Abstract
Background: The incidence and characteristics of gastric cancer have been shown to vary widely across Western and Eastern countries. Our study had two aims: to evaluate long-term trends in gastric adenocarcinoma in Japan over a period of 70 years, and to anticipate the future of gastric cancer in Japan, through comparison with data from the United States.
Methods: Japanese patient data for 19,306 incident cases of gastric adenocarcinoma from 1946 - 2014 were collected from the Gastric Cancer Database at the Cancer Institute Hospital, Tokyo, Japan (CIH-GCDB). U.S. patient data for 78,625 incident cases of gastric cancer from 1973 - 2012 were obtained from the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) database. Changes over time in patient and tumor characteristics were investigated in these two cohorts.
Results: There was a marked reduction of cancer incidence in the lower third of the stomach in the CIH-GCDB; over 70% to around 30%. The incidence in the upper third has been increasing steadily over time; 3% to 19%, although the number of cardia tumors has not changed. An increase in elderly and obese patients was also noted. In the U.S. population, there was a significant difference in the primary site across races. A notable overall increase in cardia cancer was evident in the Western population during the study period, with no similar change evident in the Japanese population over the last 15 years. In the East Asian population, the proportional frequency of tumors in the cardia was lower and that of tumors in the pyloric antrum was higher.
Conclusion: In Japan, cancer in the antrum or pylorus of the stomach has been declining, whereas cancer in the body has been increasing. Unlike the Western population in the United States, adenocarcinoma of esophago-gastric junction is not increasing in Japan.
Keywords: gastric cancer, primary site, long-term trend
Background
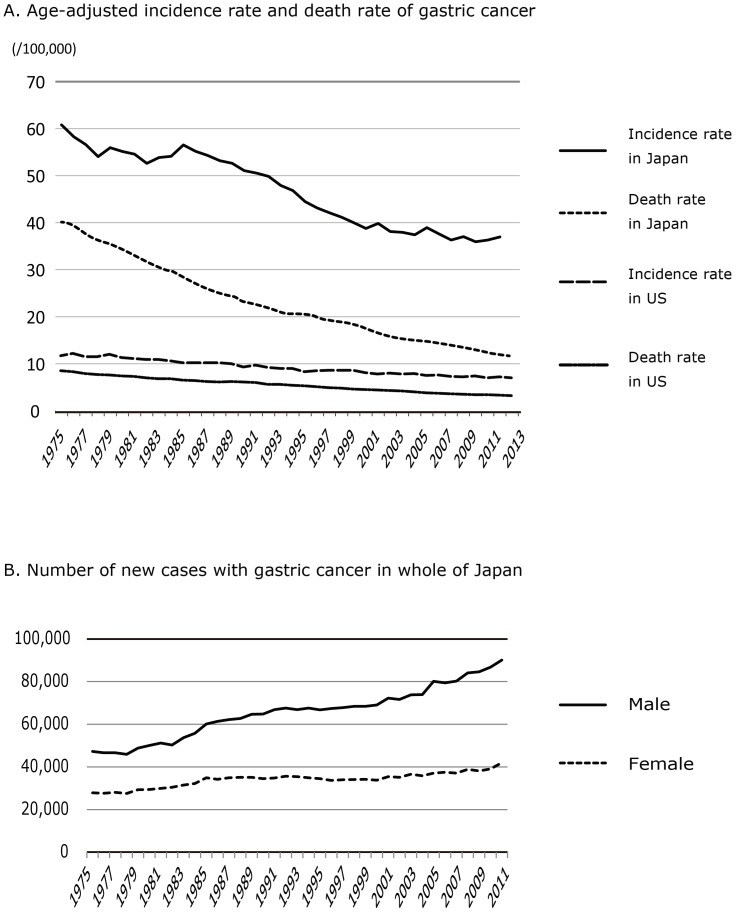
The incidence rates of gastric cancer have been shown to vary widely across countries 1,2. The highest incidence rates are in East Asia (particularly Japan, China, Mongolia and Korea) and some parts of South America, whereas the lowest rates are in North America. Over the last quarter of a century, there has been a steady decline in the age-adjusted incidence rate of gastric cancer in Japan 3-5, and also in other developed countries of North America and Europe 4,6,7 (Figure 1A). These trends may be attributable to a reduced intake of foods preserved by salting or smoking, and a reduction in the prevalence of H. pylori infection 8-10 through improvements in hygiene 3,9,11-14. However, a steady increase in the estimated number of new patients with gastric cancer has been observed in Japan with the rapid aging of society (Figure 1B) 15.
Figure 1.
Age-adjusted incident rate and death rate of Gastric cancer in Japan and the U.S. A: Age-adjusted incident rate of gastric cancer on Japanese and U.S. national statistics. Citation from Cancer Registry and Statistics, Cancer Information Service, National Cancer Center in Japan 6 and World Health Organization, GLOBOCAN 20127. B: Estimated number of new gastric cancer patients by gender in Japan. Citation from Cancer Registry and Statistics, Cancer Information Service, National Cancer Center in Japan 6
Under these circumstances, the oncological characteristics of stomach cancer have been changing. Namely, the increasing incidence of adenocarcinoma in the cardia has been strongly associated with the increase of obesity and gastro-esophageal reflux disease followed by the development of Barrett's esophagus in western countries 16-18. Under the influence of lifestyle “Westernization,” there are also concerns that a rapid increase of adenocarcinoma in the EGJ will become evident in the near future in East Asia 15,19,20, whereas cancer at the EGJ has remained rare 21-23. As some previous reports have noted, there are distinct differences in the primary site and biologic characteristics of gastric cancer between Asian patients and those from other racial and ethnic groups 24-26. Although some researchers have previously investigated differences in gastric cancer between white and black or Hispanic and non-Hispanic patients using national databases in the U.S. 25,27,28, few studies focused on the Asian population have been published recently.
This present study was conducted to delineate the near future of gastric cancer in East Asia, through comparison of the long-term trends of gastric cancer subsites between Japanese patients and the East Asian population of a national database in the U.S. Our objectives were to provide relevant information that would allow clinicians to consider suitable treatment strategies and to guide planning of clinical research.
Methods
Cohort Selection
Data for Japanese patients were identified from the Gastric Cancer Database at the Cancer Institute Hospital, Tokyo, Japan (CIH-GCDB) 29. The database includes entries for 19,306 consecutive patients who were treated for gastric cancer between 1946 and 2014 in the department of surgery at the institute. Patients who received only endoscopic resection or treated by the chemotherapy without any surgical interventions were originally excluded in CIH-GCDB. In addition, patients with histologically proven squamous cell carcinoma, adenosquamous carcinoma or non-epithelial tumors such as lymphoma, sarcoma and metastatic cancers from other primary neoplasms were excluded from the present analyses. Patient data were completely anonymized.
Data for patients in the U.S. were obtained from the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) database. The SEER database is a population-based publicly available data set that has been gathering data since 1973 and represents approximately 28% of all cancer cases in the U.S. After excluding histologically proven squamous cell carcinoma, sarcoma and lymphoma, a total of 78,625 patients who were diagnosed as having gastric cancer between 1973 and 2012 were enrolled. Because the data from both the SEER registry (http://seer.cancer.gov/) and the CIH-GCDB 29 are de-identified and publicly available, no institutional review board approval was necessary for this study.
Definition of the primary site
There is a difference in primary tumor anatomical subsite classification between the two databases (Supplemental Figure 1). The tumor location data in SEER are coded using the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) 30, whereas they are coded according to the classification of the Japanese Gastric Cancer Association (JGCA) 31 in the CIH-GCDB, being divided anatomically into three stomach regions, i.e. the upper, middle, and lower thirds defined by the lines connecting the trisected points on the lesser and greater curvatures. In addition, the area extending 2 cm above to 2 cm below the esophago-gastric junction (EGJ) is designated the EGJ area. Tumors having their epicenter in this area are designated as EGJ carcinomas. In order to account for the different anatomical subsite classifications across databases, we treated the “cardia” as synonymous with the “EGJ”, the “body and fundus” as synonymous with the “upper third and middle third” and the “antrum and pylorus” as synonymous with the lower third in the JGCA data.
Study variables
Patient-level data, including gender, age at diagnosis, ethnicity, tumor primary site or location, tumor size, TNM staging and histology were identified from each of the CIH-GCDB and SEER databases. To investigate the shifts in tumor location over time, the proportional frequencies in the CIH-GCDB were calculated every ten years, and those in the SEER every five years. As potential confounders for the primary site, patient ages, gender and body mass index (BMI) were obtained for the Japanese population. The 7th edition of the American Joint Committee on Cancer (AJCC) TNM classification was used for tumor staging based on CIH-GCDB data, whereas tumor staging was made on the basis of the 6th edition of the AJCC TNM staging system for part of the SEER database (from 2004 to 2012, n=18,092).
Statistical analysis
The descriptive statistics were evaluated with reference to the patients' backgrounds and tumor characteristics and, as necessary, continuous variables were compared using Student's t test and categorical variables by Fisher's exact test.
To estimate the odds ratio of adenocarcinoma occurring in the upper third of the stomach, logistic regression models were analyzed with the time period as a dependent variable and incidence of cancer in the upper third of the stomach as an independent variable. In addition, to compare time trends among white and black Americans, East Asian Americans (Japanese=4,320, Chinese=2,008 and Koreans=874) living in the US, and Japanese patients entered in the CIH-GCDB. All statistical tests were two-sided, and p values of 0.05 or less were considered to indicate statistical significance. All the analyses were performed with STATA version 11.1 (Stata Corp, College Station, TX).
Results
Japanese population in the CIH-GCDB
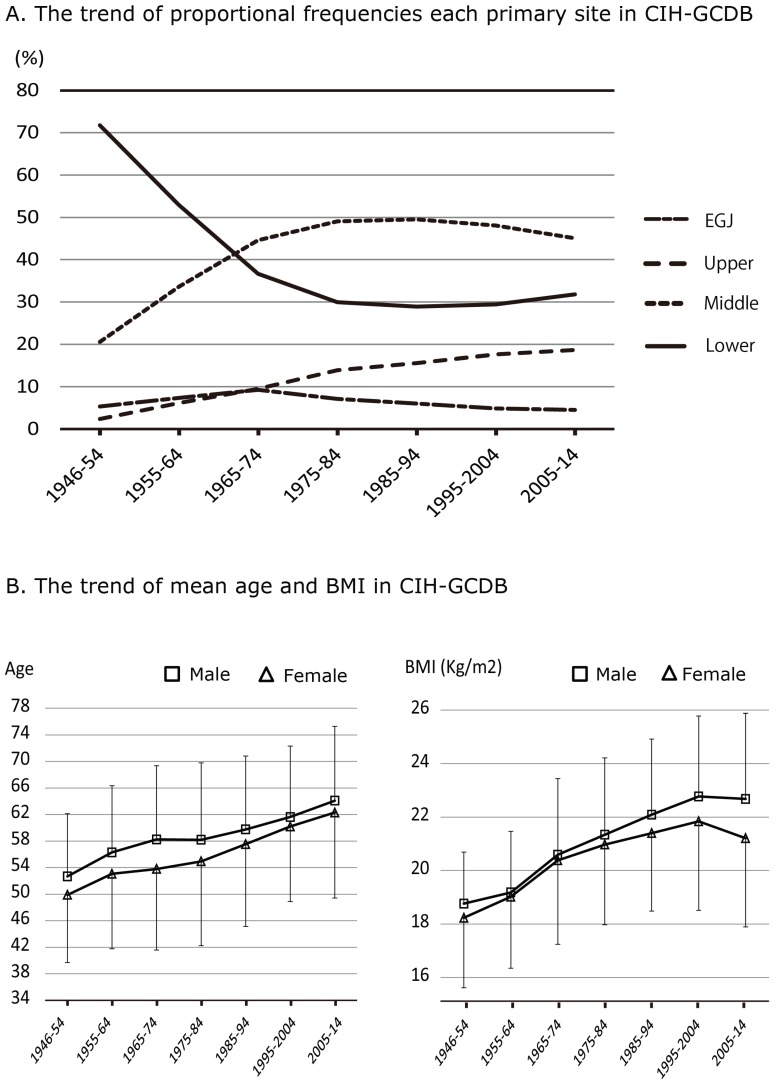
Table 1 shows the characteristics of the patients and their tumors in Japan. The mean patient age was 59.0 years, and the proportion of males was 65.2%. Figure 2A shows the change over time in the proportion of tumors according to the primary site for every ten-year period from 1946 to 2014. There was a marked reduction of cancer incidence in the lower third of the stomach, whereas incidence in the upper third has been increasing steadily up to the present. The proportion of EGJ cancers was found not to have changed markedly during the observation period. On the basis of Figure 2A, we divided the study period into three parts: 1946 to 1974 as the early period, 1975 to 1994 as the middle period, and 1995 to 2014 as the late period. Table 1 also shows the characteristics in each group. The mean age and BMI of patients at diagnosis has been increasing over time, but there have not been any significant changes in the gender ratio, histological types or cross-sectional position. Besides, the proportion of signet-ring cell carcinoma had been increasing from early to middle period.
Table 1.
Patients and tumor characteristics and the time trend in CIH-GCDB
| All Patients | 1946 to 1974 | 1975 to 1994 | 1995 to 2014 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (N = ) | 19,306 | 5,991 | 5,437 | 7,878 | ||||||||||||
| No. | % | No. | % | No. | % | No. | % | p | ||||||||
| Age at treatment | <.001 | |||||||||||||||
| Mean | 59.0 | 55.1 | 58.0 | 62.6 | ||||||||||||
| SD | 12.0 | 11.1 | 11.9 | 11.6 | ||||||||||||
| Gender | 0.029 | |||||||||||||||
| Male | 12,584 | 65.2 | 3,827 | 63.9 | 3,599 | 66.2 | 5,158 | 65.5 | ||||||||
| Female | 6,702 | 34.7 | 2,164 | 36.1 | 1,838 | 33.8 | 2,700 | 34.3 | ||||||||
| BMI (kg/m2) | ||||||||||||||||
| Mean | 21.3 | 19.8 | 21.5 | 22.3 | ||||||||||||
| SD | 3.2 | 2.8 | 2.9 | 3.3 | ||||||||||||
| Primary site* | <.001 | |||||||||||||||
| EGJ | 1,160 | 6.0 | 457 | 7.6 | 351 | 6.5 | 352 | 4.5 | ||||||||
| Upper | 2,571 | 13.3 | 401 | 6.7 | 762 | 14.0 | 1,388 | 17.6 | ||||||||
| Middle | 8,229 | 42.6 | 2,102 | 35.1 | 2,621 | 48.2 | 3,506 | 44.5 | ||||||||
| Lower | 6,843 | 35.4 | 2,927 | 48.9 | 1,565 | 28.8 | 2,351 | 29.8 | ||||||||
| Unclassified | 503 | 2.6 | 104 | 1.7 | 138 | 2.5 | 281 | 3.6 | ||||||||
| Tumor size (mm) | <.001 | |||||||||||||||
| Mean | 56.4 | 68.8 | 55.3 | 48.5 | ||||||||||||
| SD | 35.9 | 29.4 | 38.6 | 35.6 | ||||||||||||
| Stage | <.001 | |||||||||||||||
| IA | 6,225 | 32.2 | 714 | 11.9 | 2,080 | 38.3 | 3,431 | 43.6 | ||||||||
| IB | 1,483 | 7.7 | 283 | 4.7 | 451 | 8.3 | 749 | 9.5 | ||||||||
| IIA | 1,466 | 7.6 | 563 | 9.4 | 353 | 6.5 | 550 | 7.0 | ||||||||
| IIB | 1,557 | 8.1 | 630 | 10.5 | 416 | 7.7 | 511 | 6.5 | ||||||||
| IIIA | 1,390 | 7.2 | 691 | 11.5 | 308 | 5.7 | 391 | 5.0 | ||||||||
| IIIB | 1,428 | 7.4 | 699 | 11.7 | 356 | 6.5 | 373 | 4.7 | ||||||||
| IIIC | 1,108 | 5.7 | 498 | 8.3 | 352 | 6.5 | 258 | 3.3 | ||||||||
| IV | 3,072 | 15.9 | 1,304 | 21.8 | 754 | 13.9 | 1,014 | 12.9 | ||||||||
| Unknown | 1,577 | 8.2 | 609 | 10.2 | 367 | 6.8 | 601 | 7.6 | ||||||||
| Histological findings* | <.001 | |||||||||||||||
| tub/pap | 7,989 | 41.4 | 2,650 | 44.2 | 2,284 | 42.0 | 3,055 | 38.8 | ||||||||
| poor | 5,797 | 30.0 | 1,980 | 33.0 | 1,476 | 27.1 | 2,341 | 29.7 | ||||||||
| muc | 482 | 2.5 | 182 | 3.0 | 133 | 2.4 | 167 | 2.1 | ||||||||
| sig | 3,299 | 17.1 | 361 | 6.0 | 1,292 | 23.8 | 1,646 | 20.9 | ||||||||
| Unknown | 32 | 0.2 | 6 | 0.1 | 2 | 0.0 | 24 | 0.3 | ||||||||
BMI; Body Mass Index, SD: Standard Deviation, EGJ: Esophago-gastric Junction,
*; Descriptions according to the definition by Japanese Gastric Cancer Association
Figure 2.
Primary site, patients age and BMI in CIH-GCDB. A: Time trends of in the relative frequencies of cancers arising at each primary site, based on the CIH-GCDB data. B: Time trends in age and BMI of patients with gastric cancer by gender.
Figure 2B shows the changes in patient age over time, and BMI at diagnosis for each sex. Logistic regression analysis showed that gender (male), age (older), BMI (obese) and period (recent years) were significant independent risk factor for the incidence of upper third gastric cancer (Table 2).
Table 2.
Independent risk factors for upper third gastric cancer in CIH-GCDB
| Odds Ratio | SE | P-value | 95% Confidential Interval | |||
|---|---|---|---|---|---|---|
| Gender (Male/Female) | 0.745 | 0.036 | <0.001 | 0.678 | - | 0.818 |
| Time proceeds (per year) | 1.019 | 0.016 | <0.001 | 1.158 | - | 1.222 |
| Upper age (every 10 years) | 1.082 | 0.020 | <0.001 | 1.043 | - | 1.122 |
| Higher BMI(kg/m2) | 1.036 | 0.007 | <0.001 | 1.022 | - | 1.050 |
BMI; Body Mass Index
US population in the SEER database
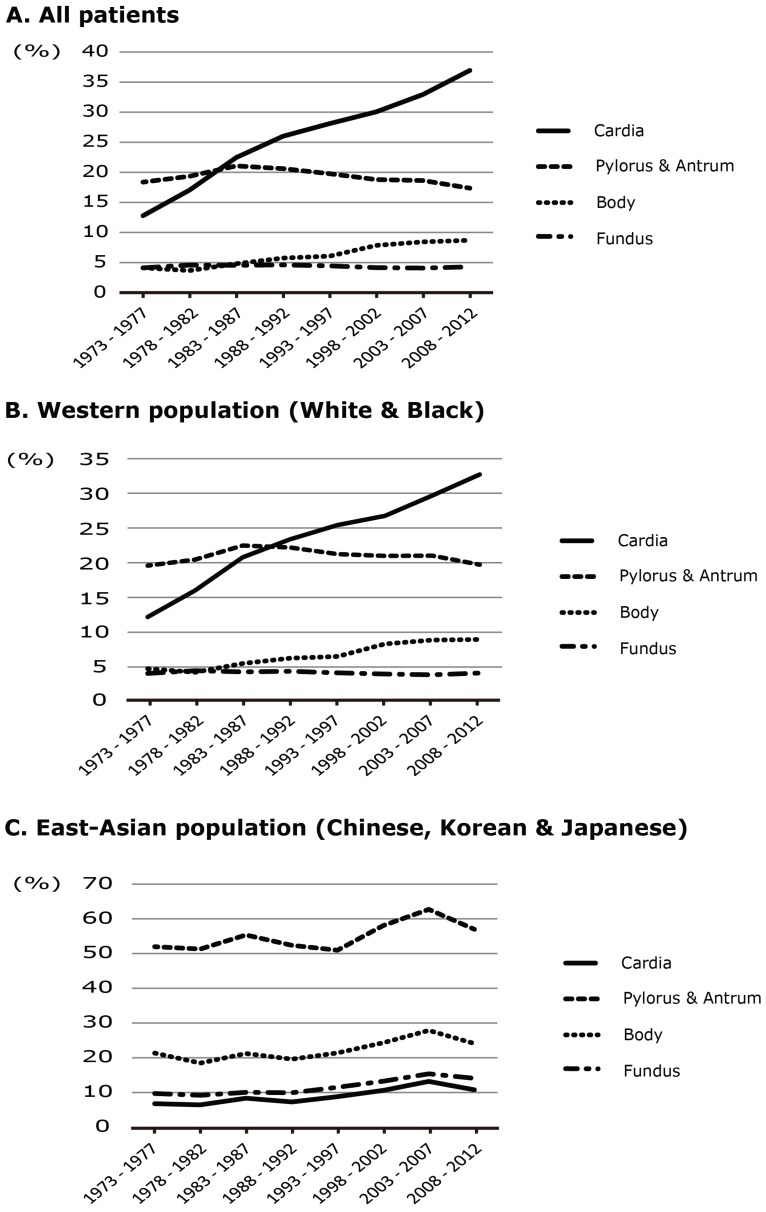
Table 3 shows the characteristics of the U.S. patients and their tumors. In this comparison between patients as a whole and the East Asian population, there was a significant difference only in the primary site, and there were no clear differences in the other items. In the East Asian population, the proportion of tumors in the cardia was lower (9.3%) and that of tumors in the pyloric antrum was higher (29.7%). Figure 3 shows the trend of the primary site for each of the white, black, and East Asian populations during this period. A notable increase of tumors in the cardia was evident in the white or black population during the study period overall, but no remarkable change was evident in the East Asian population over the last 15 years.
Table 3.
Patients and tumor characteristic of All Patients and East Asian patients with gastric cancer in SEER database
| All Patients | East Asian | |||||
|---|---|---|---|---|---|---|
| (N = ) | 78,625 | (%) | 7,202 | (%) | ||
| Diagnostic Year | ||||||
| 1973-1982 | 18,875 | 24.0 | 1,232 | 17.1 | ||
| 1983-1992 | 19,847 | 25.2 | 1,793 | 24.9 | ||
| 1993-2002 | 19,852 | 25.2 | 2,123 | 29.5 | ||
| 2003-2012 | 20,051 | 25.5 | 2,054 | 28.5 | ||
| Race | White | 58,118 | 73.9 | N.A. | ||
| Black | 9,251 | 11.8 | ||||
| East Asian | 7,202 | 9.2 | ||||
| Others | 4,054 | 5.2 | ||||
| Age at diagnosis (Year) | ||||||
| Mean | 69.4 | 70.5 | ||||
| SD | 15.7 | 13.5 | ||||
| Gender | ||||||
| Male | 48,541 | 61.7 | 4,241 | 58.9 | ||
| Female | 30,084 | 38.3 | 2,961 | 41.1 | ||
| Primary Site | ||||||
| Cardia | 18,496 | 23.5 | 673 | 9.3 | ||
| Fundus | 3,270 | 4.2 | 189 | 2.6 | ||
| Body | 5,268 | 6.7 | 767 | 10.6 | ||
| Pylorus or Antrum | 16,490 | 21.0 | 2,361 | 32.8 | ||
| Overlapping | 17,380 | 22.1 | 2,061 | 28.6 | ||
| NOS | 17,721 | 22.5 | 1,151 | 16.0 | ||
| Tumor size (mm) | ||||||
| Mean | 48.3 | 49.5 | ||||
| SD | 44.8 | 43.7 | ||||
| Histological findings | ||||||
| well differentiated | 3,851 | 4.9 | 308 | 4.3 | ||
| moderate | 14,933 | 19.0 | 1,499 | 20.8 | ||
| poor | 33,644 | 42.8 | 3,494 | 48.5 | ||
| others | 2,922 | 3.7 | 158 | 2.2 | ||
| Unknown | 23,275 | 29.6 | 1,748 | 24.3 | ||
| TNM Stage AJCC 6th (Available data from 2004 to 2012) | ||||||
| (N = ) | 18,092 | (%) | 1,840 | (%) | ||
| 0 | 182 | 1.0 | 27 | 1.5 | ||
| IA | 2,530 | 14.0 | 311 | 16.9 | ||
| IB | 1,713 | 9.5 | 198 | 10.8 | ||
| II | 1,899 | 10.5 | 194 | 10.5 | ||
| IIIA | 1,460 | 8.1 | 156 | 8.5 | ||
| IIIB | 324 | 1.8 | 46 | 2.5 | ||
| IV | 6,760 | 37.4 | 668 | 36.3 | ||
| Unknown | 3,224 | 17.8 | 240 | 13.0 | ||
SD; Standard deviation, NOS; Not otherwise specified
Figure 3.
Time trends in the relative frequencies of cancers arising at each primary site, based on the SEER database. A: Time trend of proportional frequency for patients overall. B: Time trend of proportional frequency for Western (White or Black) patients overall. C: Time trend of proportional frequency for East Asian (Chinese, Korean and Japanese) patients
Discussion
The main results of our study are threefold. First, in Japan, the incidence of gastric cancer in the lower third (antrum or pylorus) of the stomach has been steadily decreasing up to 1985, whereas that in the upper third excluding the EGJ has been steady increasing up to the present. Second, East Asians living in the United States showed a tumor location trend distinctly different from that of patients of other races living in the U.S.; their proportion of cardia cancers has not been increasing over last 15 years, unlike the trend for white and black patients. Lastly, in the Japanese population, older age, obesity, male gender and recent year of diagnosis were significant independent risk factors for upper third gastric cancer.
Whereas the incidence rate of a lot of malignant neoplasms has been rising in developed countries, these of gastric, hepatic or uterine cervix cancer showed decline tendency 1,2,6,7. The principal reason has been thought to be attributed to improvements in general public health. Namely the etiology these neoplasms were strongly associated to prevalence of specific virus or bacterial infection, and the decreasing of incident rate and advances of hygienic environment were observed in developed countries. Among environmental factors associated with the risk of gastric cancer, some researchers have regarded the endemic of H. pylori infection as one of the strongest carcinogenic factors 8,32,33. The sero-prevalence rate of H. pylori infection was approximately 40 to 70% in patients with gastric cancer in Asian countries 34, and the overall sero-prevalence rate has been decreasing with the time trend 8,34,35. Improvements in living conditions, the water supply or sewage systems are strongly correlated with the declining prevalence of H. pylori 34. The expansion of industrial refrigeration has reduced the need for salting and pickling for food preservation, and the development of transportation has allowed distribution of fresher products to consumers 3. It has also become easy for people to include potentially protective factors such as vitamins A, C, and E in their diet throughout the year 9-11, which may counteract carcinogenesis in the stomach linked to intake of N-nitroso compounds in smoked or grilled meat 36,37. The decline in the incidence of gastric cancer in Japan has been attributed to the overall improvement of public health accompanying the high economic growth following the severe poverty after World War II. Significantly, however, our results indicate that the above factors have been particularly associated with cancer in the antrum or pylorus of the stomach, rather than with gastric cancer as a whole. The number of patients with cancer in the upper third of the stomach has, in fact, been increasing over time, and therefore it can be considered that the risk factors for adenocarcinoma in the upper third are differ from those for cancer in the lower third. In term of histopathological type that has strong association with primary site arising adenocarcinoma, the fact that the proportion of signet ring cell carcinoma has been rapidly increasing from early to middle period was not inconsistent with this sift of cancer location (Table 1).
From a physician's viewpoint, the current trend suggests that in East Asian populations a larger number of patients will need to be treated for cancer in the upper third of the stomach as time passes. In addition, as the proportion of obese and elderly patients will continue to increase in the near future, treatment of gastric cancer may become more difficult than it has been in the past. In this connection, a crucial difference between Japanese and Western patients is that the incidence of adenocarcinoma at the EGJ has remained consistently low in Japan for the last 70 years. Despite the marked increase of EGJ adenocarcinoma in Western patients documented in the SEER data and previous studies, the proportion of EGJ cancer in East Asians in the SEER has remained consistently low. Considering the trends evident in the two databases, it seems unlikely that the incidence of adenocarcinoma in the EGJ will increase rapidly in East Asian countries as a result of westernization in the near future.
This large-scale database study has some limitations. First, there is the difficulty in classifying adenocarcinoma as being present in the upper third, cardia or EGJ. In general, two types of adenocarcinoma can arise in the EGJ; one is derived from the fundic glands of the stomach, and the other from Barrett's epithelium. These two cancers ought to be essentially distinguished from each other, i.e. upper gastric cancer with esophageal invasion, and lower esophageal cancer with gastric invasion. However, in most cases, it is clinicopathologically difficult to identify whether the origin of a neoplasm is the gastric gland or the metaplastic columnar epithelium of the esophagus. Therefore, both gastric cancer with esophageal invasion and adenocarcinoma from Barrett's esophagus have been registered under the same category in all clinical databases. In addition, the Japanese definition of cancer in cardia is a slightly different from that of ICD-10 (Supplemental figure 1). Second, the SEER database contains no information about the lifestyle of the East Asian population, such as how long they have lived in the US or the types of foods they usually eat. Therefore, unknown or unmeasurable - but important - potential confounders might be overlooked. Another problem is that there is no guarantee that patients entered in the CIH-GCDB are a representative sample of Japanese patients with gastric cancer. The CIH-GCDB is a database representing patients seen at a single institution, although the hospital handles the highest volume of cancer patients in Japan and the registration system has been established for nearly 70 years. The multi-center national cancer database system in Japan is still in its early stages and is tightly restricted to access for general researchers. Therefore, using the CIH-GCDB was the best resource for addressing our research aims, enabling us to analyze a large number of patients over a long time period.
In conclusion, our findings suggest that cancer in the lower third of the stomach has been declining, whereas cancer in the upper third has been increasing in recent years in Japan. Unlike the situation in Western patients, currently there is no evidence of any increase of EGJ adenocarcinoma in the Japanese population. However, from a clinical viewpoint, it may become more difficult to treat gastric cancer in Japanese patients in comparison with the past, in view of the increase of elderly and obese patients presenting with adenocarcinoma in the upper third of the stomach.
Supplementary Material
Supplementary figure 1.
Acknowledgments
We would like to thank Mrs. Yoshiko Kobayashi for management of the CIH-GCDB system and her suggestions for our research. This study was supported, in part, by a grant-in-aid of The Public Trust Fund For Clinical Cancer Research.
Abbreviations
- CIH-GCDB
Gastric Cancer Database at the Cancer Institute Hospital
- SEER
Surveillance, Epidemiology, and End Results
- H. pylori
Helicobactor pylori
- EGJ
esophago-gastric junction
- ICD-O-3
International Classification of Diseases for Oncology, 3rd edition
- JGCA
Japanese Gastric Cancer Association (JGCA)
- BMI
body mass index
- AJCC
American Joint Committee on Cancer
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.AmericanCancerSociety. Global Cancer Facts & Figures 3rd Edition. http: //wwwcancerorg/research/cancerfactsstatistics/global 2013:Accessed in 10th November; 2015. [Google Scholar]
- 3.Howson CP, Hiyama T, Wynder EL. The decline in gastric cancer: epidemiology of an unplanned triumph. Epidemiol Rev. 1986;8:1–27. doi: 10.1093/oxfordjournals.epirev.a036288. [DOI] [PubMed] [Google Scholar]
- 4.AmericanCancerSociety. Cancer Facts & Figures 2015. http: //wwwcancerorg/research/cancerfactsstatistics/cancerfactsfigures2015/index; 2015. [Google Scholar]
- 5.Malvezzi M, Bonifazi M, Bertuccio P. et al. An age-period-cohort analysis of gastric cancer mortality from 1950 to 2007 in Europe. Ann Epidemiol. 2010;20:898–905. doi: 10.1016/j.annepidem.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Vital_Statistics_Japan_(Ministry_of_Health LaW. Cancer Registry and Statistics. Cancer Information Service, National Cancer Center, Japan. http: //ganjohojp/reg_stat/statistics/dl/indexhtml 2015:Accessed in 10th November; 2015. [Google Scholar]
- 7.World_Health_Organization. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. http: //globocaniarcfr/Defaultaspx 2012:Accessed in 10th November; 2015. [Google Scholar]
- 8.Kamada T, Haruma K, Ito M. et al. Time Trends in Helicobacter pylori Infection and Atrophic Gastritis Over 40 Years in Japan. Helicobacter. 2015;20:192–8. doi: 10.1111/hel.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galanis DJ, Kolonel LN, Lee J, Nomura A. Intakes of selected foods and beverages and the incidence of gastric cancer among the Japanese residents of Hawaii: a prospective study. Int J Epidemiol. 1998;27:173–80. doi: 10.1093/ije/27.2.173. [DOI] [PubMed] [Google Scholar]
- 10.Shimazu T, Wakai K, Tamakoshi A. et al. Association of vegetable and fruit intake with gastric cancer risk among Japanese: a pooled analysis of four cohort studies. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2014;25:1228–33. doi: 10.1093/annonc/mdu115. [DOI] [PubMed] [Google Scholar]
- 11.Hirayama T. Epidemiology of stomach cancer in Japan. With special reference to the strategy for the primary prevention. Japanese journal of clinical oncology. 1984;14:159–68. [PubMed] [Google Scholar]
- 12.Katanoda K, Hori M, Matsuda T. et al. An updated report on the trends in cancer incidence and mortality in Japan, 1958-2013. Japanese journal of clinical oncology. 2015;45:390–401. doi: 10.1093/jjco/hyv002. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez CA, Jakszyn P, Pera G. et al. Meat intake and risk of stomach and esophageal adenocarcinoma within the European Prospective Investigation Into Cancer and Nutrition (EPIC) J Natl Cancer Inst. 2006;98:345–54. doi: 10.1093/jnci/djj071. [DOI] [PubMed] [Google Scholar]
- 14.Engel LS, Chow WH, Vaughan TL. et al. Population attributable risks of esophageal and gastric cancers. J Natl Cancer Inst. 2003;95:1404–13. doi: 10.1093/jnci/djg047. [DOI] [PubMed] [Google Scholar]
- 15.Matsuda A, Matsuda T, Shibata A. et al. Cancer incidence and incidence rates in Japan in 2008: a study of 25 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Japanese journal of clinical oncology. 2014;44:388–96. doi: 10.1093/jjco/hyu003. [DOI] [PubMed] [Google Scholar]
- 16.Blot WJ, Devesa SS, Kneller RW, Fraumeni JF Jr. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287–9. [PubMed] [Google Scholar]
- 17.Botterweck AA, Schouten LJ, Volovics A, Dorant E, van Den Brandt PA. Trends in incidence of adenocarcinoma of the oesophagus and gastric cardia in ten European countries. Int J Epidemiol. 2000;29:645–54. doi: 10.1093/ije/29.4.645. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong RW, Borman B. Trends in incidence rates of adenocarcinoma of the oesophagus and gastric cardia in New Zealand, 1978-1992. Int J Epidemiol. 1996;25:941–7. doi: 10.1093/ije/25.5.941. [DOI] [PubMed] [Google Scholar]
- 19.Olefson S, Moss SF. Obesity and related risk factors in gastric cardia adenocarcinoma. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2015;18:23–32. doi: 10.1007/s10120-014-0425-4. [DOI] [PubMed] [Google Scholar]
- 20.Hongo M, Nagasaki Y, Shoji T. Epidemiology of esophageal cancer: Orient to Occident. Effects of chronology, geography and ethnicity. J Gastroenterol Hepatol. 2009;24:729–35. doi: 10.1111/j.1440-1746.2009.05824.x. [DOI] [PubMed] [Google Scholar]
- 21.Shibata A, Matsuda T, Ajiki W, Sobue T. Trend in incidence of adenocarcinoma of the esophagus in Japan, 1993-2001. Japanese journal of clinical oncology. 2008;38:464–8. doi: 10.1093/jjco/hyn064. [DOI] [PubMed] [Google Scholar]
- 22.Blaser MJ, Saito D. Trends in reported adenocarcinomas of the oesophagus and gastric cardia in Japan. Eur J Gastroenterol Hepatol. 2002;14:107–13. doi: 10.1097/00042737-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Kaneko S, Sobue T. Trends in reported incidences of gastric cancer by tumour location, from 1975 to 1989 in Japan. Int J Epidemiol. 2004;33:808–15. doi: 10.1093/ije/dyh053. [DOI] [PubMed] [Google Scholar]
- 24.Strong VE, Song KY, Park CH. et al. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Annals of surgery. 2010;251:640–6. doi: 10.1097/SLA.0b013e3181d3d29b. [DOI] [PubMed] [Google Scholar]
- 25.Yao JC, Tseng JF, Worah S. et al. Clinicopathologic behavior of gastric adenocarcinoma in Hispanic patients: analysis of a single institution's experience over 15 years. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23:3094–103. doi: 10.1200/JCO.2005.08.987. [DOI] [PubMed] [Google Scholar]
- 26.Yamada T, Yoshikawa T, Taguri M, The survival difference between gastric cancer patients from the UK and Japan remains after weighted propensity score analysis considering all background factors. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association; 2015. [DOI] [PubMed] [Google Scholar]
- 27.Anderson WF, Camargo MC, Fraumeni JF Jr, Correa P, Rosenberg PS, Rabkin CS. Age-specific trends in incidence of noncardia gastric cancer in US adults. Jama. 2010;303:1723–8. doi: 10.1001/jama.2010.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camargo MC, Anderson WF, King JB. et al. Divergent trends for gastric cancer incidence by anatomical subsite in US adults. Gut. 2011;60:1644–9. doi: 10.1136/gut.2010.236737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakajima T, Yamaguchi T, Sano T. Gastric Cancer Database in Cancer Institute Hospital of JFCR 1946 - 2007. Kanehara, Tokyo, Japan; 2012. p. 2. [Google Scholar]
- 30.Fritz A, Percy C, Jack A, International Classification of Diseases for Oncology; 3rd ed. Geneva: World Health Organization; 2000. http://apps.who.int/iris/bitstream/10665/96612/1/9789241548496_eng.pdf?ua=2. [Google Scholar]
- 31.Japanese Gastric Cancer A. Japanese classification of gastric carcinoma: 3rd English edition. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2011;14:101–12. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 32.Nakajima S, Nishiyama Y, Yamaoka M, Yasuoka T, Cho E. Changes in the prevalence of Helicobacter pylori infection and gastrointestinal diseases in the past 17 years. J Gastroenterol Hepatol. 2010;25(Suppl 1):S99–S110. doi: 10.1111/j.1440-1746.2009.06214.x. [DOI] [PubMed] [Google Scholar]
- 33.Xia HH, Phung N, Altiparmak E, Berry A, Matheson M, Talley NJ. Reduction of peptic ulcer disease and Helicobacter pylori infection but increase of reflux esophagitis in Western Sydney between 1990 and 1998. Dig Dis Sci. 2001;46:2716–23. doi: 10.1023/a:1012731614075. [DOI] [PubMed] [Google Scholar]
- 34.Fock KM, Katelaris P, Sugano K. et al. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009;24:1587–600. doi: 10.1111/j.1440-1746.2009.05982.x. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi T, Kikuchi S, Lin Y. et al. Trends in the incidence of gastric cancer in Japan and their associations with Helicobacter pylori infection and gastric mucosal atrophy. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2004;7:233–9. doi: 10.1007/s10120-004-0297-0. [DOI] [PubMed] [Google Scholar]
- 36.Sobala GM, Schorah CJ, Sanderson M. et al. Ascorbic acid in the human stomach. Gastroenterology. 1989;97:357–63. doi: 10.1016/0016-5085(89)90071-1. [DOI] [PubMed] [Google Scholar]
- 37.Keszei AP, Goldbohm RA, Schouten LJ, Jakszyn P, van den Brandt PA. Dietary N-nitroso compounds, endogenous nitrosation, and the risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Am J Clin Nutr. 2013;97:135–46. doi: 10.3945/ajcn.112.043885. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1.