Abstract
Objective:
With the changes in healthcare, patients with cancer now have to assume greater responsibility for their own care. Oral cancer medications with complex regimens are now a part of cancer treatment. Patients have to manage these along with the management of medications for their other chronic illnesses. This results in medication burden as patients assume the self-management.
Methods:
This paper describes the treatment burdens that patients endured in a randomized, clinical trial examining adherence for patients on oral cancer medications. There were four categories of oral agents reported. Most of the diagnoses of the patients were solid tumors with breast, colorectal, renal, and gastrointestinal.
Results:
Patients had 1–4 pills/day for oral cancer medications as well as a number for comorbidity conditions (>3), for which they also took medications (10–11). In addition, patients had 3.7–5.9 symptoms and side effects. Patients on all categories except those on sex hormones had 49%–57% drug interruptions necessitating further medication burden.
Conclusions:
This study points out that patients taking oral agents have multiple medications for cancer and other comorbid conditions. The number of pills, times per day, and interruptions adds to the medication burden that patients’ experience. Further study is needed to determine strategies to assist the patients on oral cancer medications to reduce their medication burden.
Keywords: Burden of treatment, cancer, comorbidity, oral cancer medication

Introduction
With the rapid changes in health care and more focus on ambulatory cancer care, patients and their families are expected to assume greater responsibility for their cancer care. Because of renewed interest in quality of care, the patient's experience and engagement are gaining increased attention. The medication burden associated with the management and treatment of chronic illnesses and the increased responsibilities they impose for patients has only recently been described in the literature and remains elusive and confusing.[1,2,3,4,5,6,7,8] The purpose of this paper is to describe the treatment burdens that patients are expected to manage during their treatment with oral cancer medications.
The “burden of treatment” is a dynamic process with subjective and objective components that require patient's time and energy to actively manage their illness. Burden of treatment is defined as the patient workload to carry out the therapeutic recommendation of healthcare professionals and its impact on the patient's functioning and well-being.[1,2,8] Burden of treatment includes physical, financial, temporal, and psychosocial time demands on patients to follow the recommended treatment plan. To follow therapeutic recommendations, patients have to carry out a wide variety of tasks that include visits to clinics and laboratories, managing complex medication regimens, managing diet and exercise regimens, managing medical devices, and completing other treatments such as the use of dressings, catheters, and injections.
Treatment recommendations for cancer patients require much involvement, but clinicians often have little understanding of the burden that patients are experiencing. One of the components of treatment burden is medication burden. The focus of this paper is on the “medication burden” associated with initiating oral cancer medications for the treatment of cancer. We examine the challenges that patients’ experience as they seek to incorporate oral cancer medications into their existing treatment regimens. Oral cancer medications have become a standard of care for cancer treatment. It is now vital to understand the increased burden and responsibility these medications impose on the patient. Due to the volume, variation, complexity, difficulty of the treatment regimen, and cost, patients must assume increased responsibility and address the challenges associated with this form of treatment. Healthcare professionals have not considered what the patient's preexisting medication workload is from existing comorbidities and what negative outcomes could result from adding additional medications to their medication “burden.”[3]
In cancer care, the advent of oral cancer medications represents a paradigm shift in treatment away from intravenous (IV) chemotherapy. Patients with late-stage disease for solid tumors (such as lung, breast, pancreas, kidney, prostate, and colorectal) are now often prescribed oral cancer medications rather than or in addition to IV chemotherapy. With the oral cancer medications, the patient has the responsibility for the actual medication administration. Patients with solid tumors are often 55 years of age or older and may have other comorbid conditions, for which they also take medications.
Oral cancer medications might be viewed as good news for cancer patients as they would require fewer visits to clinics to receive infusion therapy, fewer laboratory tests, and fewer physician visits. However, the responsibility for medication acquisition, administration, side effect monitoring, and management has now been transferred to patients and their families. In essence, we have added to the patient's treatment workload, and now, cancer treatment responsibility is part of the patient's routine daily activities. Increasing treatment burden has the potential to induce nonadherence, especially with those who have frequent changes or alteration in their medication regimens – this occurs frequently among patients on oral cancer medications. Eton et al. showed that the medication burden might help explain adherence, increased healthcare service use, and poorer overall patient-reported quality of life.[1,9,10,11]
Prescribing oral cancer medications for cancer patients with solid tumors requires careful assessment of the benefit and risks of the cancer treatment and the effect of the increased workload on the patients’ preexisting comorbid conditions. Health-care professionals should acknowledge that oral cancer medications have been added to the existing treatment burden.
Patient difficulties and challenges with oral cancer medications seldom receive attention from a healthcare professional beyond the need for adherence. When prescribing medications, clinicians are seldom aware of the additional challenges that patients experience given the medication burden and other therapeutic activities from their preexisting chronic illness treatment regimens. Little has been described in the literature about the medication associated burden brought on by the use of oral cancer medications. Given the serious illness of patients when oral cancer medications are prescribed, patients’ capacity to carry out the requirements (i.e., follow the recommendation) of medication administration has to be considered. A beginning description of some of these challenges as it affects a group of patients who are on oral medications will be the focus of this paper.
Medication burden
There are several elements of medication burden. Patients on oral cancer medications have to learn details about their medication, understand why and how to take the medication, and be clear to differentiate them from their current medications for their other chronic health problem(s). The time required to acquire, plan, and organize medication administration, take the medication, monitor treatment, and manage side effects all lead to medication burden for a patient.[12,13]
Once patients obtain their oral cancer medications, they will have to determine how they will best integrate taking these medications into their daily lives along with the medications for their other chronic disease(s). There are numerous activities to consider when attempting to fully describe medication burden for patients on oral cancer medications. These activities are briefly described.
Learning about each oral cancer medication: Its name, purpose, dosage frequency, and how and when to take the medication
Dealing with insurance and payments for oral cancer medications becomes a major issue given the costs of the medications, especially among patients with large pharmacy co-pays which make out-of-pocket expenditures a concern. In the United States, preapproval by health insurance companies is essential since some of these oral cancer medications cost $10,000 or more per month and the patients co-pays vary depending on insurance. Finally, in the United States for those who are eligible, qualifying for drug assistance programs can add another level of burden to the process of obtaining oral cancer medications. These issues may not occur in other countries
Acquiring the oral cancer medication. Often, there is a delay in getting approval from insurers before shipping oral cancer medications to patients. Patients in the United States with oral cancer medications often deal with specialty pharmacies to obtain the medications after they obtain approval from their insurance carrier. These delays may create an additional type of burden in that patients may be instructed on how to take their medication when they leave their oncologist's office but fail to remember those details by the time they receive the medications from the specialty pharmacy
Routines for the administration of oral cancer medications must be established taking into consideration medications for their other chronic conditions and integrating them with those daily schedules and the mode of administration with the oral agents. Some oral cancer medications need to be taken on an empty stomach while others are taken with food and fluids, but some food must be avoided
Dealing with characteristics of the medication protocol: Number of pills, number of times per day, number of days per month. For some cancer patients, protocols might be continuous, but many are discontinuous with 7 days on and 7 days off or 21 days on and 7 days off. Several are taken more than once a day; numerous regimens have rest periods with 1 or 2 weeks with no medication, followed by resuming for 2 to 3 weeks (there are 21 and 28 days cycles). In some cases, oral cancer medications are accompanied by other medications, such as a steroid that is to be taken for a few days at the beginning of each cycle
Side effects are common with oral cancer medication just as IV administration, requiring strategies for managing side effects such as nausea, rash, and diarrhea. Managing these symptoms may include additional medications. Some of these have known strategies for management, and others are not known as the assumption may be that oral agents are less toxic. Adverse events occur that require medical attention, which may lead to emergency room visits, urgent care visits, or hospitalization. Patients need to know what events require urgent attention
Scheduling appointments and communicating with physicians for follow-up for altering dosage when side effects or adverse effects occur and/or follow-up laboratory work to monitor the effect on the blood cells
Storing and handling the medications may become an issue as many of the oral cancer medications should not be removed from the bottle, in which they are delivered. Wearing gloves to handle the oral cancer medication may be necessary.
These are examples of the activities patients assume with oral cancer medications. Two examples of how these components contribute to medication burden are in the case examples [Box 1].
Box 1.
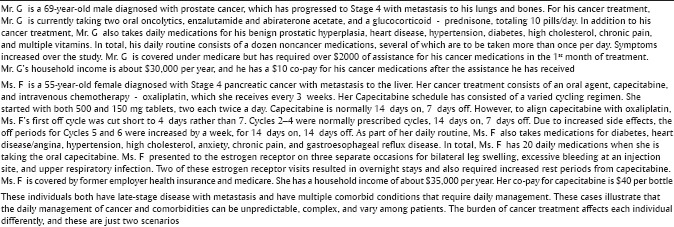
Case study examples of medication burden
In addition to medications for ongoing comorbid conditions, patients need to incorporate into their daily lives the variations in oral cancer medication administration. Examples are below.
Lapatinib may be given with capecitabine for metastatic breast cancer. When lapatinib is prescribed alone the patient is to take five tablets to reach 1250 mg once a day the same time each day, 1 h before or 1 h after a meal for 21 days. Capecitabine is taken as 2000 mg/m2/day in two doses 12 h apart on days 1–14, adding to the medication complexity
If capecitabine is used for breast cancer, it is often given with docetaxel which is administered IV; thus, patients must visit their oncology clinic at prescribed intervals which adds to the overall medication burden
Abiraterone (taken for metastatic prostate cancer) may be taken four tablets one time per day, with prednisone twice per day. Abiraterone is to be taken on an empty stomach with no food (1 h after the medication and 2 h before). High-fat meals can increase the systemic exposure of this medication. Grapefruit or grapefruit juice may affect the drug action and should be avoided
Capecitabine for colon cancer is to be taken for 6 months at intervals of 2 weeks with 1 week off and then repeated in 3-week cycles. It is to be taken within 30 min of a meal. Common side effects are diarrhea, nausea, vomiting, and hand-foot syndrome. Gloves should be worn to avoid drug dust contact with hands
Sorafenib, a tyrosine kinase inhibitor, is commonly used for renal or hepatocellular cancer. Patients take this medication two tablets twice daily with food and 1 h before or 2 h after a meal (avoiding all grapefruit) until disease progression.
From the examples above, one can see that nurses and other healthcare professionals need to know and understand the drugs, so they can help guide the patient to understand these medications and help moderate and reduce the medication burden.
When caring for patients taking oral cancer medications, treatment burdens accumulate incrementally and ironically, intensify as patients become more compromised. This may occur through deteriorating chronic conditions; from oral agent side effects requiring added doses of medications; interruptions to the oral cancer medications that subsequently are likely to require changes in dosing. These changes in conditions are managed by still additional medications. Further, this sequence of increasing burden is likely to be moderated by patients’ age, sex, characteristics, and stage of the disease, treatment, physical function, and duration of the illness before initiating oral cancer medication therapy. These should all be components of the assessment when oral cancer medications are prescribed.
To elaborate on these treatment burdens, we present descriptive findings from an ongoing study of oral cancer medications among patients who have new prescriptions for oral cancer medications for advanced cancer to demonstrate how patients experience these medication burdens.
Methods
Study design
Data are from a multi-site randomized 12-week randomized trial testing medication adherence and symptom management strategies for patients with solid tumors who have a newly prescribed oral cancer medication. Patients who agreed to participate had their oral cancer medication regimen recorded including name, dosing, number of pills per dose, number of times per day, and number of cycles. Patients completed telephone interviews at baseline and 4, 8, and 12 weeks to determine if medications were continued, interrupted, or stopped. Patients’ symptoms and associated symptom severity and interference were assessed during automated weekly calls as well as during the baseline and 4, 8, and 12 weeks interviews. Patients assigned to the experimental group were referred to a Medication Adherence and Symptom Management Toolkit (Toolkit) when symptom severity was reported at a four or higher (on a 10-point scale). Medical record audits were completed for all patients for the period 1 month before and through 1 month after the time they were actively involved in the study.
Recruitment
Staff from several comprehensive cancer centers located in the Midwest United States were trained to identify patients who met eligibility criteria and enroll them into the study. Informed consent was obtained, baseline interviews were conducted, and patients were randomized to an adherence/symptom management intervention or to a standard care control group.
Sample
Patient eligibility criteria included being 21 years or older with a new prescription for an oral cancer medication, cognitively intact, English speaking; able/willing to complete phone calls, and Eastern Cooperative Oncology Group performance score of 0–2 or Karnofsky score ≥50. Fourteen cancer types were represented, with breast, colorectal, prostate, and pancreatic cancer being the most prevalent. Site and stage of disease were collected. As these patients had advanced cancer, attrition rates were approximately 30% after baseline for reasons of death, too ill, and entered hospice.
Measures
Sociodemographic
Patient characteristics were collected at screening and enrollment and included sex, age, race, ethnicity, education, and income. A modified Bayliss Comorbidity Scale evaluated nine other diagnosed comorbid conditions using a “yes/no” response and whether medication was used to treat the comorbid condition.[14]
Oral cancer medication regimen
At enrollment in the study, the drug prescription protocol was recorded to include name of the medication, dosage, number of pills per day, frequency per day, and number of cycles. Dose reductions, increases, interruptions, or addition of new agents to the drug protocols was obtained from the medical records.
Classification of drugs
Oral cancer medications were classified into four classes and included cytotoxics, kinase inhibitors, sex hormone inhibitors, and a general classification of mixed drugs. There were different protocols for patients. For example, capecitabine was twice daily for 2 weeks and 1 week off for a 3-week cycle. Sunitinib for renal and gastrointestinal cancers is recommended once daily for 4 weeks on and 2 weeks off. Regorafenib is once daily 21 days on and 7 days off.
The cancer symptom experience inventory
Symptom assessment measure was administered at intake and weekly for 12 weeks, which included the baseline and 4, 8 and 12 weeks. The cancer symptom experience inventory (CSEI) contains 18 patients reported symptoms as present or absent and then at their worst within the past 7 days due to cancer or its treatment with symptom severity rated on a 1–9 scale (1 = very little to 9 = worst possible). Symptom interference with daily activities in the past 7 days due to cancer or its treatment on a 0–9 scale (0 = did not interfere to 9 = interfered completely). Internal consistency reliability for the CSEI was >0.80.
Results
Table 1 describes the sociodemographic variables at baseline according to major classes of drugs included in the study. Breast cancer hormones were specifically not included. Examples of the specific drugs are listed in the tables’ footnote. The mean age was comparable across drug categories except sex hormones which were for advanced prostate cancer patients. Those on sex hormones on average were a decade older. The site of cancer varied across drug categories other than hormones. Most patients, other than the leukemia patients, had advanced disease and for those with advanced disease, the oral cancer medications were prescribed after other treatment approaches had failed. Most of the patients with solid tumor included were on second- or third-line treatment. Sociodemographics showed nearly an even split of men and women with the sex hormones limited to men for prostate cancer.
Table 1.
Descriptive statistics for the sample by category of oral cancer medication at baseline (n=274)
| Drug category | ||||
|---|---|---|---|---|
| Cytotoxics* (n=95), n (%) | Kinase inhibitors† (n=129), n (%) | Sex hormone inhibitors‡ (n=27), n (%) | Other§ (n=23), n (%) | |
| Sex | ||||
| Male (n=138) | 45 (47) | 58 (45) | 27 (100) | 8 (35) |
| Female (n=136) | 50 (53) | 71 (55) | 0 | 15 (65) |
| Site of cancer | ||||
| Breast | 16 (16.84) | 36 (27.91) | 0 | 5 (21.74) |
| Colorectal | 30 (31.58) | 8 (6.20) | 0 | 0 |
| GI | 20 (21.05) | 3 (2.33) | 0 | 1 (4.35) |
| Leukemia | 0 | 13 (10.08) | 0 | 2 (8.7) |
| Lymphoma | 0 | 2 (1.55) | 0 | 2 (8.7) |
| Liver | 1 (1.05) | 10 (7.75) | 0 | 0 |
| Lung | 2 (2.11) | 12 (9.30) | 0 | 0 |
| Melanoma | 1 (1.05) | 7 (5.43) | 0 | 0 |
| Myeloma | 0 | 0 | 0 | 7 (30.43) |
| Pancreatic | 22 (23.16) | 1 (0.78) | 0 | 4 (17.39) |
| Prostate | 0 | 1 (0.78) | 26 (96.30) | 0 |
| Sarcoma | 1 (1.05) | 13 (10.08) | 0 | 1 (4.35) |
| Brain | 2 (2.11) | 0 | 0 | 0 |
| Renal | 0 | 23 (17.83) | 1 (3.70) | 1 (4.35) |
| Completed week 8 | 81 (85.26) | 110 (85.27) | 26 (96.30) | 19 (82.61) |
| Age at baseline, mean (SD) | 59.8 (12.5) | 60.33 (11.00) | 70 (8.50) | 63.08 (11.00) |
*Cytotoxics included capecitabine, temozolomide, tipiracil, trifluridine, †Kinase inhibitors included crizotinib, erlotinib, gefitinib, imatinib, lapatinib, pazopanib, regorafenib, sorafenib, sunitinib, ‡Sex hormones included enzalutamide, letrozole, and abiraterone acetate, §Other category: Everolimus, olaparib, vorinostat. SD: Standard deviation, GI: Gastrointestinal
Data regarding medications are for patients who completed 8 weeks of the study. Table 2 shows the medical record audit that the patients had on average of 3.2 (1.89)–3.85 (2.14) comorbid conditions, for which they were also taking medications. Patients were taking 10–12 medications for their comorbid conditions. The oral cancer medications per day, both at baseline and 8 weeks, varied from an average of 1 (1.21) to 4 pills, once daily. This was in addition to the medications for their comorbid conditions. Patients at baseline as well as throughout the 8 weeks experienced 3.7–5.8 symptoms.
Table 2.
Comorbidity, oral cancer medications dosing and symptom severity among patients completing 8 weeks
| Drug category | ||||
|---|---|---|---|---|
| Cytotoxics* (n=95), Mean (SD) | Kinase inhibitors† (n=129), Mean (SD) | Sex hormone inhibitors‡ (n=27), Mean (SD) | Other§ (n=23), Mean (SD) | |
| Number of medications for comorbid conditions | 11.98 (5.73) | 10.58 (5.39) | 11.46 (6.20) | 11.64 (5.96) |
| Number of comorbid conditions based on MRA medications baseline | 3.2 (1.89) | 3.46 (2.01) | 3.85 (2.14) | 3.26 (2.09) |
| Number oral agent pills per day at baseline | 2.79 (0.96) | 1.96 (1.21) | 4 (0) | 1.21 (1.04) |
| Number oral agent pills per day at week 8 | 2.72 (0.98) | 1.82 (1.17) | 4 (0) | 1.35 (1.22) |
| Number of times per day at baseline, oral agents | 1.96 (0.17) | 1.22 (0.43) | 1 (0) | 1.04 (0.20) |
| Number of times per day at week 8, oral agents | 1.98 (0.12) | 1.20 (0.40) | 1 (0) | 1.05 (0.24) |
| Summed symptom severity at baseline | 27.94 (22.57) | 24.33 (22.62) | 14.29 (16.46) | 26.26 (18.78) |
| Summed symptom severity at week 8 | 21.32 (12.72) | 18.85 (19.12) | 13.12 (13.31) | 16.41 (12.60) |
| Number of symptoms at baseline | 5.86 (3.40) | 5.52 (3.64) | 3.70 (3.03) | 5.30 (3.05) |
| Number of symptoms at week 8 | 5.27 (2.21) | 4.77 (3.39) | 4.12 (3.60) | 4.11 (2.20) |
*Cytotoxics included capecitabine, temozolomide, tipiracil, trifluridine, †Kinase inhibitors included crizotinib, erlotinib, gefitinib, imatinib, lapatinib, pazopanib, regorafenib, sorafenib, sunitinib, ‡Sex hormones included enzalutamide, letrozole, and abiraterone acetate, §Other category: Everolimus, olaparib, vorinostat. SD: Standard deviation, MRA: Magnetic resonance angiogram
Kinase inhibitors were the largest category of drugs in this study [Table 3]. Table 3 shows that the classification of oral agents the patient was on did not change over the study period even though there were many drug interruptions. Patients on these drugs had on average 3.46 comorbid conditions for which they took medications. Thus, looking at this category, one can see that patients might be on 10–12 medications/day. This however is complicated as medications for cancer treatment varied in frequency and days per month based on the protocol of the oral cancer medication. This adds to the patient's burden as they schedule, stop, start the oral cancer medication integrating them with their medications for their comorbid conditions.
Table 3.
Oral cancer medications prescribed, n=274 at baseline, n=236 at 8 weeks
| Oral cancer medications | Baseline, n (%) | 8 weeks, n (%) |
|---|---|---|
| Cytotoxics* | 95 (35) | 81 (34) |
| Kinase inhibitors† | 129 (47) | 110 (47) |
| Sex hormone inhibitors‡ | 27 (10) | 26 (11) |
| Other: IMIDs, PARP inhibitors, and mTOR inhibitors§ | 23 (8) | 19 (8) |
*Cytotoxics included capecitabine, temozolomide, tipiracil, trifluridine, †Kinase inhibitors included crizotinib, erlotinib, gefitinib, imatinib, lapatinib, pazopanib, regorafenib, sorafenib, sunitinib, ‡Sex hormones included enzalutamide, letrozolec and abiraterone acetate, §Other category: Everolimus, olaparib, vorinostat. PARP: Ribose polymerase, IMIDs: Immunomodulatory drugs, mTOR: Mammalian target of rapamycin
Table 4 summarizes interruptions in taking oral cancer medications based on patient self-report and medical record documentation.
Table 4.
Drug interruptions by drug category across the study period
| Drug category | ||||
|---|---|---|---|---|
| Cytotoxics* (n=95), n (%) | Kinase inhibitors† (n=129), n (%) | Sex hormone inhibitors‡ (n=27), n (%) | Other§ (n=23), n (%) | |
| Interruptions during 8 weeks | ||||
| Yes | 41 (57.00) | 50 (48.50) | 2 (8.70) | 10 (55.50) |
| No | 31 (43.00) | 53 (51.50) | 21 (91.30) | 8 (44.50) |
| Interruption at week 4 only | 10 (29.41) | 21 (61.76) | 0 | 3 (8.82) |
| Interruption at week 8 only | 24 (58.54) | 13 (31.71) | 2 (4.88) | 2 (4.88) |
| Interruption at both 4 and 8 weeks | 7 (25.00) | 16 (57.14) | 0 | 5 (17.86) |
*Cytotoxics included capecitabine, temozolomide, tipiracil, trifluridine, †Kinase inhibitors included crizotinib, erlotinib, gefitinib, imatinib, lapatinib, pazopanib, regorafenib, sorafenib, sunitinib, ‡Sex hormones included enzalutamide, letrozole, and abiraterone acetate, §Other category: Everolimus, olaparib, vorinostat
Except men taking sex hormones, nearly 50% of patients self-reported interruptions in taking their oral cancer medication. When interruptions happened, patients had to readjust their medication schedule for the interruption and then again when they resumed taking medications. Most of the interruptions for cytotoxics occurred after patients had been on the drug for more than 4 weeks (for gastrointestinal, colorectal, and pancreas cancers).
Based on self-report, patients on cytotoxics had most of the interruptions between 4 and 8 weeks of starting the medications, while kinase inhibitors had interruptions (62%) within the first 4 weeks, but 57% of the kinase inhibitors had interruptions throughout the entire 8 weeks follow-up period.
Thus, one can see many changes and much variability in what patients on oral agents experienced and how the actual oral agent medication demand has to be considered in the context of their comorbid conditions to adequately understand the full medication burden on the patient.
Discussion
There is a difference among the classifications of oral cancer medications in terms of the medication burden they produce and in the frequency and duration of the interruptions patients experience. If the cancer medications were interrupted along with a change in the medication (dose and or frequency) and a change in disease status, this increases the medication burden for the patient. Further, patients on kinase inhibitors report frequent side effects such as nausea and vomiting, diarrhea, rash, headache, heartburn, and fluid retention which may require supportive care drugs in addition to manage these symptoms. Side effects and symptoms might result in additional medications to manage the symptoms such as pain, nausea, or diarrhea further adding to their medication burden. Thus, patients must adjust their oral cancer medications in response to medication interruptions or reductions, continue with medications for their comorbid conditions, and manage symptoms and side effects all at a time when they may be more compromised due to progressing disease.
Research is needed to determine if and how patients prioritize which medications they continue to take and which they forego. Some patients in this study indicated they prioritized their medications and did not take cancer or comorbid drugs on the same day. Moreover, patterns that emerge as patients discount the value of some medications have not been studied. The cumulation of changes in medication dosage and increasing symptoms and side effects help sharpen the issues of medication treatment burden.
A large proportion of patients experienced drug interruptions, thus threatening the patients’ clinical outcomes related to the disease and treatment. Consequently, frequent changes in dosing and interruptions produce significant increases in medication burden which are problematic for patients. For those with multi-morbidity, there will be not only consequences for cancer and cancer treatment but also consequences for each of the chronic diseases (adherence with medications, recurrence, exacerbation, and/or progression of the disease). Thus, we need to carefully access the burden of treatment for each patient and individualize a plan of care that considers simplifying their medication regimens. It will be essential to use clinical practice guidelines for patient instruction and guidance. Such guidelines are necessary to ease the burden, promote adherence, and enable patients to stay on their medication so that they have a chance for a therapeutic effect.
Limitations
These data presented here are from patient interviews and medical record documentation from studies completed in the United States of America. Issues related to medication cost coverage, insurance, medical, financial assistance, and co-payments will be different in other countries.
We have not investigated the discrepancies between the two sources (patient reports and medical records) for the purpose of this manuscript. Some patients were receiving IV chemotherapy in addition to the oral cancer medications which would be an additional burden as patients would need to travel to clinics for that. We review key factors of patient medication burden, but we are aware that this probably underreports the total medication burdens that patients’ experience.
Implications for Practice
Nurses need to assess the medication burden and challenges that patients on oral agents might experience. They need to know the demands and regimens of the other chronic illnesses that the patient has as well and consider how to assist the patient. What is the patient's capacity to manage the medication burden they experience? It is important to implement the education and the guidelines needed to manage the medication adherence based on the protocol details and then help the patient deal with the side effects. Strategies to simplify the regimen, increase adherence, and manage symptom should be considered. Patients need information and guidance to be able to plan and integrate their medications into their daily lives. Nurses need help reduce the medication burden. This must be a component of the teaching for all patients on oral agents and their families.
Implications for Research
A more comprehensive detailed approach to include specific comorbidities and all medications for patients on oral agents is needed. All dose changes over several months need to be determined as oral agents bring new challenges to determining adherence rates in patients. Interventions need to be tested to see if they can be effective at reducing or eliminating overall medication burden and if reducing medication burden can result in improved patient quality of life.
Financial support and sponsorship
This work was supported by the National Cancer Institute, an institute of the National Institutes of Health (Grant No. 1R01CA162401-01A1. 2013-2017).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Eton DT, Ramalho de Oliveira D, Egginton JS, Ridgeway JL, Odell L, May CR, et al. Building a measurement framework of burden of treatment in complex patients with chronic conditions: A qualitative study. Patient Relat Outcome Meas. 2012;3:39–49. doi: 10.2147/PROM.S34681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eton DT, Ridgeway JL, Egginton JS, Tiedje K, Linzer M, Boehm DH, et al. Finalizing a measurement framework for the burden of treatment in complex patients with chronic conditions. Patient Relat Outcome Meas. 2015;6:117–26. doi: 10.2147/PROM.S78955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallacher K, May CR, Montori VM, Mair FS. Understanding patients’ experiences of treatment burden in chronic heart failure using normalization process theory. Ann Fam Med. 2011;9:235–43. doi: 10.1370/afm.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sav A, Kendall E, McMillan SS, Kelly F, Whitty JA, King MA, et al. ’You say treatment, I say hard work’: Treatment burden among people with chronic illness and their carers in Australia. Health Soc Care Community. 2013;21:665–74. doi: 10.1111/hsc.12052. [DOI] [PubMed] [Google Scholar]
- 5.Sav A, King MA, Whitty JA, Kendall E, McMillan SS, Kelly F, et al. Burden of treatment for chronic illness: A concept analysis and review of the literature. Health Expect. 2015;18:312–24. doi: 10.1111/hex.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tran VT, Montori VM, Eton DT, Baruch D, Falissard B, Rvaud P. Development and description of measurement properties of an instrument to assess treatment burden among patients with multiple chronic conditions. BMC Med. 2012;10:68. doi: 10.1186/1741-7015-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran VT, Harrington M, Montori VM, Barnes C, Wicks P, Ravaud P. Adaptation and validation of the Treatment Burden Questionnaire (TBQ) in English using an internet platform. BMC Med. 2014;12:109. doi: 10.1186/1741-7015-12-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran VT, Barnes C, Montori VM, Falissard B, Ravaud P. Taxonomy of the burden of treatment: A multi-country web-based qualitative study of patients with chronic conditions. BMC Med. 2015;14(13):115. doi: 10.1186/s12916-015-0356-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brod M, Hammer M, Christensen T, Lessard S, Bushnell DM. Understanding and assessing the impact of treatment in diabetes: The treatment-related impact measures for diabetes and devices (TRIM-Diabetes and TRIM-Diabetes Device) Health Qual Life Outcomes. 2009;7:83. doi: 10.1186/1477-7525-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eton DT, Elraiyah TA, Yost KJ, Ridgeway JL, Johnson A, Egginton JS, et al. A systematic review of patient-reported measures of burden of treatment in three chronic diseases. Patient Relat Outcome Meas. 2013;4:7–20. doi: 10.2147/PROM.S44694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vijan S, Hayward RA, Ronis DL, Hofer TP. Brief report: The burden of diabetes therapy: Implications for the design of effective patient-centered treatment regimens. J Gen Intern Med. 2005;20:479–82. doi: 10.1111/j.1525-1497.2005.0117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernhard J, Maibach R, Thürlimann B, Sessa C, Aapro MS. Swiss Group for Clinical Cancer Research. Patients’ estimation of overall treatment burden: Why not ask the obvious? J Clin Oncol. 2002;20:65–72. doi: 10.1200/JCO.2002.20.1.65. [DOI] [PubMed] [Google Scholar]
- 13.Moss L, Crane PB. Exploring polypharmacy in elderly women after myocardial infarction. J Women Aging. 2010;22:22–33. doi: 10.1080/08952840903488948. [DOI] [PubMed] [Google Scholar]
- 14.Bayliss EA, Steiner JF, Fernald DH, Crane LA, Main DS. Descriptions of barriers to self-care by persons with comorbid chronic diseases. Ann Fam Med. 2003;1:15–21. doi: 10.1370/afm.4. [DOI] [PMC free article] [PubMed] [Google Scholar]


