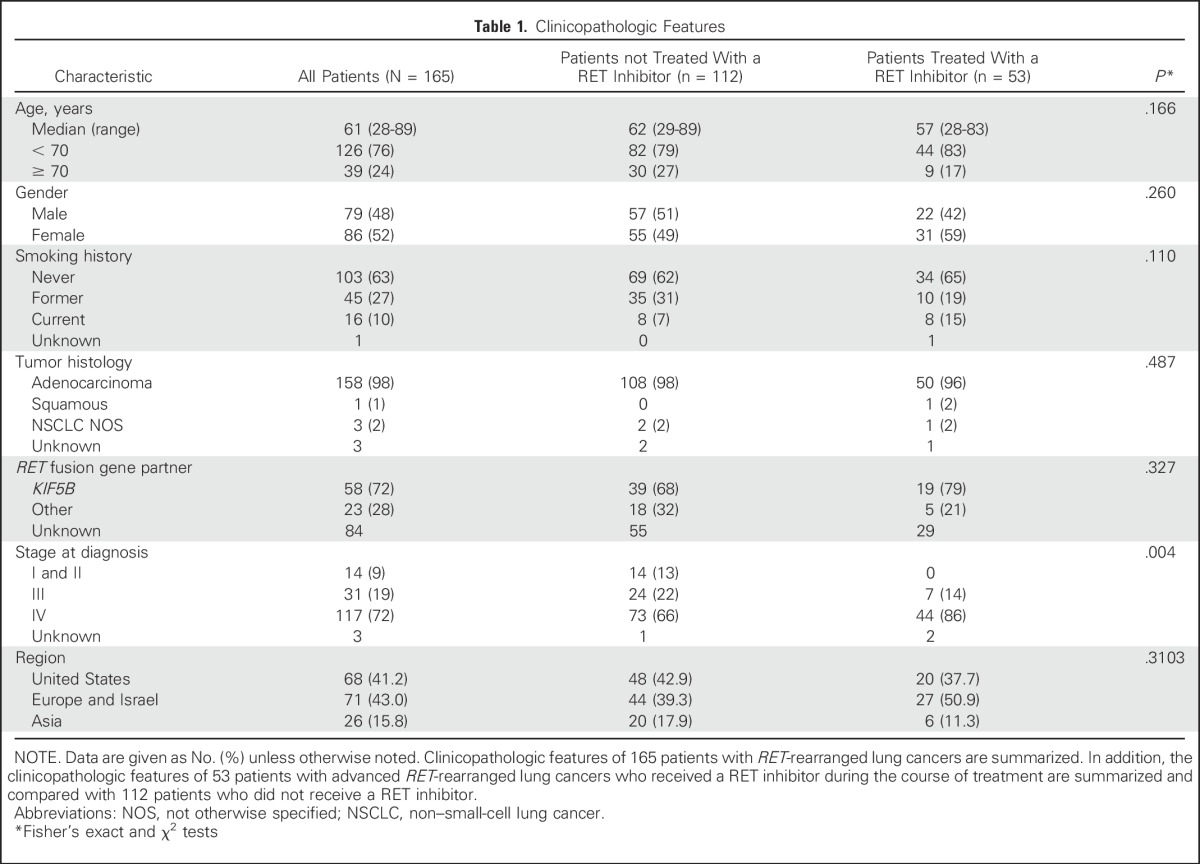
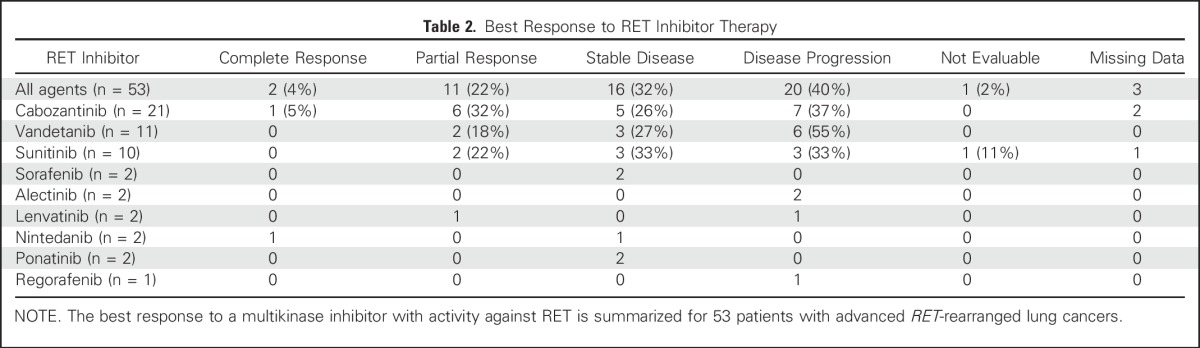
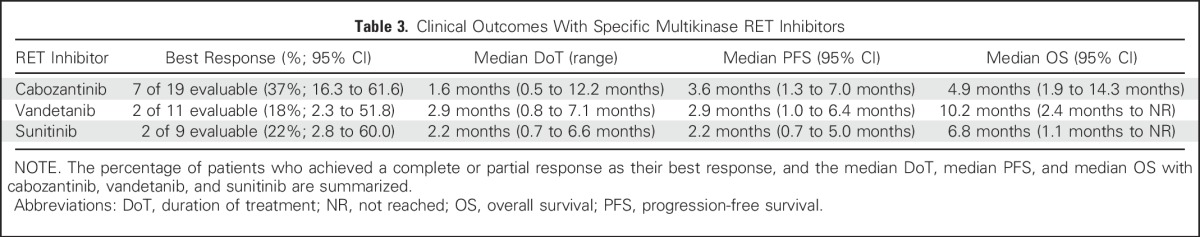
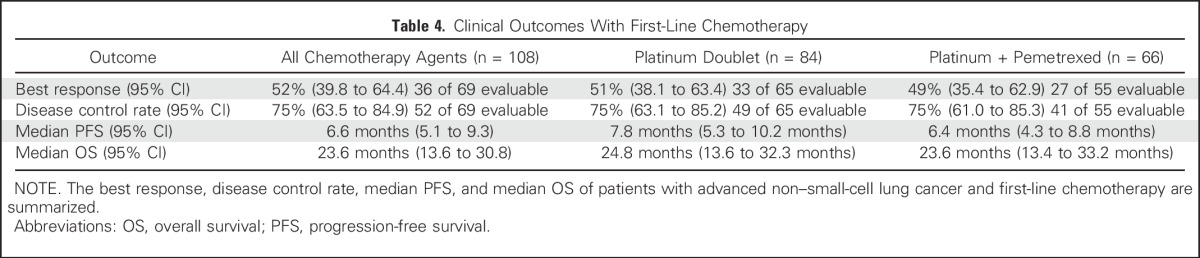
Oliver Gautschi
Oliver Gautschi
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,✉,
Julie Milia
Julie Milia
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Thomas Filleron
Thomas Filleron
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Juergen Wolf
Juergen Wolf
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
David P Carbone
David P Carbone
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Dwight Owen
Dwight Owen
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Ross Camidge
Ross Camidge
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Vignhesh Narayanan
Vignhesh Narayanan
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Robert C Doebele
Robert C Doebele
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Benjamin Besse
Benjamin Besse
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Jordi Remon-Masip
Jordi Remon-Masip
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Pasi A Janne
Pasi A Janne
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Mark M Awad
Mark M Awad
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Nir Peled
Nir Peled
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Chul-Cho Byoung
Chul-Cho Byoung
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Daniel D Karp
Daniel D Karp
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Michael Van Den Heuvel
Michael Van Den Heuvel
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Heather A Wakelee
Heather A Wakelee
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Joel W Neal
Joel W Neal
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Tony SK Mok
Tony SK Mok
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
James CH Yang
James CH Yang
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Sai-Hong Ignatius Ou
Sai-Hong Ignatius Ou
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Georg Pall
Georg Pall
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Patrizia Froesch
Patrizia Froesch
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Gérard Zalcman
Gérard Zalcman
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
David R Gandara
David R Gandara
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Jonathan W Riess
Jonathan W Riess
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Vamsidhar Velcheti
Vamsidhar Velcheti
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Kristin Zeidler
Kristin Zeidler
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Joachim Diebold
Joachim Diebold
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Martin Früh
Martin Früh
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Sebastian Michels
Sebastian Michels
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Isabelle Monnet
Isabelle Monnet
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Sanjay Popat
Sanjay Popat
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Rafael Rosell
Rafael Rosell
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Niki Karachaliou
Niki Karachaliou
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Sacha I Rothschild
Sacha I Rothschild
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Jin-Yuan Shih
Jin-Yuan Shih
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Arne Warth
Arne Warth
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Thomas Muley
Thomas Muley
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Florian Cabillic
Florian Cabillic
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Julien Mazières
Julien Mazières
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1,
Alexander Drilon
Alexander Drilon
1Oliver Gautschi, Kristin Zeidler, and Joachim Diebold, Lucerne Cantonal Hospital, Luzern; Patrizia Froesch, Ente Ospedaliero Cantonale, Bellinzona; Martin Früh, Kantonsspital St Gallen, St Gallen; Sacha I. Rothschild, University Hospital Basel, Basel, Switzerland; Julie Milia and Julien Mazières, Hôpital Larrey; Thomas Filleron, Institut Universitaire du Cancer, Claudius Regaud, Toulouse; Benjamin Besse and Jordi Remon-Masip, Institute Gustave Roussy, Villejuif; Gérard Zalcman, University Hospital Bichat, Paris; Isabelle Monnet, Centre Hospitalier Intercommunal de Creteil, Creteil; Florian Cabillic, Université de Rennes 1, Rennes, France; Juergen Wolf and Sebastian Michels, Center for Integrated Oncology, Cologne; Georg Pall, Fachkliniken Wangen, Wangen Im Allgäu; Arne Warth, Heidelberg University Hospital; Thomas Muley, Thoraxklinik at University of Heidelberg and Translational Lung Research Center, Heidelberg, Germany; David P. Carbone and Dwight Owen, Ohio State University Comprehensive Cancer Center, Columbus; Vamsidhar Velcheti, Cleveland Clinic, Pepper Pike, OH; Ross Camidge and Vignhesh Narayanan, University of Colorado, Denver; Robert C. Doebele, University of Colorado, Aurora, CO; Pasi A. Janne and Mark M. Awad, Dana-Farber Cancer Institute, Boston, MA; Daniel D. Karp, The University of Texas MD Anderson Cancer Center, Houston, TX; Heather A. Wakelee and Joel W. Neal, Stanford University, Stanford; Sai-Hong Ignatius Ou, University of California, Irvine, Orange; David R. Gandara and Jonathan W. Riess, University of California, Davis Cancer Center, Sacramento, CA; Alexander Drilon, Memorial Sloan Kettering Cancer Center, New York, NY; Nir Peled, Davidoff Cancer Center, Petach Tiqwa, Israël; Chul-Cho Byoung, Yonseï Cancer Center, Seoul, Republic of Korea; Michael Van Den Heuvel, Netherlands Cancer Institute, Amsterdam, the Netherlands; Tony S.K. Mok, The Chinese University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; James C.H. Yang and Jin-Yuan Shih, National Taiwan University Hospital, Taipei, Republic of China; Sanjay Popat, Royal Marsden Hospital, London, United Kingdom; and Rafael Rosell and Niki Karachaliou, Catalan Institute of Oncology, Barcelona, Spain.
1