Abstract
Previous studies have demonstrated the ability of bone morphogenetic proteins (BMPs) to promote chondrogenic differentiation in vitro. However, the in vivo role of BMP signaling during chondrogenesis has been unclear. We report here that BMP signaling is essential for multiple aspects of early chondrogenesis. Whereas mice deficient in type 1 receptors Bmpr1a or Bmpr1b in cartilage are able to form intact cartilaginous elements, double mutants develop a severe generalized chondrodysplasia. The majority of skeletal elements that form through endochondral ossification are absent, and the ones that form are rudimentary. The few cartilage condensations that form in double mutants are delayed in the prechondrocytic state and never form an organized growth plate. The reduced size of mutant condensations results from increased apoptosis and decreased proliferation. Moreover, the expression of cartilage-specific extracellular matrix proteins is severely reduced in mutant elements. We demonstrate that this defect in chondrocytic differentiation can be attributed to lack of Sox9, L-Sox5, and Sox6 expression in precartilaginous condensations in double mutants. In summary, our study demonstrates that BMPR1A and BMPR1B are functionally redundant during early chondrogenesis and that BMP signaling is required for chondrocyte proliferation, survival, and differentiation in vivo.
Keywords: bone morphogenetic protein, cartilage, endochondral ossification, Sox proteins, skeletal development
During endochondral ossification, mesenchymal cells condense and differentiate into chondrocytes. The chondrocytes undergo a highly organized differentiation program, forming the template for bone formation (1, 2). Bone morphogenetic proteins (BMPs) were identified by their ability to promote ectopic cartilage and bone formation (3). BMPs are members of the TGFβ superfamily and transduce signals by binding to heteromeric complexes of type 1 and type 2 serine/threonine kinase receptors. The binding of BMPs to the receptor complex results in the phosphorylation of intracellular Smads, which then translocate to the nucleus, where they regulate transcription (4). The differential affinities of distinct BMP ligands for the three type 1 receptors, BMPR1A (BMP receptor type 1A), BMPR1B (BMP receptor type 1B), and ActR1 (activin receptor type 1), are thought to contribute to the diversity of actions during development. Several lines of evidence suggest that Bmpr1a, Bmpr1b, and ActR1 have distinct roles during chondrogenesis. In the chick, Bmpr1a is expressed at low levels throughout the limb bud mesenchyme, whereas Bmpr1b is expressed in precartilaginous condensations, suggesting that these receptors have different roles in chondrogenesis. Furthermore, whereas constitutively active forms of either BMPR1A or BMPR1B promote chondrogenesis, only the overexpression of dominant negative (DN)-BMPR1B, and not DN-BMPR1A or DN-ActR1, blocks these events, suggesting that BMPR1B is the major transducer of BMP signals in limb condensations (5–8). However, Bmpr1b null mice exhibit a restricted skeletal phenotype with defects confined to phalangeal elements, suggesting either overlapping functions or a more prominent role for BMPR1A during chondrogenesis in mice (9, 10).
We generated mice that are null for both Bmpr1a and Bmpr1b in chondrocytes to examine the possibility of functional redundancy and/or unique functions for Bmpr1a and Bmpr1b and to clarify the role of BMPs during chondrogenesis. We show that like Bmpr1b null mice, Bmpr1a conditional knockouts (CKOs) have few skeletal defects. However, Bmpr1aCKO; Bmpr1b–/– mice develop a severe and generalized chondrodysplasia, demonstrating overlapping functions for BMPR1A and BMPR1B during early chondrogenesis. Furthermore, our analysis reveals that BMP signaling is required for chondrocyte proliferation, survival, and differentiation. These results provide a genetic in vivo demonstration of the essential role of BMPs during cartilage development.
Materials and Methods
Generation of Bmpr1aCKO; Bmpr1b–/– Mice. Generation of Bmpr1b-deficient, Bmpr1a floxed, and Col2-Cre mice was as described in refs. 10 through 12. To generate cartilage-specific Bmpr1a null mice, Bmpr1a floxed mice were crossed with Col2-Cre mice. Bmpr1a fx/+; Bmpr1b+/–; Col2-Cre mice were then intercrossed to generate Bmpr1a fx/fx; Bmpr1b–/–; Col2-Cre (Bmpr1aCKO; Bmpr1b–/–) mice.
Skeletal Preparation and Histology. Skeletal preparations were performed as described in ref. 13. For histology, embryos were fixed in 4% paraformaldehyde, decalcified, and embedded in paraffin. Sections were stained with alcian blue and counter-stained with nuclear fast red by using standard protocols (14).
Immunohistochemistry (IHC) and in Situ Hybridization. For IHC, sections were microwaved in citrate buffer or pretreated with chondroitinase ABC (Sigma) or hyaluronidase (Calbiochem) for antigen demasking. Sections were blocked with 5% goat serum, incubated with primary antibody (BMPR1A and BMPR1B from Orbigen, San Diego; type 2 collagen from Research Diagnostics, Flanders, NJ; aggrecan and link protein from Developmental Studies Hybridoma Bank, Iowa City, IA; phospo-Smad1, 5, and 8 from Cell Signaling Technology, Beverly, MA) overnight at 4°C, and then incubated with the appropriate secondary antibody at room temperature. Color was developed with diaminobenzidine or with the Zymed kit chromogen, and sections were counterstained with hematoxylin.
For in situ hybridization, digoxygenin-labeled probes were generated as described in ref. 13. Sections were pretreated with proteinase K and hybridized with the appropriate probe. Slides were washed in 50% formamide/2× SSC and treated with RnaseA. Color was detected by using BM purple (Roche).
Cell Proliferation and TUNEL Labeling. To measure proliferation, IHC for proliferating cell nuclear antigen (PCNA) was performed. Anti-PCNA antibody (Zymed) was used as described above for IHC. For TUNEL labeling, the fluorescein In Situ Cell Death Detection Kit (Roche) was used according to the manufacturer's instructions.
Results
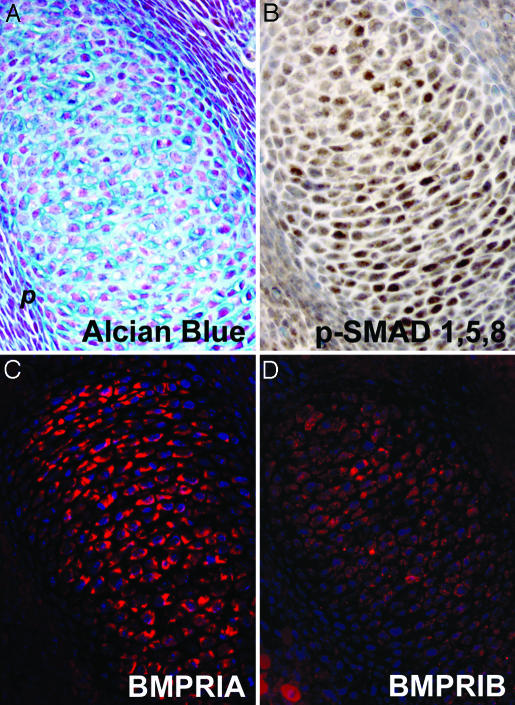
BMPR1A and BMPR1B Are Expressed During Early Chondrogenesis. IHC was performed to examine the expression pattern of BMPR1A and BMPR1B in mice during early chondrogenesis. By embyronic day (E)12.5, cells in chondrocyte condensations are enlarged and produce cartilage-specific proteoglycans, as shown by alcian blue staining (Fig. 1A). Similar to its expression pattern in the chick (5, 6), BMPR1B is expressed in these condensations (Fig. 1D). However, unlike the broad mesenchymal distribution reported in the chick, BMPR1A is highly expressed in chondrocyte condensations in mice (Fig. 1C). The higher intensity of BMPR1A immunostaining compared with that for BMPR1B raises the possibility that Bmpr1a is more highly expressed than Bmpr1b in early cartilage condensations. However, differential affinities of the antibodies cannot be ruled out. Interestingly, both BMPR1A and BMPR1B are absent in the surrounding perichondrium, suggesting that BMP signaling is not active in this tissue. IHC to detect phosphorylated forms of BMP-specific Smads confirmed that BMP signaling pathways are active in chondrocyte condensations but not in the perichondrium (Fig. 1B). Therefore, BMPR1A and BMPR1B are coexpressed in a pattern consistent with the possibility of redundant roles during early chondrogenesis in mice.
Fig. 1.
Expression of BMPR1A and BMPR1B in E12.5 cartilage condensations. (A) Alcian blue staining of E12.5 cartilage condensations. (B) IHC of phosphorylated Smad1, 5, and 8 showing active BMP signaling in cartilage condensations but not in surrounding perichondrium. (C and D) IHC of BMPR1A (C) and BMPR1B (D) showing overlapping expression patterns of the two type 1 receptors in cartilage condensations. p, perichondrium.
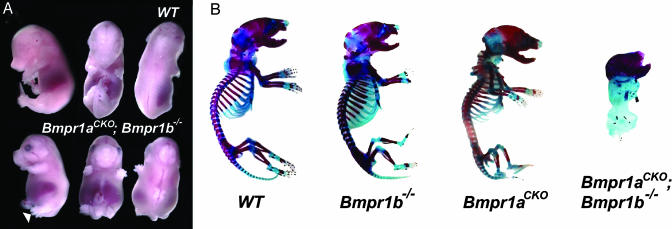
BMPR1A and BMPR1B Are Functionally Redundant and Are Essential for Endochondral Ossification. Because Bmpr1a-deficient mice die during gastrulation (15), mice carrying a Bmpr1a floxed allele were crossed with Col2-Cre transgenic mice to inactivate BMPR1A in chondrocytes, generating Bmpr1a fx/fx; Col2-Cre (Bmpr1ACKO) mice. In contrast to Bmpr1b–/– mice, where defects are primarily restricted to phalangeal elements and appendicular joints (9, 10), Bmpr1aCKO mice exhibit generalized chondrodysplasia (Fig. 2). In Bmpr1aCKO mice, long bones are shortened, ossification is delayed, and the rib cage is smaller and flattened, leading to respiratory distress and subsequent postnatal death. Because loss of neither Bmpr1a nor Bmpr1b led to major alterations in chondrogenesis, Bmpr1aCKO; Bmpr1b–/– embryos were generated to investigate the possibility of functional redundancy. These embryos are recovered at expected ratios up until E16.5 (data not shown), but, starting from E13.5, double conditional null embryos are reduced in size and develop shortened limbs, tails, and snouts. All five digits appear to be patterned correctly, suggesting that BMP signaling within the chondrogenic condensations is not required for specification (Fig. 2 A). Skeletal preparations of Bmpr1aCKO; Bmpr1b–/– embryos confirm a severe chondrodysplasia. The majority of skeletal elements that form through endochondral ossification, and hence express the Col2-Cre transgene, are absent, and the elements that do form are rudimentary and malformed. The clavicles and the craniofacial bones, which form through cartilage-independent intramembranous ossification, are present and stain strongly for alizarin red (Fig. 2B). The profound disturbance in skeletal development results in lethality between E17.5 and birth, due to compression of internal organs and eventual heart failure. This severe chondrodysplasia clearly demonstrates that BMP signaling is essential and that Bmpr1a and Bmpr1b are functionally redundant at early stages of chondrogenesis in the mouse.
Fig. 2.
BMPR1A and BMPR1B are functionally redundant during chondrogenesis. (A) Gross appearance of E14.5 WT and Bmpr1aCKO; Bmpr1b–/– embryos. Mutant embryos have widened and flattened thoracic cavities, shortened limbs, and exposed neural tissue but have correctly patterned digits (arrow).(B) Skeletal preparations of postnatal day 0 embryos. Bmpr1aCKO mice have a mild generalized chondrodysplasia, which leads to a smaller thoracic cavity and shortening of long bones. In contrast, skeletal elements that form through endochondral ossification are completely absent or rudimentary in Bmpr1aCKO; Bmpr1b–/– mice.
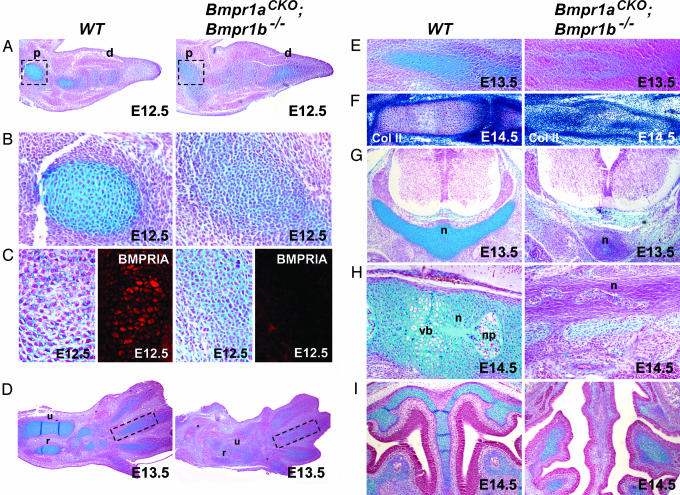
Impaired Chondrocyte Differentiation in Bmpr1aCKO; Bmpr1b–/– Mice. At E12.5, the most distal condensations of WT forelimbs stain lightly for alcian blue and are composed of small and tightly packed prechondocytes. More proximal, and hence more mature, condensations stain intensely for alcian blue and contain larger, cobblestone-like, overtly differentiated chondrocytes (Fig. 3 A and B). Within the limb, the Col2-Cre transgene is first expressed in condensed prechondrocytes (12). The ability of Col2-Cre to induce excision of the floxed allele of Bmpr1a in prechondrocytes was examined by IHC for BMPR1A. This analysis revealed that the Col2-Cre transgene mediated excision in essentially all condensed prechondrocytes in appendicular elements (Fig. 3C). In forelimbs of Bmpr1aCKO; Bmpr1b–/– mice, both proximal and distal condensations are composed of less mature cells, exhibiting characteristics seen in prechondrocytes at the onset of Col2-Cre expression (Fig. 3 A and B). Thus, condensations from double mutants are able to differentiate into prechondrocytes, but upon activation of the Col2-Cre transgene, these cells fail to differentiate further. By E13.5, cartilaginous condensations in WT forelimbs have elongated to form the radius and ulna. However, in mutant forelimbs, the radius and ulna are significantly smaller, and cells remain prechondrocytic (Fig. 3D). This impairment in differentiation is more severe in digits. In E13.5 mutant digits, shells of prechondrocytes enclose cells that do not stain for alcian blue (3E). By E14.5, in contrast to the fully differentiated and segmented digits of WT mice, digits of mutant embryos have fully formed perichondria, but cells within the cores do not exhibit characteristics of prechondrocytes (Fig. 3F). This phenotype is unlikely to be a reflection of differential levels of expression of the Col2-Cre transgene in peripheral vs. internal cells within condensations, because it also arises in the most distal phalanges of Bmpr1b null mice (9, 10).
Fig. 3.
Histological analysis of Bmpr1aCKO; Bmpr1b–/– mice. (A and B) Alcian blue staining of cartilage condensations in E12.5 limb buds. Images in B are magnifications of the boxed region in A. In the WT limb, cells in the most proximal element have differentiated into overt chondrocytes; cells in the distal elements are prechondrocytic. In the mutant limb, all condensations are delayed in a prechondrocytic state. (C) IHC of BMPR1A at E12.5 demonstrating that BMPR1A is not expressed in mutant condensations. (D) Alcian blue staining of E13.5 limbs. Condensations in mutant limbs are reduced and remain in a prechondrocytic state. (E) Magnification of boxed areas in D. In contrast to the fully differentiated WT digits, shells of prechondrocytes surround cells that do not stain for alcian blue in mutants. (F) IHC of type 2 collagen (brown) counterstained with hematoxylin (blue) of E14.5 digits. A dense perichondrium is present, but core cells do not differentiate into chondrocytes. (G and H) Alcian blue staining of thoracic vertebral bodies in E13.5 (G) and E14.5 (H) littermates. (I) Alcian blue staining of neural crest-derived nasal cavity in E14.5 embryos. p, proximal; d, distal; u, ulna; r, radius; n, notochord; vb, vertebral body; np, nucleus pulposi.
Col2-Cre is expressed in the sclerotome compartment of the somite (12), ablating Bmpr1a before condensation in vertebral bodies. Consequently, vertebral elements in double mutants are more severely impaired than limb elements. By E13.5, no vertebrae have formed in Bmpr1aCKO; Bmpr1b–/– embryos (Fig. 3G). Midsagittal sections of E14.5 WT embryos reveal a vertebral column containing nuclei pulposi, vertebral bodies, and a regressing notochord. In contrast, the vertebral column is completely absent in double mutants, and a disorganized notochord is embedded within a layer of dense fibroblasts (Fig. 3H). The development of craniofacial endochondral elements is also impaired in Bmpr1aCKO; Bmpr1b–/– embryos. As in the limb bud, condensations within the nasal cavity are delayed in a prechondrocytic state (Fig. 3I). In summary, histological analysis of Bmpr1aCKO; Bmpr1b–/– embryos reveals an essential role for BMP signaling in both condensation and differentiation of chondrocytes.
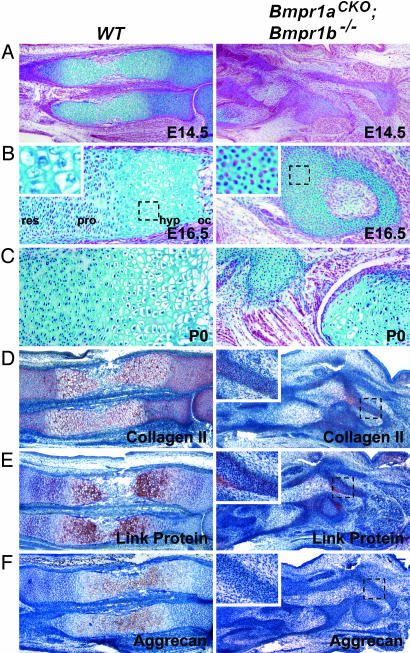
Cartilage Elements of Bmpr1aCKO; Bmpr1b–/– Are Severely Disorganized and Do Not Produce a Cartilage-Specific Extracellular Matrix (ECM). During later stages of endochondral ossification, chondrocytes undergo a highly organized program of differentiation to form a growth plate. Chondrocytes flatten and proliferate, resulting in formation of chondrocyte columns. These cells then exit the cell cycle and differentiate into prehypertrophic chondrocytes, which in turn terminally differentiate to form the hypertrophic zone. Cells in the hypertrophic zone enlarge and undergo apoptosis, leaving an environment that promotes the invasion of blood vessels and osteoblasts (1, 2). In E14.5 WT embryos, an organized growth plate is present containing resting, proliferative, prehypertrophic, and hypertrophic chondrocytes. In contrast, in Bmpr1aCKO; Bmpr1b–/– mice, appendicular elements are underdeveloped because most cells remain prechondrocytic (Fig. 4A). By E16.5, the growth plates of the radius and ulna are undergoing ossification. In mutants, cells featuring a chondrocytic morphology are finally present in the core regions of disorganized skeletal elements. However, these elements do not display any characteristics of a growth plate. The cell morphology and lack of type X collagen expression, a hypertrophic specific ECM protein, demonstrate that these elements do not contain prehypertrophic or hypertrophic cells (Fig. 4B and data not shown). In postnatal day 0 Bmpr1aCKO; Bmpr1b–/– embryos, the overall size of the cartilaginous elements is no different from that at E16.5, but chondrocytes have undergone random and disorganized hypertrophy (Fig. 4C). Therefore, although cells in prechondrocytic condensations eventually differentiate in double mutants, they do not undergo the organized differentiation program found in the growth plate.
Fig. 4.
Impaired chondrogenesis and ECM expression in mutants. (A–C) Alcian blue staining of E14.5 (A), E16.5 (B), and postnatal day 0 (C) growth plates. In WT embryos, a growth plate has formed, composed of resting, proliferative, and hypertrophic zones. Mutants do not form an organized growth plate. Chondrocytes undergo random hypertrophy in mutants. (D–F) IHC for type 2 collagen (D), link protein (E), and aggrecan (F). Expression of ECM proteins is significantly reduced in mutant mice. Interestingly, some ECM production does take place at the peripheries of mutant condensations (Insets). res, resting zone; pro, proliferative zone; hyp, hypertrophic zone; oc, ossification center.
As prechondrocytes differentiate into chondrocytes, they up-regulate the production of cartilage ECM proteins. BMPs promote the production of these ECM proteins in vitro (16–18). To address the importance of BMP signaling in cartilage ECM production in vivo, we examined the production of three major cartilage matrix components: type 2 collagen, link protein, and aggrecan. The expression of all three proteins is significantly reduced in E14.5 Bmpr1aCKO; Bmpr1b–/– mice in comparison with their WT littermates (Fig. 4 D–F). Furthermore, areas of matrix expression in mutants are limited to regions at the peripheries of cartilaginous elements (Fig. 4 D–F Insets). Thus, analysis of Bmpr1aCKO; Bmpr1b–/– embryos reveals an essential role of BMP signaling in the production of cartilage ECM proteins in vivo.
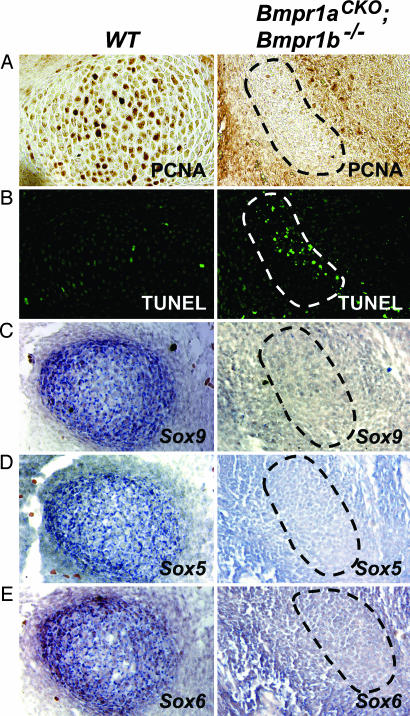
Cartilage Elements in Bmpr1aCKO; Bmpr1b–/– Embryos Have Reduced Rates of Proliferation and Cell Survival. The reduced sizes of Bmpr1aCKO; Bmpr1b–/– cartilaginous elements throughout development suggest that BMP signaling is essential for chondrocyte proliferation and/or survival. Staining for PCNA demonstrated significantly reduced proliferation in mutant condensations from E13.5 to E16.5 (Fig. 5A and data not shown). The reduced proliferation rate observed in E16.5 mutant mice, in which chondrocytes have differentiated (Fig. 4B), demonstrates that proliferation is not inhibited, because cells are delayed in the prechondrocytic state. TUNEL staining was performed to determine whether altered rates of apoptosis also contribute to the reduction and deformation of cartilaginous elements in mutant mice. As expected, very few cells undergo apoptosis in WT condensations. However, significant numbers of apoptotic cells are present in prechondrocytic condensations in mutants (Fig. 5B). Therefore, in addition to defects in differentiation, skeletal elements in Bmpr1aCKO; Bmpr1b–/– embryos exhibit impaired proliferation and survival during early chondrogenesis.
Fig. 5.
Proliferation, apoptosis, and expression of Sox family proteins in Bmpr1aCKO; Bmpr1b–/– mice. (A) PCNA labeling of E12.5 condensations showing essentially no proliferation in mutants. (B) TUNEL labeling reveals an increase in apoptosis in mutant condensations. (C–E) In situ hybridizations for Sox9 (C), L-Sox5 (D), and Sox6 (E) show that expression is significantly reduced in Bmpr1aCKO; Bmpr1b–/– condensations. All images are from sections adjacent to those shown in Fig. 3B.
Loss of Sox9, L-Sox5, and Sox6 Expression in Bmpr1aCKO; Bmpr1b–/– Cartilage. The transcription factor Sox9 has an essential role in commitment to and maintenance of early chondrogenic differentiation and is the earliest known marker of cells committed to the chondrogenic fate. L-Sox5 and Sox6 are downstream targets of Sox9 that are expressed in chondrocytic condensations, and deficiency of Sox9 or both L-Sox5 and Sox6 results in severe chondrodysplasia (19–22). In vitro studies have shown that BMP signaling can regulate Sox protein expression in mesenchymal cell types (23–26). Therefore, we examined whether impaired chondrocyte differentiation in Bmpr1aCKO; Bmpr1b–/– mice was the result of reduced Sox9, L-Sox5, and Sox6 expression. All three Sox proteins are expressed in prechondrocytic and chondrocytic condensations in WT embryos at E12.5, as previously reported (19). In contrast, expression of Sox9, L-Sox5, and Sox6 is undetectable in prechondrocytic condensations in Bmpr1aCKO; Bmpr1b–/– mice (Fig. 5 C–E). Thus, these results demonstrate an essential role for BMP signaling in maintenance of Sox protein expression in vivo, and impaired differentiation of mutant cartilaginous elements can be attributed to loss of Sox9, L-Sox5, and Sox6 expression.
Discussion
Essential Overlapping Functions of BMPR1A and BMPR1B. The differential affinities of distinct BMP ligands for the three type 1 receptors, BMPR1A, BMPR1B, and ActR1, are thought to contribute to the diversity of BMP actions during development. For instance, in vitro reporter assays demonstrate that BMPR1B is more effective than BMPR1A in transducing GDF5 signals (27). Moreover, the different effects on cartilage nodule formation observed after DN-BMPR1A and DN-BMPR1B overexpression in the chick suggest that these receptors have different quantitative and/or qualitative signaling abilities in early chondrogenic differentiation (5, 6). However, other studies raise the possibility of functional redundancy. For example, BMPR1A, BMPR1B, and ActR1 activate the same downstream signaling components, Smad1, 5, and 8 (28, 29). Moreover, the mild skeletal phenotype in Bmpr1b null mice contrasts the potent inhibitory effects of DN-BMPR1B in the chick. Our analysis of Bmpr1aCKO and Bmpr1aCKO; Bmpr1b–/– mice confirms that BMPR1A and BMPR1B are functionally redundant during chondrogenic differentiation in mammals. Bmpr1b–/– mice are viable, and defects in chondrogenesis are primarily restricted to distal phalanges (9, 10). Bmpr1aCKO mice exhibit a generalized skeletal dysplasia. In contrast, in double conditional nulls, all bones that form through endochondral ossification are either absent or malformed, and differentiation of prechondrocytes into chondrocytes is severely impaired. The overlapping functions of BMPR1A and BMPR1B in early chondrogenesis are consistent with the overlapping expression patterns of these receptors in early condensations and with the activation of intracellular BMP Smads at this stage. The more predominant role for Bmpr1b in the chick than in the mouse may reflect a difference in relative levels of expression of Bmpr1a and Bmpr1b between birds and mammals. Interestingly, in E16.5 mutant condensations, although BMPR1A and BMPR1B are absent (Fig. 3C), some cells are able to differentiate into chondrocytes. One possible explanation is that ActR1 is able to transduce enough BMP signaling to allow these cells to differentiate. The role of ActR1 during chondrogenesis is unclear. CA-ActR1 can synergize with either BMPR1A or BMPR1B to promote chondrogenesis, but, acting alone, ActR1 is a weak inducer of chondrogenesis and in some cases inhibits differentiation (30, 31). Further studies are needed to clarify the role ActR1 plays in cartilage and to determine whether it has overlapping roles with BMPR1A and BMPR1B. Although BMPR1A and BMPR1B have largely overlapping functions, the distinct skeletal phenotypes of Bmpr1aCKO and Bmpr1b–/– mice also provide evidence for nonoverlapping functions. BMPR1B plays an essential role in the development of the distal phalanges (9, 10). Similarly, the shortened long bones of Bmpr1aCKO mice suggest a distinct role for BMPR1A at later stages of development, possibly in the growth plate. In conclusion, our analysis reveals that BMPR1A and BMPR1B are functionally redundant and essential transducers of BMP signaling during early chondrogenic differentiation.
BMPs Are Essential for Cartilage Development. Various studies have demonstrated the ability of BMP signaling to promote chondrogenic differentiation in vitro (32, 33). However, the in vivo role of BMP signaling in early stages of chondrogenesis has not been described previously in genetic models. Our analysis demonstrates a requirement for BMP signaling in three phases of early chondrogenesis: proliferation, cell survival, and differentiation. First, the absence of PCNA staining in mutant condensations demonstrates the necessity of BMP signaling for chondrocyte proliferation. Second, the increase in TUNEL labeling in mutant mice confirms a requirement for BMP signaling in chondrocyte survival. Finally, we show that impaired BMP signaling inhibits the differentiation of prechondrocytes into overt chondrocytes. The effects of BMP signaling on chondrogenic differentiation are mediated through Sox9, L-Sox5, and Sox6 regulation. However, the more severe phenotype observed in Bmpr1aCKO; Bmpr1b–/– mice compared with the cartilage-specific Sox9 null mice and the L-Sox5 and Sox6 double null mice (19–22) suggests that BMP signaling regulates chondrogenesis through both Sox-dependent and Sox-independent mechanisms.
Our analysis of Bmpr1aCKO; Bmpr1b–/– mice may suggest different requirements for BMPR1A and BMPR1B and possibly for BMP signaling in different regions of cartilaginous elements. For example, alcian blue staining of digits and IHC for ECM proteins in double mutants reveal that peripheral chondrogenesis in digits is affected to a lesser extent than is chondrogenesis in the cores of developing skeletal elements. Similarly, cells at the peripheries of other appendicular elements in double mutants are able to express cartilage-specific ECM proteins, such as type 2 collagen and link protein, whereas cells in the core cannot. In situ hybridization studies have shown that many BMPs are expressed in the perichondrium of developing skeletal elements (34). It is thus conceivable that chondrocytes closest to this concentrated source of BMPs can undergo limited chondrogenic differentiation, perhaps through ActR1. Alternatively, cells at the periphery may be responding to other chondrogenic signals. For example, FGFs and their receptors are expressed in the perichondrium, and FGF signaling pathways are important regulators of chondrogenesis (35). Understanding how loss of BMP signaling impacts other regulatory pathways in early condensations is an important area for future studies.
Acknowledgments
We thank Dr. Véronique Lefebvre (Cleveland Clinic Foundation, Cleveland) for the kind gifts of plasmids. This work was supported by National Institutes of Health Grants AR44528 (to K.M.L.) and AR42919 (to R.R.B.) and Vascular Biology Training Grant NHLBI-T32-69766 (to B.S.Y.).
Author contributions: B.S.Y. and K.M.L. designed research; B.S.Y. and I.Y. performed research; D.A.O., Y.M., and R.R.B. contributed new reagents/analytic tools; B.S.Y. and K.M.L. analyzed data; and B.S.Y. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: BMP, bone morphogenetic protein; BMPR1A, BMP receptor type 1A; BMPR1B, BMP receptor type 1B; ActR1, activin receptor type 1; ECM, extracellular matrix; DN, dominant negative; CKO, conditional knockout; IHC, immunohistochemistry; PCNA, proliferating cell nuclear antigen; En, embyronic day n.
References
- 1.Kronenberg, H. M. (2003) Nature 423, 332–336. [DOI] [PubMed] [Google Scholar]
- 2.Olsen, B. R., Reginato, A. M. & Wang, W. (2000) Annu. Rev. Cell Dev. Biol. 16, 191–220. [DOI] [PubMed] [Google Scholar]
- 3.Wozney, J. M. (1989) Prog. Growth Factor Res. 1, 267–280. [DOI] [PubMed] [Google Scholar]
- 4.Shi, Y. & Massague, J. (2003) Cell 113, 685–700. [DOI] [PubMed] [Google Scholar]
- 5.Zou, H., Wieser, R., Massagué, J. & Niswander, L. (1997) Genes Dev. 11, 2191–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawakami, Y., Ishikawa, T., Shimabara, M., Tanda, N., Enomoto-Iwamoto, M., Iwamoto, M., Kuwana, T., Ueki, A., Noji, S. & Nohno, T. (1996) Development (Cambridge, U.K.) 122, 3557–3566. [DOI] [PubMed] [Google Scholar]
- 7.Enomoto-Iwamoto, M., Iwamoto, M., Mukudai, Y., Kawakami, Y., Nohno, T., Higuchi, Y., Takemoto, S., Ohuchi, H., Noji, S. & Kurisu, K. (1998) J. Cell. Biol. 140, 409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujii, M., Takeda, K., Inamura, T., Aoki, H., Sampath, T. K., Enomoto, S., Kawabata, M., Kato, M., Ichijo, H. & Miyazono, K. (1999) Mol. Biol. Cell 10, 3801–3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baur, S. T., Mai, T. T. & Dymecki, S. M. (2000) Development (Cambridge, U.K.) 127, 605–619. [DOI] [PubMed] [Google Scholar]
- 10.Yi, S. E., Daluiski, A., Pederson, R., Rosen, V. & Lyons, K. M. (2000) Development (Cambridge, U.K.) 127, 627–630. [DOI] [PubMed] [Google Scholar]
- 11.Mishina, Y., Hanks, M. C., Miura, S., Tallquist, M. D. & Behringer, R. R. (2002) Genesis 32, 69–72. [DOI] [PubMed] [Google Scholar]
- 12.Ovchinnikov, D. A., Deng, J. M., Ogunrinu, G. & Behringer, R. R. (2000) Genesis 26, 145–146. [PubMed] [Google Scholar]
- 13.Ivkovic, S., Yoon, B. S., Popoff, S. N., Safadi, F. F., Libuda, D. E., Stephenson, R. C., Daluiski, A. & Lyons, K. M. (2003) Development (Cambridge, U.K.) 130, 2779–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luna, L. G. (1992) Histopathologic Methods and Color Atlas of Special Stains and Tissue Artifacts (American Histolabs, Gaithersburg, MD).
- 15.Mishina, Y., Suzuki, A., Ueno, N. & Behringer, R. R. (1995) Genes Dev. 9, 3027–3037. [DOI] [PubMed] [Google Scholar]
- 16.Denker, A. E., Haas, A. R., Nicoll, S. B. & Tuan, R. S. (1999) Differentiation 64, 67–76. [DOI] [PubMed] [Google Scholar]
- 17.Haas, A. R. & Tuan, R. S. (1999) Differentiation 64, 77–89. [DOI] [PubMed] [Google Scholar]
- 18.Ju, W., Hoffmann, A., Verschueren, K., Tylzanowski, P., Kaps, C., Gross, G. & Huylebroeck, D. (2000) J. Bone Miner. Res. 15, 1889–1899. [DOI] [PubMed] [Google Scholar]
- 19.Smits, P., Dy, P., Mitra, S. & Lefebvre, V. (2001) Dev. Cell 1, 277–290. [DOI] [PubMed] [Google Scholar]
- 20.Bi, W., Deng, J. M., Zhang, Z., Behringer, R. R. & de Crombrugghe, B. (1999) Nat. Genet. 22, 85–89. [DOI] [PubMed] [Google Scholar]
- 21.Bi, W., Huang, W., Whitworth, D. J., Deng, J. M., Zhang, Z., Behringer. R. R. & de Crombrugghe, B. (2001) Proc. Natl. Acad. Sci. USA 98, 6698–6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akiyama, H., Chaboissier, M., Martin, J. F., Schedl, A. & de Crombrugghe, B. (2002) Genes Dev. 16, 2813–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zehentner, B. K., Dony, C. & Burtscher, H. (1999) J. Bone Miner. Res. 14, 1734–1741. [DOI] [PubMed] [Google Scholar]
- 24.Pizette, S. & Niswander, L. (2000) Dev. Biol. 219, 237–249. [DOI] [PubMed] [Google Scholar]
- 25.Chimal-Monroy, J., Rodrigues-Leon, J., Montero, J. A., Ganan, Y., Macias, D., Merino, R. & Hurle, J. M. (2003) Dev. Biol. 257, 292–301. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez-Lloris, R., Vinals, F., Lopez-Rovira, T., Harley, V., Bartrons, R., Rosa, J. L. & Ventura, F. (2003) Mol. Endocrinol. 17, 1332–1343. [DOI] [PubMed] [Google Scholar]
- 27.Nishitoh, H., Ichijo, H., Kimura, M., Matsumoto, T., Makishima, F., Yamaguchi, A., Yamashita, H., Enomoto, S. & Miyazono, K. (1996) J. Biol. Chem. 271, 21345–21352. [DOI] [PubMed] [Google Scholar]
- 28.Kretzschmar, M., Liu, F., Hata, A., Doody, J. & Massagué, J. (1997) Genes Dev. 11, 984–995. [DOI] [PubMed] [Google Scholar]
- 29.Macias-Silva, M., Hoodless, P. A., Tang, S. J., Buchwald, M. & Wrana, J. L. (1998) J. Biol. Chem. 273, 25628–25636. [DOI] [PubMed] [Google Scholar]
- 30.Zhang, H., Schwarz, E. M., Rosier, R. N., Zuscik, M. J., Puzas, J. E. & O'Keefe, R. J. (2003) J. Bone Miner. Res. 18, 1593–1604. [DOI] [PubMed] [Google Scholar]
- 31.Aoki, H., Fujii, M., Inamura, T., Yagi, K., Takehara, K., Kato, M. & Miyazona, K. (2001) J. Cell Sci. 114, 1483–1489. [DOI] [PubMed] [Google Scholar]
- 32.Kramer, J., Hegert, C., Guan, K., Wobus, A. M., Muller, P. K. & Rohwedel, J. (2000) Mech. Dev. 92, 193–205. [DOI] [PubMed] [Google Scholar]
- 33.Majumdar, M. K., Wang, E. & Morris, E. A. (2001) J. Cell. Physiol. 189, 275–284. [DOI] [PubMed] [Google Scholar]
- 34.Yoon, B. S. & Lyons, K. M. (2004) J. Cell. Biochem. 93, 93–103. [DOI] [PubMed] [Google Scholar]
- 35.Ornitz, D. M. & Marie, P. J. (2002) Genes Dev. 16, 1446–1465. [DOI] [PubMed] [Google Scholar]