Abstract
Purpose
Oral targeted therapies represent a significant advance for the treatment of patients with chronic lymphocytic leukemia (CLL); however, their high cost has raised concerns about affordability and the economic impact on society. Our objective was to project the future prevalence and cost burden of CLL in the era of oral targeted therapies in the United States.
Methods
We developed a simulation model that evaluated the evolving management of CLL from 2011 to 2025: chemoimmunotherapy (CIT) as the standard of care before 2014, oral targeted therapies for patients with del(17p) and relapsed CLL from 2014, and for first-line treatment from 2016 onward. A comparator scenario also was simulated where CIT remained the standard of care throughout. Disease progression and survival parameters for each therapy were based on published clinical trials.
Results
The number of people living with CLL in the United States is projected to increase from 128,000 in 2011 to 199,000 by 2025 (55% increase) due to improved survival; meanwhile, the annual cost of CLL management will increase from $0.74 billion to $5.13 billion (590% increase). The per-patient lifetime cost of CLL treatment will increase from $147,000 to $604,000 (310% increase) as oral targeted therapies become the first-line treatment. For patients enrolled in Medicare, the corresponding total out-of-pocket cost will increase from $9,200 to $57,000 (520% increase). Compared with the CIT scenario, oral targeted therapies resulted in an incremental cost-effectiveness ratio of $189,000 per quality-adjusted life-year.
Conclusion
The increased benefit and cost of oral targeted therapies is projected to enhance CLL survivorship but can impose a substantial financial burden on both patients and payers. More sustainable pricing strategies for targeted therapies are needed to avoid financial toxicity to patients.
INTRODUCTION
Chronic lymphocytic leukemia (CLL) is the most prevalent leukemia in the western world. In the United States, approximately 130,000 patients live with CLL, and approximately 15,000 new cases occur every year.1 Although most patients with CLL have early-stage disease at the time of initial diagnosis and are recommended for watchful waiting,2 the majority eventually require treatment, typically after a few years of observation, and often experience prolonged survival.3,4
Chemoimmunotherapy (CIT) regimens, such as fludarabine, cyclophosphamide, and rituximab (FCR), have been the standard first-line treatment of young patients with CLL.5,6 In a single-center experience, the FCR regimen led to a complete remission rate of 72% and a median progression-free survival (PFS) of 80 months.7,8 Subsequently, the German CLL8 trial established FCR as the standard first-line therapy.6 For patients older than 65 years or with comorbidities, the combination of chlorambucil and obinutuzumab is considered standard of care on the basis of the results of the CLL11 trial.9
In the past few years, major strides have been made in understanding the biology of CLL, which have led to significant advances in the treatment of CLL. In particular, oral targeted agents, such as ibrutinib and idelalisib, have demonstrated remarkable outcomes in patients with CLL. In the relapsed setting, ibrutinib showed an overall response rate (ORR) of 90% and estimated PFS of 69% at 30 months10; idelalisib showed an ORR of 72% and median PFS of 15.8 months as monotherapy in a phase I study11 and an ORR of 81% and estimated overall survival (OS) of 92% at 12 months when used in combination with rituximab in a phase III study.12 In 2014, the Food and Drug Administration approved two oral targeted therapies: ibrutinib for patients with relapsed/refractory CLL and for patients with del(17p) and idelalisib in combination with rituximab for patients with relapsed/refractory CLL. In March 2016, ibrutinib was approved for first-line management of CLL. In addition, several other targeted therapies are expected to become available in the near future.13 Venetoclax was approved for patients with relapsed CLL with del(17p) in April 2016.14 These novel therapies have revolutionized the CLL treatment paradigm.
However, the high cost of these targeted therapies raises concerns for payers as well as for patients.15,16 Both ibrutinib and idelalisib are priced at approximately $130,000 per year and are recommended until patients have progressive disease or significant toxicities. In contrast, the costs for CIT-based treatments range from $60,000 to $100,000 for a finite duration, that is, a typical six-cycle course that lasts for approximately 6 months. Therefore, novel targeted therapies could strain the budget of both private and government payers, such as Medicare, and copayments and other expenses can be a substantial burden to patients, yet the budget impact and cost-effectiveness of these therapies are not well understood. Our objective for this study was to project the changing economic as well as disease burden of CLL in the United States in the era of targeted therapies and to evaluate the affordability and value of these new therapies.
METHODS
We developed a microsimulation model, simCLL (simulation model of CLL management), that simulated the dynamics of the CLL patient population under given management strategies in the United States from 2011 to 2025.
Patient Population
Patient characteristics were defined by age, phase of CLL treatment (watchful waiting, first line, or relapse), and del(17p) status. Age at diagnosis for each individual patient was sampled from the age distribution on the basis of SEER data between 2000 and 2011.17 Del(17p) was assumed to be present in 7% of the CLL patient population.18 New CLL cases were added to the simulated population in each year on the basis of published annual incidence estimates from the American Cancer Society1 (Data Supplement). The prevalence of CLL from the simulation model was calibrated to SEER data between 2000 and 2011 (Data Supplement).
Simulated Clinical Pathways
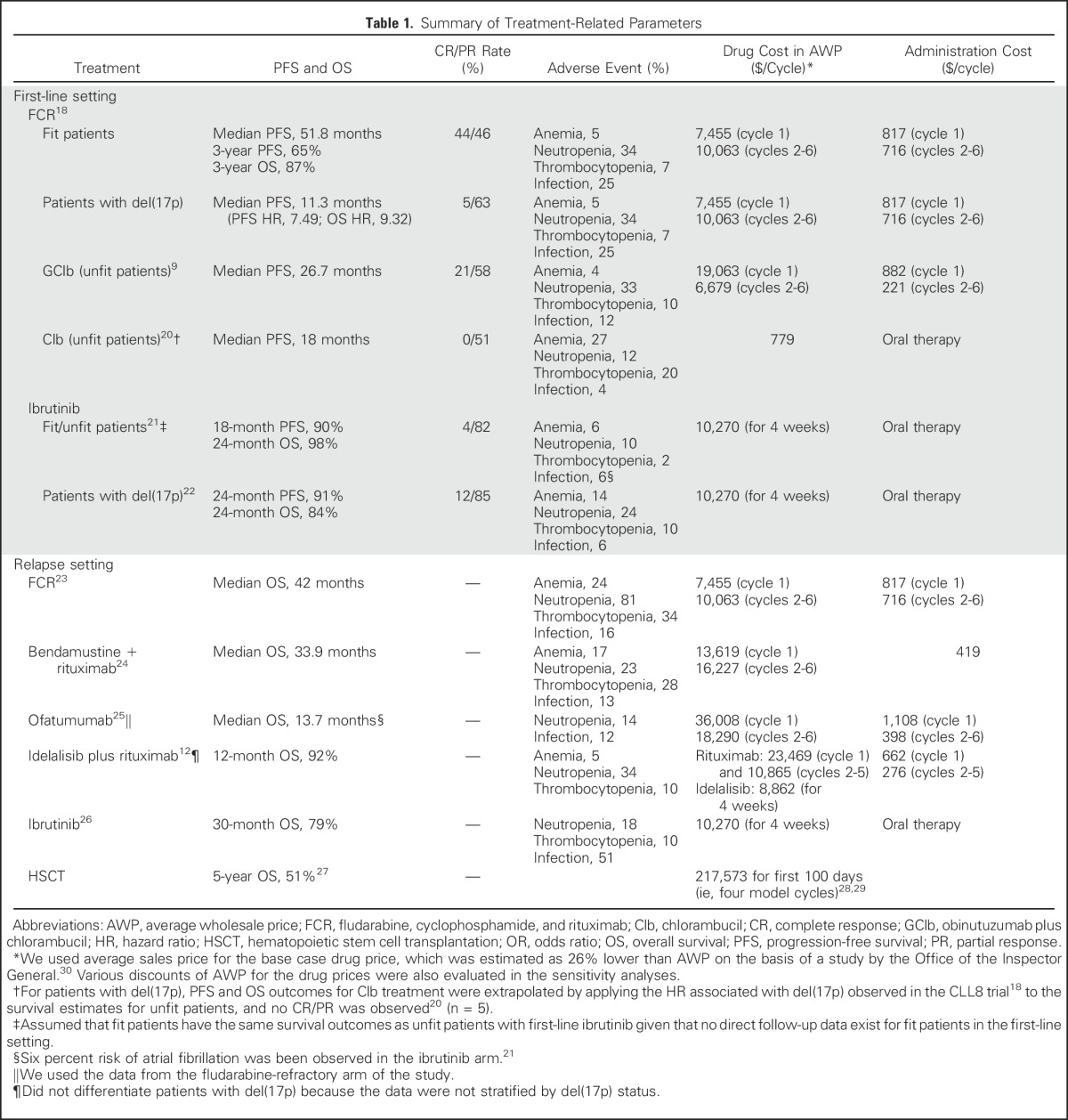
We modeled the clinical course of patients with CLL by using a patient-level state-transition model, which included the following health states: watchful waiting, first-line treatment, relapse, and death (Data Supplement). The majority of patients with a new diagnosis of CLL do not need immediate treatment4 and were assumed to start in the watchful waiting state.19 (The probability was determined through the model calibration.) Upon failure of first-line treatment, patients entered the relapse state. The probabilities of health state transitions were estimated on the basis of time to treatment, PFS, and OS data from clinical trials (Table 1; Data Supplement). We selected the trials that represent the best available evidence (eg, phase III trials, large observational studies) for the major regimens in general practice, with reference to clinical guidelines4 and expert opinions. We also validated our model by comparing the simulated survival curves with observed survival data (Data Supplement). Finally, we applied age-specific background mortality on the basis of US life tables.31
Table 1.
Summary of Treatment-Related Parameters
Treatment Strategies
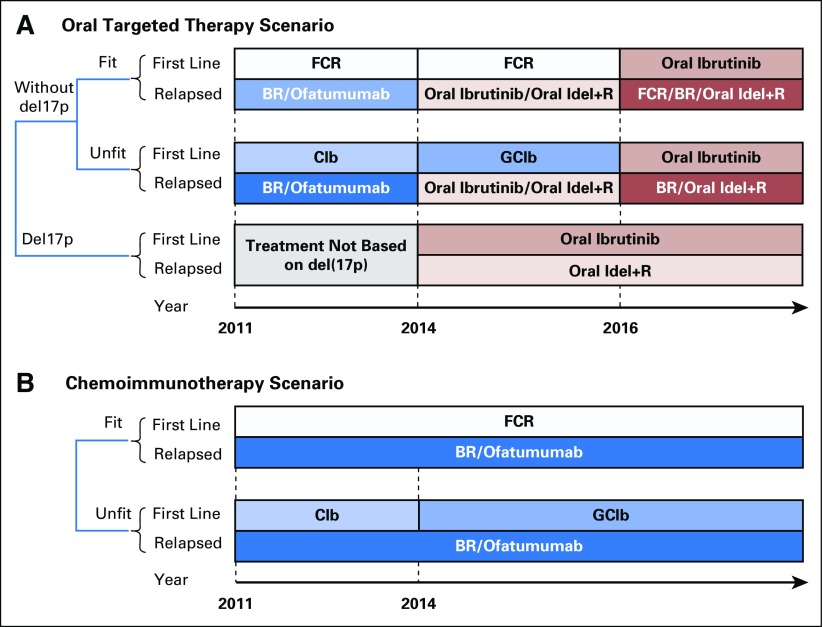
A treatment strategy defined the specific therapy for a patient by status of relapse, fitness (determined by age), del(17p), and year of treatment (Fig 1). We first simulated a clinical scenario that considered the current standard of care and emerging treatment options (Fig 1A). In particular, before 2014, CIT was the mainstay treatment of patients with CLL. The most common choices for first-line treatment were FCR for fit patients and chlorambucil for unfit patients. From 2014 on, oral targeted therapies were approved for patients with relapsed CLL and for patients with del(17p).12,32 From 2016 on, oral targeted therapies became the standard of care in the first-line setting.21 This scenario is referred to as the oral targeted therapy scenario.
Fig 1.
Management strategies for patients with chronic lymphocytic leukemia (CLL). (A) The oral targeted therapy scenario with evolving therapeutic options for patients with CLL. (B) The chemoimmunotherapy scenario, which continues to use chemoimmunotherapy as the standard of care. We assumed equal allocation to multiple therapies if more than one therapy is considered available for patients in the same condition. For example, for fit patients in the relapse setting during 2014 to 2016, 50% of patients received ibrutinib and 50% received idelalisib plus rituximab. Moreover, 5% of patients in the relapse setting were assumed to receive hematopoietic stem-cell transplantation (data not shown). BR, bendamustine plus rituximab; Clb, chlorambucil; FCR, fludarabine, cyclophosphamide, and rituximab; GClb, obinutuzumab plus chlorambucil; Idel+R, idelalisib plus rituximab.
For comparison, we simulated a scenario where CIT would have remained the standard of care in the future (Fig 1B). In this scenario, first-line therapy for unfit patients would be obinutuzumab plus chlorambucil after 2014.9 This scenario is referred to as the CIT scenario.
Costs
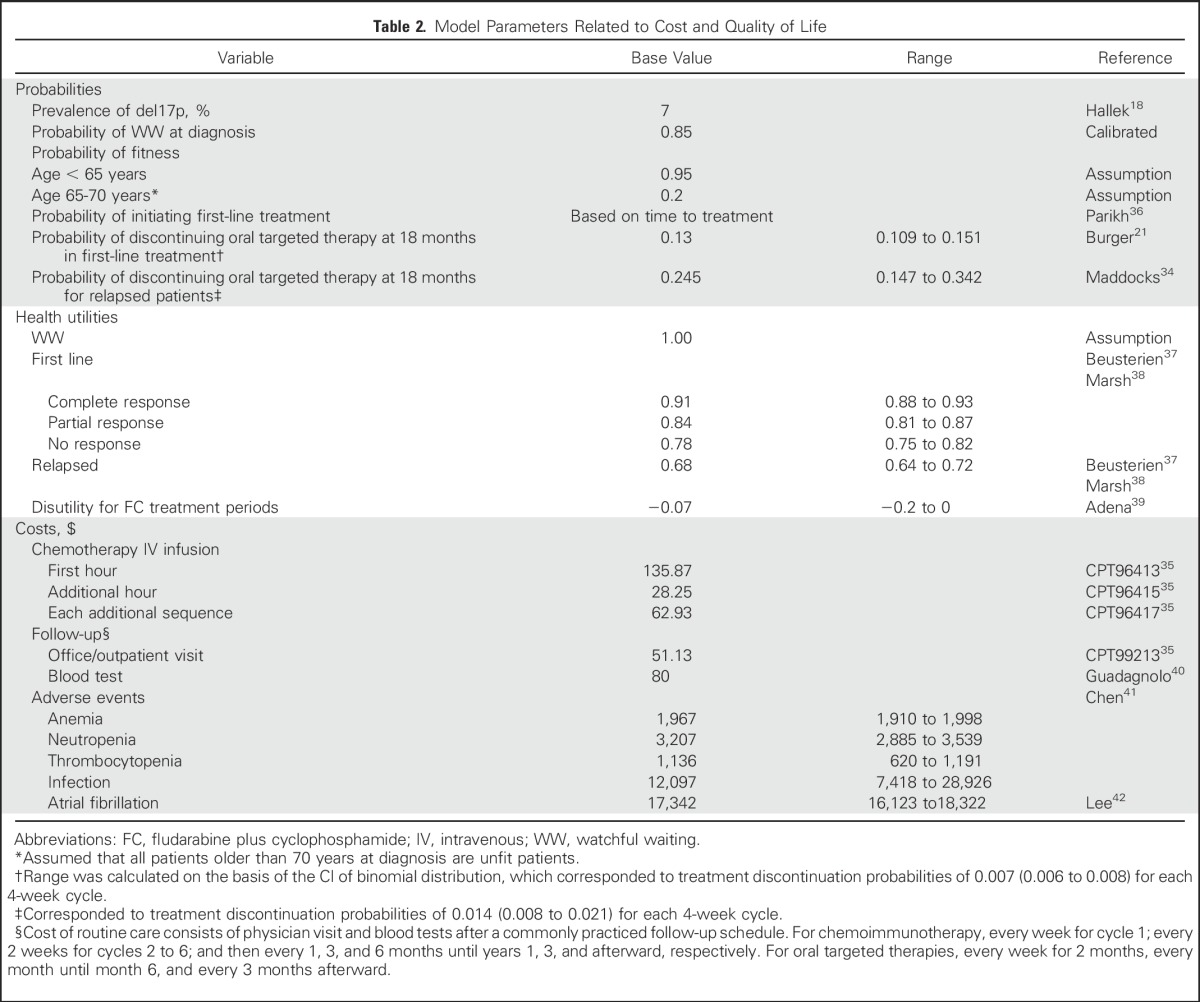
Direct medical costs were considered, including the cost of drugs and administration, routine follow-up, and management of adverse events. Drug costs were calculated on the basis of doses of the standard regimen and average sales price of each drug. Average sales price was estimated as 26% lower than the average wholesale price (AWP; Table 1, Data Supplement),33 as suggested by an Office of the Inspector General study.30 For oral targeted agents, drug costs were accumulated for an indefinite period until treatment was discontinued. As observed in the clinical studies with ibrutinib, 87% of patients in the first-line setting and 75% in the relapsed setting would continue oral targeted therapy beyond 18 months.21,34
Administration costs, such as for physician visits and chemotherapy infusions, were calculated on the basis of the Medicare physician fee schedule35 (Table 2; Data Supplement) by using an approach described elsewhere.43 Common serious adverse events, including grade 3/4 infection and hematologic toxicities, were simulated on the basis of the reported incidence for each therapy (Table 1), and the management cost for each was derived from the corresponding treatment and hospitalization costs41 (Table 2). We also considered the risk and the cost of atrial fibrillation with oral targeted therapy because atrial fibrillation has emerged as an important concern with ibrutinib treatment.43
Table 2.
Model Parameters Related to Cost and Quality of Life
Health-Related Quality of Life
The health-related quality-of-life weights (utilities) were adjusted by health states and patient age.37,44 We assumed a utility of 1 in the watchful waiting state. For the first-line treatment state, utility was determined by response type (ie, complete, partial, no response), which was sampled according to the response rate of the treatment. In addition, the utilities were adjusted on the basis of patient age,45 and disutility was applied to fludarabine and cyclophosphamide–containing regimens.39,46
Model Outcomes
We projected the number of people living with CLL and the annual cost of CLL management in the United States from 2011 to 2025. In addition, we calculated per-patient lifetime cost with oral targeted therapies as well as with CIT as the standard of care. Because the majority of patients are older than 65 years at the time of CLL diagnosis and are covered by Medicare, we also estimated the lifetime out-of-pocket cost of the oral targeted therapies for patients enrolled in Medicare Part D (Data Supplement). We estimated total discounted person-life-years (LYs) and person-quality-adjusted LYs (QALYs) as the total health outcomes at the population level from 2011 to 2025 and finally estimated the incremental cost-effectiveness ratio (ICER) of oral targeted therapies compared with the CIT scenario. All costs were converted to 2015 US dollars. In the cost-effectiveness analysis, future outcomes were discounted to the value in 2015 at 3% per year. For simplicity, we present the rounded values of all numerical results.
Sensitivity Analysis
To evaluate the robustness of outcomes against uncertainty in model inputs, we performed one-way deterministic sensitivity analyses. Utilities and probabilities of discontinuation of oral targeted therapies were varied within their reported CI, other transition probabilities and costs were varied within a 20% range, and survival distributions were adjusted with hazard ratios between 0.8 and 1.2. We also performed a probabilistic sensitivity analysis that accounted for joint uncertainty in all model inputs.
In addition, we performed two scenario analyses. First, with consideration of the aging US population (Data Supplement),47 we adjusted the CLL incidence with the US population projection data (Data Supplement) and simulated a scenario with a higher CLL incidence rate. Second, we evaluated a scenario that simulated partial uptake of oral targeted therapies, which represents a gradual transition from CIT. Specifically, we assumed 25% use of oral targeted therapies in first-line treatment for fit patients after approval in 2016 and increased by 25% every year until a full use in 2019 and beyond.
RESULTS
Disease Burden
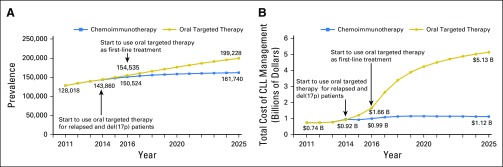
The total number of people living with CLL is projected to increase from 128,000 in 2011 to 199,000 (55% increase) in 2025 due to improved survival with the use of oral targeted therapies. In contrast, if CIT remains the standard of care, the number of people living with CLL would be 162,000 (26% increase) by 2025 (Fig 2A).
Fig 2.
Trend in disease and cost burden of chronic lymphocytic leukemia (CLL) for the chemoimmunotherapy and the oral targeted therapy scenarios. (A) The number of patients with CLL under the chemoimmunotherapy and oral targeted therapy scenarios. The use of oral targeted therapies is projected to increase the number of people living with CLL from 128,000 in 2011 to 199,000 (55% increase) in 2025 due to improved survival with the use of oral targeted therapies. (B) Annual management cost of CLL for the chemoimmunotherapy and the oral targeted therapy scenarios. The use of oral targeted therapies is projected to increase the annual cost in CLL management from $0.74 billion in 2011 to $5.13 billion (593% increase) in 2025, which is mainly driven by high drug prices, prolonged treatment duration of oral agents, and increased number of patients living with CLL.
Cost Burden
Annual cost of CLL care.
Under the oral targeted therapy scenario, the annual cost of CLL management is projected to increase from $0.74 billion in 2011 to $5.13 billion (593% increase) in 2025 (Fig 2B). The first surge in the annual cost occurred in 2014 when oral targeted therapies became available for patients who relapsed, and the second surge will occur in 2016 because of the approval of oral targeted therapy in the first-line setting. In contrast to the increasing cost trend with oral targeted therapies, the annual cost under the CIT scenario would have remained relatively stable from 2014 on, reaching $1.12 billion in 2025. Compared with the CIT scenario, oral targeted therapies would result in an additional spending of $29 billion from their availability in 2014 until 2025. Among the total cost of CLL management, drug costs constituted 96% in the oral targeted therapy scenario and 86% in the CIT scenario.
Lifetime cost of CLL treatment.
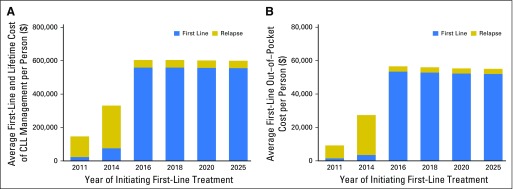
The per-person lifetime cost of CLL treatment of patients initiating therapy in 2011 was $147,000, which increased to $331,000 (125% increase) for patients who initiated therapy in 2014 (Fig 3A). For patients who initiated oral therapy (now approved in the first-line setting) in 2016, the lifetime cost of CLL treatment is projected to reach $604,000 (310% increase from 2011).
Fig 3.
Lifetime treatment cost grouped by the year of initiating first-line treatment of the oral targeted therapy scenario. (A) Lifetime treatment cost to payers. (B) Lifetime out-of-pocket cost for Medicare patients.
Out-of-pocket cost for Medicare patients.
The majority of patients with CLL in the United States are covered by Medicare and have drug coverage through Medicare Part D. The out-of-pocket cost of oral agents for Medicare patients was estimated on the basis of deductible and coverage limits in Medicare Part D plan (Data Supplement)48 and was estimated to be $9,200 for those initiating therapy in 2011, which increased to $27,000 (193% increase) for patients who initiated therapy in 2014 and to $57,000 (519% increase) for those who initiate treatment from 2016 on (Fig 3B). Use of oral targeted therapies in the first-line setting after 2016 also substantially increased the first-line treatment cost, which constituted the major proportion of the lifetime cost.
Cost-effectiveness Analysis
From 2011 to 2025, the total discounted health outcomes were 1,850,000 person-QALYs (2,193,000 person-LYs) under the oral targeted therapy scenario and 1,743,000 person-QALYs (2,044,000 person-LYs) under the CIT scenario. Compared with the CIT scenario, the oral targeted therapy scenario resulted in an increase of 107,000 person-QALYs (149,000 person-LYs), with additional discounted costs of $20.2 billion. The ICER of oral targeted therapies was $189,000/QALY ($136,000/LY).
Sensitivity Analysis
We examined the sensitivity of results to oral drug cost discounts. With consideration of a 37% discount off the AWP as the lowest price paid by private sector payers for drug products,49 the total incremental cost was $24 billion, lifetime out-of-pocket cost for patients with first-line oral targeted therapy was $52,000, and ICER of oral targeted therapy was $161,000/QALY (Data Supplement). When the oral targeted agent cost is reduced to 50% of AWP, the ICER reduces to $107,000/QALY. A threshold cost analysis showed that the cost of oral targeted therapies needs to be at least 69% lower than the current AWP to bring the ICER below $50,000/QALY threshold.
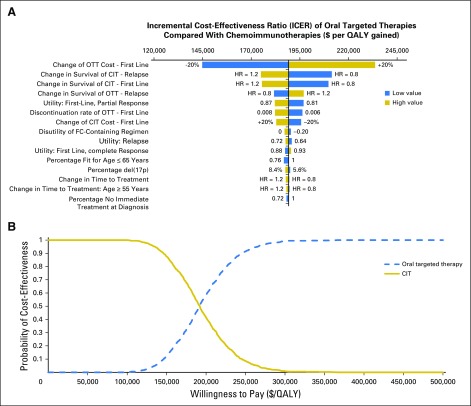
One-way sensitivity analysis showed that the cost of CLL management is sensitive to treatment cost, discontinuation rate of oral targeted therapies, immediate treatment probability at initial visit, and time to first-line treatment from the watchful waiting state (Data Supplement). The ICER was most sensitive to survival distributions of treatments as well as to health-related utilities for partial response in first-line treatment and in the relapse setting (Fig 4A). Probabilistic sensitivity analysis showed that oral targeted therapy was deemed cost-effective with a very low probability even at a willingness-to-pay as high as $150,000/QALY (Fig 4B). We also found that an increase in CLL incidence because of the aging US population would further escalate the cost burden and reduce the cost-effectiveness of oral targeted therapies, and the partial uptake of first-line oral targeted therapies for fit patients resulted in limited changes to all model results and would not change the conclusions (Data Supplement).
Fig 4.
Sensitivity analysis of the cost-effectiveness of the oral targeted therapy (OTT) scenario compared with the chemoimmunotherapy (CIT) scenario. (A) Tornado diagram for one-way sensitivity analysis of incremental cost-effectiveness ratio. (B) Cost-effectiveness acceptability curves from the probabilistic sensitivity analysis. HR, hazard ratio; QALY, quality-adjusted life-year.
DISCUSSION
Oral targeted therapies represent a major advance for patients with CLL, with improvement in OS compared with conventional therapies.21 Our study projected an increase in the number people living with CLL over time, largely due to improved survival in the era of oral targeted therapies; we also projected a substantial increase in the cost of CLL management. The annual cost of CLL management is projected to reach $5.13 billion by 2025, a 590% increase from that in 2011. The cost of new therapies will add considerable financial burden to both patients and payers. At the current price, oral therapies are not deemed cost-effective on the basis of the willingness-to-pay threshold of $100,000/QALY.
This study provides a comprehensive view and analysis of the changing burden of CLL care in the United States. To our knowledge, no study has evaluated the cost-effectiveness of oral targeted cancer therapies from a population level. Earlier studies on the cost of CLL did not consider recent data and changing population dynamics. One study found that the lifetime cost of CLL treatment of Medicare patients is $87,000 based on data from older drug regimens from 1999 to 2007.50 Another study by Shanafelt et al15 estimated the annual societal cost of CLL to be $0.73 billion with CIT and $2.63 and $1.24 billion with ibrutinib in the first- and second-line settings, respectively. However, their estimates were lower than our projections because they did not account for the growing disease population due to improved survival. Our results highlight the expected societal impact of the rising disease burden from CLL that would compound the increased cost associated with long-term oral therapy.
Our study has several limitations. First, the model did not consider all possible treatment sequences in practice. We also did not account for individual practice patterns that deviate from standard of care and guidelines because no data exist for comprehensive utilization estimates for each treatment option. However, we believe that our approach is sufficient to capture the most commonly accepted practice patterns and population-level trends in costs and prevalence of CLL. Second, we considered constant drug prices and did not capture the possible fluctuation of drug prices over time in reality. We performed a series of sensitivity analyses on drug prices and found that the results remain valid across a wide range of drug price discounts and that the oral therapies could be deemed cost-effective if the prices were at least 69% lower than our current AWP estimates.
Although the cost of cancer care is rising, the results indicate that the rising trend in the cost of CLL management will outpace that of other cancers. The annual cost of cancer care in the United States is expected to increase by 27%-50% from $143 billion in 2010 to $180 billion in 2020.51 For breast and prostate cancers, the annual cost of care is expected to increase by 24%-38% from 2010 to 2020. In contrast, the annual cost of the CLL is estimated to increase by 500% from $0.7 billion in 2011 to $4.2 billion in 2020. The substantial increase in the cost of CLL management is mainly driven by the high cost of oral targeted drugs and prolonged treatment duration along with improved survival. Although the current analysis suggests that the cost of CLL management will rise faster than that of other cancers, future advances in treatments could increase the costs of care of other cancers as well. Such an increase could strain the budget of private as well as government payers.
Patients also will experience the escalating cost burden of expensive treatments because the higher overall cost could translate into higher health insurance premiums and cost sharing for individual patients.52 One study found that medical bankruptcies ranks number one (67%) of all US family bankruptcies because out-of-pocket costs range from $18,000 to $27,000.53 The current results show that the lifetime out-of-pocket costs of CLL treatment for Medicare patients is expected to increase approximately fourfold to $57,000 for those who initiate first-line oral targeted therapy after 2016, which could further exacerbate the likelihood of medical bankruptcy and result in discontinuation of treatment. The high out-of-pocket costs not only causes material financial hardship as one survey showed that 12% of patients with cancer could not cover their share of medical care cost, but also could lead to psychological financial hardship.16 Furthermore, high out-of-pocket costs could result in disparities in access to these therapies. For example, patients with CLL with lower income levels may not be able to afford these therapies, which will adversely affect their outcomes. Their health could remain suboptimal, even in the era of oral targeted therapies.54,55
High drug prices have been a disturbing concern not only in the area of CLL management but also in the setting of cancer care in general. The average annual cost of cancer treatment before 2000 was < $10,000, which has now increased to > $100,000.56,57 A recently published systematic review found that the majority of drugs for hematologic malignancies are not cost-effective at their current prices.58 A similar trend is observed for other cancer treatments.59-61 The cost of care has become an important component of delivering high-quality care.62-64
We do not recommend that clinicians choose less-effective management strategies; instead, we propose that the price of oral targeted therapies be reduced such that the treatment becomes cost-effective and more affordable. Besides a price reduction, strategies to optimize the drug and dose schedule are needed. Minimal residual disease–negative remissions have been reported with drugs such as venetoclax. Clinical trials are needed to ascertain whether drug discontinuation in patients who meet certain parameters (eg, minimal residual disease–negative remission) would be an effective approach. Similar approaches have been used in patients with chronic myeloid leukemia who have received imatinib.65
In conclusion, this study provides a comprehensive analysis of the changing prevalence and cost of CLL care in the United States. Oral targeted therapies will increase survival rates substantially; however, with the current price structure, they will dramatically increase the cost of CLL management for both patients and payers. Such an economic impact could result in financial toxicity, limited access, and lower adherence to the oral therapies, which may undermine their clinical effectiveness. A more sustainable pricing strategy is needed for oral targeted therapies.
Footnotes
Presented at the XVI International Workshop on Chronic Lymphocytic Leukaemia, Sydney, New South Wales, Australia, September 7-9, 2015, and 57th American Society of Hematology Annual Meeting and Exposition, Orlando, FL, December 5-8, 2015.
Listen to the podcast by Dr Flowers at ascopubs.org/jco/podcasts
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Administrative support: Jagpreet Chhatwal
Collection and assembly of data: Qiushi Chen, Nitin Jain, Hagop M. Kantarjian, Jagpreet Chhatwal
Data analysis and interpretation: Qiushi Chen, Nitin Jain, Turgay Ayer, William G. Wierda, Christopher R. Flowers, Susan M. O’Brien, Hagop M. Kantarjian, Jagpreet Chhatwal
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Economic Burden of Chronic Lymphocytic Leukemia in the Era of Oral Targeted Therapies in the United States
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Qiushi Chen
No relationship to disclose
Nitin Jain
Honoraria: Pharmacyclics, Novartis, ADC Therapeutics, Pfizer, Servier, Novimmune
Consulting or Advisory Role: Pharmacyclics, Novartis, ADC Therapeutics, Pfizer, Servier, Novimmune
Research Funding: AbbVie (Inst), Genentech (Inst), Pharmacyclics (Inst), Infinity Pharmaceuticals (Inst), Bristol-Myers Squibb (Inst), Pfizer (Inst), ADC Therapeutics (Inst), Seattle Genetics (Inst), Incyte (Inst), Servier (Inst), Celgene (Inst)
Turgay Ayer
No relationship to disclose
William G. Wierda
Consulting or Advisory Role: Sanofi, Genetech, Roche, Pharmacyclics, Celgene, Gilead Sciences, GlaxoSmithKline, Novartis, Genzyme, Merck, AbbVie, Emergent BioSolutions
Research Funding: GlaxoSmithKline, Novartis, AbbVie, Genetech, Karyopharm Therapeutics, Pharmacyclics, Acerta Pharma, Gilead Sciences, Janssen Pharmaceuticals, Emergent BioSolutions, Juno Therapeutics, Kite Pharma
Christopher R. Flowers
Consulting or Advisory Role: OptumRx, Seattle Genetics
Research Funding: Acerta Pharma (Inst), Infinity Pharmaceuticals (Inst), Onyx Pharmaceuticals (Inst), Janssen Pharmaceuticals (Inst), Gilead Sciences (Inst), Celgene (Inst), TG Therapeutics (Inst), Genetech (Inst), Roche (Inst), Pharmacyclics (Inst), AbbVie (Inst)
Travel, Accommodations, Expenses: Gilead Sciences, Celgene, Genentech, Roche
Susan M. O’Brien
Honoraria: Celgene, Janssen Pharmaceuticals, ProNAi, Pharmacyclics, Regeneron Pharmaceuticals, Gilead Sciences, Pfizer, Amgen
Consulting or Advisory Role: Amgen, Celgene
Research Funding: Acerta Pharma, Regeneron Pharmaceuticals, Gilead Sciences
Travel, Accommodations, Expenses: Celgene, Janssen Pharmaceuticals, Gilead Sciences, Regeneron Pharmaceuticals
Michael J. Keating
Consulting or Advisory Role: Roche, Celgene
Hagop M. Kantarjian
Research Funding: Pfizer (Inst), Bristol-Myers Squibb (Inst), Novartis (Inst), Amgen (Inst), Ariad Pharmaceutical (Inst)
Jagpreet Chhatwal
Honoraria: Merck
Consulting or Advisory Role: Gilead Sciences
Research Funding: Gilead Sciences (Inst)
Travel, Accommodations, Expenses: Gilead Sciences
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Jain N, O’Brien S. Initial treatment of CLL: Integrating biology and functional status. Blood. 2015;126:463–470. doi: 10.1182/blood-2015-04-585067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gribben JG, O’Brien S. Update on therapy of chronic lymphocytic leukemia. J Clin Oncol. 2011;29:544–550. doi: 10.1200/JCO.2010.32.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hallek M. Chronic lymphocytic leukemia: 2015 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2015;90:446–460. doi: 10.1002/ajh.23979. [DOI] [PubMed] [Google Scholar]
- 5. doi: 10.1182/blood-2015-09-667675. Thompson PA, Tam CS, O’Brien SM, et al: Fludarabine, cyclophosphamide and rituximab achieves long-term disease-free survival in IGHV-mutated chronic lymphocytic leukemia. Blood 127:303-309, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: A randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 7.Keating MJ, O’Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 8.Tam CS, O’Brien S, Wierda W, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–980. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370:1101–1110. doi: 10.1056/NEJMoa1313984. [DOI] [PubMed] [Google Scholar]
- 10.Byrd JC, Furman RR, Coutre SE, et al. Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125:2497–2506. doi: 10.1182/blood-2014-10-606038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown JR, Byrd JC, Coutre SE, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123:3390–3397. doi: 10.1182/blood-2013-11-535047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. doi: 10.1016/j.blre.2015.12.002. Jain N, O’Brien S: Targeted therapies for CLL: Practical issues with the changing treatment paradigm. Blood Rev 30:233-244, 2016. [DOI] [PubMed] [Google Scholar]
- 14. Food and Drug Administration: FDA approves new drug for chronic lymphocytic leukemia in patients with a specific chromosomal abnormality, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm495253.htm.
- 15. Shanafelt TD, Borah BJ, Finnes HD, et al: Impact of ibrutinib and idelalisib on the pharmaceutical cost of treating chronic lymphocytic leukemia at the individual and societal levels. J Oncol Pract11:252-258, 2015. [DOI] [PubMed]
- 16.Yabroff KR, Dowling EC, Guy GP, Jr, et al. Financial hardship associated with cancer in the United States: Findings from a population-based sample of adult cancer survivors. J Clin Oncol. 2016;34:259–267. doi: 10.1200/JCO.2015.62.0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. SEER: Research Data (1973-2011), based on the November 2013 submission, in Surveillance Research Program SSB (ed), National Cancer Institute, DCCPS, 2014. http://seer.cancer.gov/data/citation.html.
- 18.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: A randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 19.Wierda WG, O’Brien S, Wang X, et al. Multivariable model for time to first treatment in patients with chronic lymphocytic leukemia. J Clin Oncol. 2011;29:4088–4095. doi: 10.1200/JCO.2010.33.9002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eichhorst BF, Busch R, Stilgenbauer S, et al. First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood. 2009;114:3382–3391. doi: 10.1182/blood-2009-02-206185. [DOI] [PubMed] [Google Scholar]
- 21.Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373:2425–2437. doi: 10.1056/NEJMoa1509388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farooqui MZ, Valdez J, Martyr S, et al. Ibrutinib for previously untreated and relapsed or refractory chronic lymphocytic leukaemia with TP53 aberrations: A phase 2, single-arm trial. Lancet Oncol. 2015;16:169–176. doi: 10.1016/S1470-2045(14)71182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wierda W, O’Brien S, Wen S, et al. Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4070–4078. doi: 10.1200/JCO.2005.12.516. [DOI] [PubMed] [Google Scholar]
- 24.Fischer K, Cramer P, Busch R, et al. Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: A multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2011;29:3559–3566. doi: 10.1200/JCO.2010.33.8061. [DOI] [PubMed] [Google Scholar]
- 25.Wierda WG, Kipps TJ, Mayer J, et al. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2010;28:1749–1755. doi: 10.1200/JCO.2009.25.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Byrd JC, Furman RR, Coutre SE, et al: Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood 125:2437-2506, 2015. [DOI] [PMC free article] [PubMed]
- 27.Khouri IF, Bassett R, Poindexter N, et al. Nonmyeloablative allogeneic stem cell transplantation in relapsed/refractory chronic lymphocytic leukemia: Long-term follow-up, prognostic factors, and effect of human leukocyte histocompatibility antigen subtype on outcome. Cancer. 2011;117:4679–4688. doi: 10.1002/cncr.26091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Majhail NS, Mau L-W, Denzen EM, et al. Costs of autologous and allogeneic hematopoietic cell transplantation in the United States: A study using a large national private claims database. Bone Marrow Transplant. 2013;48:294–300. doi: 10.1038/bmt.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khera N, Zeliadt SB, Lee SJ. Economics of hematopoietic cell transplantation. Blood. 2012;120:1545–1551. doi: 10.1182/blood-2012-05-426783. [DOI] [PubMed] [Google Scholar]
- 30. Levinson DR: Medicaid Drug Price Comparison: Average Sales Price to Average Wholesale Price. Washington, DC, Office of the Inspector General, 2005. [Google Scholar]
- 31.Arias E. United States life tables, 2010. Natl Vital Stat Rep. 2014;63:1–63. [PubMed] [Google Scholar]
- 32.Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213–223. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. UpToDate: Drug informations, 2015. https://www.uptodate.com/
- 34.Maddocks KJ, Ruppert AS, Lozanski G, et al. Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol. 2015;1:80–87. doi: 10.1001/jamaoncol.2014.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Centers for Medicare & Medicaid Services: Medicare physician fee schedule, 2015. https://www.cms.gov/apps/physician-fee-schedule/overview.aspx.
- 36.Parikh SA, Rabe KG, Kay NE, et al. Chronic lymphocytic leukemia in young (≤ 55 years) patients: A comprehensive analysis of prognostic factors and outcomes. Haematologica. 2014;99:140–147. doi: 10.3324/haematol.2013.086066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beusterien KM, Davies J, Leach M, et al. Population preference values for treatment outcomes in chronic lymphocytic leukaemia: A cross-sectional utility study. Health Qual Life Outcomes. 2010;8:50. doi: 10.1186/1477-7525-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marsh K, Xu P, Orfanos P, et al. Model-based cost-effectiveness analyses for the treatment of chronic lymphocytic leukaemia: A review of methods to model disease outcomes and estimate utility. Pharmacoeconomics. 2014;32:981–993. doi: 10.1007/s40273-014-0187-1. [DOI] [PubMed] [Google Scholar]
- 39.Adena M, Houltram J, Mulligan SP, et al. Modelling the cost effectiveness of rituximab in chronic lymphocytic leukaemia in first-line therapy and following relapse. Pharmacoeconomics. 2014;32:193–207. doi: 10.1007/s40273-013-0125-7. [DOI] [PubMed] [Google Scholar]
- 40.Guadagnolo BA, Punglia RS, Kuntz KM, et al. Cost-effectiveness analysis of computerized tomography in the routine follow-up of patients after primary treatment for Hodgkin’s disease. J Clin Oncol. 2006;24:4116–4122. doi: 10.1200/JCO.2006.07.0409. [DOI] [PubMed] [Google Scholar]
- 41.Chen Q, Ayer T, Nastoupil LJ, et al. Comparing the cost-effectiveness of rituximab maintenance and radioimmunotherapy consolidation versus observation following first-line therapy in patients with follicular lymphoma. Value Health. 2015;18:189–197. doi: 10.1016/j.jval.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee WC, Lamas GA, Balu S, et al. Direct treatment cost of atrial fibrillation in the elderly American population: A Medicare perspective. J Med Econ. 2008;11:281–298. doi: 10.3111/13696990802063425. [DOI] [PubMed] [Google Scholar]
- 43. Tumeh JW, Moore SG, Shapiro R, et al: Practical approach for using Medicare data to estimate costs for cost-effectiveness analysis. Expert Rev Pharmacoeconomics Outcomes Res 5:153-162, 2005. [DOI] [PubMed]
- 44.Ferguson J, Tolley K, Gilmour L, et al. PCN79 Health state preferences study mapping the change over the course of the disease process in chronic lymphocytic leukemia (CLL) Value Health. 2008;11:A485. [Google Scholar]
- 45.Hanmer J, Lawrence WF, Anderson JP, et al. Report of nationally representative values for the noninstitutionalized US adult population for 7 health-related quality-of-life scores. Med Decis Making. 2006;26:391–400. doi: 10.1177/0272989X06290497. [DOI] [PubMed] [Google Scholar]
- 46.Catovsky D, Richards S, Matutes E, et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): A randomised controlled trial. Lancet. 2007;370:230–239. doi: 10.1016/S0140-6736(07)61125-8. [DOI] [PubMed] [Google Scholar]
- 47. Ortman JM, Velkoff VA, Hogan H: An Aging Nation: The Older Population in the United States. Washington, DC, US Census Bureau, 2014, p 25. [Google Scholar]
- 48. The Henry J. Kaiser Family Foundation: The Medicare Part D Prescription Drug Benefit, 2016. http://kff.org/medicare/fact-sheet/the-medicare-prescriptiondrug-benefit-fact-sheet.
- 49. Congressional Budget Office: Prices for Brand-Name Drugs Under Selected Federal Programs. Washington, DC, The Congress of the United States, 2005. https://www.cbo.gov/sites/default/files/109th-congress-2005-2006/reports/06-16-prescriptdrug.pdf. [Google Scholar]
- 50.Lafeuille M-H, Vekeman F, Wang S-T, et al. Lifetime costs to Medicare of providing care to patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2012;53:1146–1154. doi: 10.3109/10428194.2011.643405. [DOI] [PubMed] [Google Scholar]
- 51.Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. The Kaiser Family Foundation; Health Research & Educational Trust: Employer Health Benefits Survey, 2014. http://files.kff.org/attachment/2014-employer-health-benefits-survey-full-report.
- 53.Himmelstein DU, Thorne D, Warren E, et al. Medical bankruptcy in the United States, 2007: Results of a national study. Am J Med. 2009;122:741–746. doi: 10.1016/j.amjmed.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 54. Kale HP, Carroll NV: Self-reported financial burden of cancer care and its effect on physical and mental health-related quality of life among US cancer survivors. Cancer 122:283-289, 2016. [DOI] [PubMed]
- 55.Ramsey SD, Bansal A, Fedorenko CR, et al. Financial insolvency as a risk factor for early mortality among patients with cancer. J Clin Oncol. 2016;34:980–986. doi: 10.1200/JCO.2015.64.6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kantarjian H, Steensma D, Rius Sanjuan J, et al. High cancer drug prices in the United States: Reasons and proposed solutions. J Oncol Pract. 2014;10:e208–e211. doi: 10.1200/JOP.2013.001351. [DOI] [PubMed] [Google Scholar]
- 57.Light DW, Kantarjian H. Market spiral pricing of cancer drugs. Cancer. 2013;119:3900–3902. doi: 10.1002/cncr.28321. [DOI] [PubMed] [Google Scholar]
- 58.Chhatwal J, Mathisen M, Kantarjian H. Are high drug prices for hematologic malignancies justified? A critical analysis. Cancer. 2015;121:3372–3379. doi: 10.1002/cncr.29512. [DOI] [PubMed] [Google Scholar]
- 59. Goldstein DA, Ahmad BB, Chen Q, et al: Cost-effectiveness analysis of regorafenib for metastatic colorectal cancer. J Clin Oncol 33:3727-3732, 2015. [DOI] [PMC free article] [PubMed]
- 60.Goldstein DA, Chen Q, Ayer T, et al. First- and second-line bevacizumab in addition to chemotherapy for metastatic colorectal cancer: A United States-based cost-effectiveness analysis. J Clin Oncol. 2015;33:1112–1118. doi: 10.1200/JCO.2014.58.4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shih V, Ten Ham RM, Bui CT, et al: Targeted therapies compared to dacarbazine for treatment of BRAF(V600E) metastatic melanoma: A cost-effectiveness analysis. J Skin Cancer 10.1155/2015/525302 [Epub ahead of print on June 10, 2015] [DOI] [PMC free article] [PubMed]
- 62.Meropol NJ, Schrag D, Smith TJ, et al. American Society of Clinical Oncology guidance statement: The cost of cancer care. J Clin Oncol. 2009;27:3868–3874. doi: 10.1200/JCO.2009.23.1183. [DOI] [PubMed] [Google Scholar]
- 63.Bach PB. Limits on Medicare’s ability to control rising spending on cancer drugs. N Engl J Med. 2009;360:626–633. doi: 10.1056/NEJMhpr0807774. [DOI] [PubMed] [Google Scholar]
- 64.Bach PB. New math on drug cost-effectiveness. N Engl J Med. 2015;373:1797–1799. doi: 10.1056/NEJMp1512750. [DOI] [PubMed] [Google Scholar]
- 65.Mahon F-X, Réa D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: The prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11:1029–1035. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]