Abstract
Objective:
The objective of this study is to examine the impact of vary doses of buprenorphine on anxiety symptoms in opioid-dependent inpatients over a 7 days period, using a randomized controlled trial design.
Design:
Patients were randomized to three groups.
Patients and Methods:
Fourteen men who met the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition criteria for both opioid use disorder and generalized anxiety disorder and were seeking for treatment.
Intervention:
Patients obtain dosages of 32 mg or 64 mg or 96 mg of buprenorphine as a single dose only and were treated in a psychiatric inpatient unit. Of 14 subjects; 5 (35.7%) obtained 32 mg, 4 (28.6%) obtained 64 mg, and 5 (35.7%) obtained 96 mg of buprenorphine.
Measurements:
Administering daily Hamilton Anxiety Rating Scale and interview.
Results:
All the patients ended the 7-day treatment time. The results showed a significant reduction in anxiety symptoms within each of the three groups (P = 0.00), but no difference in outcome between the groups (P = 0.605).
Conclusions:
The outcome suggests a single high dose of buprenorphine can supply a speedy, safe, simple, and suitable means of anxiety treatment. The single high dose of buprenorphine could be a novel mechanism medication that provides a rapid and sustained improvement for generalized anxiety disorder in opioid dependent patients. Placebo-controlled trials of longer duration are needed to evaluate ability, safety, and psychological and physiological influence of extended exposure to this medication.
Keywords: Anxiety, buprenorphine, opioid-dependent
INTRODUCTION
A significant number of studies and clinical trials reveal that patients with substance use disorders have high rates of anxiety and depression,[1,2,3,4] personality disorders,[5,6] and also minor psychopathology.[7,8]
Some studies disclosed that success in the detoxification therapy of patients with opioid dependence could be prognosticated by primary psychiatric symptoms.[9] Coexisting of mental problems principally depression and anxiety can interfere with the course and prognosis of substance use disorders. Studies illuminate that opioid-dependent patients associated with anxiety or depression in the beginning of treatment might have reduced the chance to be in remission and have clean urines at follow-up than opioid dependents with a normal mood.[10]
Buprenorphine, ketamine, or ayahuasca can reduce the level of depression or anxiety.[10,11,12,13,14,15,16,17,18,19,20]
An Amazonian botanical hallucinogenic substance - Ayahuasca - includes dimethyltryptamine, which is an agonist of 5-HT2A receptor, and also harmine, which is a monoamine-oxidase A inhibitor. Ayahuasca lower the severity of depression and anxiety. A single dose of ayahuasca lessens the level of depression and anxiety very promptly.[11,12]
Likewise, buprenorphine medication can reduce the level of anxiety and depression straightaway.[13,14]
Buprenorphine medication which is a partial agonist of μ-opioid receptors has a high affinity for these receptors. Likewise, it binds more firmly to these receptors than full opioid agonists. Buprenorphine has pharmacological properties that are more features of an antagonist. Hence, can have considerable denotation for clinical practice. Buprenorphine has the dual influence of causing opioid reactions while obstructing the effects of extra opioid use. It is usually administered for the management of pain and opioid withdrawal symptoms.
Although some investigations indicated a reduction of depression, however, buprenorphine is not Food and Drug Administration approved nor planned, to manage depression. Clinical trials required to test its effects have not been conducted yet. Buprenorphine should be considered as possibly addicting itself. Thus, it should not be usually used for this aim. Significant investigations and also clinical trials are needed to recognize this field. We are now optimistic that research workers will open the basis for treatment of depression and anxiety in opioid dependents.[13,15]
Buprenorphine is regarded to treat opioids withdrawals and pain.[16]
We are now using a single dose of sublingual buprenorphine only as a new approach for the treatment of anxiety symptoms because we consider and theorize that (our rationale) the biochemistry in anxiety is more or less similar to opioid dependence.[16,17,18,19,20]
We could not find published controlled findings on this issue (single high dose buprenorphine for the therapy of anxiety symptoms in opioid dependents). Hence, our report may display a new finding.
The essential goal of the current pilot study was to test the impact of vary single high doses of buprenorphine (32, 64, and 96 mg buprenorphine) for the treatment of anxiety symptoms in opioid-dependent population.
PATIENTS AND METHODS
Subjects
All patients admitted consecutively to an inpatient psychiatric hospital in Shiraz city during 2016 entered the study. Fourteen unpaid male patients were enrolled in the study. Only males were selected for the study because the rate of opioid abuse is thought to be negligible among females. Shiraz is the Capital City of Fars Province with a population of over 1.5 million, in the South Iran. At screening, patients were examined by a physician to establish eligibility. Before each interview, we explained the aims of the research study, guaranteed confidentiality, and obtained informed consent. The interviews and examinations were done on the premises of the treatment hospital because it seemed a nonthreatening and suitable setting. Family members, relatives, or friends accompanied most patients to the hospital; this attendance provided a condition to confirm some of the data obtained from the patients. They had to meet the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria for both opioid use disorder and generalized anxiety disorder. Daily use of an opioid for at least 1 year was a requisite. Patients were kept out from the trial if they had a substance use disorder other than opioid. Patients who were not interested in participation at the beginning of the study were excluded.
Buprenorphine (as a single dose only) was administered gradually when the opioid use disorder patients were moderately in withdrawal from opioids.
Procedure
The current research was a three group, randomized, double-blind study. Both investigators and patients were blind to the study. Subjects were randomized to treatment groups in consecutive numerical order by the investigational team (patients could not decide, to which group to be enrolled). The research team included addiction psychiatrist, general physician, and psychologist.
The Hamilton Anxiety Rating Scale (HAM-A) was used to rate the level of anxiety in opioid-dependent men, having generalized anxiety disorder as well.
The HAM-A was applied to the inpatients before beginning of opioid withdrawal symptoms.
Randomization to research study was carried out by appointing the patients to the medication. Patients were enrolled onto 32 mg or 64 mg or 96 mg of buprenorphine as a single dose only. Out of 14 patients; 5 (35.7%) received 32 mg, 4 (28.6%) received 64 mg, and 5 (35.7%) received 96 mg of buprenorphine.
Subjects were studied for 7 days. Outcome was calculated by daily measurement of anxiety score based on the HAM-A, and interview.
Statistical Analysis
Statistical analyses included both descriptive statistics and inference statistical procedures. Data analysis was done using SPSS version 18 for windows (USA). Chi-square analyses were used to test for differences in frequencies, and ANOVA or t-test analyses were used to test for differences in means among the three groups. These were two-sided with significance set at P < 0.05.
RESULTS
All the 14 inpatients completed the 7-day period study. Hence, the data were gathered from 14 male opioid dependents whose mean age was 31.87 ± 8.15 year; with age range of 17–48 years.
Out of 14 patients; 5 (35.7%) obtained 32 mg, 4 (28.6%) obtained 64 mg and also 5 (35.7%) obtained 96 mg of buprenorphine.
Regarding marital status, age, education, and employment, we could not find any statistically significant differences in groups.
Table 1 demonstrates demographic features of the patients. As we note, there are not any significant differences in their demographic features.
Table 1.
Demographic characteristics of 14 subjects
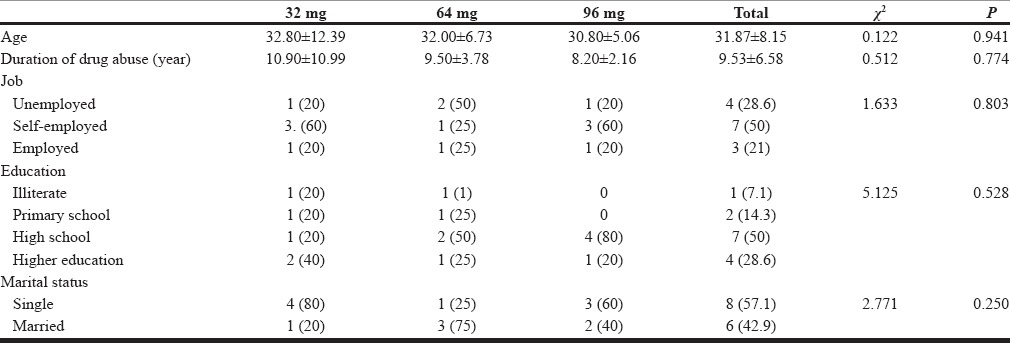
Table 2 represents anxiety scores of the groups during 7 days of treatment time. As we look in 32-mg group, we note that there are significant statistical differences in anxiety scores between day 1 and day 7 (P = 0.001). Similarly, there are significant differences in anxiety scores between day 1 and day 7 both in 64-mg group (P = 0.000) and 96-mg group (P = 0.00).
Table 2.
Hamilton Anxiety Rating Scale
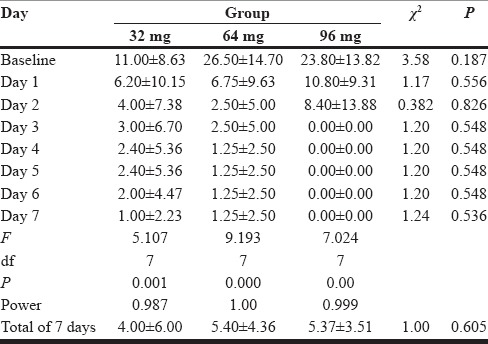
Comparing the mean anxiety scores in three groups, we cannot see any significant differences.
Figure 1 portrays the anxiety scores from day 1 to day 7 in all groups.
Figure 1.
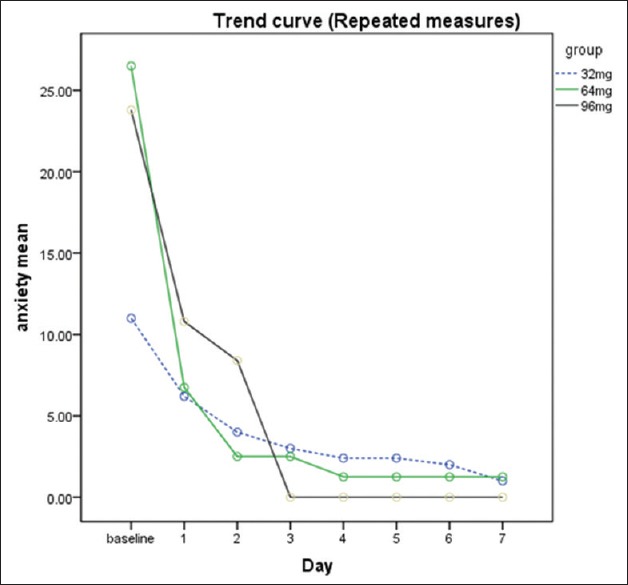
Hamilton Anxiety Rating Scale
DISCUSSION
The results showed a significant reduction in anxiety symptoms within each of the three groups (P = 0.00), but no difference in outcome between the groups (P = 0.605).
The current clinical study displayed that a single high dose buprenorphine appears to be significantly efficient, safe and helpful. This trial suggests that buprenorphine can supply a simple, speedy, safe, and suitable means of treatment of anxiety. Giving buprenorphine medication as single high dose looks to lower concerns of compliance, in addition to decreasing the possibility of buprenorphine being diverted for dependency and abuse. Likewise, the cost benefits appear to be favorable, primarily while considering the chance of using for outpatients without a necessity for admission to a hospital. Still, the findings of this research require to be repeated in a larger sample with a control group receiving a conventional mode of treatment and in an outpatient center.
CONCLUSIONS
The results illustrated a significant reduction in anxiety symptoms within each of the three groups, but no difference in outcome between the groups.
Our findings documented that giving a single high dose of buprenorphine might provide a simple, speedy, safe, and suitable means of anxiety reduction. A single dose of buprenorphine could be a new mechanism medication that provides a rapid and sustained improvement for anxiety symptoms in opioid-dependent patients. Placebo-controlled clinical trials of longer duration are required to test efficacy, safety, and psychological and physiological effect of extended exposure to this medication.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This research proposal was approved on August 21, 2016, by Ethic Research Committee of Shiraz University of Medical Sciences No. IR.SUMS.REC.1395.102, and was granted by Vice Chancellery for Research, Shiraz University of Medical Sciences, Shiraz, Iran.
REFERENCES
- 1.Karp JF, Butters MA, Begley AE, Miller MD, Lenze EJ, Blumberger DM, et al. Safety, tolerability, and clinical effect of low-dose buprenorphine for treatment-resistant depression in midlife and older adults. J Clin Psychiatry. 2014;75:e785–93. doi: 10.4088/JCP.13m08725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorus W, Senay EC. Depression, demographic dimensions, and drug abuse. Am J Psychiatry. 1980;137:699–704. doi: 10.1176/ajp.137.6.699. [DOI] [PubMed] [Google Scholar]
- 3.Ross HE, Glaser FB, Germanson T. The prevalence of psychiatric disorders in patients with alcohol and other drug problems. Arch Gen Psychiatry. 1988;45:1023–31. doi: 10.1001/archpsyc.1988.01800350057008. [DOI] [PubMed] [Google Scholar]
- 4.Rounsaville BJ, Weissman MM, Kleber H, Wilber C. Heterogeneity of psychiatric diagnosis in treated opiate addicts. Arch Gen Psychiatry. 1982;39:161–8. doi: 10.1001/archpsyc.1982.04290020027006. [DOI] [PubMed] [Google Scholar]
- 5.DeJong CA, van den Brink W, Harteveld FM, van der Wielen EG. Personality disorders in alcoholics and drug addicts. Compr Psychiatry. 1993;34:87–94. doi: 10.1016/0010-440x(93)90052-6. [DOI] [PubMed] [Google Scholar]
- 6.Nace EP, Davis CW, Gaspari JP. Axis II comorbidity in substance abusers. Am J Psychiatry. 1991;148:118–20. doi: 10.1176/ajp.148.1.118. [DOI] [PubMed] [Google Scholar]
- 7.Darke S, Wodak A, Hall W, Heather N, Ward J. Prevalence and predictors of psychopathology among opioid users. Br J Addict. 1992;87:771–6. doi: 10.1111/j.1360-0443.1992.tb02723.x. [DOI] [PubMed] [Google Scholar]
- 8.Swift W, Williams G, Neill O, Grenyer B. The prevalence of minor psychopathology in opioid users seeking treatment. Br J Addict. 1990;85:629–34. doi: 10.1111/j.1360-0443.1990.tb03523.x. [DOI] [PubMed] [Google Scholar]
- 9.Kosten TR, Rounsaville BJ, Kleber HD. DSM-III personality disorders in opiate addicts. Compr Psychiatry. 1982;23:572–81. doi: 10.1016/0010-440x(82)90050-5. [DOI] [PubMed] [Google Scholar]
- 10.Rounsaville BJ, Kosten T, Kleber H. Success and failure at outpatient opioid detoxification. Evaluating the process of clonidine- and methadone-assisted withdrawal. J Nerv Ment Dis. 1985;173:103–10. doi: 10.1097/00005053-198502000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Osório Fde L, Sanches RF, Macedo LR, Santos RG, Maia-de-Oliveira JP, Wichert-Ana L, et al. Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: A preliminary report. Rev Bras Psiquiatr. 2015;37:13–20. doi: 10.1590/1516-4446-2014-1496. [DOI] [PubMed] [Google Scholar]
- 12.Sanches RF, de Lima Osório F, Dos Santos RG, Macedo LR, Maia-de-Oliveira JP, Wichert-Ana L, et al. Antidepressant Effects of a Single Dose of Ayahuasca in patients with recurrent depression: A SPECT study. J Clin Psychopharmacol. 2016;36:77–81. doi: 10.1097/JCP.0000000000000436. [DOI] [PubMed] [Google Scholar]
- 13.Ahmadi J. Fast treatment of methamphetamine related anxiety and depressive disorders: A novel approach. J Addict Med Ther Sci. 2016;1:44–6. Doi: 1017352/2455-3484000011. [Google Scholar]
- 14.Ahmadi J. Instant detoxification of heroin with high dose of buprenorphine. J Addict Prev. 2016;4:3. [Google Scholar]
- 15.Gracer R. The Buprenorphine Effect on Depression. The National Alliance of Advocates for Buprenorphine Treatment (NAABT); February. 2007 Newsletter. [Google Scholar]
- 16.Sadock B, Sadock V, Ruiz P, editors. Kaplan and Sadock's Synopsis of Psychiatry. Philadelphia, USA: Lippincott Williams & Wilkins; 2015. [Google Scholar]
- 17.Gerra G, Leonardi C, D’Amore A, Strepparola G, Fagetti R, Assi C, et al. Buprenorphine treatment outcome in dually diagnosed heroin dependent patients: A retrospective study. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:265–72. doi: 10.1016/j.pnpbp.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Ipser JC, Terburg D, Syal S, Phillips N, Solms M, Panksepp J, et al. Reduced fear-recognition sensitivity following acute buprenorphine administration in healthy volunteers. Psychoneuroendocrinology. 2013;38:166–70. doi: 10.1016/j.psyneuen.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Maremmani AG, Rovai L, Pani PP, Pacini M, Lamanna F, Rugani F, et al. Do methadone and buprenorphine have the same impact on psychopathological symptoms of heroin addicts? Ann Gen Psychiatry. 2011;10:17. doi: 10.1186/1744-859X-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falcon E, Maier K, Robinson SA, Hill-Smith TE, Lucki I. Effects of buprenorphine on behavioral tests for antidepressant and anxiolytic drugs in mice. Psychopharmacology (Berl) 2015;232:907–15. doi: 10.1007/s00213-014-3723-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


