Abstract
Aims: The levels of coagulation system tests have been studied in various cancers. In this study, our aim is to evaluate the prognostic value of pretreatment plasma coagulation tests in hepatocellular carcinoma (HCC) patients.
Patient and methods: A retrospective study was performed in 539 patients with HCC, and follow-up period was at least 60 months until recurrence or death. The prognostic significance of coagulation system tests (prothrombin time, activated partial thromboplastin time, thrombin time, fibrinogen) were determined by univariate and multivariate Cox hazard models. Then, according to the results of the multivariate analyses, we proposed the coagulation-Based Stage, which combined the independent risk factors (prothrombin time and fibrinogen).
Results: Coagulation system tests including prothrombin time (PT), activated partial thromboplastin time (APTT), thrombin time (TT), fibrinogen (Fbg) were analyzed. Patients with prolonged PT (≥12.1 sec) levels had significantly poor overall survival (OS) and disease-free survival (DFS), not only in the entire cohort (HR: 1.661, 95%CI: 1.125-2.451, p=0.011 vs. HR: 1.660, 95%CI: 1.125-2.451, p=0.011), but also in the subgroups stratified by pathological stage (stage I-II and stage III-IV). Additionally, high Fbg (≥2.83 g/L) levels experienced significantly decreased OS and DFS (HR: 2.158, 95%CI: 1.427-3.263, p<0.001 vs. HR: 2.161, 95%CI: 1.429-3.267, p<0.001), not only in the entire cohort but also in the subgroups stratified by pathological stage (stage I-II and stage III-IV). All the patients were then stratified (based on combined PT and Fbg) into three groups, The OS for HCC patients were (41.37±17.76), (31.83±19.84) and (18.68±18.41) months, and the DFS for HCC patients were (41.15±17.88), (31.65±19.81) and (18.66±18.39) months.
Conclusions: Our findings suggest that the combination of plasma PT and Fbg levels should be evaluated as the valuable predictor of survival in patients with HCC.
Keywords: HCC, prothrombin time, fibrinogen, prognosis, overall survival, disease-free survival
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies and the third most frequent cause of cancer-related death worldwide 1. More than half of the patients with HCC are diagnosed in China. In the past several decades, substantial developments and advances have been made in the screening, diagnosis and staging, especially the improvement of imaging techniques. However, in the worldwide, the 5-year overall survival (OS) rate of HCC is only 7% 2. To date, many serum biomarkers, such as α-fetoprotein (AFP), γ-glutamyl transferase (GGT), and (carcino-embryonic antigen) CEA, have been used to predict the survival of HCC patents 3, 4. Especially, AFP is the widely used tumor biomarker for HCC prognosis. However, due to limited sensitivity and specificity, these markers are not entirely reliable. Thus, more effective biomarkers are needed to identify the biological characteristics and guide individualized comprehensive treatments of HCC to improve patient survival.
The association between cancer and the coagulation system is well recognized 5. Importantly, cancer-induced hemostatic activity has been shown to promote tumor development, progression and metastasis 6-8. In fact, abnormal coagulation parameters that represent hemostatic and fibrinolytic systems have been associated with tumor progression and dissemination, which could decrease overall survival (OS) of tumors 9. In our previous study, the decreased pre-treatment thrombin time (TT) was reported to be associated with the shorter ESCC survival 10. Research was conducted by Tas et al. 11, who reported the prognostic values of the prothrombin time (PT) and international normalized ratio (INR) in lung cancer. Moreover, in the original studies, hyperfibrinogenemia was also identified as independent prognostic predictors in melanoma 12 and gallbladder cancer 13. The liver plays an important role in the metabolism and synthesis of clotting factors, which could regulate the blood coagulation and anticoagulation system. The ability to synthesize clotting factors and anticoagulation proteins is damaged by various liver diseases, such as hepatitis, liver cirrhosis and HCC 13. Furthermore, the patients in advanced HCC often have abnormal coagulation, involving coagulation, fibrinolysis and other complex pathological changes, which is closely related to the tumor progression, the prognosis and survival of cancer patients. Prothrombin time (PT), activated partial thromboplastin time (APTT), thrombin time (TT), fibrinogen (Fbg) are the most common coagulation tests used in the laboratory, but few studies focused on the prognostic value in cancer. Therefore, there is little evidence about the impact on hemostatic system in clinical outcomes with HCC patients.
Given this, we aim to elucidate a retrospective cohort analysis to examine the association between PT, Fbg, overall survival (OS) and disease-free-survival (DFS) in HCC patients.
Methods
Patients
A total of 539 eligible patients, who received histologically confirmed diagnosed with HCC between January 2009 and December 2012 at the Sun Yat-sen University Cancer Center were collected and enrolled into this retrospective study. The demographic details are described in Table 1. All of the patients met the diagnostic criteria for HCC. Cancer staging was based on the American Joint Committee on Cancer Staging system (AJCC, 2002; Greene, American Joint Committee on Cancer., American Cancer Society, 2002). Exclusion criteria were as follows: (1) patients treated with surgery or received any antitumor treatment drugs before plasma collection; (2) patients who regularly took procoagulant or anticoagulant therapy or took blood transfusions within 1 month of study onset; (4) patients with concomitant diseases associated with influenced plasma coagulation levels (i.e., VTE, pulmonary embolism, or disseminated intravascular coagulation (DIC) within 1 month of study onset or during the subsequent treatment); (5) other types of malignancy. All patients received treatment. Clinical information, including demographic data, pathological tumor node metastasis (pTNM) stage, alcohol consumption, BMI, family history of cancer, overall survival (OS) and disease-free survival (DFS) data were available for all patients. Prior to use of these plasma, informed consent was obtained from each of the patients. This study was approved by the medical ethics committee of Sun Yat-Sen University Cancer Center (Guangdong, China).
Table 1.
Characteristics and parameters of the HCC patients
| Characteristics | no. (%) | 5-year OS (Months) Mean ± SD |
p value | 5-year DFS (Months) Mean ± SD |
p value |
|---|---|---|---|---|---|
| Gender(n) | |||||
| Male | 480(89.05) | 30.82 ±20.74 | 0.524 | 30.65 ±20.71 | 0.566 |
| Female | 59(10.95) | 29.71 ±20.21 | 19.71 ±20.21 | ||
| Age (years) | |||||
| ≥53 | 275(51.02) | 32.05 ±20.57 | 0.167 | 31.75 ±20.55 | 0.226 |
| < 53 | 264(48.98) | 29.30 ±20.71 | 29.30 ±20.71 | ||
| Stage(n) | |||||
| I and II | 306(56.77) | 38.16 ±17.84 | 0.000 | 37.92 ±17.87 | 0.000 |
| III and IV | 233(43.23) | 20.90 ±20.06 | 20.87 ±20.07 | ||
| Tumor size(cm) | |||||
| ≥5 | 220(40.82) | 20.17±19.68 | 0.000 | 20.14±19.69 | 0.000 |
| <5 | 319(59.18) | 37.96±18.06 | 37.73±18.09 | ||
| Tumor number | |||||
| Single | 335(62.15) | 33.71±20.02 | 0.001 | 33.55±20.04 | 0.001 |
| Multiple | 192(35.62) | 26.74±20.75 | 26.60±20.66 | ||
| Node stage | |||||
| N0 | 502(93.14) | 31.35±20.34 | 0.016 | 31.19±20.32 | 0.017 |
| N1-2 | 37(6.86) | 21.92±23.30 | 21.92±23.30 | ||
| Distant metastases | |||||
| Yes | 31(5.75) | 24.52±23.08 | 0.102 | 24.52±23.08 | 0.108 |
| No | 508(94.25) | 31.08±20.48 | 30.92±20.45 | ||
| BMI (kg/m2) | |||||
| ≥24.0 | 174(32.28) | 32.70±20.70 | 0.234 | 32.44±20.58 | 0.292 |
| 18.5-23.9 | 319(59.18) | 29.94±20.74 | 29.83±20.77 | ||
| <18.5 | 45(8.35) | 28.80±19.90 | 28.80±19.90 | ||
| Alcohol behavior | |||||
| Yes | 200(37.11) | 30.02±21.18 | 0.683 | 29.66±21.08 | 0.535 |
| No | 339(62.89) | 31.10±20.38 | 21.08±20.40 | ||
| Family history of cancer | |||||
| Yes | 138(25.60) | 32.21±20.52 | 0.362 | 32.21±20.52 | 0.303 |
| No | 401(74.40) | 30.18±20.72 | 29.98±20.68 | ||
| HBV DNA (copies/mL) | |||||
| ≥103 | 281(52.13) | 30.23±19.87 | 0.000 | 30.22±19.87 | 0.000 |
| <103 | 128(23.75) | 39.98±18.05 | 39.77±18.05 | ||
| AFP (ng/mL) | |||||
| ≥400 | 195(36.18) | 24.35±20.46 | 0.000 | 24.19±20.40 | 0.000 |
| <400 | 323(59.53) | 35.85±19.18 | 35.70±19.19 | ||
| PT(sec) | |||||
| ≥12.1 | 275(51.02) | 25.75±19.89 | 0.000 | 25.66±19.87 | 0.000 |
| <12.1 | 263(48.79) | 35.99±20.17 | 35.77±20.17 | ||
| APTT(sec) | |||||
| ≥25.6 | 271(50.28) | 27.66±21.34 | 0.000 | 27.55±21.35 | 0.000 |
| <25.6 | 267(49.54) | 33.90±19.47 | 33.70±19.42 | ||
| Fbg (g/L) | |||||
| ≥2.83 | 269(49.91) | 23.88±20.43 | 0.000 | 23.76±20.33 | 0 |
| <2.83 | 269(49.91) | 37.62±18.51 | 37.44±18.58 | ||
| TT (sec) | |||||
| ≥18.4 | 275(51.02) | 30.71±20.57 | 0.995 | 30.63±20.52 | 0.943 |
| <18.4 | 263(48.79) | 30.80±20.77 | 30.58±20.78 | ||
| PLT (109/L) | |||||
| ≥178 | 250(46.38) | 26.60±20.20 | 0.000 | 26.44±20.04 | 0.000 |
| <178 | 248(46.01) | 34.95±20.36 | 34.78±20.37 |
Laboratory Measurements
As part of the physical examination, peripheral blood was collected in tubes containing sodium citrate prior to the initiation of any treatment to measure coagulation parameters between 7 and 8 am, and centrifuged at 3000 r/min for 10 min for plasma separation. The levels of PT, APTT, TT and Fbg were tested using a Sysmex CA-7000 automatic coagulation analyzer (Sysmex Corporation, Kobe, Japan). All reagents used in this study were provided by kinetic nephelometric detection system using a Diagon Dia-Timer 4 (Diagon Ltd, Budapest, Hungary). The platelet count (PLT) was measured from the blood count using a Sysmex XE-5000 automatic blood-cell counter (Sysmex Corporation, Kobe, Japan)
Follow-up
After completion of primary treatment, patients were generally followed up every 3 months in the first 2 years, every 6 months for the following 3 to 5 years, and annually thereafter for patients without evidence of recurrence. The last follow-up was in December 2016. The survival status was verified through checking clinical attendance records or direct telecommunication with the patient or their family (performed by The Medical Information Unit in our Cancer Center).
Statistical analysis
One outcome of our study was OS, which was calculated between the first diagnosis of HCC and death, or the date of the last follow-up. The other outcome was DFS, defined as the time from first diagnosis to the date of disease recurrence. Data were expressed as the mean and standard deviation (mean ± SD). Continuous variables were categorized using median values as cut-off point. Univariate and multivariate analyses of clinical variables were performed using Cox proportional hazards regression models. The results of this survey were analyzed using the Kaplan-Meier survival curves with the log-rank test and proportional hazard model. The correlation between PT, Fbg and Clinical Characteristics was analyzed using the Mann-Whitney U test and χ2 test. P values were derived from two-sided tests and P values <0.05 were regarded as statistically significant. All statistical tests were performed with SPSS 16.0 for Windows software (SPSS, Chicago, IL, USA).
Results
Patient Characteristics
From January 2009 to December 2012, 539 patients with a pathologically confirmed diagnosis of HCC were enrolled in this analysis, and their characteristics are presented in Table 1. There were 480 men (89.05%) and 59 women (10.95%), the median age was 53 years (range: 19 to 88 years). Of all patients, the pathological stage of I - II and III-IV were observed in 306 (56.77%), 233 (43.23%) of the patients, respectively. A total of 409 patients (75.88%) had hepatic B virus infection. In the entire cohort of clinical characteristics, pTNM status, tumor size, tumor number, node stage, distant metastases, HBV DNA, AFP, PT and Fbg had influence on OS and DFS of HCC patients.
Univariate and multivariate analyses of plasma hemostatic system in HCC
To determine the prognostic value of the pre-treatment hemostatic system in HCC, the clinical characteristics (including age, gender, alcohol index, BMI, family history of cancer, pTNM status, tumor size, tumor number, node stage, distant metastases, HBV DNA, AFP) and the hemostatic system profiles were subjected to univariate and multivariate analysis. With univariate analysis, TNM stage (P=0.000), tumor size (P=0.000), tumor number (P=0.000), node stage (P=0.000), distance metastases (P=0.009), BMI (P=0.042), AFP (P=0.000), HBV DNA (P=0.001), PT (P=0.000), APTT (P=0.011), Fbg (P=0.000), and PLT (P=0.000) were found to be associated with significantly overall survival (Table 2), also TNM stage (P=0.000), tumor size (P=0.000), tumor number (P=0.000), node stage (P=0.000), distance metastases (P=0.010), BMI (P=0.043), AFP (P=0.000), HBV DNA (P=0.001), PT (P=0.000), APTT (P=0.011), Fbg (P=0.000), and PLT (P=0.000) had effect on DFS (Table 3).
Table 2.
Univariate and multivariate cox hazards analysis for overall survival in 539 patients with HCC
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95%CI | p value* | HR | 95%CI | p value* |
| Gender | ||||||
| Male vs. Female | 1.041 | 0.681-1.191 | 0.854 | 1.214 | 0.657-2.242 | 0.537 |
| Age (years) | ||||||
| <53 vs.≥53 | 0.911 | 0.697-1.190 | 0.492 | 1.206 | 0.818-1.778 | 0.345 |
| TNM stage | ||||||
| III-IV vs. I-II | 4.387 | 3.290-5.849 | 0.000 | |||
| Tumor size(cm) | ||||||
| <5 vs.≥5 | 4.267 | 3.220-5.654 | 0.000 | 4.399 | 2.838-6.817 | 0.000 |
| Tumor number | ||||||
| Single vs. Multiple | 1.853 | 1.410-2.436 | 0.000 | 0.737 | 0.488-1.115 | 0.149 |
| Node stage | ||||||
| N0 vs. N1-2 | 2.293 | 1.487-3.536 | 0.000 | 1.513 | 0.753-3.040 | 0.245 |
| Distant metastases | ||||||
| Yes vs. No | 1.899 | 1.172-3.077 | 0.009 | 1.211 | 0.522-2.811 | 0.655 |
| BMI (kg/m2) | ||||||
| ≥24.0 vs. 18.5-23.9 vs. <18.5 | 0.793 | 0.634-0.992 | 0.042 | 0.977 | 0.721-1.324 | 0.880 |
| Alcohol behavior | ||||||
| Yes vs. No | 1.000 | 0.757-1.321 | 0.999 | |||
| Family history of cancer | ||||||
| Yes vs. No | 0.732 | 0.527-1.017 | 0.063 | |||
| HBV DNA | ||||||
| <103 vs. ≥103 | 2.010 | 1.349-2.993 | 0.001 | 1.830 | 1.190-2.814 | 0.006 |
| AFP | ||||||
| <400 vs. ≥400 | 2.066 | 1.564-2.729 | 0.000 | 1.519 | 1.038-2.224 | 0.031 |
| PT | ||||||
| <12.1 vs. ≥12.1 | 1.787 | 1.359-2.350 | 0.000 | 1.661 | 1.125-2.451 | 0.011 |
| APTT | ||||||
| <25.6 vs. ≥25.6 | 1.416 | 1.082-1.854 | 0.011 | 1.457 | 0.982-2.161 | 0.061 |
| Fbg | ||||||
| <2.83 vs. ≥2.83 | 2.794 | 2.102-3.714 | 0.000 | 2.158 | 1.427-3.263 | 0.000 |
| TT | ||||||
| <18.4 vs. ≥18.4 | 1.009 | 0.772-1.319 | 0.948 | |||
| PLT | ||||||
| <178 vs. ≥178 | 1.850 | 1.389-2.464 | 0.000 | 1.081 | 0.706-1.656 | 0.720 |
HR, Hazard ratio; 95% CI, 95% confidence interval;
*Cox hazard regression model.
Table 3.
Univariate and multivariate cox hazards analysis for Disease-free survival in 539 patients with HCC
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95%CI | p value* | HR | 95%CI | p value* |
| Gender | ||||||
| Male vs. Female | 1.038 | 0.679-1.587 | 0.863 | 1.212 | 0.656-2.240 | 0.537 |
| Age (years) | ||||||
| <53 vs.≥53 | 0.916 | 0.701-1.197 | 0.519 | 1.207 | 0.819-1.780 | 0.342 |
| TNM stage♯ | ||||||
| III-IV vs. I-II | 4.366 | 3.275-5.821 | 0.000 | |||
| Tumor size(cm) | ||||||
| <5 vs.≥5 | 4.247 | 3.206-5.628 | 0.000 | 4.388 | 2.831-6.802 | 0.000 |
| Tumor number | ||||||
| Single vs. Multiple | 1.850 | 1.407-2.432 | 0.000 | 0.738 | 0.488-1.116 | 0.150 |
| Node stage | ||||||
| N0 vs. N1-2 | 2.282 | 1.480-3.519 | 0.000 | 1.513 | 0.753-3.041 | 0.245 |
| Distant metastases | ||||||
| Yes vs. No | 1.893 | 1.1682-3.067 | 0.010 | 1.212 | 0.522-2.812 | 0.655 |
| BMI (kg/m2) | ||||||
| ≥24.0 vs. 18.5-23.9 vs. <18.5 | 0.794 | 0.635-0.993 | 0.043 | 0.977 | 0.721-1.325 | 0.881 |
| Alcohol behavior | ||||||
| Yes vs. No | 1.006 | 0.762-1.328 | 0.968 | |||
| Family history of cancer | ||||||
| Yes vs. No | 0.729 | 0.525-1.013 | 0.060 | |||
| HBV DNA | ||||||
| <103 vs. ≥103 | 2.005 | 1.346-2.987 | 0.001 | 1.829 | 1.190-2.812 | 0.006 |
| AFP | ||||||
| <400 vs. ≥400 | 2.065 | 1.563-2.728 | 0.000 | 1.518 | 1.037-2.222 | 0.032 |
| PT | ||||||
| <12.1 vs. ≥12.1 | 1.784 | 1.357-2.346 | 0.000 | 1.660 | 1.125-2.451 | 0.011 |
| APTT | ||||||
| <25.6 vs. ≥25.6 | 1.416 | 1.082-1.854 | 0.011 | 1.457 | 0.982-2.161 | 0.061 |
| Fbg | ||||||
| <2.83 vs. ≥2.83 | 2.787 | 2.096-3.705 | 0.000 | 2.161 | 1.429-3.267 | 0.000 |
| TT | ||||||
| <18.4 vs. ≥18.4 | 1.004 | 0.768-1.313 | 0.975 | |||
| PLT | ||||||
| <178 vs. ≥178 | 1.848 | 1.387-2.462 | 0.000 | 1.083 | 0.707-1.659 | 0.714 |
HR, Hazard ratio; 95% CI, 95% confidence interval;
*Cox hazard regression model.
All of the potentially important parameters identified in univariate analysis were further included in the multivariate analysis, and we exclude the factors of TNM stage in the multivariate analysis because of the influence of statistical colinearity. The result showed that tumor size (P=0.000), HBV DNA (P=0.006), AFP (P=0.031), PT (P=0.011) and Fbg (P=0.000) were identified as significantly independent predictors of OS of all patients (Table 2), and tumor size (P=0.000), HBV DNA (P=0.006), AFP (P=0.032), PT (P=0.011) and Fbg (P=0.000) were also independent prognostic indicators of DFS (Table 3).
Associations between plasma hemostatic system and HCC patients' survival
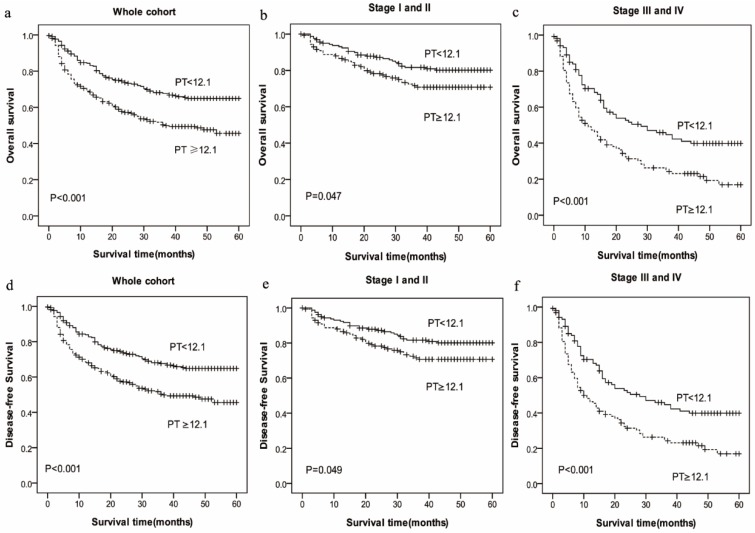
In the Kaplan-Meier analysis, PT was closely associated with OS and DFS. Among all the HCC patients, the mean OS in high-PT group (PT≥12.1sec, mean: 25.75 months) was shorter than low-PT group (PT<12.1sec, mean: 35.99 months) (P=0.000), and the mean DFS in high-PT group (PT≥12.1sec, mean: 25.66months) also was shorter than low-PT group (PT<12.1sec, mean: 35.77 months) (P=0.000) (Figure 1).
Figure 1.
Prognostic significance of prolonged PT in HCC in whole cohort and different pathological stages by Kaplan-Meier survival curves. OS and DFS of HCC patients showing significantly short survival with prolonged PT not only in the entire cohort (a, d) but also in the subgroups stratified by pathological stage (stage I-II (b, e), stage III-IV (c, f)).
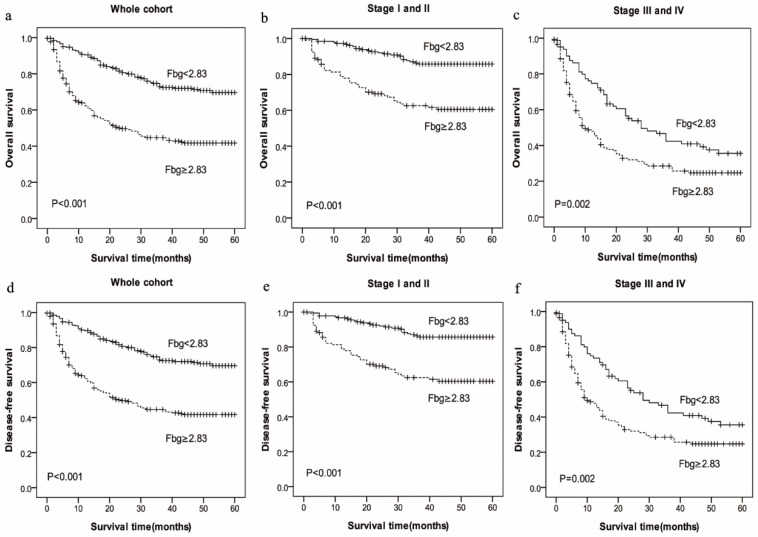
Meanwhile, the mean OS in high-Fbg group (Fbg≥2.83 g/L, mean: 23.88 months) was shorter than low-PT group (Fbg<2.83g/L, mean: 37.62 months) (P=0.000), and the mean DFS in high-PT group (mean: 23.76 months) also was shorter than low-PT group (mean: 37.44 months) (P=0.000). The results show that patients with increased PT and Fbg levels were significantly associated with shorter OS (P=0.000) and RFS (P=0.000) (Figure 2).
Figure 2.
Prognostic significance of increased Fbg levels in HCC in whole cohort and different pathological stages by Kaplan-Meier survival curves. OS and DFS of HCC patients showing significantly short survival with increased Fbg levels not only in the entire cohort (a, d) but also in the subgroups stratified by pathological stage (stage I-II(b, e), stage III-IV(c, f) ).
To further explore the relationship between PT and survival, we analyzed the prognostic effect of the PT level in subgroups based on the pathological stage. HCC patients with a higher PT level indicated significantly shorter OS and DFS than patients with a lower PT level in the both in stage I-II (P=0.047 vs. P=0.049) and stage III-IV (P=0.000 vs. P=0.000) subgroup, and the survival rate of patients with increased Fbg level had a significantly shorter OS and DFS compared with those patients with decreased Fbg levels both in stage I-II (P=0.000 vs. P=0.000) and stage III-IV subgroup (P=0.002 vs. P=0.002).
The Correlation between PT, Fbg concentrations and Clinicopathologic Characteristics in HCC patients
The associations between plasma PT, Fbg levels and clinicopathological variables in 539 HCC patients are presented in our study. The PT was associated with pathological stage and HBV DNA copies (P=0.038, P=0.000, respectively). Furthermore, all the patients were divided into two groups by the median PT (12.1sec). The PT levels were not significantly different between the two groups that were classified by age, gender, tumor size, tumor number, node stage, distant metastasis, BMI, alcohol behavior, family history of cancer, AFP. However, patients in advanced stages had longer PTs than those in the early stages of HCC (P=0.014), and patients with much more copies of HBV DNA also had longer PTs than those with less (P=0.000) (Table 4). Meanwhile, The Fbg was associated with pathological stage, tumor size, tumor number (P=0.0000, P=0.000, P=0.000, respectively). Furthermore, all the patients were divided into two groups by the median Fbg (2.83 g/L). The Fbg levels were not significantly different between the two groups that were classified by age, gender, distant metastasis, BMI, alcohol behavior, family history of cancer, HBV DNA copies, AFP. However, patients in advanced stages had higher Fbg levels than those in early stages of HCC (P=0.000) (Table 5).
Table 4.
Relationship between the PT levels and the clinical characteristics in patients with HCC
| Characteristics | PT | |||||
|---|---|---|---|---|---|---|
| Cases(n) | <12.1 | ≥12.1 | p value* | Mean ± SD | p value** | |
| Gender | ||||||
| Male | 479 | 231 | 248 | 0.383 | 12.25 ±1.13 | 0.079 |
| Female | 59 | 32 | 27 | 12.03 ±1.33 | ||
| Age (years) | ||||||
| ≥53 | 275 | 132 | 143 | 0.675 | 12.27±1.16 | 0.433 |
| < 53 | 263 | 131 | 132 | 12.36±1.16 | ||
| Stage | ||||||
| I and II | 305 | 161 | 144 | 0.038 | 12.13 ±1.14 | 0.014 |
| III and IV | 233 | 102 | 131 | 20.87 ±20.07 | ||
| Tumor size(cm) | ||||||
| ≥5 | 220 | 98 | 122 | 0.094 | 12.35±1.17 | 0.033 |
| <5 | 318 | 165 | 153 | 12.14±1.14 | ||
| Tumor number | ||||||
| Single | 335 | 168 | 167 | 0.748 | 12.20±1.13 | 0.770 |
| Multiple | 192 | 93 | 98 | 12.23±1.15 | ||
| Node stage | ||||||
| N0 | 501 | 246 | 255 | 0.711 | 12.22±1.13 | 0.659 |
| N1-2 | 37 | 17 | 20 | 12.38±1.39 | ||
| Distant metastases | ||||||
| Yes | 31 | 14 | 17 | 0.677 | 12.42±1.28 | 0.454 |
| No | 507 | 248 | 258 | 12.22±1.15 | ||
| BMI (kg/m2) | ||||||
| ≥24.0 | 174 | 91 | 83 | 0.000 | 12.19±1.21 | 0.156 |
| 18.5-23.9 | 318 | 152 | 166 | 12.21±1.13 | ||
| <18.5 | 45 | 19 | 26 | 12.49±1.10 | ||
| Alcohol behavior | ||||||
| Yes | 199 | 97 | 102 | 0.96 | 12.17±1.15 | 0.358 |
| No | 339 | 166 | 173 | 12.26±1.16 | ||
| Family history of cancer | ||||||
| Yes | 138 | 69 | 69 | 0.761 | 12.15±1.08 | 0.516 |
| No | 400 | 194 | 206 | 12.26±1.18 | ||
| HBV DNA (copies/mL) | ||||||
| ≥103 | 280 | 121 | 159 | 0.000 | 12.36±1.10 | 0.000 |
| <103 | 128 | 81 | 47 | 11.81±1.03 | ||
| AFP (ng/mL) | ||||||
| ≥400 | 194 | 88 | 106 | 0.125 | 12.35±1.12 | 0.018 |
| <400 | 323 | 169 | 154 | 12.13±1.11 | ||
Mean ± SD, Mean ± standard deviation.
*P values were calculated using the chi-squared test (χ2 test), p <0.05 indicated significant differences.
**P values were calculated using unpaired Student's t-tests or Mann-Whitney U test, p <0.05 indicated significant differences.
Table 5.
Relationship between the Fbg levels and the clinical characteristics in patients with HCC
| Characteristics | Fbg | |||||
|---|---|---|---|---|---|---|
| Cases(n) | <2.83 | ≥2.83 | p value* | Mean ± SD | p value* | |
| Gender | ||||||
| Male | 479 | 246 | 233 | 0.073 | 3.20 ±1.21 | 0.371 |
| Female | 59 | 23 | 36 | 3.21 ±1.06 | ||
| Age (years) | ||||||
| ≥53 | 275 | 132 | 143 | 0.000 | 3.24±1.25 | 0.253 |
| < 53 | 263 | 137 | 126 | 3.13±1.13 | ||
| Stage | ||||||
| I and II | 305 | 185 | 120 | 0.000 | 2.90 ±1.06 | 0.000 |
| III and IV | 233 | 84 | 149 | 3.55 ±1.26 | ||
| Tumor size(cm) | ||||||
| ≥5 | 220 | 77 | 143 | 0.000 | 3.58±1.27 | 0.000 |
| <5 | 318 | 192 | 126 | 2.91±1.05 | ||
| Tumor number | ||||||
| Single | 335 | 189 | 146 | 0.000 | 3.04±1.14 | 0.000 |
| Multiple | 191 | 77 | 114 | 3.39±1.23 | ||
| Node stage | ||||||
| N0 | 501 | 256 | 245 | 0.061 | 3.16±1.20 | 0.044 |
| N1-2 | 37 | 13 | 24 | 3.45±1.07 | ||
| Distant metastases | ||||||
| Yes | 31 | 15 | 16 | 0.853 | 3.26±1.20 | 0.709 |
| No | 507 | 254 | 253 | 3.18±1.19 | ||
| BMI (kg/m2) | ||||||
| ≥24.0 | 174 | 95 | 79 | 0.319 | 3.15±1.20 | 0.671 |
| 18.5-23.9 | 318 | 152 | 166 | 3.19±1.19 | ||
| <18.5 | 45 | 21 | 24 | 3.29±1.19 | ||
| Alcohol behavior | ||||||
| Yes | 199 | 103 | 96 | 0.532 | 3.24±1.29 | 0.791 |
| No | 339 | 166 | 173 | 3.15±1.13 | ||
| Family history of cancer | ||||||
| Yes | 138 | 71 | 67 | 0.693 | 3.15±1.19 | 0.524 |
| No | 400 | 198 | 202 | 3.19±1.19 | ||
| HBV DNA (copies/mL) | ||||||
| ≥103 | 280 | 139 | 141 | 0.275 | 3.18±1.18 | 0.886 |
| <103 | 128 | 71 | 57 | 3.19±1.35 | ||
| AFP (ng/mL) | ||||||
| ≥400 | 194 | 93 | 101 | 0.271 | 3.23±1.16 | 0.181 |
| <400 | 323 | 171 | 152 | 3.15±1.24 | ||
Mean ± SD, Mean ± standard deviation.
*P values were calculated using the chi-squared test (χ2 test), p <0.05 indicated significant differences.
**P values were calculated using unpaired Student's t-tests or Mann-Whitney U test, p <0.05 indicated significant differences.
A Novel hemostatic-Based Stage Predicts Overall Survival and of Patients with HCC
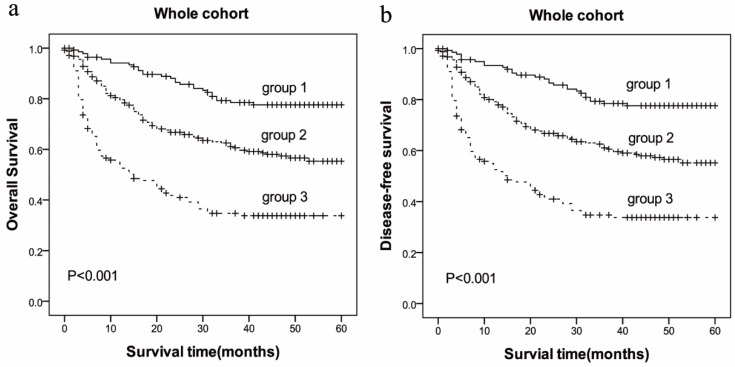
The prolonged PT and increased Fbg concentrations, which reflects poor OS and DFS of HCC, were prompted to develop a prognostic grouping system and evaluate the prognostic significance in HCC. Patients were assigned as follows: group 1 (low risk: PT<12.1sec and Fbg<2.83 g/L), group 2 (medium risk: PT≥12.1sec or Fbg≥2.83 g/L), group 3 (high risk: PT≥12.1sec and Fbg≥2.83 g/L). There were 139 (25.84%) allocated in group 1, 254 (47.21%) allocated in group 2, and 145 (26.95%) allocated in group 3. The 5-year OS for HCC patients in group 1, group 2 and group 3 were (41.37±17.76) months, (31.83±19.84) months, (18.68±18.41) months, respectively (P=0.000, Figure 3), and the 5-year DFS for HCC patients in group 1, group 2 and group 3 were (41.15±17.88) months, (31.65±19.81) months, (18.66±18.39) months, respectively (P=0.000, Figure 3).
Figure 3.
Patients with HCC were divided into three group by incorporating two independent prognostic variables (PT and Fbg). Group 1(low risk: PT<12.1sec and Fbg<2.83 g/L), group 2(medium risk: PT≥12.1sec or Fbg≥2.83 g/L), group 3(high risk: PT≥12.1sec and Fbg≥2.83 g/L).
Discussion
Hepatocellular carcinoma is a heterogeneous solid tumor affecting the main detoxifying organ in our body. And the treatment selection is tough because no diagnostic tool has been developed to detect HCC in its early stages, which lead to a short OS and DFS. Therefore, prognostic biomarkers are needed in understanding the development of cancer and choosing individual therapeutic strategies. Thus, it is urgent to find inexpensive and convenient prognostic biomarkers for this disease.
Coagulation parameters are routine laboratory tests, which are widely used to detect the function of coagulation, anticoagulation and fibrinolytic system. And many earlier investigations have noted the relationship between coagulation parameters and the prognostic significance of malignances. A systematic activation of clotting system has been observed in cancer patients which is usually reflected by subclinical abnormalities of conventional coagulation tests 15. In this study, we defined the cut-off value of PT and Fbg as the median, and demonstrated that pretreatment plasma PT and Fbg levels were associated with OS and DFS in HCC patients. In this retrospective study, we observed that patients with increased PT levels had significantly shorter OS and DFS compared with those patients with decreased PT levels, not only in the entire cohort (HR: 1.661, 95%CI: 1.125-2.451, p=0.011 vs. HR: 1.660, 95%CI: 1.125-2.451, p=0.011) but also in the subgroups stratified by pathological stage (stage I-II and stage III-IV). We also found that patients with a higher Fbg experienced significantly shorter OS and DFS than patients with a lower Fbg, not only in the entire cohort (HR: 2.158, 95%CI: 1.427-3.263, p<0.001 vs. HR: 2.161, 95%CI: 1.429-3.267, p<0.001), but also in the subgroups stratified by pathological stage (stage I-II and stage III-IV). Therefore, PT and Fbg are the independent risk factors for HCC prognosis. Our evidence shows that with advanced HCC pathological stage, the coagulation parameters were more impaired: patients with advanced HCC exhibited longer PT levels than patients with early-stage disease in the current study; Higher Fbg was found to be positively correlated with pathological stage, tumor size, tumor number, node metastasis in HCC.
The PT and Fbg tests are widely used in clinical laboratories to demonstrate the abnormal coagulation and fibrinolysis system. The system consists of extrinsic and intrinsic coagulation cascades, which could activate the prothrombin to thrombin, and formatted the fibrin clot. Meanwhile, the fibrin clot was lysed by the activated fibrinolysis system. And the associations between coagulation system and cancer have been frequently studied 16. First of all, many studies have demonstrated that coagulation system has an effect on the solid tumors, such as ESCC 10, penile cancer 17, HCC 18, lung cancer 11 and so on. Secondly, hematological system diseases have been reported related to this 19, 20. The last, Zhu et. suggested that abnormal coagulation system was an independent prognostic factor for the brain metastases of NSCLC 21. But, the function of coagulation and fibrinolysis system in carcinogenesis is complex and not well understood, which might be involved in advanced age, tumor-inflammatory activity, and cancer treatment (including chemotherapeutic agents, hormonal therapy, operative treatment).
As we all known, the liver is a vital organ of the body metabolism, which is play an important role in synthesizing clotting factors. Liver cells is damaged by various diseases, especially in patients with liver cancer, which lead to the reduction of protein synthesis including clotting factors, and the function of removal of tissue coagulation enzymes and fibrinolytic factors declined 21. Furthermore, tumor cells directly, produce various procoagulant activities (PCAs) and proinflammatory cytokines, among which, the best defined are the tissue factor (TF), the cancer procoagulant (CP), tumor necrosis factor (TNF-a), interleukin-1 (IL-1b) and vascular endothelial growth factor (VEGF). TF known as coagulation factor III, is a transmembrane glycoprotein, which comprise complex with factor VII, activating the extrinsic pathway of blood coagulation. Overexpression of TF in the blood of cancer patients not only originates from monocytes and other host cells, but also directly synthesized and released from malignant cells 23, which supports tumor metastatic development and dissemination 24. CP known as a cysteine proteinase, which exhibits procoagulant activity through directly activating factor X and is found principally in malignant tissues 25, but the precise role that CP plays in mediating the hypercoagulable state in malignancy remains speculative. TNF-α, IL-1β and VEGF are proinflammatory cytokines, in which, TNF-α and IL-1β are play a role in reducing activation of the C protein system, one of the principal endogenous anticoagulant defense systems 26. And the VEGF could be influenced by TF, is associated with tumor neovascularization 27. That is to say, the imbalance of tumor, coagulation and inflammation, not only function of blood coagulation disorders, but also promote the growth of tumor invasion and metastasis.
For prolonged PT, the exact mechanisms underlying the association remains unclear, some possible explanations as follows: Firstly, PT prolongation reflected the biosynthetic capacity of the liver, hepatic insufficiency may lead to decreased the coagulation factors (including FXIII, FX, FV and so on) in plasma, and hepatic congestion might also affect the production of coagulation factors; Secondly, activation of the coagulation system may result in consumption of coagulation factors. The increased inflammation and activated neurohormonal activity in tumor microenvironment, have been previously suggested to cause activated coagulation in HCC patients 28; Thirdly, some specific inhibitor of coagulation factors and lots of fibrin split products have been produced by tumor, which could delay the fibrin formation. Fbg is recognized as multiple integrin and nonintegrin receptors among tumor cells, stromal cells and inflammatory cells. The cellular interactions of Fbg mediated by specific receptors function as control cell proliferation, cell migration, apoptosis, and the expression of inflammatory mediators. Fbg may also play a major role in tumor metastasis, though the mechanism remains unclear 29. The associations between increased Fbg and cancer, several potential mechanisms have been postulated: 1. The function of synthesizing fibrinolytic enzyme inhibitor and removing of fibrin degradation products was damaged; 2. In blood circulation, the tumor cells, endothelial cells and platelets interacted with each other, which activated the platelets through releasing various bioactive substances, and the molecules in platelets particle, such as Fbg, may dilute into the blood circulation to participate in tumor metastasis 29; 3. The natural killer cytotoxicity with thrombin was blocked by Fbg layers, which can protect tumor cells from the innate immune system 32.
In summary, to our knowledge, this is the retrospective study investigating the relationship between pre-therapy plasma coagulation system and HCC. Based on the 539 patients, our study provides strong evidence supporting that pretreatment plasma PT and Fbg levels are prognostic factors for OS and DFS in HCC, both in the entire cohort and in groups stratified by pathological stage, which presents a high reproducibility and it can easily be measured in all diagnostic laboratories. As this study is a retrospective analysis, it is only valid for generating hypotheses and the value of PT and Fbg should be validated in larger prospective trials.
Acknowledgments
We thank the staff of the biochemical lab of Sun Yat-sen University Cancer Center, who provided various biochemical markers, and all the staff who supported our study.
Authors' contributions
Xueping Wang and Minjie Mao carried out the main work and contributed equally. They participated in the design of the study and drafted the manuscript. Lin Zhang and Peidong Chi performed the statistical analysis. Wanli Liu and Shuqin Dai conceived the study and participated in its design and coordination, Zhonglian He and Jiarui Su helped to draft the manuscript. All authors read and approved the final manuscript.
Abbreviations
- HCC
Hepatocellular carcinoma
- OS
overall survival
- DFS
disease-free survival
- AFP
α-fetoprotein
- GGT
γ-glutamyl transferase
- CEA
carcino-embryonic antigen
- PT
Prothrombin time
- APTT
activated partial thromboplastin time
- TT
thrombin time
- Fbg
fibrinogen
- INR
international normalized ratio.
References
- 1.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 3.Kabbach G, Assi HA, Bolotin G. et al. Hepatobiliary Tumors: Update on Diagnosis and Management. J Clin Transl Hepatol. 2015;3:169–181. doi: 10.14218/JCTH.2015.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J, Xia Y, Shi L. et al. Elevated Serum Carcinoembryonic Antigen Is Associated with a Worse Survival Outcome of Patients After Liver Resection for Hepatocellular Carcinoma: a Propensity Score Matching Analysis. J Gastrointest Surg. 2016;20:2063–2073. doi: 10.1007/s11605-016-3295-8. [DOI] [PubMed] [Google Scholar]
- 5.Loreto MF, De Martinis M, Corsi MP. et al. Coagulation and cancer: implications for diagnosis and management. Pathol Oncol Res. 2000;6:301–312. doi: 10.1007/BF03187336. [DOI] [PubMed] [Google Scholar]
- 6.Amirkhosravi A, Meyer T, Amaya M. et al. The role of tissue factor pathway inhibitor in tumor growth and metastasis. Semin Thromb Hemost. 2007;33:643–652. doi: 10.1055/s-2007-991531. [DOI] [PubMed] [Google Scholar]
- 7.Langer F, Holstein K, Eifrig B, Bokemeyer C. [Haemostatic aspects in clinical oncology] Hamostaseologie. 2008;28:472–480. [PubMed] [Google Scholar]
- 8.Falanga A, Marchetti M, Vignoli A, Balducci D. Clotting mechanisms and cancer: implications in thrombus formation and tumor progression. Clin Adv Hematol Oncol. 2003;1:673–678. [PubMed] [Google Scholar]
- 9.McAlister RK, Ito S. Minimal Prolongation of Prothrombin Time with Extended Exposure to Argatroban. Pharmacotherapy. 2015;35:e122–e126. doi: 10.1002/phar.1613. [DOI] [PubMed] [Google Scholar]
- 10.Li XH, Wang XP, Gu WS. et al. Clinical Significance of Preoperative Thrombin Time in Patients with Esophageal Squamous Cell Carcinoma following Surgical Resection. PLoS One. 2015;10:e140323. doi: 10.1371/journal.pone.0140323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tas F, Kilic L, Serilmez M. et al. Clinical and prognostic significance of coagulation assays in lung cancer. Respir Med. 2013;107:451–457. doi: 10.1016/j.rmed.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Bottasso B, Mari D, Coppola R. et al. Hypercoagulability and hyperfibrinolysis in patients with melanoma. Thromb Res. 1996;81:345–352. doi: 10.1016/0049-3848(96)00006-0. [DOI] [PubMed] [Google Scholar]
- 13.Shu YJ, Weng H, Bao RF. et al. Clinical and prognostic significance of preoperative plasma hyperfibrinogenemia in gallbladder cancer patients following surgical resection: a retrospective and in vitro study. BMC Cancer. 2014;14:566. doi: 10.1186/1471-2407-14-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vlodavsky I, Ilan N, Nadir Y. et al. Heparanase, heparin and the coagulation system in cancer progression. Thromb Res. 2007;120(Suppl 2):S112–S120. doi: 10.1016/S0049-3848(07)70139-1. [DOI] [PubMed] [Google Scholar]
- 15.Falanga A. Thrombophilia in cancer. Semin Thromb Hemost. 2005;31:104–110. doi: 10.1055/s-2005-863812. [DOI] [PubMed] [Google Scholar]
- 16.Prandoni P, Lensing AW, Buller HR. et al. Deep-vein thrombosis and the incidence of subsequent symptomatic cancer. N Engl J Med. 1992;327:1128–1133. doi: 10.1056/NEJM199210153271604. [DOI] [PubMed] [Google Scholar]
- 17.Ma C, Zhou Y, Zhou S, Preoperative peripheral plasma fibrinogen level is an independent prognostic marker in penile cancer. Oncotarget; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao H, Zhu F, Wang M, Preoperatively staging liver fibrosis using noninvasive method in Hepatitis B virus-infected hepatocellular carcinoma patients. Oncotarget; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bottasso B, Mari D, Coppola R. et al. Hypercoagulability and hyperfibrinolysis in patients with melanoma. Thromb Res. 1996;81:345–352. doi: 10.1016/0049-3848(96)00006-0. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh BC, Cliffton EE. Malignant tumors with superior vena cava obstruction. N Y State J Med. 1973;73:283–289. [PubMed] [Google Scholar]
- 21.Zhu JF, Cai L, Zhang XW. et al. High plasma fibrinogen concentration and platelet count unfavorably impact survival in non-small cell lung cancer patients with brain metastases. Chin J Cancer. 2014;33:96–104. doi: 10.5732/cjc.012.10307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aytac S, Turkay C, Bavbek N, Kosar A. Hemostasis and global fibrinolytic capacity in chronic liver disease. Blood Coagul Fibrinolysis. 2007;18:623–626. doi: 10.1097/MBC.0b013e328285d80e. [DOI] [PubMed] [Google Scholar]
- 23.Rak J, Milsom C, May L. et al. Tissue factor in cancer and angiogenesis: the molecular link between genetic tumor progression, tumor neovascularization, and cancer coagulopathy. Semin Thromb Hemost. 2006;32:54–70. doi: 10.1055/s-2006-933341. [DOI] [PubMed] [Google Scholar]
- 24.Francis JL, Biggerstaff J, Amirkhosravi A. Hemostasis and malignancy. Semin Thromb Hemost. 1998;24:93–109. doi: 10.1055/s-2007-995829. [DOI] [PubMed] [Google Scholar]
- 25.Donati MB, Gambacorti-Passerini C, Casali B. et al. Cancer procoagulant in human tumor cells: evidence from melanoma patients. Cancer Res. 1986;46:6471–6474. [PubMed] [Google Scholar]
- 26.Dittman WA, Majerus PW. Structure and function of thrombomodulin: a natural anticoagulant. Blood. 1990;75:329–336. [PubMed] [Google Scholar]
- 27.Mackman N. Role of tissue factor in hemostasis and thrombosis. Blood Cells Mol Dis. 2006;36:104–107. doi: 10.1016/j.bcmd.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Cugno M, Mari D, Meroni PL. et al. Haemostatic and inflammatory biomarkers in advanced chronic heart failure: role of oral anticoagulants and successful heart transplantation. Br J Haematol. 2004;126:85–92. doi: 10.1111/j.1365-2141.2004.04977.x. [DOI] [PubMed] [Google Scholar]
- 29.Jones JM, McGonigle NC, McAnespie M. et al. Plasma fibrinogen and serum C-reactive protein are associated with non-small cell lung cancer. Lung Cancer. 2006;53:97–101. doi: 10.1016/j.lungcan.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Jones JM, McGonigle NC, McAnespie M. et al. Plasma fibrinogen and serum C-reactive protein are associated with non-small cell lung cancer. Lung Cancer. 2006;53:97–101. doi: 10.1016/j.lungcan.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Palumbo JS, Kombrinck KW, Drew AF. et al. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood. 2000;96:3302–3309. [PubMed] [Google Scholar]
- 32.Zheng S, Shen J, Jiao Y. et al. Platelets and fibrinogen facilitate each other in protecting tumor cells from natural killer cytotoxicity. Cancer Sci. 2009;100:859–865. doi: 10.1111/j.1349-7006.2009.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]