Understanding the inner workings of an ion channel has, in the last several years, boiled down to a hunt for dynamic structural cartoons that capture the essence of the channel's biophysical behavior. The main approach to this end has been the “structure–function” study in which selected residues are mutated and the functional consequences are explored electrophysiologically. Functionally important regions of the ion channel protein are often revealed from this type of experiment. Nevertheless, these studies are typically devoid of anything approaching real structural data. Therefore, the cartoon models depicting, for example, the way ion channels open and close are largely fantasies, however much insight they provide. This deficiency changed dramatically with the first crystal structure of a bacterial potassium channel (1). The significance of this scientific breakthrough included the sobering realization that standard structure–function studies can result in absurd structural predictions, highlighting the importance of atomic level structures to accompany functional studies.
The most captivating property of the ion channels responsible for action potentials in excitable cells (nerve and muscle cells) is their exquisite sensitivity to small changes of membrane potential. This is arguably the most highly scrutinized feature of these proteins since Hodgkin and Huxley (2) first described the sodium and potassium currents of the squid giant axon in 1952. However, despite a wealth of electrophysiological studies and a surfeit of discarded cartoons, the atomic structure of a voltage-gated ion channel remained elusive until 2003, when the MacKinnon laboratory obtained a crystal structure of the potassium channel KvAP (3). A functional study of KvAP by the same group led to a proposal, known as the “voltage sensor paddle model” (4), that was so surprising and apparently contradictory with a wide variety of previous experimental data that it generated an extensive controversy (e.g., see refs. 5–7) and a flurry of new experimental studies. The results of most of these studies are at variance with the paddle model, including those in the article by Gonzalez et al. (8) in this issue of PNAS.
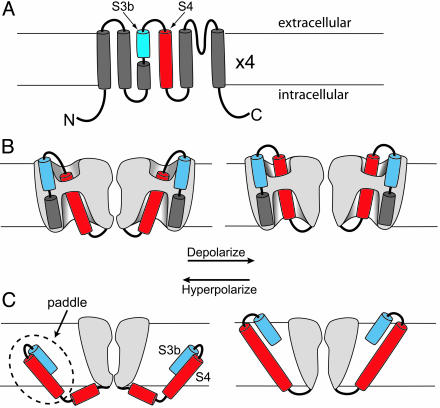
In the BP (before paddle) era, voltage-gated potassium channels were shown to be tetramers in which each subunit had six transmembrane segments (Fig. 1A). The fourth transmembrane segment, S4, is the principal voltage sensor of the channel (9). Every third residue of S4 is basic, usually an arginine, and neutralizing any of the first four of these arginines reduces the channel's sensitivity to changes of membrane potential. The consensus opinion is that the positively charged S4 segment can be electrophoresed across the membrane electric field in response to changes of membrane potential; this movement controls whether the permeation pathway is available for the flux of ions. Exactly how the S4 segment moves distinguishes the paddle model from all others. In many BP-era models, the S4 segment is largely surrounded by aqueous solutions and moves its charge through a hydrophobic gasket made primarily of protein (Fig. 1B). Although several variations on this theme have been proposed, all of them involve a small physical movement of the outer four arginines through an electric field highly focused by the aqueous vestibules surrounding the S4 segment (9). In all of these models, the side chains of the S4 arginines transfer their charges through proteinaceous terrain. In the paddle model, the S4 segment and the C-terminal helix of the S3 segment (S3b) form a compact structure (the voltage sensor paddle) that extends into lipid at the periphery of the channel protein (Fig. 1C). In response to a change of membrane potential, the paddle moves a large distance (15–28 Å; refs. 4 and 10) through the lipid, schlepping its cargo of positive charges along with S3b.
Fig. 1.
Models of topology and voltage sensor movement. The membrane is indicated by horizontal lines. (A) Topology of a potassium channel subunit with six transmembrane segments (S1–S6). The S3 segment is divided into two helices, S3a and S3b. The S4 segment is in red. (B) Conventional model of voltage sensor movement. Two of the four subunits are shown (sliced open to expose the “gating pore”) with the ion permeation path between them. The foreground and background subunits are removed for clarity. Gray represents protein. Depolarization (Right) moves the extracellular portion of the S4 segment (red) outward through a short hydrophobic gating pore, opening the permeation pathway. Most of the S4 segment is surrounded by hydrophilic vestibules. The transmembrane electric field falls mainly across the gating pore. (C) Paddle model, which is adapted from ref. 4. Two opposing subunits are shown. Depolarization moves the paddle outward through lipid, pulling the cytoplasmic activation gate open. The transmembrane electric field felt by S4 arginines falls mainly across the lipid. B and C are adapted from ref. 11.
What's so contentious about the paddle model? That depends on how it is defined. In the originally published version (figure 5 in ref. 4), the S2 and S3a segments were oriented in the same plane as the bilayer, with the S1–S2 and S2–S3 linkers buried within lipid. In a modified version (10), the S1–S4 segments were all rotated into transmembrane orientations, consistent with experimental data on potassium channel topology (5–7). The original paddle model also advanced the energetically unpalatable idea that the side chains of charge-carrying arginines cross the membrane in intimate association with the hydrocarbon core of the bilayer rather than with protein. Subsequent experimental data suggest otherwise (11–13), and a newer variant of the model, although only partially fleshed out, acknowledges this accommodation (10). Other controversial aspects of the paddle model are discussed elsewhere (5–7).
In the space of 2 years, the paddle model has morphed into something more akin to the previous models. To be fair, these older models have also taken on one feature of the paddle model. In many of the previous proposals, including several from my laboratory, the S4 segment was completely insulated from lipid. We and others now acknowledge that, although the business district through which S4 transports its charges is proteinaceous, at least some of S4's backside is exposed to lipid. Nevertheless, the two classes of models continue to differ in one key aspect, the physical magnitude of S4 movement. The paddle model proposes a substantially larger transmembrane displacement of the S4 segment, because the critical arginine side chains must each move at least 80% of the distance across the wide electric field of the bilayer. In conventional models, these charges only need to cross a focused electric field (e.g., Fig. 1B), a distance that may extend over only a few angstroms (9). This discriminating feature was tested experimentally by Gonzalez et al. (8).
A large movement of the paddle has consequences. Membrane potential moves the N terminus of S4 between aqueous compartments on opposite sides of the membrane (14, 15). If this S4 movement is large, it should also drag S3b with it. Thus, if S3b residues are accessible to the extracellular solution at depolarized voltages, then, at hyperpolarized voltages, these residues should either become buried in lipid or in the extreme traverse to the cytoplasmic side. Moreover, because S4 is moving its outermost charges through the electric field, a physically large S4 movement should carry any charged residues on S3b at least partway through the electric field. Small-movement conventional models, by contrast, predict that S3b will remain near the extracellular surface at all voltages and that charged residues on S3b will not contribute to the load carried by the S4 arginines. Gonzalez et al. (8) tested the voltage-dependent accessibility of S3b residues by cysteine scanning with extracellular methanethiosulfonate-ethyltrimethylammonium (MTSET), a permanently charged cysteine reagent that is excluded from the hydrophobic lipid. Also, they tested whether the side chains of S3b residues move through the electric field by altering the charge of these residues and determining whether this affects the total number of charges coupled to channel opening.
The voltage-dependent accessibility of cysteines introduced into S3b was examined previously (16, 17), and, in both of these studies, the cysteines were accessible to extracellular MTSET at hyperpolarized voltages. Gonzalez et al. (8) extended the scan all of the way down to the C terminus of S3a (residue I315) in Shaker potassium channels. The mutant I315C was equally reactive to extracellular MTSET at –110 mV as at +100 mV. It could be argued, however, that these results are not in direct contradiction with the paddle model developed originally for the KvAP channel, which has a very short (3-aa) linker between S3b and S4. With such a short linker, it is difficult to imagine how S4 could move a large distance without dragging S3b with it. However, Shaker's S3–S4 linker has 26 residues, leaving open the possibility that S4 could drag the linker and leave the S3b segment relatively stationary. Gonzalez et al. (8) eliminated this criticism by lopping off most of the linker, leaving only three residues, as in KvAP. This construct exhibits relatively normal gating with as much voltage dependence as a wild-type Shaker channel (18). The results with the short-linker channel, including those for the I315C mutant, are nearly indistinguishable from those with the long-linker channel and argue against a large transmembrane movement of the S4 segment during charge movement.
Gonzalez et al. (8) applied a second test of the paddle model. If S4 shuttles its charges a long distance across the electric field, then it should also move other paddle residues, whether charged or not, through the electric field. Therefore, any changes in the total charge of paddle residues should influence the voltage dependence of gating. The number of charges moved completely through the electric field during gating was estimated by the steepness of the voltage dependence of channel opening at very negative voltages, the “limiting-slope” method (19, 20). Gonzalez et al. (8) found that even drastic changes in total charge, e.g., neutralization of four consecutive acidic residues at the extracellular end of S3b (a total of 16 elementary charges for the four subunits), had no effect on the number of charges coupled to gating. Moreover, charge alterations in the middle of S3b had no effect on limiting slope in the short-linker construct. These results are consistent with an earlier study (11) in which the charge of paddle residues was altered not by mutation but by attaching charged adducts to introduced cysteines. All of these results are inconsistent with a large movement of the S4 segment during gating, a hallmark feature of the paddle model.
Despite extensive efforts to understand voltage sensor movement, there is no consensus model. In part, this is because of the difficulty of obtaining crystallographic data for a membrane protein designed to be pliant enough to respond rapidly to small changes of membrane potential. Moreover, understanding the movement of charges through the electric field depends on some grasp of the “structure” of the electric field in a protein–lipid complex invaded by aqueous crevices, which themselves may change shape dramatically when the protein undergoes conformational changes. The good news is that a motivated cadre of researchers is actively attacking this problem with an impressive array of experimental and theoretical tools.
Acknowledgments
I thank Chris Ahern (Jefferson Medical College) for making Fig. 1 and Kenton Swartz and all members of the Institute of Hyperexcitability for critiquing the manuscript.
See companion article on page5020.
References
- 1.Doyle, D. A., Cabral, J. M., Pfuetzner, R. A., Kuo, A. L., Gulbis, J. M., Cohen, S. L., Chait, B. T. & MacKinnon, R. (1998) Science 280, 69–77. [DOI] [PubMed] [Google Scholar]
- 2.Hodgkin, A. L. & Huxley, A. F. (1952) J. Physiol. (London) 116, 176–195. [Google Scholar]
- 3.Jiang, Y. X., Lee, A., Chen, J. Y., Ruta, V., Cadene, M., Chait, B. T. & MacKinnon, R. (2003) Nature 423, 33–41. [DOI] [PubMed] [Google Scholar]
- 4.Jiang, Y. X., Ruta, V., Chen, J. Y., Lee, A. & MacKinnon, R. (2003) Nature 423, 42–48. [DOI] [PubMed] [Google Scholar]
- 5.Cohen, B. E., Grabe, M. & Jan, L. Y. (2003) Neuron 39, 395–400. [DOI] [PubMed] [Google Scholar]
- 6.Ahern, C. A. & Horn, R. (2004) Trends Neurosci. 27, 303–307. [DOI] [PubMed] [Google Scholar]
- 7.Swartz, K. J. (2004) Nat. Rev. Neurosci. 5, 905–916. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez, C., Morera, F. J., Rosenmann, E., Alvarez, O. & Latorre, R. (2005) Proc. Natl. Acad Sci. USA 102, 5020–5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bezanilla, F. (2000) Physiol. Rev. 80, 555–592. [DOI] [PubMed] [Google Scholar]
- 10.MacKinnon, R. (2004) Science 306, 1304–1305. [DOI] [PubMed] [Google Scholar]
- 11.Ahern, C. A. & Horn, R. (2004) J. Gen. Physiol. 123, 205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuello, L. G., Cortes, D. M. & Perozo, E. (2004) Science 306, 491–495. [DOI] [PubMed] [Google Scholar]
- 13.Tombola, F., Pathak, M. M. & Isacoff, E. Y. (2005) Neuron 45, 379–388. [DOI] [PubMed] [Google Scholar]
- 14.Yang, N., George, A. L., Jr., & Horn, R. (1996) Neuron 16, 113–122. [DOI] [PubMed] [Google Scholar]
- 15.Larsson, H. P., Baker, O. S., Dhillon, D. S. & Isacoff, E. Y. (1996) Neuron 16, 387–397. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen, T. P. & Horn, R. (2002) J. Gen. Physiol. 120, 419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gandhi, C. S., Clark, E., Loots, E., Pralle, A. & Isacoff, E. Y. (2003) Neuron 40, 515–525. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez, C., Rosenman, E., Bezanilla, F., Alvarez, O. & Latorre, R. (2000) J. Gen. Physiol. 115, 193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Almers, W. (1978) Rev. Physiol. Biochem. Pharmacol. 82, 96–190. [DOI] [PubMed] [Google Scholar]
- 20.Sigg, D. & Bezanilla, F. (1997) J. Gen. Physiol. 109, 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]