes.24 Furthermore, although we did not collect information on the primary site of mucosal melanomas in our patient population, it is possible that response to treatment may have differed depending on anatomic location.
The distinct biologic characteristics of melanoma subtypes are likely to be explained, at least in part, by differences in genetic alterations.25-27 BRAF gene mutations occur at a much lower rate in mucosal melanomas than in cutaneous melanomas without chronic sun damage.25 Conversely, gene copy number and structural variations (eg, in KIT) are much more common in mucosal melanoma than in cutaneous melanoma.26 Patients were not selected for mutational status in our analyses; however, the results suggest that nivolumab may be effective in mucosal melanoma regardless of the tumor molecular profile, similar to the demonstrated efficacy of nivolumab in cutaneous melanoma regardless of BRAF mutation status.28
In our study population, it is interesting to note that more patients with cutaneous melanoma had tumor PD-L1 expression ≥ 5% than patients with mucosal melanoma. The reasons for this finding remain unclear, but one hypothesis is that mucosal melanomas may be less immunogenic due to a lower mutational burden.26 Despite differences in the proportion of patients with tumor PD-L1 expression ≥ 5%, ORR was similar between subtypes for nivolumab monotherapy and combination therapy. In contrast, lower activity in mucosal melanoma was observed across treatment groups for patients with tumor PD-L1 expression < 5%. However, an ORR of 33.3% with nivolumab plus ipilimumab in patients with mucosal melanoma and tumor PD-L1 expression < 5% suggests clinical activity of the combination regardless of PD-L1 status. The role of PD-L1 as a biomarker for nivolumab alone or in combination with ipilimumab remains unclear in any melanoma subtype, but the availability of mature OS data may help answer this question.
Poor outcomes have been reported with conventional therapies for mucosal melanoma, and there remains a high unmet need for effective systemic treatments for this subtype.12 Due to its rarity, mucosal melanoma has not been studied in large, randomized clinical trials. Thus, data supporting the efficacy of new systemic therapies is mostly based on anecdotal evidence and small retrospective analyses. Imatinib has demonstrated efficacy in patients with mucosal melanoma, but treatment is limited to the subset of patients with KIT mutations.14,15 The results of our current analyses support prior reports showing an ORR with ipilimumab of 7% to 12% and a median PFS of 2.3 to 4.3 months in patients with mucosal melanoma.16-18 Although there are no studies directly comparing agents, the median PFS of 5.9 months and ORR of 37.1% with nivolumab plus ipilimumab suggest that this combination may provide a greater outcome in patients with mucosal melanoma than previously reported with other therapies.
In summary, this large, pooled analysis of data from six clinical studies provides evidence for the efficacy and safety of anti–PD-1–based therapy in an aggressive melanoma subtype with a poor prognosis. Patients may benefit from anti–PD-1–based therapy regardless of the presence of poor prognostic factors, tumor PD-L1 expression, and prior therapy. The results of our analyses, pending mature OS data, suggest that nivolumab alone and in combination with ipilimumab are promising treatment options for mucosal melanoma.
ACKNOWLEDGMENT
Professional medical writing and editorial assistance were provided by Ward A. Pedersen, PhD and Cara Hunsberger at StemScientific, an Ashfield Company, funded by Bristol-Myers Squibb.
Appendix
Fig A1.
Time to and duration of response in patients with mucosal melanoma. IPI, ipilimumab alone; NIVO, nivolumab alone, NIVO+IPI, combination therapy.
Fig A2.
Progression-free survival by programmed death-1 receptor ligand 1 (PD-L1) status in patients with mucosal melanoma. (A) PD-L1 expression ≥ 5%; (B) PD-L1 expression < 5%. IPI, ipilimumab alone; NIVO, nivolumab alone, NIVO+IPI, combination therapy.
Table A1.
Best Overall Response by PD-L1 Status
Footnotes
Supported by Bristol-Myers Squibb, Princeton, NJ. J.L. is supported by the Royal Marsden/Institute of Cancer Research Biomedical Research Centre for Cancer.
Presented at the 12th International Congress of the Society for Melanoma Research, San Francisco, CA, November 18-21, 2015.
Clinical trial information: NCT00730639, NCT01621490, NCT01927419, NCT01721772, NCT01721746, and NCT01844505.
AUTHOR CONTRIBUTIONS
Conception and design: Sandra P. D’Angelo, Celeste Lebbé, Mary Ruisi, Jedd D. Wolchok
Provision of study materials or patients: Jeffrey A Sosman, Jessica C. Hassel, Wilson H. Miller Jr, Julie Charles, Jeffrey S. Weber
Collection and assembly of data: Sandra P. D'Angelo, Celeste Lebbé, Benjamin Brady, Bart Neyns, Jessica C. Hassel, F. Stephen Hodi, Kerry J. Savage, Peter Mohr, Ivan Marquez-Rodas, Martin Kaatz, Mario Sznol, Jeffrey S. Weber, Mary Ruisi
Data analysis and interpretation: Sandra P. D'Angelo, James Larkin, Jeffrey A. Sosman, Celeste Lebbé, Henrik Schmidt, F. Stephen Hodi, Paul Lorigan, Kerry J. Savage, Wilson H. Miller Jr, Julie Charles, Mario Sznol, Alexander N. Shoushtari, Mary Ruisi, Joel Jiang, Jedd D. Wolchok
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Efficacy and Safety of Nivolumab Alone or in Combination With Ipilimumab in Patients With Mucosal Melanoma: A Pooled Analysis
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Sandra P. D'Angelo
Consulting or Advisory Role: EMD Serono, Amgen
James Larkin
Research Funding: Pfizer (Inst), Novartis (Inst), Merck Sharp & Dohme Oncology (Inst), Bristol-Myers Squibb (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Merck Sharp & Dohme Oncology, Pfizer, Novartis, Eisai, GlaxoSmithKline, Roche, Genentech
Jeffrey A. Sosman
Honoraria: Array, Genentech, Merck, Novartis
Consulting or Advisory Role: Array, Genentech, Merck, Novartis
Research Funding: Novartis (Inst), Bristol-Myers Squibb (Inst), Genentech (Inst)
Celeste Lebbé
Honoraria: Amgen, Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Roche
Consulting or Advisory Role: Roche
Research Funding: Roche
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Roche
Benjamin Brady
Consulting or Advisory Role: Merck, Novartis
Speakers' Bureau: Bristol-Myers Squibb, Merck
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Bart Neyns
Consulting or Advisory Role: Bristol-Myers Squibb
Speakers' Bureau: Bristol-Myers Squibb
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Henrik Schmidt
Consulting or Advisory Role: Bristol-Myers Squibb, Merck Sharp & Dohme, Roche, GlaxoSmithKline
Speakers' Bureau: Bristol-Myers Squibb, GlaxoSmithKline
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Amgen
Jessica C. Hassel
Honoraria: Bristol-Myers Squibb, Merck Sharp & Dohme, Roche, GlaxoSmithKline, Novartis, Amgen
Consulting or Advisory Role: Merck Sharp & Dohme, Amgen
Research Funding: Bristol-Myers Squibb (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Merck Sharp & Dohme, Amgen, GlaxoSmithKline, Novartis, Roche
F. Stephen Hodi
Consulting or Advisory Role: Bristol-Myers Squibb, EMD Serono, Genentech, Merck, Novartis, Synta
Research Funding: Bristol-Myers Squibb (Inst)
Patents, Royalties, Other Intellectual Property: Tumor antigens and uses therof as per institutional policy, patent pending royalties received on MICA-related disorders application to institution per institutional IP policy
Paul Lorigan
Honoraria: Bristol-Myers Squibb, Merck, Roche, Novartis, Amgen
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, Roche, Novartis, Amgen
Speakers' Bureau: Bristol-Myers Squibb, Merck, Novartis
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Merck
Kerry J. Savage
Honoraria: Seattle Genetics, Bristol-Myers Squibb, Celgene
Consulting or Advisory Role: Seattle Genetics, Bristol-Myers Squibb
Speakers' Bureau: Seattle Genetics
Research Funding: Roche (Inst)
Wilson H. Miller Jr
Honoraria: Bristol-Myers Squibb, Merck, Roche, Novartis, GlaxoSmithKline
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, Roche, Novartis
Research Funding: Argos (Inst), AstraZeneca (Inst), Bayer (Inst), Bristol-Myers Squibb (Inst), GlaxoSmithKline (Inst), MedImmune (Inst), Merck (Inst), Novartis (Inst), Roche (Inst)
Stock or Other Ownership: Bristol-Myers Squibb
Peter Mohr
Honoraria: Merck Sharp & Dohme, Bristol-Myers Squibb, Roche, Novartis, Amgen
Consulting or Advisory Role: Bristol-Myers Squibb, Merck Sharp & Dohme, Roche, Novartis
Research Funding: Merck Sharp & Dohme
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Bristol-Myers Squibb, Novartis, Roche, Amgen
Ivan Marquez-Rodas
Honoraria: Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Roche, Amgen
Consulting or Advisory Role: Bristol-Myers Squibb, Amgen, Merck Sharp & Dohme, Novartis, Roche
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Merck Sharp & Dohme
Julie Charles
Travel, Accommodations, Expenses: Roche
Martin Kaatz
Honoraria: Roche, Bristol-Myers Squibb, Novartis, Merck Sharp & Dohme
Consulting or Advisory Role: Roche, Bristol-Myers Squibb, Novartis, Merck Sharp & Dohme
Research Funding: Federal Ministry of Education and Research
Expert Testimony: Roche, Novartis
Mario Sznol
Stock or Other Ownership: Amphivena, Intensity Therapeutics, Adaptive Biotechnologies
Consulting or Advisory Role: Alexion, Adaptive Biotechnologies, Amphivena, AstraZeneca/Medimmune, Biodesix, Bristol-Myers Squibb, Genentech-Roche, Immune Design, Intensity, Janssen/Johnson and Johnson, Kyowa-Kirin, Lilly, Lion Biotechnologies, Merck, Nektar, Novartis, Pfizer, Pierre-Fabre, Prometheus, Symphogen, Theravance, Vaccinex
Jeffrey S. Weber
Stock or Other Ownership: Altor BioScience, Celldex, cCam Biotherapeutics, CytomX Therapeutics
Honoraria: Abbvie, Alkermes, AstraZeneca, Bristol-Myers Squibb, cCAM, Celldex, CytomX Therapeutics, Daiichi Sankyo, EMD Serono, Genentech, GlaxoSmithKline, Lion Biotechnologies, Merck, Nektar
Consulting or Advisory Role: Abbvie, Alkermes, AstraZeneca, Bristol-Myers Squibb, cCAM, Celldex, CytomX, Daiichi Sankyo, EMD Serono, Genentech, GlaxoSmithKline, Lion Biotechnologies, Merck, Nektar
Research Funding: Acetylon, Bristol-Myers Squibb, Genentech, GlaxoSmithKline, MacroGenics, Merck, Mirati
Travel, Accommodations, Expenses: Abbvie, Alkermes, AstraZeneca, Bristol-Myers Squibb, cCAM, Celldex, CytomX, Daiichi Sankyo, EMD Serono, Genentech, GlaxoSmithKline, Lion Biotechnologies, Merck, Nektar
Alexander N. Shoushtari
Consulting or Advisory Role: Vaccinex, Castle Biosciences
Research Funding: Bristol-Myers Squibb
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Mary Ruisi
Employment: Bristol-Myers Squibb
Stock or Other Ownership: Bristol-Myers Squibb
Joel Jiang
Employment: Bristol-Myers Squibb
Stock or Other Ownership: Bristol-Myers Squibb
Jedd D. Wolchok
Stock or Other Ownership: Potenza Therapeutics, Tizona Pharmaceuticals
Consulting or Advisory Role: Bristol-Myers Squibb, Genentech, MedImmune, Merck
Research Funding: Bristol-Myers Squibb (Inst), MedImmune (Inst), Genentech, Merck (Inst)
Patents, Royalties, Other Intellectual Property: I am a co-inventor on an issued patent for DNA vaccines for treatment of cancer in companion animals
Travel, Accommodations, Expenses: Bristol-Myers Squibb
REFERENCES
- 1.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33:1889–1894. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 4.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomized, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 5.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmad SS, Qian W, Ellis S, et al. Ipilimumab in the real world: The UK expanded access programme experience in previously treated advanced melanoma patients. Melanoma Res. 2015;25:432–442. doi: 10.1097/CMR.0000000000000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tas F, Keskin S, Karadeniz A, et al. Noncutaneous melanoma have distinct features from each other and cutaneous melanoma. Oncology. 2011;81:353–358. doi: 10.1159/000334863. [DOI] [PubMed] [Google Scholar]
- 9.Mihajlovic M, Vlajkovic S, Jovanovic P, et al. Primary mucosal melanomas: A comprehensive review. Int J Clin Exp Pathol. 2012;5:739–753. [PMC free article] [PubMed] [Google Scholar]
- 10.DeMatos P, Tyler DS, Seigler HF. Malignant melanoma of the mucous membranes: A review of 119 cases. Ann Surg Oncol. 1998;5:733–742. doi: 10.1007/BF02303485. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Si L, Guo J. Treatment algorithm of metastatic mucosal melanoma. Chin Clin Oncol. 2014;3:38. doi: 10.3978/j.issn.2304-3865.2014.08.04. [DOI] [PubMed] [Google Scholar]
- 12. Bitas C, Shoushtari AN, Bluth MJ, et al: The Memorial Sloan Kettering Cancer Center (MSKCC) experience of systemic therapy in mucosal melanoma. J Clin Oncol 32:5s, 2014 (suppl 5s; abstr 9073)
- 13.Yi JH, Yi SY, Lee HR, et al. Dacarbazine-based chemotherapy as first-line treatment in noncutaneous metastatic melanoma: Multicenter, retrospective analysis in Asia. Melanoma Res. 2011;21:223–227. doi: 10.1097/CMR.0b013e3283457743. [DOI] [PubMed] [Google Scholar]
- 14.Carvajal RD, Antonescu CR, Wolchok JD, et al. KIT as a therapeutic target in metastatic melanoma. JAMA. 2011;305:2327–2334. doi: 10.1001/jama.2011.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodi FS, Corless CL, Giobbie-Hurder A, et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol. 2013;31:3182–3190. doi: 10.1200/JCO.2012.47.7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Postow MA, Luke JJ, Bluth MJ, et al. Ipilimumab for patients with advanced mucosal melanoma. Oncologist. 2013;18:726–732. doi: 10.1634/theoncologist.2012-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexander M, Mellor JD, McArthur G, et al. Ipilimumab in pretreated patients with unresectable or metastatic cutaneous, uveal and mucosal melanoma. Med J Aust. 2014;201:49–53. doi: 10.5694/mja13.10448. [DOI] [PubMed] [Google Scholar]
- 18.Del Vecchio M, Di Guardo L, Ascierto PA, et al. Efficacy and safety of ipilimumab 3mg/kg in patients with pretreated, metastatic, mucosal melanoma. Eur J Cancer. 2014;50:121–127. doi: 10.1016/j.ejca.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Zimmer L, Eigentler TK, Kiecker F, et al. Open-label, multicenter, single-arm phase II DeCOG-study of ipilimumab in pretreated patients with different subtypes of metastatic melanoma. J Transl Med. 2015;13:351. doi: 10.1186/s12967-015-0716-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Min L, Hodi FS. Anti-PD1 following ipilimumab for mucosal melanoma: Durable tumor response associated with severe hypothyroidism and rhabdomyolysis. Cancer Immunol Res. 2014;2:15–18. doi: 10.1158/2326-6066.CIR-13-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Urba WJ, Martin-Algarra S, Callahan M, et al: Immunomodulatory activity of nivolumab monotherapy in patients with advanced melanoma. Presented at Am Assoc Cancer Res Annual Meeting, Philadelphia, PA, April 18-22, 2015 (abstr 2855) [Google Scholar]
- 23.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Del Prete V, Chaloupka K, Holzmann D, et al. Noncutaneous melanomas: A single-center analysis. Dermatology. 2016;232:22–29. doi: 10.1159/000441444. [DOI] [PubMed] [Google Scholar]
- 25.Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 26.Furney SJ, Turajlic S, Stamp G, et al. Genome sequencing of mucosal melanomas reveals that they are driven by distinct mechanisms from cutaneous melanoma. J Pathol. 2013;230:261–269. doi: 10.1002/path.4204. [DOI] [PubMed] [Google Scholar]
- 27.Bastian BC. The molecular pathology of melanoma: An integrated taxonomy of melanocytic neoplasia. Annu Rev Pathol. 2014;9:239–271. doi: 10.1146/annurev-pathol-012513-104658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larkin J, Lao CD, Urba WJ, et al. Efficacy and safety of nivolumab in patients with BRAF V600 mutant and BRAF wild-type advanced melanoma: A pooled analysis of 4 clinical trials. JAMA Oncol. 2015;1:433–440. doi: 10.1001/jamaoncol.2015.1184. [DOI] [PubMed] [Google Scholar]