Abstract
Purpose
Pneumonitis is an uncommon but potentially fatal toxicity of anti–programmed death-1 (PD-1)/programmed death ligand 1 (PD-L1) monoclonal antibodies (mAbs). Clinical, radiologic, and pathologic features are poorly described.
Methods
Patients who received anti–PD-1/PD-L1 monotherapy or in combination with anti–cytotoxic T-cell lymphocyte-4 mAb were identified at two institutions (Memorial Sloan Kettering Cancer Center: advanced solid cancers, 2009 to 2014, and Melanoma Institute of Australia: melanomas only, 2013 to 2015). Pneumonitis was diagnosed by the treating investigator; cases with confirmed malignant lung infiltration or infection were excluded. Clinical, radiologic, and pathologic features of pneumonitis were collected. Associations among pneumonitis incidence, therapy received, and underlying malignancy were examined with Fisher’s exact test as were associations between pneumonitis features and outcomes.
Results
Of 915 patients who received anti–PD-1/PD-L1 mAbs, pneumonitis developed in 43 (5%; 95% CI, 3% to 6%; Memorial Sloan Kettering Cancer Center, 27 of 578 [5%]; Melanoma Institute of Australia, 16 of 337 [5%]). Time to onset of pneumonitis ranged from 9 days to 19.2 months. The incidence of pneumonitis was higher with combination immunotherapy versus monotherapy (19 of 199 [10%] v 24 of 716 [3%]; P < .01). Incidence was similar in patients with melanoma and non–small-cell lung cancer (overall, 26 of 532 [5%] v nine of 209 [4%]; monotherapy, 15 of 417 v five of 152 [P = 1.0]; combination, 11 of 115 v four of 57 [P = .78]). Seventy-two percent (31 of 43) of cases were grade 1 to 2, and 86% (37 of 43) improved/resolved with drug holding/immunosuppression. Five patients worsened clinically and died during the course of pneumonitis treatment; proximal cause of death was pneumonitis (n = 1), infection related to immunosuppression (n = 3), or progressive cancer (n = 1). Radiologic and pathologic features of pneumonitis were diverse.
Conclusion
Pneumonitis associated with anti–PD-1/PD-L1 mAbs is a toxicity of variable onset and clinical, radiologic, and pathologic appearances. It is more common when anti–PD-1/PD-L1 mAbs are combined with anti–cytotoxic T-cell lymphocyte-4 mAb. Most events are low grade and improve/resolve with drug holding/immunosuppression. Rarely, pneumonitis worsens despite immunosuppression, and may result in infection and/or death.
INTRODUCTION
Anti–programmed death-1 (anti–PD-1) and anti–programmed death ligand 1 (anti–PD-L1) monoclonal antibodies (mAbs) for patients with multiple malignancies are now Food and Drug Administration–approved therapies, which include nivolumab and pembrolizumab for melanoma1,2 and non–small-cell lung cancer (NSCLC),3-6 nivolumab for renal cell carcinoma7 and Hodgkin lymphoma,8 atezolizumab for bladder cancer,9 and nivolumab plus ipilimumab for melanoma.10 These agents also have been studied in other diseases11-13 along with durvalumab (PD-L1 mAb) and tremelimumab (cytotoxic T-cell lymphocyte-4 [CTLA-4] mAb).14,15 One of the remarkable characteristics of anti–PD-1/PD-L1 mAbs is their relatively mild toxicity profile. However, immune-related adverse events can occur and may be severe.16,17 Pneumonitis is an immune-related adverse event that accounted for three deaths in an early-phase study with an anti–PD-1 mAb.18
Pneumonitis is defined as a focal or diffuse inflammation of the lung parenchyma,19 and its incidence in studies with anti–PD-1/PD-L1 mAbs has ranged from 0% to 10%.20 Drug-related pneumonitis can also occur with chemotherapy (docetaxel,21 gemcitabine,22 bleomycin23), targeted therapy (epidermal growth factor receptor inhibitors,24,25 mammalian target of rapamycin inhibitors26), and radiation therapy.27,28 Previous experience with these pneumonitides highlighted that clinical, radiologic, and pathologic characterization may facilitate early recognition, treatment optimization, and improved outcomes. The underlying etiology and mechanisms of pneumonitis associated with anti–PD-1/PD-L1 mAbs are unknown.
With the recent approval of anti–PD-1/PD-L1 mAbs, and several other anticipated indications, use is expected to expand rapidly. A critical need exists to gain familiarity with the clinical features of pneumonitis and to optimize management. The clinical experience of patients with anti–PD-1/PD-L1–associated pneumonitis has not been comprehensively described, and data are sparse with regard to management and outcomes. We describe the clinical, radiologic, and pathologic features and management of 43 cases of pneumonitis as a result of anti–PD-1/PD-L1 mAbs from two separate institutions.
METHODS
Patients
After institutional review board approval, patients treated with anti–PD-1/PD-L1 mAbs either as monotherapy or in combination with anti–CTLA-4 mAb were identified from Memorial Sloan Kettering Cancer Center (MSKCC; January 2009 to September 2014; all advanced cancers) and the Melanoma Institute of Australia (MIA) and affiliated hospitals (January 2013 to August 2015; melanomas only). Anti–PD-1/PD-L1 mAbs were delivered either as part of an institutional review board–approved therapeutic study or as an expanded access program. Patients treated concurrently with chemotherapy, targeted therapy, and immunotherapy other than anti–CTLA-4 mAb, and in whom the treatment received was still blinded, were excluded. Cases were identified and reviewed retrospectively (MSKCC, J.N., H.R., X.H., M.D.H.; MIA, X.W., A.M.M., A.D.G., M.S.C., B.Y.K., G.V.L.). Those with a clear alternative etiology, such as proven malignant lung infiltration or active lung infection, were excluded. Grading was performed by the treating investigator in real time by using Common Toxicity Criteria for Adverse Events (version 4.0); the reported grade refers to the highest grade of pneumonitis experienced.
Methods
For all patients, the treatment regimen received (anti–PD-1/PD-L1 monotherapy or combination with anti–CTLA-4 mAb) and primary tumor site were recorded. In patients with pneumonitis, the following data were collected retrospectively: demographics, prior oncologic therapy, clinical features of pneumonitis, and pneumonitis treatment. Clinical and radiologic outcomes were classified as completely resolved, improved, or worsened. Patients in whom recurrent pneumonitis developed, either with or without drug rechallenge, were noted. Assessment of final clinical outcomes as well as highest grade of pneumonitis included periods of recurrent pneumonitis, if applicable.
Retrospective radiology review of the serial chest computed tomography (CT) scans of patients with pneumonitis was performed in the MSKCC cohort by two independent radiologists (T.I., J.C.) blinded to patient clinical data followed by a consensus read if there was disagreement. Each radiologist described the phenotypic appearance and severity of pneumonitis by using criteria for interstitial lung diseases.29-31 When available, chest x-rays at the time of pneumonitis were also reviewed. Lung biopsy specimens obtained at the time of pneumonitis at MSKCC were reviewed by thoracic pathologists (C.L., N.R.) and phenotypically described.
Outcomes Analysis
For all patients treated with anti–PD-1/PD-L1 mAbs, associations between development of pneumonitis and treatment received (anti–PD-1 versus anti–PD-L1 mAb; monotherapy versus combination) and disease type (melanoma versus NSCLC stratified by monotherapy versus combination) were assessed by Fisher’s exact test. In patients with pneumonitis, best objective response rates to anti–PD-1/PD-L1 mAbs were calculated with exact 95% CIs. Associations among clinical or radiologic features of pneumonitis, treatment course, and outcomes from pneumonitis treatment were examined. In the radiologic assessment, agreement between individual radiologists was evaluated by Cohen κ-coefficient such that a score of 1 indicated complete agreement and 0 indicated no agreement other than what would be expected by chance. All statistical tests were two sided, and 5% was set as the level of significance. Statistical analyses were performed with R version 3.1.1 software (R Development Core Team), including the irr and Hmisc packages.
RESULTS
Incidence of Pneumonitis
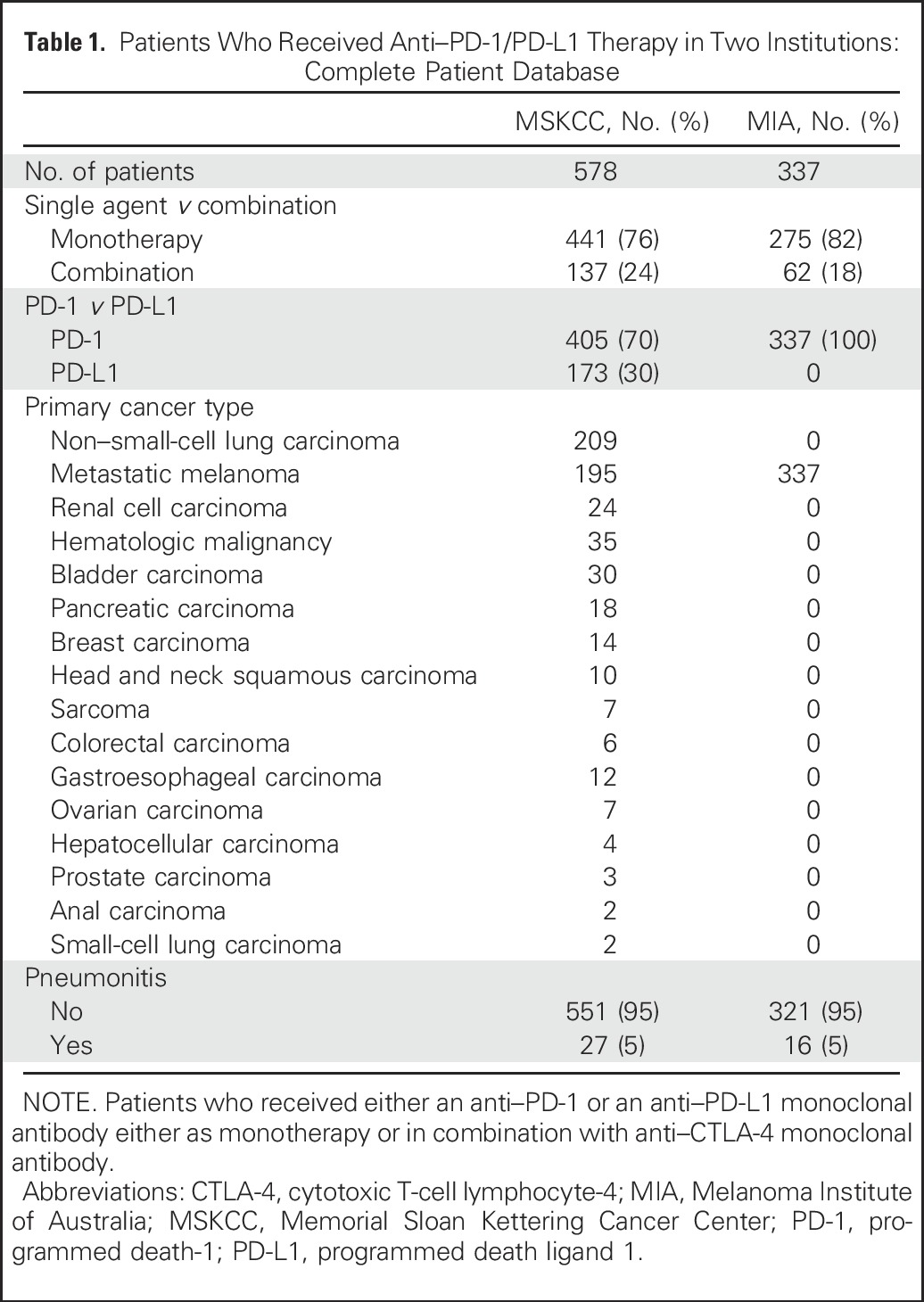
Nine hundred fifteen patients received anti–PD-1/PD-L1 mAbs as monotherapy or in combination with anti–CTLA-4 mAb (Table 1). The overall incidence of pneumonitis was 5% (43 of 915; 95% CI, 3% to 6%) and was similar within institutional cohorts (MSKCC, 27 of 578 [5%]; 95% CI, 3% to 7%; MIA, 16 of 337 [5%]; 95% CI, 3% to 8%). The incidence of pneumonitis was greater in patients who received combination therapy than in those who received monotherapy (19 of 199 [10%] v 24 of 716 [3%], P < .001) and was not statistically different in those treated with anti–PD-1 compared with anti–PD-L1 mAb (monotherapy, 22 of 564 [4%] v two of 152 [1%], P = .13; combination, 18 of 178 [10%] v one of 21 [5%], P = .70).
Table 1.
Patients Who Received Anti–PD-1/PD-L1 Therapy in Two Institutions: Complete Patient Database
Patients With Pneumonitis
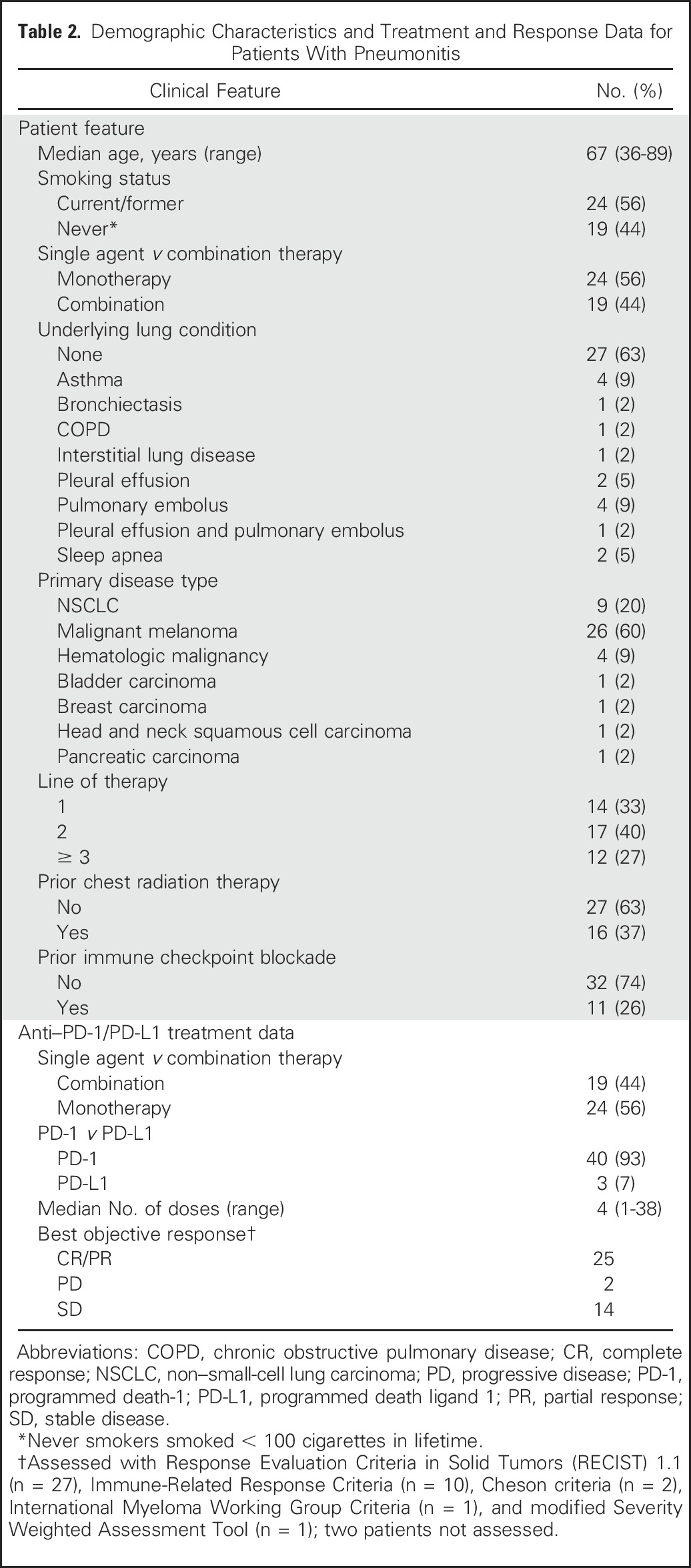
Pneumonitis occurred in patients with metastatic melanoma (26 of 532 [5%]) and NSCLC (nine of 209 [4%]), hematologic malignancies (four of 35 [11%]), bladder carcinoma (one of 30 [3%]), breast carcinoma (one of 14 [7%]), pancreatic carcinoma (one of 18 [6%]), and head and neck squamous carcinoma (one of 10 [10%]; Table 2). Incidence was similar among patients with melanoma and NSCLC for monotherapy (15 of 417 [3.6%] v five of 152 [3.3%], P = 1.0) and combination therapy (11 of 115 [9.6%] v four of 57 [7.0%], P = .78). Pneumonitis developed in both former/current smokers (24 of 43 [56%]) and never smokers (19 of 43 [44%]); the majority had not received prior chest radiation therapy (27 of 43 [63%]). Pneumonitis occurred irrespective of line of therapy in which immunotherapy was received (first line, 32%; second line, 40%; third line or more, 28%). Across all evaluable patients (n = 41), the best objective response to therapy was 61% (25 of 41; 95% CI, 45% to 76%; Table 2). Among patients with melanoma, the response rate to monotherapy was 73% (11 of 15; 95% CI, 45% to 91%) and to combination therapy, 73% (eight of 11; 95% CI, 39% to 94%).
Table 2.
Demographic Characteristics and Treatment and Response Data for Patients With Pneumonitis
Clinical Features
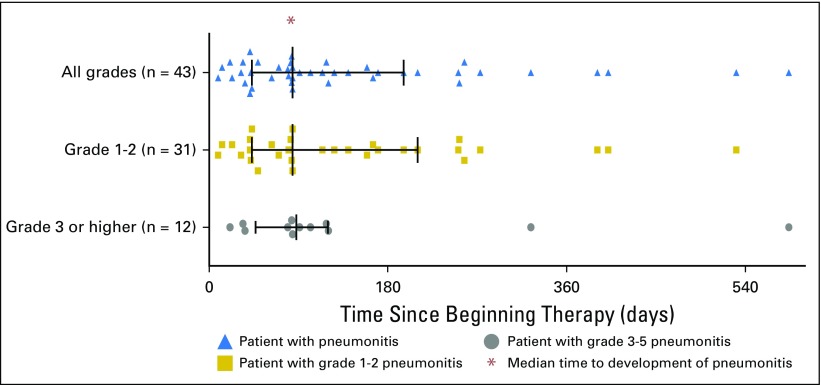
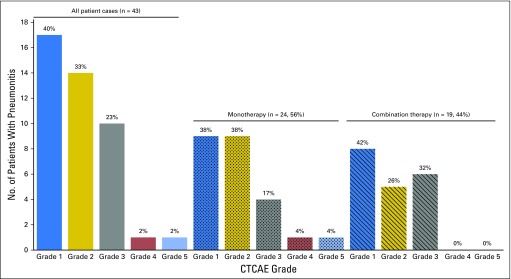
Median time to onset of pneumonitis was 2.8 months, with a wide range (9 days to 19.2 months; Fig 1). Onset tended to be earlier in patients who received combination therapy than in those who received monotherapy (median, 2.7 months [range, 9 days to 6.9 months] v 4.6 months [range, 21 days to 19.2 months]; P = 0.02). Seventeen (40%) patients experienced grade 1 pneumonitis, 14 (33%) experienced grade 2, 10 (23%) experienced grade 3, one (2%) experienced grade 4, and one (2%) experienced grade 5; no difference in distribution of severity between monotherapy and combination therapy was found (Fig 2).
Fig 1.
Time from first dose of anti–programmed death-1/programmed death ligand 1 therapy to date of pneumonitis event stratified by grade, with interquartile range and median values shown.
Fig 2.
Patients in whom pneumonitis developed stratified by highest Common Terminology Criteria for Adverse Events (version 4.0; CTCAE) grade, including whether patients received anti–programmed death-1/programmed death ligand 1 monotherapy versus in combination with anti–cytotoxic T-cell lymphocyte-4 monoclonal antibody.
The most common presenting symptoms of pneumonitis were dyspnea (23 of 43 [53%]) and cough (15 of 43 [35%]). Fever (five of 43 [12%]) and chest pain (three or 43 [7%]) were less common. One third of patients were asymptomatic at the onset of pneumonitis (14 of 43 [33%]). Three patients recorded as having grade 1 pneumonitis had symptoms on retrospective chart review, but grading was not changed retrospectively.
More than one half of patients with pneumonitis experienced additional immune-related toxicity (25 of 43 [58%]), which included skin rash (n = 8); colitis (n = 6); hypophysitis, arthritis, and thyroiditis (n = 3 each); and hepatitis, esophagitis, duodenitis, hyperthyroidism, nephritis, myositis, vitiligo, pernicious anemia, and hemolytic anemia (n = 1 each).
Pneumonitis Management
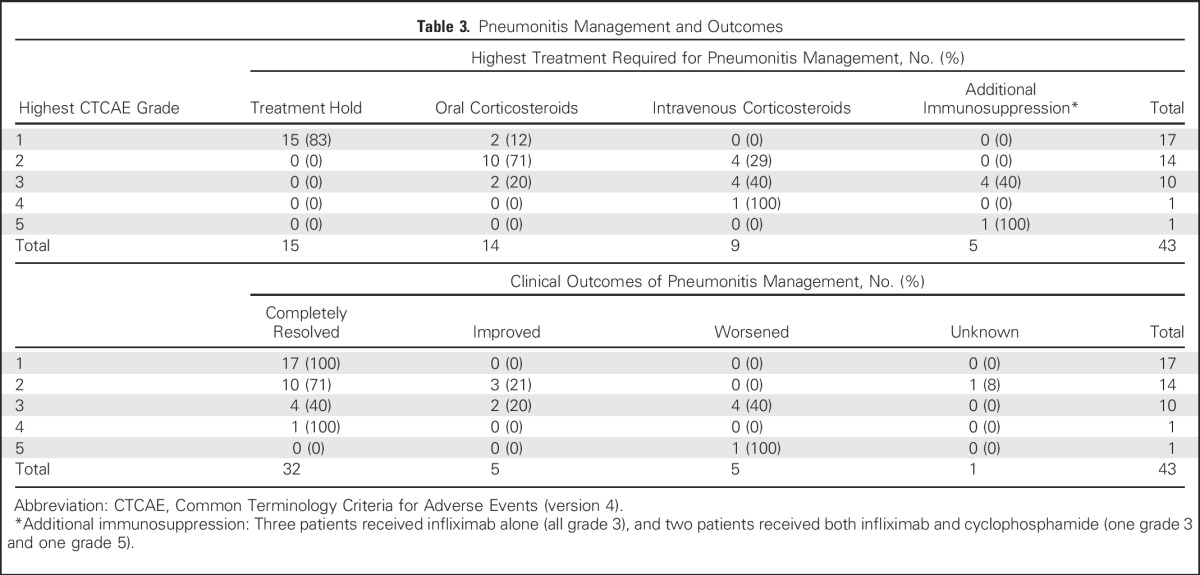
Most patients with grade 1 to 2 pneumonitis were managed as outpatients (25 of 31 [81%]), whereas 19% (six of 31, all grade 2) were hospitalized. All grade 3 and higher cases (n = 12) required hospitalization. In patients with grade 1 pneumonitis, maximum treatment received was drug holding (15 of 17 [88%]) or oral corticosteroids (two of 17 [12%]; Table 3). All patients with grade 2 pneumonitis were treated initially with oral/intravenous corticosteroids (n = 14). All patients with grade 3 or higher pneumonitis received oral/intravenous corticosteroids initially (n = 12), five (42%) of whom required additional immunosuppression (three with infliximab and two with both infliximab plus cyclophosphamide).
Table 3.
Pneumonitis Management and Outcomes
Among all patients who received corticosteroids (28 of 43 [65%]), 61% (17 of 28) began with oral treatment, and 39% (11 of 28) began with intravenous treatment. For most patients who began oral corticosteroids, this was the maximum immunosuppression used (14 of 17 [82%]), with a median starting dose of prednisone of 50 mg (range, 20 to 80 mg) and median duration of corticosteroid treatment of 68 days (range, 20 to 154 days).
Clinical Outcomes and Mortality Associated With Pneumonitis
Pneumonitis improved/resolved in 88% (37 of 43) of cases (Table 3), which included all grade 1 (17 of 17), 93% (13 of 14; one patient lost to follow-up and outcome unknown) of grade 2, and 64% (seven of 12) of grade 3 and higher events.
Five (12%) patients clinically worsened during treatment of pneumonitis. All had grade 3 and higher pneumonitis, were treated with additional immunosuppression beyond corticosteroids, and ultimately died. Although one patient’s death was solely attributable to pneumonitis, the cause of clinical worsening and death was multifactorial in most cases. Three patients had infections associated with immunosuppression, and one patient had progressive cancer, which seemed to be the most proximal contributors to death.
The patient with grade 5 pneumonitis had initially presented with grade 2 pneumonitis after six doses of anti–PD-1 monotherapy for the treatment of metastatic NSCLC. Oral corticosteroids were initiated, with initial clinical and radiologic improvement. However, pneumonitis recurred during corticosteroid taper and did not improve with high-dose intravenous corticosteroids, infliximab, and cyclophosphamide, and the patient died.
Three patients died as a result of infection in the context of immunosuppression for pneumonitis. One patient received one dose of the anti–PD-1 plus anti–CTLA-4 mAb for melanoma and then was treated for grade 3 pneumonitis with a prolonged course of oral/intravenous corticosteroids over 2 months as well as infliximab. Although the pneumonitis initially seemed to improve, the patient developed pseudomonas pneumonia during corticosteroid treatment and subsequently died. A second patient received two doses of anti–PD-1 mAb for NSCLC and grade 3 pneumonitis developed, which was treated with methylprednisone and infliximab. The patient’s condition was complicated by herpes simplex virus 1 sepsis, which resulted in death. A third patient with NSCLC received 38 doses of anti–PD-1 mAb before grade 3 pneumonitis developed that required long-term corticosteroids, infliximab, and cyclophosphamide treatment. The patient died, and on autopsy, fulminant necrotizing fungal pneumonia (mucormycosis) was identified and attributed as the cause of death; no residual cancer was identified.
Recurrent Pneumonitis: With or Without T-Cell Checkpoint Rechallenge
Eleven patients experienced recurrent pneumonitis during drug holding/corticosteroid therapy after initial clinical improvement (Appendix Table A1, online only). Of these patients in whom recurrence occurred without drug rechallenge, eight experienced resolution/improvement with further management and three worsened and died despite immunosuppression.
Twelve patients underwent rechallenge with immunotherapy after an initial pneumonitis event (nine grade 1 and three grade 2; nine treated with drug holding only and three treated with corticosteroids for initial event; Appendix Table A2, online only). All rechallenged cases occurred after complete clinical resolution of pneumonitis. Nine patients did not experience a second pneumonitis event on rechallenge (eight grade 1 and one grade 2), whereas three experienced recurrent pneumonitis after rechallenge of whom one patient initially had grade 1 pneumonitis treated with drug hold only, and had recurrent pneumonitis that was again grade 1 and resolved with drug holding. The other two patients had grade 2 pneumonitis initially, were treated with corticosteroids, and had recurrent grade 2 pneumonitis that again resolved with oral corticosteroids.
Radiologic Features
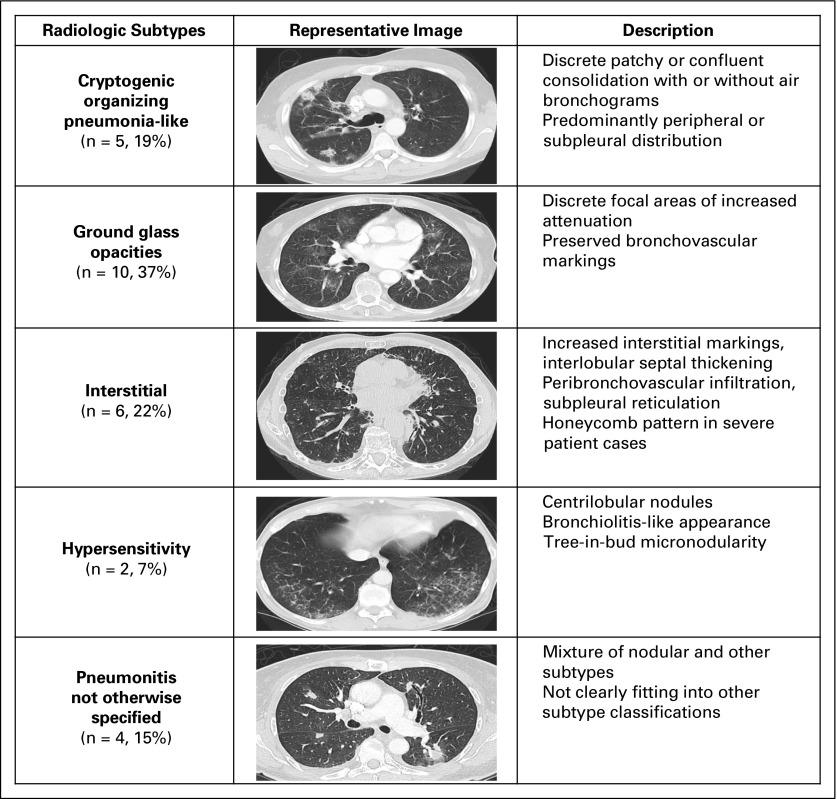
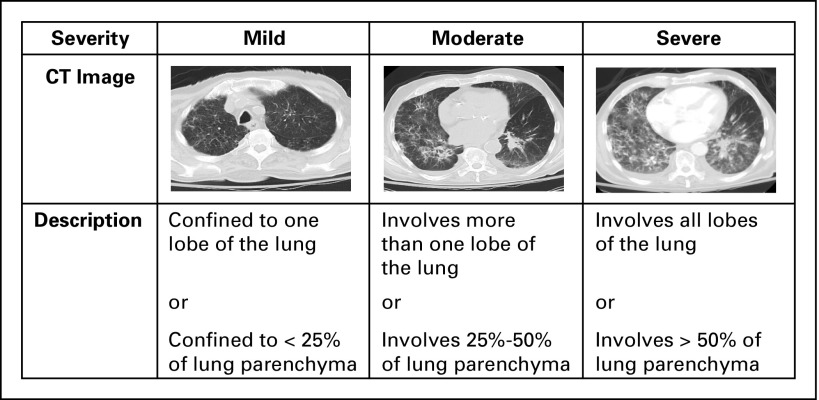
Radiologic and clinical outcomes of pneumonitis were aligned in all patients who experienced clinical improvement/resolution (37 of 37), and four of five patients who experienced clinical worsening of their pneumonitis. Serial chest CT scans were available for all MSKCC patients. Radiologic features of pneumonitis were classified into five subtypes (Fig 3): cryptogenic organizing pneumonia (COP) like (five of 27 [19%]), ground glass opacities (GGO; 10 of 27 [37%]), interstitial (two of 27 [7%]), hypersensitivity (six of 27 [22%]), and pneumonitis not otherwise specified (four of 27 [15%]). Radiologic severity at the time of pneumonitis was classified as mild (15 of 27 [56%]), moderate (six of 27 [22%]), or severe (six of 27 [22%]; Appendix Fig A1, online only). Cohen κ-coefficients were 0.66 for radiologic subtype and 0.88 for radiologic severity grading, respectively. Radiologic subtypes were consistent throughout a patient’s clinical course, except in two cases where COP-like pneumonitis evolved into a severe GGO type, and where GGO type developed additional interstitial appearances. COP-like appearance was more common among patients with NSCLC than among patients with other cancers (four of nine v one of 18, P = .03). In addition, patients with the COP-like subtype were more likely to require treatment of pneumonitis (beyond drug hold) than those with other radiologic subtypes (five of five v 11 of 22, P = .06). Chest x-rays obtained at the time of pneumonitis (n = 9) demonstrated possible pneumonitis in 67% of cases (six of nine), possible progressive cancer in 11% (one of nine), and no new radiographic abnormality in 22% (two of nine).
Fig 3.
Radiologic features of pneumonitis associated with anti–programmed death-1/programmed death ligand 1 therapy stratified into five distinct phenotypes.
Pathologic Features
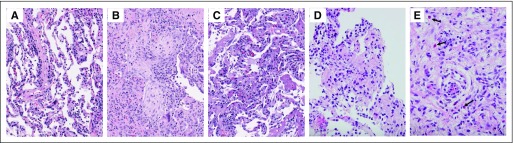
Eleven of 27 (41%) patients at MSKCC underwent lung biopsy at the time of pneumonitis (eight bronchoscopic, two core biopsies, one wedge resection; Appendix Table A3, online only). Histopathologic findings were cellular interstitial pneumonitis (four of 11; Appendix Fig A2A, online only), organizing pneumonia (three of 11; Appendix Fig A2B), diffuse alveolar damage (one of 11; Appendix Fig A2C), and no abnormalities identified (three of 11). The interstitial inflammatory infiltrate included poorly formed granulomas in three cases (Appendix Fig A2D) and eosinophils in two cases (Appendix Fig A2E).
Associations Between Clinical Features and Pneumonitis Outcomes
Worsening clinical outcomes with pneumonitis were more frequent in current versus former smokers (five of 23 v zero of 19, P = .053) and those with underlying lung conditions versus no lung conditions (four of 15 v one of 27, P = .047; Appendix Table A4, online only). Forced expiratory volume in 1 second and diffusing capacity of lung for carbon monoxide adjusted for hemoglobin were completed in a subset of patients at the time of pneumonitis (16 of 43 and 12 of 43, respectively); no associations between these parameters and clinical outcomes were seen.
DISCUSSION
We describe the first large series of pneumonitis to our knowledge associated with anti–PD-1/PD-L1 mAbs in patients with advanced cancers and comprehensively characterize the clinical, radiologic, and pathologic features, and management of this toxicity. Any-grade pneumonitis developed in approximately 5% of patients treated with anti–PD-1/PD-L1 mAbs, and grade 3 and higher pneumonitis developed in 1%. Pneumonitis was more common in patients treated with anti–PD-1/PD-L1 mAbs plus anti–CTLA-4 mAb compared with anti–PD-1/PD-L1 monotherapy. Rates of pneumonitis with anti–PD-1 versus anti–PD-L1 mAb in this series were not statistically different; however, larger data sets that include meta-analyses across tumor types are needed to determine conclusively whether a difference exists. Most cases of pneumonitis were mild, which is reassuring, but with the increasing use of anti–PD-1/PD-L1 mAbs in many disease settings, the absolute burden of pneumonitis undoubtedly will rise.
Most patients with pneumonitis were symptomatic at presentation, with one third of cases identified incidentally by imaging. Because timing of pneumonitis onset varied widely, constant vigilance for the signs and symptoms of this toxicity is required.
The majority of patients with pneumonitis in this study were also responders to immunotherapy, although they also had a variety of diseases, treatments, and systems of assessment. Additionally, there may be confounding between increased time on therapy and the association between both risk of pneumonitis and likelihood of benefit from immunotherapy. Nevertheless, it is intriguing to consider a possible mechanistic association between benefit and toxicity with anti–PD-1/PD-L1 agents.
We also describe the varied clinical, radiologic, and pathologic features of anti–PD-1/PD-L1 pneumonitis. Unlike bleomycin-induced pneumonitis, which is characterized by specific radiologic appearances23 and changes in diffusing capacity of lung for carbon monoxide,32 we did not identify any pathognomonic radiographic or pathologic features of anti–PD-1/PD-L1 pneumonitis. To provide a common language for this toxicity, we describe distinct radiologic phenotypes by CT26,33 and found acceptable concordance between independent radiologic reviewers. Because radiology review was only performed in clinically determined pneumonitis cases, the real-world interobserver concordance of radiologic assessments may be lower. Of note, chest x-ray did not detect a new radiographic abnormality in nearly one quarter of pneumonitis cases, which suggests that it may be an inadequate tool for evaluating suspected pneumonitis.
Clinically, nearly all cases of pneumonitis improved/resolved with drug holding and/or immunosuppression. However, some cases worsened and were fatal. In this series, worsening cases were restricted to current and former smokers and were more common in patients with underlying lung conditions; such patients may require particularly careful management. Among patients in whom pneumonitis improved/resolved, 12 (all with grade 1 to 2) underwent rechallenge with anti–PD-1/PD-L1 mAbs, and recurrent pneumonitis occurred in three (25%). This suggests that in mild cases, one may cautiously resume therapy after pneumonitis has improved/resolved and after careful discussion with the patient.
Although most instances of pneumonitis were not severe, five deaths occurred, and in three cases, infection from prolonged immunosuppression contributed to death. No patient who received immunosuppression beyond corticosteroids (infliximab with or without cyclophosphamide) recovered from pneumonitis. Improvement is needed in the choice, dose, and duration of therapies for pneumonitis with consideration of the role of antimicrobial prophylaxis in the context of prolonged immunosuppression.
Drug-induced pneumonitis remains a diagnosis of exclusion and requires consideration of competing diagnoses, including infection and malignant lung infiltration. Diagnostic bronchoscopy with lung biopsy may play an important role in excluding competing diagnoses. We attempted to exclude cases with alternative etiologies and evidence of pulmonary infection or infiltrative cancer. However, the excluded cases may have represented true pneumonitis or mixed presentation. In addition some cases of pneumonitis may not have been detected radiologically, so the true incidence of pneumonitis may be higher than that described here. This series largely comprised patients with melanoma and NSCLC; a larger series that investigates pneumonitis as a result of anti–PD-1/PD-L1 mAbs in other malignancies are needed. Finally, this series describes the features of pneumonitis, but did not seek to identify risk factors for its development, which remains an open question for future investigation.
This study fills an important gap in the literature because the only published data on anti–PD-1/PD-L1 pneumonitis are from two small case reports34,35 and two larger case series reported at ASCO 2016.36,37 Collectively, these reports affirm the variable timing of onset and potential for recurrent pneumonitis during pneumonitis management. Although these studies cannot make firm recommendations about optimal pneumonitis management, grade 1 pneumonitis seems reasonable to treat with drug holding alone, with close clinical and radiologic follow-up (2 to 4 weeks) for resolution/improvement. If symptoms arise or no radiologic improvement is seen, corticosteroids are appropriate. Grade 2 and higher pneumonitis should be treated with corticosteroids in addition to drug holding, with continued close clinical and radiologic follow-up. Patients in whom worsening pneumonitis develops can be resistant to traditional immunosuppression and may benefit from studies of early additional or new/alternative immunosuppression.
In summary, pneumonitis is an uncommon but potentially serious toxicity that occurs in 5% of patients who receive anti–PD-1/PD-L1 mAbs. Treating physicians should be aware of its diverse clinical, radiologic, and pathologic features and that it may develop at any time during a patient’s treatment course. Most cases are mild and managed successfully with favorable outcomes. However, worsening pneumonitis may develop in a subset of patients despite additional immunosuppression, and they may suffer from the immunosuppressive consequences of pneumonitis treatment. Improvements in the treatment and understanding of the biology of pneumonitis are needed to optimize management.
Appendix
Table A1.
Recurrent Pneumonitis Without Rechallenge
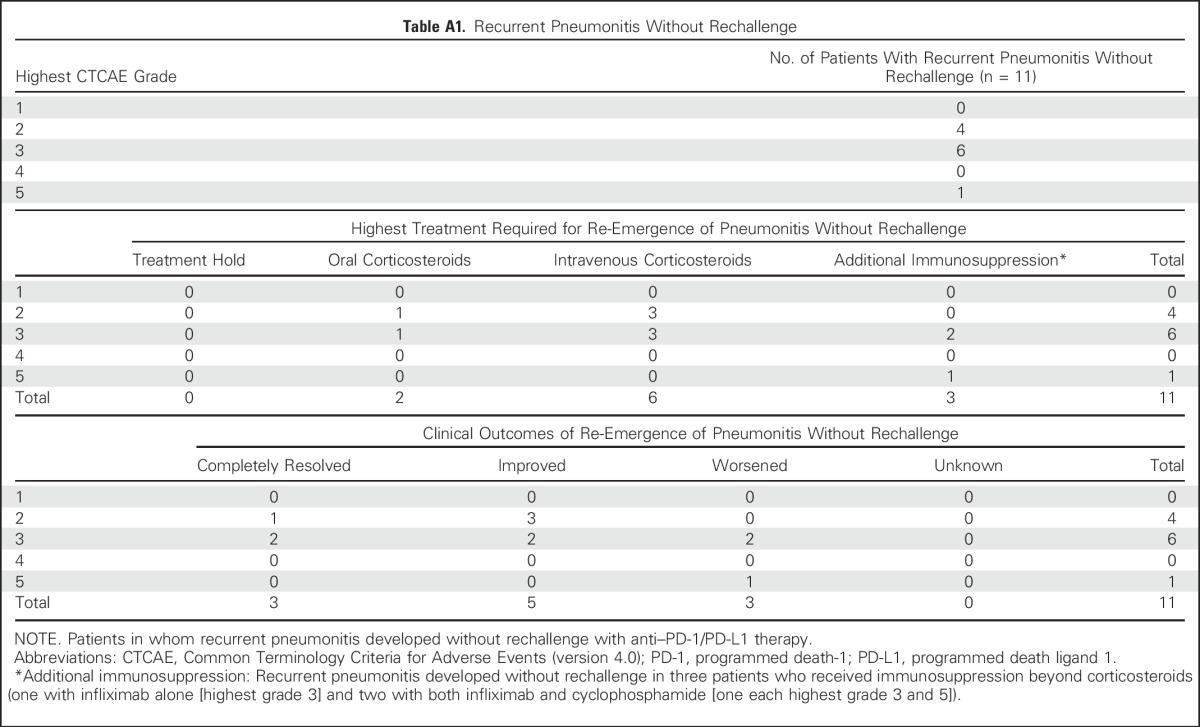
Table A2.
Recurrent Pneumonitis With Rechallenge: Treatment and Outcomes
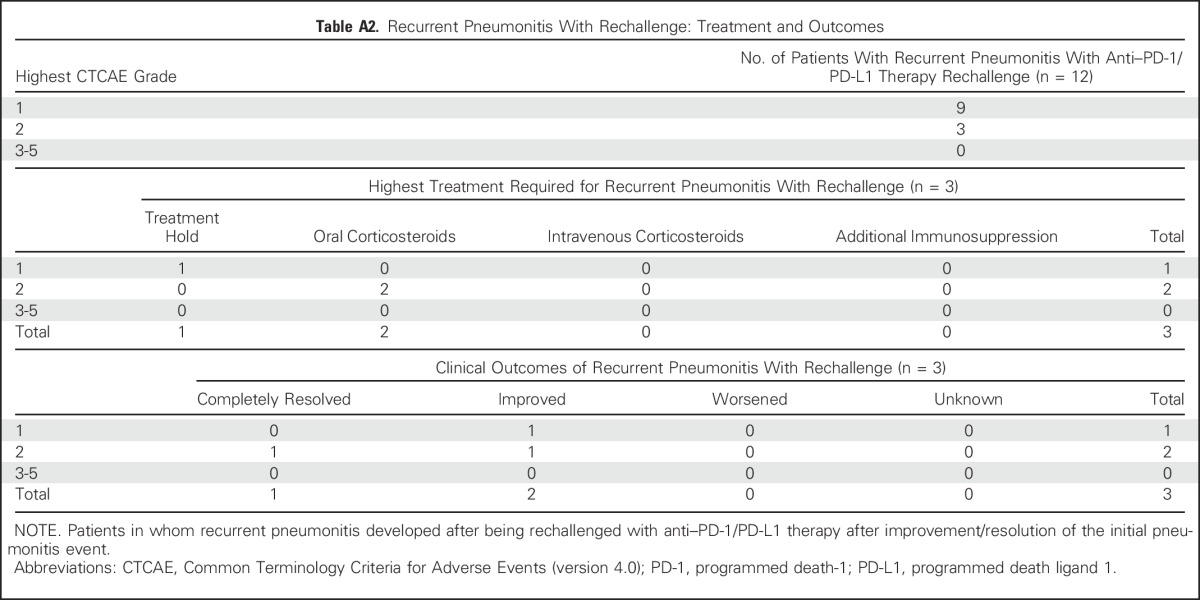
Table A3.
Pathologic Features of Pneumonitis
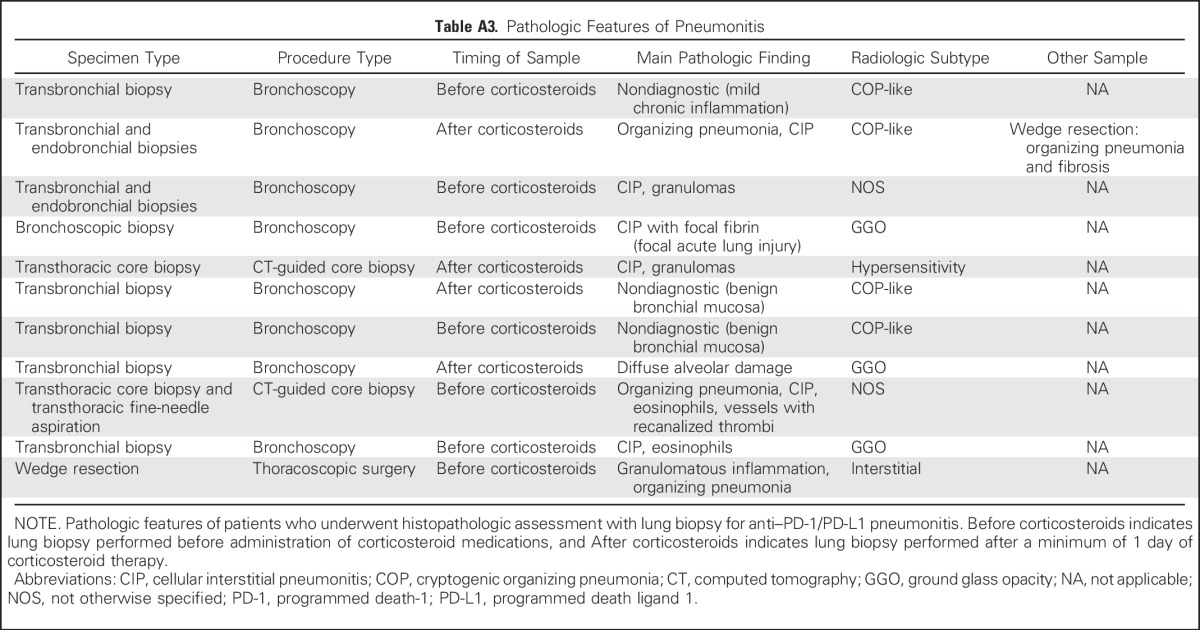
Table A4.
Association Between Clinicopathologic Features and Treatment Data With Clinical Outcomes of Pneumonitis
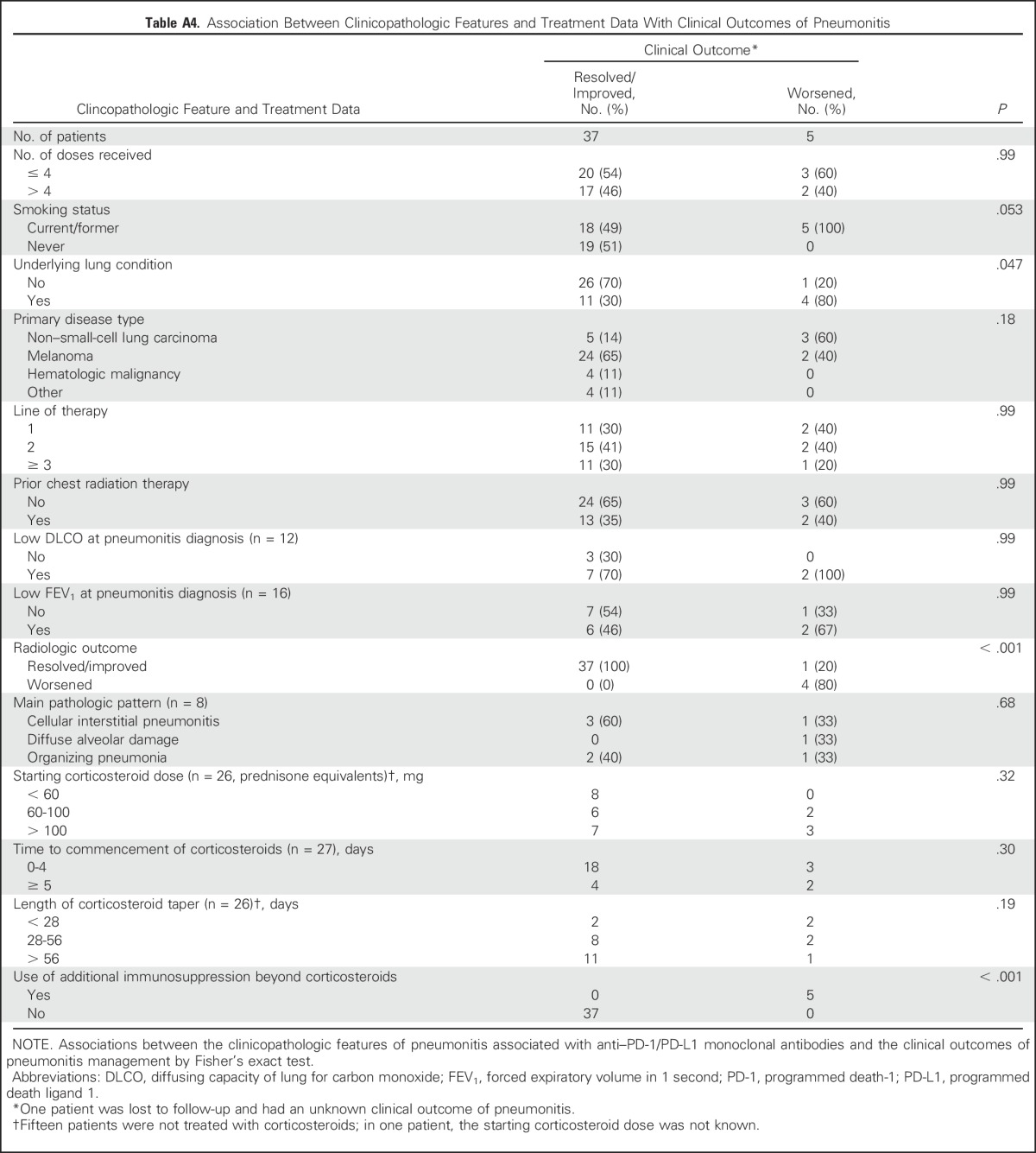
Fig A1.
Radiologic severity of pneumonitis associated with anti–programmed death-1/programmed death ligand 1 therapy stratified into mild, moderate, and severe. CT, computed tomography.
Fig A2.
Histologic patterns of pneumonitis associated with anti–programmed death-1/programmed death ligand 1 therapy on lung biopsy (hematoxylin and eosin [HE] stain magnification, ×200) included (A) cellular interstitial pneumonitis (mild case shown), (B) organizing pneumonia, and (C) diffuse alveolar damage. Additional findings (HE stain magnification, ×400) include (D) poorly formed granulomas, and (E) eosinophils (arrows).
Footnotes
Processed as a Rapid Communication manuscript.
Supported by Memorial Sloan Kettering Cancer Center Support Grant/Core Grant No. P30 CA008748.
Presented in part at the European Cancer Congress 2015, Vienna, Austria, September 25-29 2015.
See accompanying article on page 705
AUTHOR CONTRIBUTIONS
Conception and design: Jarushka Naidoo, Tunc Iyriboz, Jane Cunningham, Charles Leduc, Georgina V. Long, Matthew D. Hellmann
Provision of study materials or patients: Jamie E. Chaft, Neil H. Segal, Margaret K. Callahan, Alexander M. Lesokhin, Jonathan Rosenberg, Martin H. Voss, Charles M. Rudin, Ruth-Ann Gordon, Matteo S. Carlino, Jedd D. Wolchok, Michael A. Postow, Georgina V. Long, Matthew D. Hellmann
Collection and assembly of data: Jarushka Naidoo, Xuan Wang, Tunc Iyriboz, Darragh Halpenny, Jane Cunningham, Jamie E. Chaft, Neil H. Segal, Margaret K. Callahan, Alexander M. Lesokhin, Jonathan Rosenberg, Martin H. Voss, Charles M. Rudin, Hira Rizvi, Xue Hou, Katherine Rodriguez, Melanie Albano, Ruth-Ann Gordon, Charles Leduc, Natasha Rekhtman, Alexander M. Menzies, Alexander D. Guminski, Matteo S. Carlino, Benjamin Y. Kong, Georgina V. Long, Matthew D. Hellmann
Data analysis and interpretation: Jarushka Naidoo, Xuan Wang, Kaitlin M. Woo, Darragh Halpenny, Jane Cunningham, Jamie E. Chaft, Neil H. Segal, Margaret K. Callahan, Alexander M. Lesokhin, Jonathan Rosenberg, Martin H. Voss, Charles M. Rudin, Hira Rizvi, Katherine Rodriguez, Melanie Albano, Ruth-Ann Gordon, Charles Leduc, Natasha Rekhtman, Bianca Harris, Alexander M. Menzies, Alexander D. Guminski, Matteo S. Carlino, Benjamin Y. Kong, Jedd D. Wolchok, Michael A. Postow, Georgina V. Long, Matthew D. Hellmann
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Pneumonitis in Patients Treated With Anti–Programmed Death-1/Programmed Death Ligand 1 Therapy
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Jarushka Naidoo
Honoraria: Bristol-Myers Squibb, AstraZeneca
Consulting or Advisory Role: Bristol-Myers Squibb, AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca, Bristol-Myers Squibb
Xuan Wang
No relationship to disclose
Kaitlin M. Woo
No relationship to disclose
Tunc Iyriboz
No relationship to disclose
Darragh Halpenny
No relationship to disclose
Jane Cunningham
No relationship to disclose
Jamie E. Chaft
Honoraria: DAVA Oncology
Consulting or Advisory Role: Myriad Genetics, Biodesix, Genentech, Roche, Clovis Oncology, AstraZeneca, MedImmune
Research Funding: Genentech (Inst), Roche (Inst), Bristol-Myers Squibb (Inst), AstraZeneca (Inst), MedImmune (Inst)
Neil H. Segal
Honoraria: MedImmune
Consulting or Advisory Role: Bristol-Myers Squibb, Pfizer, AstraZeneca, MedImmune, MacroGenics, Imugene, Roche, Genentech, Kyocera, Amgen, Calithera Biosciences
Research Funding: MedImmune, Bristol-Myers Squibb, Pfizer, Roche, Genentech, Merck
Margaret K. Callahan
Employment: Bristol-Myers Squibb (I), Celgene (I)
Consulting or Advisory Role: AstraZeneca
Research Funding: Bristol-Myers Squibb (Inst)
Alexander M. Lesokhin
Honoraria: Bristol-Myers Squibb, Janssen Pharmaceuticals, Gilead Sciences (I), Novartis
Consulting or Advisory Role: Bristol-Myers Squibb, Foundation Medicine (Inst), Janssen Pharmaceuticals, Novartis
Research Funding: Bristol-Myers Squibb (Inst), Janssen Pharmaceuticals (Inst), Genentech (Inst), Roche (Inst)
Patents, Royalties, Other Intellectual Property: Serametrix (Inst)
Jonathan Rosenberg
Stock or Other Ownership: Merck, Illumina
Honoraria: UpToDate
Consulting or Advisory Role: Boehringer Ingelheim, Bristol-Myers Squibb, Dendreon, Janssen Pharmaceuticals, Johnson & Johnson, OncoGenex Pharmaceuticals, Onyx Pharmaceuticals, Eli Lilly, Merck, Agensys, Roche, Genentech, Sanofi
Research Funding: Genentech (Inst), OncoGenex Pharmaceuticals (Inst), Agensys (Inst), Mirati Therapeutics (Inst), Novartis (Inst)
Patents, Royalties, Other Intellectual Property: ERCC2-predicting cisplatin sensitivity
Travel, Accommodations, Expenses: Genentech, Roche
Martin H. Voss
Honoraria: Novartis
Consulting or Advisory Role: Novartis, Calithera Biosciences, Natera, GlaxoSmithKline, Exelixis
Research Funding: Pfizer, Bristol-Myers Squibb, Genentech, Roche
Travel, Accommodations, Expenses: Novartis, Takeda Pharmaceuticals
Charles M. Rudin
Consulting or Advisory Role: Novartis, Bristol-Myers Squibb, Medivation
Hira Rizvi
No relationship to disclose
Xue Hou
No relationship to disclose
Katherine Rodriguez
No relationship to disclose
Melanie Albano
No relationship to disclose
Ruth-Ann Gordon
Consulting or Advisory Role: AstraZeneca
Charles Leduc
No relationship to disclose
Natasha Rekhtman
No relationship to disclose
Bianca Harris
No relationship to disclose
Alexander M. Menzies
Honoraria: Bristol-Myers Squibb, Novartis
Consulting or Advisory Role: MSD, Chugai Pharmaceutical
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Alexander D. Guminski
Consulting or Advisory Role: Bristol-Myers Squibb, Novartis, Merck
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Astellas Pharma, Novartis, Merck
Matteo S. Carlino
Honoraria: Novartis, Merck Sharp & Dohme, Bristol-Myers Squibb
Consulting or Advisory Role: Novartis, Bristol-Myers Squibb, Merck Sharp & Dohme, Amgen
Benjamin Y. Kong
No relationship to disclose
Jedd D. Wolchok
Stock or Other Ownership: Potenza Therapeutics, Vesuvius Pharmaceuticals
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, MedImmune, ZIOPHARM Oncology, Polynoma, Polaris Pharmaceuticals, Jounce Therapeutics, Genentech
Research Funding: Bristol-Myers Squibb (Inst), MedImmune (Inst), GlaxoSmithKline (Inst), Merck (Inst)
Patents, Royalties, Other Intellectual Property: Coinventor on an issued patent for DNA vaccines for the treatment of cancer in companion animals
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Michael A. Postow
Honoraria: Bristol-Myers Squibb, Merck
Consulting or Advisory Role: Amgen, Bristol-Myers Squibb
Research Funding: Bristol-Myers Squibb (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Georgina V. Long
Honoraria: Bristol-Myers Squibb, Merck, Roche, Genentech
Consulting or Advisory Role: Bristol-Myers Squibb, Roche, Genentech, Amgen, Merck, Provectus Pharmaceuticals, Novartis
Matthew D. Hellmann
Consulting or Advisory Role: Third Rock Ventures, Bristol-Myers Squibb, Merck, Genentech, Alexion Pharmaceuticals, Inovio Biomedical, AstraZeneca, MedImmune
Research Funding: Bristol-Myers Squibb
REFERENCES
- 1.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 2.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: A randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 3.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 6. doi: 10.1016/S0140-6736(15)01281-7. Herbst RS, Baas P, Kim DW, et al: Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet, 387:1540-1550, 2015. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillison ML, Blumenschein G, Fayette J, et al. Nivolumab (nivo) vs investigator’s choice (IC) for recurrent or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): CheckMate-141. Presented at the American Association for Cancer Research Annual Meeting, New Orleans, LA, April 16-20, 2016. [Google Scholar]
- 14.Antonia S, Goldberg SB, Balmanoukian A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: A multicentre, phase 1b study. Lancet Oncol. 2016;17:299–308. doi: 10.1016/S1470-2045(15)00544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rizvi NA, Gettinger SN, Goldman JW, et al. Safety and efficacy of first-line nivolumab (NIVO; anti-programmed death-1 [PD-1]) and ipilimumab in non-small cell lung cancer (NSCLC). Presented at the International Association for the Study of Lung Cancer Annual Meeting, Denver, CO, September 6-9, 2015. [Google Scholar]
- 16.Weber JS, Yang JC, Atkins MB, et al. Toxicities of immunotherapy for the practitioner. J Clin Oncol. 2015;33:2092–2099. doi: 10.1200/JCO.2014.60.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book. 2015;35:76–83. doi: 10.14694/EdBook_AM.2015.35.76. [DOI] [PubMed] [Google Scholar]
- 18.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Disayabutr S, Calfee CS, Collard HR, et al. Interstitial lung diseases in the hospitalized patient. BMC Med. 2015;13:245. doi: 10.1186/s12916-015-0487-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26:2375–2391. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Genestreti G, Di Battista M, Trisolini R, et al. A commentary on interstitial pneumonitis induced by docetaxel: Clinical cases and systematic review of the literature. Tumori. 2015;101:e92–e95. doi: 10.5301/tj.5000275. [DOI] [PubMed] [Google Scholar]
- 22.Poole BB, Hamilton LA, Brockman MM, et al. Interstitial pneumonitis from treatment with gemcitabine. Hosp Pharm. 2014;49:847–850. doi: 10.1310/hpj4909-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Comis RL: Bleomycin pulmonary toxicity: current status and future directions. Semin Oncol 19:64-70, 1992 (suppl 5) [PubMed] [Google Scholar]
- 24.Ando M, Okamoto I, Yamamoto N, et al. Predictive factors for interstitial lung disease, antitumor response, and survival in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2006;24:2549–2556. doi: 10.1200/JCO.2005.04.9866. [DOI] [PubMed] [Google Scholar]
- 25.Liu V, White DA, Zakowski MF, et al. Pulmonary toxicity associated with erlotinib. Chest. 2007;132:1042–1044. doi: 10.1378/chest.07-0050. [DOI] [PubMed] [Google Scholar]
- 26.White DA, Camus P, Endo M, et al. Noninfectious pneumonitis after everolimus therapy for advanced renal cell carcinoma. Am J Respir Crit Care Med. 2010;182:396–403. doi: 10.1164/rccm.200911-1720OC. [DOI] [PubMed] [Google Scholar]
- 27.Bradley J, Movsas B. Radiation pneumonitis and esophagitis in thoracic irradiation. Cancer Treat Res. 2006;128:43–64. doi: 10.1007/0-387-25354-8_4. [DOI] [PubMed] [Google Scholar]
- 28.Weshler Z, Breuer R, Or R, et al. Interstitial pneumonitis after total body irradiation: Effect of partial lung shielding. Br J Haematol. 1990;74:61–64. doi: 10.1111/j.1365-2141.1990.tb02538.x. [DOI] [PubMed] [Google Scholar]
- 29.Palmucci S, Roccasalva F, Puglisi S, et al. Clinical and radiological features of idiopathic interstitial pneumonias (IIPs): A pictorial review. Insights Imaging. 2014;5:347–364. doi: 10.1007/s13244-014-0335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johkoh T, Fukuoka J, Tanaka T. Rare idiopathic intestinal pneumonias (IIPs) and histologic patterns in new ATS/ERS multidisciplinary classification of the IIPs. Eur J Radiol. 2015;84:542–546. doi: 10.1016/j.ejrad.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 31.Kadoch MA, Cham MD, Beasley MB, et al. Idiopathic interstitial pneumonias: A radiology-pathology correlation based on the revised 2013 American Thoracic Society-European Respiratory Society classification system. Curr Probl Diagn Radiol. 2015;44:15–25. doi: 10.1067/j.cpradiol.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Comis RL. Detecting bleomycin pulmonary toxicity: A continued conundrum. J Clin Oncol. 1990;8:765–767. doi: 10.1200/JCO.1990.8.5.765. [DOI] [PubMed] [Google Scholar]
- 33.Endo M, Johkoh T, Kimura K, et al. Imaging of gefitinib-related interstitial lung disease: Multi-institutional analysis by the West Japan Thoracic Oncology Group. Lung Cancer. 2006;52:135–140. doi: 10.1016/j.lungcan.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Nishino M, Sholl LM, Hodi FS, et al. Anti-PD-1-related pneumonitis during cancer immunotherapy. N Engl J Med. 2015;373:288–290. doi: 10.1056/NEJMc1505197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishino M, Chambers ES, Chong CR, et al. Anti-PD-1 inhibitor-related pneumonitis in non-small cell lung cancer. Cancer Immunol Res. 2016;4:289–293. doi: 10.1158/2326-6066.CIR-15-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishino M, Ramaiya NH, Awad MM, et al. PD-1 inhibitor-related pneumonitis in advanced cancer patients. Presented at the American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 3-7, 2016. [Google Scholar]
- 37.Gettinger SN, Zhang X, Homer R, et al. Pneumonitis in non-small cell lung cancer (NSCLC) patients treated with programmed death 1 (PD1) axis inhibitors. Presented at the American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 3-7, 2016. [Google Scholar]