Abstract
Background
Platelet crosslinking during arterial thrombosis involves von Willebrand Factor (VWF) multimers. Therefore, proteolysis of VWF appears promising to disaggregate platelet-rich thrombi and restore vessel patency in acute thrombotic disorders such as ischemic stroke, acute coronary syndrome or acute limb ischemia. N-Acetylcysteine (NAC, a clinically approved mucolytic drug) can reduce intrachain disulfide bonds in large polymeric proteins. In the present study, we postulated that NAC might cleave the VWF multimers inside occlusive thrombi, thereby leading to their dissolution and arterial recanalization.
Methods
Experimental models of thrombotic stroke induced by either intra-arterial thrombin injection or ferric chloride application followed by measurement of cerebral blood flow using a combination of laser Doppler flowmetry and magnetic resonance imaging were performed to uncover the effects of NAC on arterial thrombi. To investigate the effect of NAC on larger vessels, we also performed ferric chloride induced carotid artery thrombosis. In vitro experiments were performed to study the molecular bases of NAC thrombolytic effect, including platelet aggregometry, platelet-rich thrombi lysis assays, thromboelastography (ROTEM) and high shear VWF string formation using microfluidic devices. We also investigated the putative pro-hemorrhagic effect of NAC in a mouse model of intracranial hemorrhage induced by in situ collagenase type VII injection.
Results
We demonstrated that intravenous NAC administration promotes lysis of arterial thrombi that are resistant to conventional approaches such as recombinant tissue-type plasminogen activator, direct thrombin inhibitors and antiplatelet treatments. Through in vitro and in vivo experiments, we provide evidence that the molecular target underlying the thrombolytic effects of NAC is principally the VWF that crosslink platelets in arterial thrombi. Co-administration of NAC and a non peptidic GpIIb/IIIa inhibitor further improved its thrombolytic efficacy, essentially by accelerating thrombus dissolution and preventing re-thrombosis. Thus, in a new large vessel thromboembolic stroke model in mice, this co-treatment significantly improved ischemic lesion size and neurological outcome. Importantly, NAC did not worsen hemorrhagic stroke outcome, suggesting that it exerts thrombolytic effects without significantly impairing normal hemostasis.
Conclusion
We provide evidence that NAC is an effective and safe alternative to currently available antithrombotic agents to restore vessel patency after arterial occlusion.
Keywords: stroke, plasminogen activator, platelet aggregation, thrombolysis, von Willebrand factor
Additional keywords: Brain, GpIb, Thromboinflammation, ischemia/reperfusion injury/neuroprotection, ADAMTS-13
Introduction
In acute thrombotic disorders, prompt reestablishment of vessel patency can alleviate tissue injuries triggered by the thrombus-induced ischemia. Current pharmacological strategy to promote reperfusion relies on the use of tissue-type plasminogen activator (tPA) or its derivatives to activate the fibrinolytic cascade1. However, despite unquestionable clinical utility, tPA remains mainly active on fibrin and does not significantly affect the other components of the thrombus. As a result, in stroke for instance, the rate of early arterial recanalization after tPA administration is low (~30%)2, especially when occlusive thrombi are platelet-rich (~6%)3. Moreover, tPA promotes life threatening brain hemorrhages, thereby impairing the benefit/risk ratio of thrombolysis. There is therefore a clinical need for thrombolytic agents that can disaggregate arterial thrombi without significantly increasing bleeding.
Arterial thrombi often originate from atherosclerotic lesions, where they constitute under high shear rates. Interestingly, we recently demonstrated that high shear arterial thrombosis involves mainly von Willebrand Factor (VWF)-dependent platelet cross-linking4–6. Thus, proteolysis of VWF could be an efficient strategy to disaggregate arterial and platelet-rich thrombi7, 8. N-Acetylcysteine (NAC) is a widely used mucolytic drug with a simple molecular structure containing a free thiol group. Thanks to this free thiol group, NAC can reduce the disulfide bonds inside multimeric proteins, such as VWF9.
In the present study, we hypothesized that NAC may reduce the size of VWF multimers that crosslink platelets inside arterial thrombi. Thus, systemically administered NAC may promote thrombus dissolution and arterial recanalization, especially in case of platelet-rich thrombi. If right, the use of NAC will provide a new, effective and cheap thrombolytic treatment.
Methods
An extended methods section is available as a supplementary material.
Animals
Experiments were performed on male Swiss mice (35 ± 2 g; CURB, Caen, France), VWF deficient mice10 (VWF−/−) and C57BL/6 wild type mice (WT, 15 – 18 weeks old). All experiments were performed in accordance with the French (Decree 87/848) and the European Communities Council (2010/63/EU) guidelines and were approved by institutional review board (French ministry of Research). All the experiments were validated by the local ethical committee of Normandy (CENOMEXA) registered under the reference CENOMEXA-0113-03 and received the agreement number N/03-01-13/03/01-18. Anesthesia was induced using 5% isoflurane (Aerrane, Baxter) and maintained using 2% isoflurane in a mixture of O2/N2O (30%/70%).
In situ middle cerebral artery (MCA) occlusion
Three methods were used to induce occlusion of the MCA. In the first one, a piece of Whatman™ filter paper strip soaked in freshly prepared FeCl3 (20%, the lowest concentration to achieve occlusive and stable cerebral blood flow (CBF) reduction under 20% of baseline; Sigma-Aldrich) was placed on the intact dura mater over the endothelium of the MCA for 4 minutes11. CBF in the MCA territory was determined by laser Doppler flowmetry (Oxford Optronix) recording. Occlusion time was defined as the time between FeCl3 application and CBF diminution below 20% of baseline. In the second approach, the coagulation cascade was triggered by injection of thrombin, as previously described12. In this model, 1 μL of murine α-thrombin (1.0 IU) was injected in the MCA using a glass micropipette. The last method was direct electrocoagulation of the MCA13.
Large vessel thromboembolic stroke model
Mice were placed on the back and a midline incision was performed in the neck. The right common carotid artery was isolated. A piece of Whatman™ filter paper strip soaked in freshly prepared FeCl3 (20%) was placed on the artery for 5 minutes and then removed, to allow the formation of a thrombus. Thereafter, ischemic stroke was induced by mechanically promoting embolization of FeCl3-triggered thrombi to the internal carotid artery (as evidenced by the recoloration of the previously thrombosed CCA). Then anesthesia was stopped after analgesia was delivered (0.1 mg/kg of subcutaneously injected buprenorphine). Neurological assessment was performed at 1 hour and 24 hours blinded to the experimental data. Only mice presenting a neuroscore >1 were randomized. This model obtained specific institutional review board approval by the local ethic committee CENOMEXA under the title “Modèle murin d’infarctus cérébral thrombo-embolique à point de départ carotidien”.
Intracranial hemorrhage model
A unilateral striatal injection of collagenase type VII 14 (0.1 U in 0.5 μL of saline) was performed after placing Swiss male mice in a stereotaxic frame (coordinates: 0.5 mm anterior, 2.0 mm lateral, −3 mm ventral to the bregma). Solutions were injected by the use of a glass micropipette to minimize hemorrhage-unrelated tissular damage. Neurologic evaluation was performed blind to the experimental groups using the neuroscore scale, as previously described14.
Treatments
For NAC injection, mice received 400 mg/kg of NAC (N-acetylcysteine) in phosphate buffered saline (PBS). Administration of NAC was performed as a slow intravenous bolus (60 seconds) through a tail vein catheter. For fibrinolysis, animals under isoflurane anesthesia (this anesthetic regimen does not influence ischemic lesion size 15), were injected intravenously (tail vein, 200 μL, 10% bolus, 90% infusion over 40 minutes) with tPA (Actilyse®, 10 to 30 mg/kg), either early (20 min) or late (4 h) after thrombus formation. Intra-arterial administration of tPA was performed according to the same protocol as for intravenous injection but after placement of a catheter in the right common carotid artery. For the blockade of platelet GpIIb/IIIa receptors, mice were injected intravenously as a bolus with GR144053 trihydrochloride (GR, 10 mg/kg; Tocris), a non-peptidic GpIIb/IIIa inhibitor. Aspirin (100 mg/kg, Sigma-Aldrich) was dissolved in DMSO and PBS (10%/90%) and injected intravenously as a slow bolus (60 seconds). To achieve anticoagulation, argatroban (a direct thrombin inhibitor, 10 mg/kg, Sigma-Aldrich) or unfractioned heparin (200 U/kg) were injected intravenously in bolus.
Statistical analyses
Results are expressed as mean ± SD. When comparing multiple groups, statistical analyses were performed using Kruskal-Wallis test followed by Mann–Whitney test and the p-values were adjusted for multiple testing. When comparing only two groups, the Mann-Whitney test was used. Differences were considered statistically significant if probability value p <0.05.
Results
Ferric chloride-induced thrombi are platelet-rich and tPA-resistant
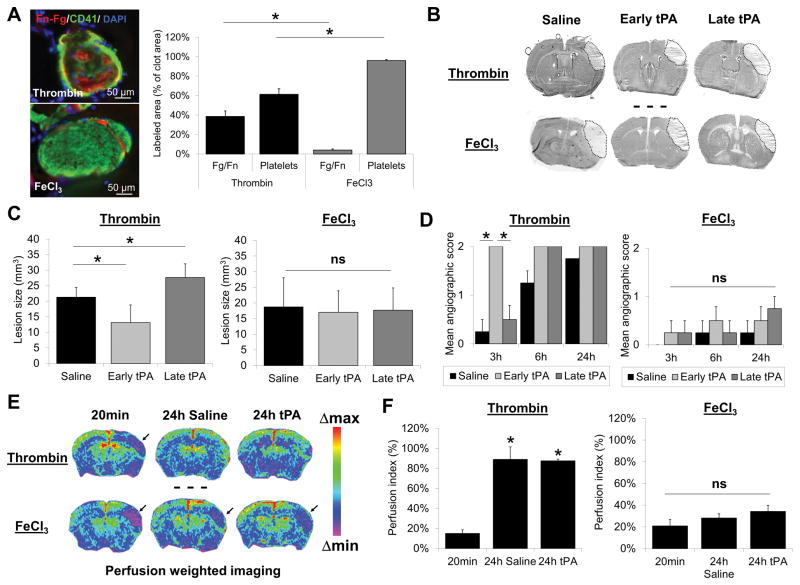
We first aimed to develop an ischemic stroke model in mice involving platelet-rich thrombi. To this aim, we selected two experimental models to induce in situ occlusive thrombus formation in the middle cerebral artery (MCA): direct intra-arterial injection of recombinant thrombin, as described by Orset et al.12, and topical application of FeCl3, as described by Karatas et al.11. We first characterized these two models in terms of thrombus composition by immunohistofluorescence. Brain samples were collected 20 minutes after thrombus formation and immunohistological analyses of the arterial thrombi were performed. Our results indicate that FeCl3 induced the formation of merely pure platelet thrombi, whereas thrombin induced the formation of mixed thrombi, containing significant amounts of both platelets and fibrin (Fig. 1A).
Figure 1. Thrombi induced by FeCl3 in the middle cerebral artery (MCA) are platelet-rich and resistant to tPA-mediated thrombolysis.
(A) Left: Representative immunohistological images of thrombi in the MCA 20 minutes after thrombin- or FeCl3-induced MAC thrombosis. Right: quantitative analysis of fibrinogen-fibrin (Fg-Fn) and platelets contents of thrombi 20 minutes after thrombosis (n=3 per group). Thrombin induced fibrin-rich thrombi, whereas FeCl3 induced platelet-rich thrombi. (B) Representative images of thionin-stained brain sections, 24 hours post-MCA occlusion (MCAo) induced by either thrombin or FeCl3 (dotted lines represent the ischemic lesions). Subsequently, 20 minutes (early) or 4 hours (late) after MCAo, mice received an intravenous infusion of tPA (10 mg/kg). (C) Mean lesion size 24 hours after MCAo in thrombin- and FeCl3-induced stroke models, with or without tPA administration (n=10 per group). Although in the thrombin model, early and late tPA were respectively beneficial and deleterious (due to the deleterious effect of late tPA mediated reperfusion), tPA had no significant effect in the FeCl3 model. (D) Mean angiographic score (see methods section) of longitudinally studied mice after thrombin- or FeCl3-induced MCAo (n=5 per group) showing spontaneous and tPA-induced recanalization in the thrombin model. No recanalization occurred in the FeCl3 model, with or without tPA treatment. (E) Representative MRI ΔR2* maps of mice immediately (20 minutes) or 24 hours after MCAo in thrombin- and FeCl3-induced stroke models. Black arrows indicate areas of perfusion defect, confirming the lack of reperfusion in the FeCl3 model. (F) Quantitative assessment of perfusion index (see methods section) in the two models (n=4–6 per group). (*=p<0.05; ns = non-significant).
Since clinical evidence suggests that tPA-mediated thrombolysis is less efficient in platelet-rich thrombi, we investigated whether the sensitivity to intravenous tPA differs in these two models. In line with clinical studies, early intravenous administration of tPA (10 mg/kg, 20 minutes after occlusive thrombus formation) diminished the lesion size by 26.2% in the thrombin model, whereas it failed to influence the lesion size in the FeCl3 model (Fig. 1B and C). The lack of efficacy of tPA in the FeCl3 model was also observed when started 4 hours (late) after thrombus formation (Fig. 1B and C). Consistently, we demonstrated that tPA promoted recanalization and subsequent reperfusion in the thrombin model, as assessed at different time points (20 min and 4 hours) by magnetic resonance angiography and perfusion-weighted imaging respectively, whereas it failed to do so in the FeCl3 model (Fig. 1D–F).
As human tPA has a lower efficacy in mouse than in human plasma, we also investigated whether higher doses of tPA (up to 30 mg/kg) or local delivery (intra-arterial administration) improved vessel patency and stroke outcome in the FeCl3 model. As shown in Figure S1A–C, neither classical nor higher doses of intravenously administered tPA induced arterial recanalization in the FeCl3 model during the 40 minutes monitoring period. In line with this resistance to tPA, there were no significant difference in ischemic lesion sizes between control (saline-treated) and tPA-treated mice as assessed by magnetic resonance imaging 24 hours after stroke onset (Supplemental Figure 1D–E). Similarly, intra-arterial administration of tPA (10 mg/kg) also failed to induce reperfusion and improve stroke outcome in this model (Supplemental Figure 2).
Altogether, these results demonstrated that FeCl3-induced thrombi in the mouse MCA are platelet-rich and resistant to classical thrombolytic treatment.
NAC induces arterial recanalization after acute thrombosis
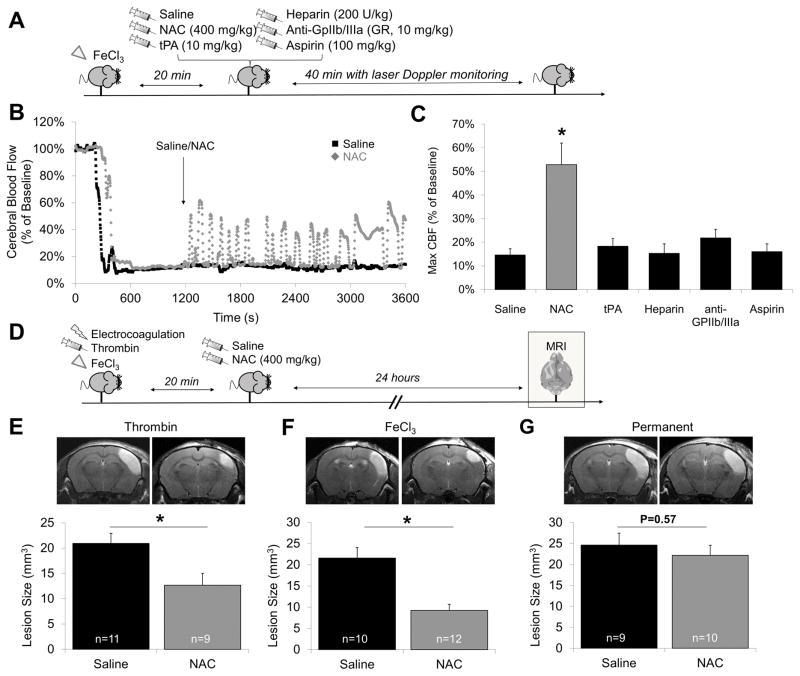
We thereafter investigated whether NAC could disaggregate FeCl3-induced platelet-rich thrombi. To this aim, we injected 400 mg/kg of NAC (we chose this dose since it is the lowest dose which has been shown to reduce VWF multimers in vivo in mice9), 20 minutes after occlusive thrombus formation (Fig. 2A). NAC administration led to a rapid and significant reperfusion reaching up to 53% of the baseline cerebral blood flow (CBF), as measured by laser Doppler flowmetry (Fig. 2B–C). This reperfusion was transient and followed by rapid re-thrombosis and subsequent cyclic flow variations. Thus, NAC as a monotherapy induces transient arterial recanalization in the FeCl3 model.
Figure 2. NAC restores vessel patency after occlusive thrombosis and improves thrombotic stroke outcome.
(A) Schematic representation of the experiments performed in B and C. (B) Representative Doppler flowmetry after FeCl3 injury on the MCA (monitoring during 1 hour) of saline and NAC (400 mg/kg) treated animals. The arrow indicates time to saline or NAC intravenous injection. (C) Mean value of cerebral blood flow in the last 10 minutes of monitoring. (D) Schematic representation of the experiments performed in E, F and G. (E) Graphs: Mean lesion size in saline and NAC-treated animals (400 mg/kg, 20 minutes after arterial occlusion). 24 hours after MCAo in (E) thrombin-, (D) FeCl3- and (E) electrocoagulation (permanent)-induced stroke models as assessed by T2-weighted imaging. Top: representative T2-weighted images of saline and NAC-treated animals. (* means significant versus saline).
Then, we investigated whether other clinically available antithrombotic agents influence arterial patency in the FeCl3 model (Fig. 2A). As shown on Figure 2C, neither tPA (10 mg/kg), unfractioned heparin (200 UI/kg), aspirin (100 mg/kg) nor GR-144053 (a fast-acting reversible GpIIb/IIIa inhibitor, analog to tirofiban, 10 mg/kg) influenced the CBF in this model. These data suggest that the thrombolytic effects of NAC are independent of plasmin generation, anticoagulation or platelet activation inhibition.
To further assess the potential of NAC as an acute ischemic stroke treatment, we injected NAC 20 minutes after ischemic onset in three different stroke models (Fig. 2D): electrocoagulation (permanent ischemia), in situ thrombin injection (mixed thrombi with significant amount of VWF, Supplemental Figure 3) or topical FeCl3 application (platelet-rich thrombi). NAC significantly reduced ischemic lesion sizes in both thrombin-induced (−39%, p<0.05, Fig. 2E) and FeCl3-induced (−57%, p<0.05, Fig. 2F) stroke models as assessed by magnetic resonance imaging (MRI) 24 hours after stroke. In contrast, NAC failed to reduce the ischemic lesion size in the electrocoagulation model (−10%, p=0.57, Fig. 2G), suggesting that its brain protective effects in our experimental conditions are related to arterial recanalization rather than direct neuroprotection. These results demonstrate that NAC administration is beneficial in acute ischemic stroke thanks to its thrombolytic properties.
Overall, these results demonstrate that NAC acts as a thrombolytic in the presence of platelet-rich (FeCl3 model) and mixed thrombi (thrombin model) and improves ischemic stroke outcome when injected as a monotherapy.
NAC prevents the formation of large VWF fibers, reduces the size of VWF multimers and displays direct thrombolytic effects on VWF- and platelet-rich thrombi
In order to get further insights into the mechanism of action of NAC underlying its ability to induce arterial recanalization, we performed additional in vitro and in vivo experiments aiming at studying NAC-mediated VWF cleavage and the impact of NAC on coagulation and tPA-mediated fibrinolysis. As shown on Figure S4A, concentrations of NAC as low as 5 mM were able to induce VWF multimers cleavage in vitro, with a stronger effect at 10 mM and 20 mM, confirming previous studies9. In another experiment, we investigated the effect of an intravenous injection of NAC (400 mg/kg) on circulation VWF multimers in mice. Interestingly, 1 hour after NAC administration, we observed a reduced concentration of circulating high molecular weight multimers of VWF in mice (Supplemental Figure 4B). Notably, NAC at concentrations up to 20 mM had no effect on the apparent size after electrophoretic migration of fibronectin and fibrinogen, two other proteins involved in platelet aggregation (Supplemental Figure 5).
Then, we performed a microfluidic assay allowing to observe the formation of VWF fibers under high shear rates16, 17. When NAC was added to citrated platelet free plasma (PFP) and perfused over collagen at 30.000 s−1 for 5 min, fewer and smaller VWF fibers formed on the collagen than in the saline-treated control (Supplemental Figure 6A). When examining the distribution of fiber sizes, the addition of NAC resulted in a downward shift in the size distribution of VWF fibers (Supplemental Figure 6B–C). It was also observed that in the presence of NAC, the total length of all fibers formed was less than half that of the control. These results confirm that NAC can alter the structure of VWF and suggests that NAC could inhibit platelet adhesion on injured vessels by preventing VWF fiber formation on collagen.
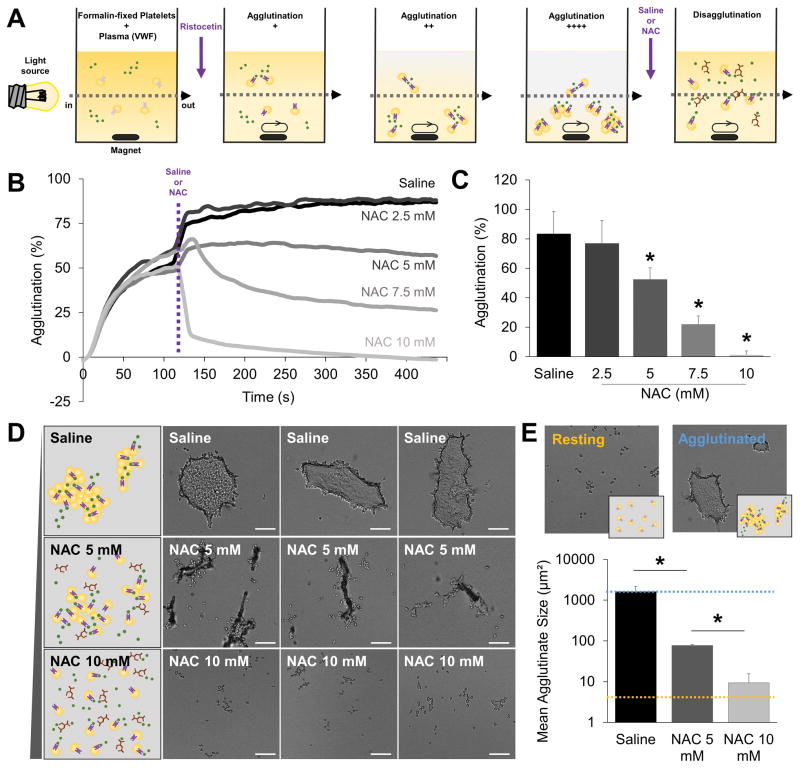
Even if NAC alters the structure of VWF, whether this is sufficient to induce disaggregation of platelet thrombi remains unproven. To test this hypothesis, we performed additional experiments in vitro, investigating the effect of NAC on in vitro-formed platelet-aggregates. VWF-dependent platelet agglutination was induced using ristocetin in an aggregometer (Fig. 3A). Once the platelet agglutinates were stably formed, we added different concentrations of NAC (0 mM, 2.5 mM, 5 mM, 7.5 mM or 10 mM) and observed whether this induced platelet disagglutination. As shown on Figure 3B–C, NAC at 10 mM induced complete disagglutination of the platelet thrombi, with a partial but significant effect starting at the 5 mM dose. We completed this experiment by performing microscopic analyses of the resulting platelet thrombi at the end of the monitoring period in the 0 mM (saline), 5mM and 10 mM samples. As shown on Figure 3D–E, we confirm that NAC has a thrombolytic effect on these VWF-rich platelet thrombi. At the highest tested dose (10 mM), virtually no platelet agglutinate was visible anymore.
Figure 3. NAC has a direct thrombolytic effect on platelet thrombi after ristocetin-induced agglutination.
(A) Schematic representation of the platelet agglutination experiments. Once platelet agglutinates were stably formed, either saline or NAC (2.5, 5, 7.5 or 10 mM) was added to the solution to observe platelet disagglutination. The small increase in platelet agglutination at the time of treatment addition (purple dotted line) is related to the small dilution of the samples due to the 30 μL volume increase. (B) Representative agglutination and disagglutination curves of lyophilized platelets in the presence of ristocetin before and after either saline or NAC treatment. (C) Corresponding quantification (n=3–5/group) at the end of the monitoring period (450 s). (D) Schematic representation (left) and three representative bright-field microscopic images (right) of the resulting platelet agglutinates after either saline or NAC treatment. White line = 20 μm. (E) Corresponding quantification (n=4/group). The size of resting lyophilized platelets and fully agglutinated platelets are shown in blue and orange respectively for comparison purpose. (* means significant versus saline).
Importantly, further experiments performed using platelets from platelet-rich plasma (PRP) after ADP or ristocetin induced platelet aggregation also support a direct effect of NAC on the stability of VWF-rich platelet aggregates (Supplemental Figure 7). We first performed platelet aggregation experiments using ADP as a platelet agonist. ADP induces platelet activation and therefore triggers the formation of GpIIb/IIIa-fibrinogen and, to a lesser extent, GpIIb/IIIa-VWF dependent platelet aggregates. Accordingly, NAC treatment (0, 5 or 10 mM) induced partial disaggregation of the platelet aggregates (up to 43% using 10 mM NAC compared to control saline-treated platelets). Second, we performed platelet aggregation experiments using ristocetin as a platelet agonist (Figure S7D–F). Ristocetin induces changes in conformation of VWF, leading to GpIbα-VWF interactions followed by platelet activation and subsequent formation of GpIIb/IIIa-fibrinogen and, to a lesser extent, GpIIb/IIIa-VWF dependent platelet aggregates. As expected and in line with the results obtained using ADP, NAC treatment (0, 5 or 10 mM) induced partial disaggregation of the platelet aggregates after ristocetin treatment (up to 27% using 10 mM NAC compared to control saline-treated platelets). In both ADP-induced and ristocetin-induced platelet aggregation experiments, we hypothesized that the resistant platelet aggregates were cross-linked by GpIIb/IIIa-fibrinogen interactions. To test this hypothesis, we induced platelet aggregation using ristocetin in the presence of a GpIIb/IIIa antagonist (GR, 50 μg/mL). In these conditions, platelet cross-linking is only dependent on GpIbα-VWF interactions (Figure S7G–I). Accordingly, NAC treatment (0, 5 or 10 mM) induced almost complete disaggregation of the platelet aggregates (up to 85% using 10 mM NAC compared to control saline-treated platelets).
Thereafter, we looked for potential effects of NAC on coagulation and fibrinolysis. As shown on Figure S8, NAC affected coagulation testing and tPA-mediated fibrinolysis in plasma clot-lysis assays18 only at the highest studied concentration (20 mM), without significant effects at lower concentrations. Similar results were obtained using thromboelastography (ROTEM, Supplemental Figure 9). In line with these results, NAC failed to induce cleavage of plasminogen and tPA (Supplemental Figure 10). Interestingly, according to pharmacokinetic studies, the plasmatic concentration of NAC after a bolus of 400 mg/kg is expected to reach a peak of 9.1 mM and thus, is able to induce VWF cleavage and VWF-rich thrombi disaggregation without significantly affecting coagulation or endogenous fibrinolysis.
NAC impacts thrombus stability but cannot prevent occlusive thrombus formation
Then we investigated whether NAC could prevent occlusive thrombus formation when injected before application of FeCl3 (Supplemental Figure 11A). This hypothesis was supported by our in vitro studies showing that NAC prevents the formation of large VWF fibers and also by additional in vivo experiments revealing that VWF knock-out mice (VWF KO19) were protected from thrombosis in this model (Supplemental Figure 11B). To this aim, we injected either NAC or saline 10 minutes before topical FeCl3 application on the MCA (Supplemental Figure 11A). As shown on Supplemental Figure 11C–F, saline pre-treated mice presented stable thrombi with a resulting CBF never exceeding 20% of its baseline value during the monitoring period. NAC pretreatment failed to prevent occlusive thrombus formation since all mice presented sudden drop in CBF with visual evidence of complete thrombosis (Supplemental Figure 11D). However, the resulting thrombi were unstable, leading to spontaneous recanalization and cyclic flow variations in most NAC-treated mice. These findings demonstrate that, when injected before topical FeCl3 application, NAC impacts thrombus stability but cannot prevent complete thrombosis.
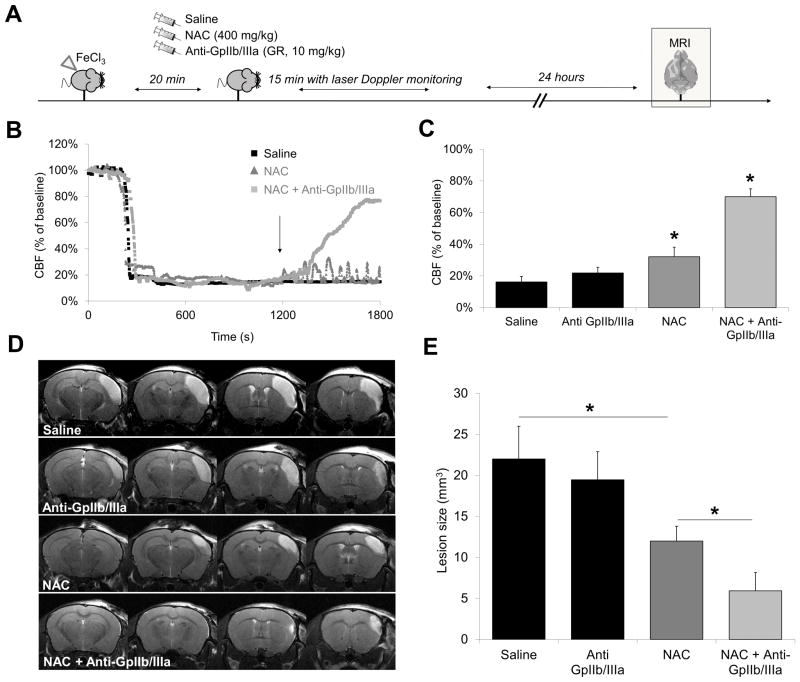
Synergistic effect of NAC and GpIIb/IIIa inhibitors on thrombolytic efficiency
As demonstrated earlier, NAC treatment induces arterial recanalization but cannot prevent re-thrombosis. Thus, we hypothesized that co-injection of NAC with an antithrombotic agent would prevent re-thrombosis after NAC-induced recanalization and, in consequence, allow a faster and more complete reperfusion. To test this hypothesis, we injected either NAC, a GpIIb/IIIa inhibitor (GR144053) or a combination of the two drugs following FeCl3-induced thrombosis of the MCA (Fig. 4A). In line with the previous results, administration of NAC alone induced reperfusion followed by cyclic flow reductions resulting in significantly reduced ischemic lesion sizes, as assessed by MRI at 24 hours (Fig. 4B–E). As previously demonstrated4, when injected alone, GpIIb/IIIa inhibitors failed to promote reperfusion and improve stroke outcome in this model. Remarkably, co-administration of NAC with GpIIb/IIIa inhibitors induced a rapid and almost complete recanalization of the MCA. Consequently, ischemic lesion sizes were reduced by a mean of 73% in NAC+Anti-GpIIb/IIIa treated mice compared to saline treated mice. Thus, co-administration of NAC and a GpIIb/IIIa inhibitor presents a synergistic effect on thrombolytic efficiency and subsequent improvement of stroke outcome.
Figure 4. Adjunctive treatment with GpIIb/IIIa inhibitors further improves NAC-induced reperfusion.
(A) Schematic representation of the performed experiments. (B) Representative Doppler flowmetry after FeCl3 injury on the MCA (monitoring during 30 minutes) of saline-, NAC− (400 mg/kg) or NAC+Anti-GpIIb/IIIa− (400 mg/kg, 10 mg/kg) treated mice. The arrow indicates time to treatment injection. (C) Mean value of cerebral blood flow in the last 5 minutes of monitoring (n=8 per group). (D) Representative T2-weighted images 24 hours after MCAo. (E) Quantification of the lesion size (n=8 per group). (* means significant versus saline or otherwise indicated).
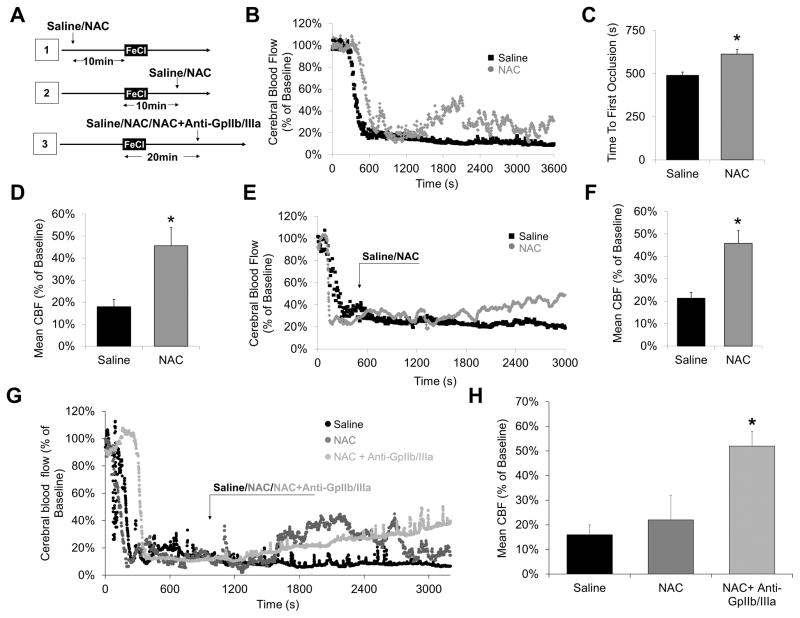
NAC restores vessel patency after large artery thrombosis
To determine whether our findings would be valid for larger vessels, we performed a model of common carotid artery (CCA) thrombosis induced by topical application of 10% FeCl3 (Fig. 5A). When injected before thrombus formation, NAC pretreated mice showed delayed time to first occlusion (614 s vs 491 s) and higher residual CBF (45.6% vs 18.0%) than saline pretreated animals (both p<0.05, Fig. 5B–D). Ten minutes after FeCl3 application, intravenous injection of 400 mg/kg NAC induced significant reperfusion of the CCA as assessed by Doppler laser flowmetry (45.8% vs 21.3% of the baseline CBF, compared to saline injection, p<0.05; Fig. 5E–F). Lastly, we injected NAC 20 minutes after FeCl3 application, when the thrombus was stabilized. As shown on Fig. 5G–H, NAC slightly enhanced the blood flow, but re-thrombosis frequently occurred, so that the mean blood flow at the end of the measurement was not significantly improved compared to control mice (Figure 5G–H). Interestingly, co-administration of NAC and a GpIIb/IIIa inhibitor led to a persistent recanalization with significantly improved blood flow, further supporting the synergistic effect of NAC and GpIIb/IIIa inhibitors to restore arterial patency.
Figure 5. NAC restores vessel patency after common carotid artery thrombosis.
(A) Schematic representation of the performed experiments. (B) Representative Doppler flowmetry after FeCl3-induced injury on the common carotid artery (monitoring during 1 hour). Mice received either NAC (400 mg/kg) or an equivalent volume of saline 10 minutes after arterial injury, when the artery was already occluded. (C) Corresponding time to first occlusion. (D) Mean blood flow in the last 10 minutes of monitoring. (n=5 per group). (E) Representative Doppler flowmetry after FeCl3-induced injury on the common carotid artery (monitoring during 1 hour). Mice received either NAC (400 mg/kg) or an equivalent volume of saline 10 minutes before arterial injury. The time to first occlusion was measured (drop of the blood flow under 30% of baseline) and the monitoring lasted 1 hour after FeCl3 treatment. (F) Mean values of the time to first occlusion. (G) Mean blood flow in the last 10 minutes of monitoring. (n=5 per group). (G) Representative Doppler flowmetry after FeCl3-induced injury on the common carotid artery (monitoring during 1 hour). Mice received either NAC (400 mg/kg) or an equivalent volume of saline 20 minutes after arterial injury, when the artery was stably occluded. (H) Corresponding mean blood flow at the end of the monitoring period. (* means significant versus saline).
NAC improves ischemic lesion size and neurological outcome in a new large vessel thromboembolic stroke model in mice
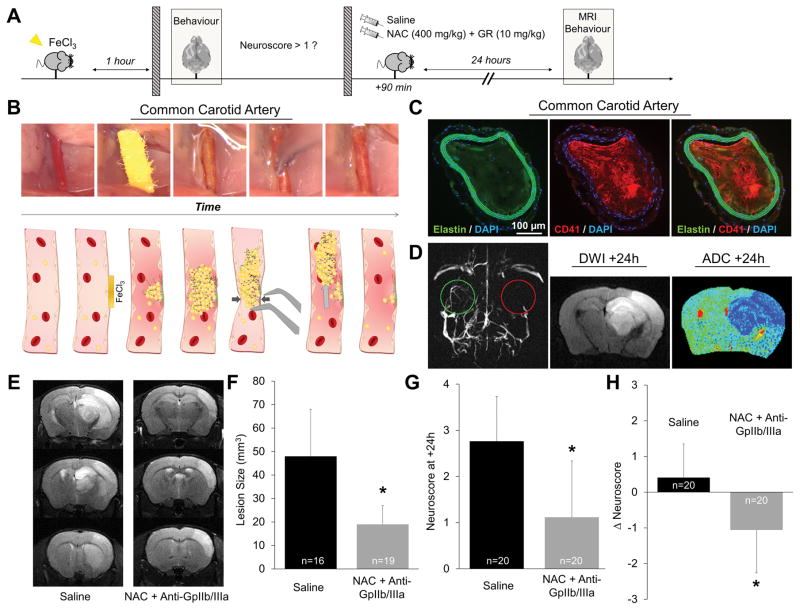
To investigate whether the reduction in ischemic lesion size translates into an improved neurological outcome after stroke, we developed an original model of large vessel thromboembolic stroke. Indeed, we failed to measure consistent neurological deficits in the thrombin and FeCl3 models, which involve distal occlusion of the MCA and induce only small cortical strokes. To overcome this limitation, we developed and characterized an original thromboembolic stroke model induced by intracranial embolization of a thrombus formed at the surface of the CCA (Fig. 6A–D). Therefore, it mimics part of the pathophysiology of human large artery atherosclerotic stroke: i.e. carotid atherosclerotic plaque rupture followed by thrombus embolization and subsequent intracranial arterial occlusion.
Figure 6. Thrombolytic treatment using NAC and an anti-GpIIb/IIIa improves neurological outcome in a mode of large vessel thromboembolic stroke.
(A) Schematic representation of the experiments in mice. Only mice presenting a neuroscore >1 were randomized. The other mice were excluded. (B) Pictures from the operator view (upper panels) and schematic representation (lower panels) of the different steps of the thromboembolic model. First, the right common carotid artery (CCA) is isolated. Then, FeCl3 (20%, mass/volume) is applied on the CCA for 3 minutes, leading to an endothelial lesion. Thereafter, clot formation on the endothelial lesion induces CCA occlusion (3). (4) Lastly, mechanical pressure is applied on the occluded CCA to detach the thrombus, thereby inducing its migration toward the intracranial circulation. (C) Representative immunohistological images of the right CCA 5 minutes after thrombus formation revealing a large occluding platelet-rich thrombus. (D) Left: representative magnetic resonance angiogram showing a patent left MCA (green) but the absence of flow in the right MCA (red) starting from its proximal segment. Right: Diffusion weighted image (DWI) and pseudo-colored apparent diffusion coefficient (ADC) map 24 hours after the thromboembolic stroke showing the typical cytotoxic edema usually observed in acute and subacute ischemic stroke encompassing a significant part of the right hemisphere. (E) Representative T2-weighted MRI at 24 hours after thromboembolic stroke in saline and NAC + Anti-GpIIb/IIIa mice showing a smaller ischemic lesion in the NAC + Anti-GpIIb/IIIa treated animal. (F) Corresponding quantification (n=16–19/group). (G) Mean neuroscore at 24 hours after thromboembolic stroke showing a significantly better neurological outcome (lower neuroscore) in NAC + Anti-GpIIb/IIIa treated mice (n=20/group). (H) Mean ΔNeuroscore corresponding to the difference between the neuroscore evaluated at +24 hours and the neuroscore evaluated at +1 hour after stroke onset, confirming the beneficial effect of NAC + Anti-GpIIb/IIIa treatment (n=20/group). (* means significant versus saline).
Using this model, we performed a controlled, randomized and blinded preclinical trial of NAC+Anti-GpIIb/IIIa (the most efficient therapeutic strategy identified in our previous experiments) versus vehicle (saline) using 20 animals per group. The onset-to-treatment delay was chosen based on the median onset-to-treatment observed in the NINDS stroke trials which demonstrated the beneficial effect of tPA in stroke patients: 90 minutes. The primary endpoint was neurological outcome at 24 hours as assessed by a previously described neuroscore14 (higher score meaning more severe neurological deficits). Secondary outcomes were mortality, lesion size and hemorrhagic transformation rate as assessed by T2-weighted and T2*-weighted imaging at 24 hours respectively. Importantly, to include only the mice with large vessel occlusion resulting from thrombus embolization, only animals presenting significant neurological deficits (Neuroscore >1 and <4 (death) 60 minutes after thrombus embolization were randomized (so that 36% of the performed mice were excluded before randomization due to failure of the surgery to induce significant neurological deficit).
NAC+Anti-GpIIb/IIIa significantly improved neurological outcome and ischemic lesion size compared to control saline-treated mice when administered intravenously 90 minutes after stroke onset (Fig. 6E–H). No significant difference was observed for mortality (20% in the saline group vs 5% in the NAC+Anti-GpIIb/IIIa group, p=0.1567 using Chi-squared test) and hemorrhagic transformation rates (0% in both groups). Thus, these data demonstrate that this thrombolytic strategy improves neurological functional status after stroke.
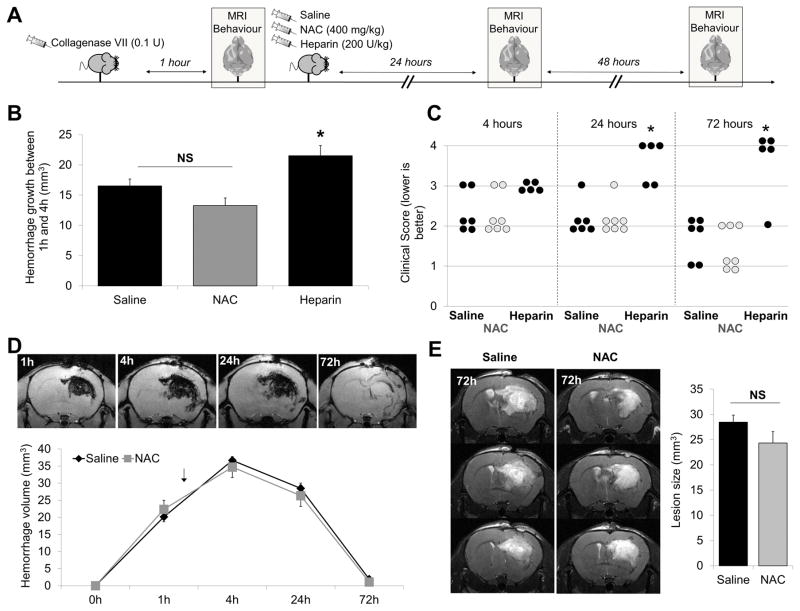
NAC displays a favorable safety profile in a hemorrhagic stroke model
In order to further evidence the safety of NAC regarding the risk of hemorrhagic transformations, we determined whether NAC treatment worsened the outcome of mice subjected to intracranial hemorrhage (ICH) induced by intrastriatal administration of 0.1UI type VII collagenase (Fig. 7A)14. Unlike intravenous heparin administration (200 UI/kg in bolus, 75 minutes after collagenase administration) that led to hematoma expansion and high mortality rate, NAC treatment (400 mg/kg in bolus, 75 minutes after collagenase administration) failed to promote hematoma expansion, worsen clinical score or increase lesion sizes at any time points as compared to saline treated animals (Fig. 7B–E).
Figure 7. NAC does not aggravate collagenase-induced hemorrhage.
(A) Schematic representation of the performed experiments. Mice received intravenous NAC (400 mg/kg), heparin (200 IU/kg) or an equivalent volume of saline 75 min after intrastriatal administration of collagenase (0.1 U). (B) Hemorrhage growth between 1h and 4h after collagenase injection as assessed by T2* imaging (n=5–7 per group). (C) Clinical score 4 hours, 24 hours and 72 hours post collagenase administration. (D) Time course of hematoma size as assessed by T2* weighted imaging (deoxyhemoglobin) in saline and NAC-treated mice. The black arrow indicates time of treatment injection. (E) Right: mean final hematoma volume as assessed by T2-weighted imaging at 72-hour post-collagenase injection. Left: Representative T2-weighted images of saline- and NAC-treated animals (n=6–7 per group). (* means significant versus saline).
Discussion
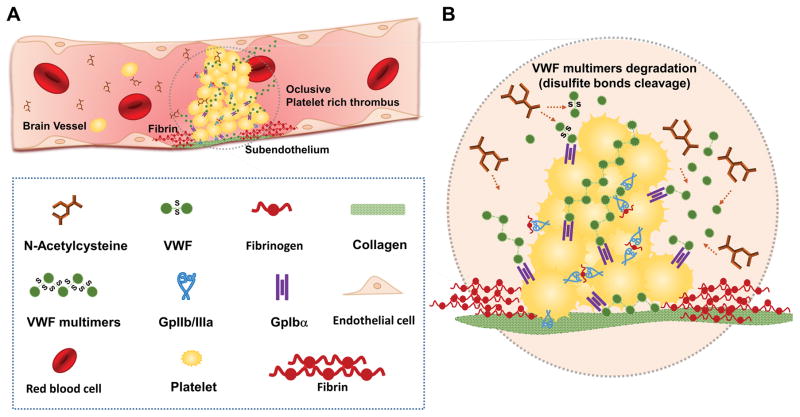
In the present study, we have shown that NAC has a potent thrombolytic activity in the presence of arterial thrombi (Fig. 8). This thrombolytic effect is essentially mediated by the cleavage of VWF that crosslinks platelets. Moreover, using three different ischemic stroke models, we demonstrated that NAC alleviates ischemic lesions and improves neurological outcome when injected intravenously in combination with a GpIIb/IIIa antagonist. Importantly, NAC did not worsen outcome nor induced hematoma expansion in a model of intracranial hemorrhage suggesting that it displays a favorable benefit-risk ratio.
Figure 8. Schematic representation of the main findings.
(A) Schematic representation (B) NAC reduces the disulfide bonds of the VWF multimers that maintain platelets linked in arterial thrombi, thereby inducing thrombus dissolution.
The dose injected in the present study is only slightly higher (but more rapidly injected) than the dose recommended to treat acetaminophen poisoning in human (300 mg/kg), supporting the potential for future clinical translation of our findings. Importantly, GpIIb/IIIa inhibitors have been widely used in acute coronary syndrome patients and tirofiban, a non peptidic GpIIb/IIIa inhibitor similar to GR155053, has recently been shown to be safe in the acute phase of stroke20, thereby opening the possibility to co-administrate NAC and a GpIIb/IIIa inhibitor to further improve its thrombolytic effect. The clinical relevance of our results is also supported by the fact that NAC has been successfully used as a VWF degrading agent in small series of patients presenting thrombotic thrombocytopenic purpura21, 22, a condition characterized by abnormally large circulating VWF multimers. Moreover, NAC has been shown to improve outcome in patients with acute myocardial infarction treated with streptokinase and nitroglycerine23. In this study, the authors detected a trend towards faster coronary reperfusion in NAC treated patients (−39%), albeit the reason for this effect remained elusive. More recently, the NACIAM trial demonstrated that early use of N-acetylcysteine in STEMI patients resulted in accelerated tissue reperfusion, more rapid chest pain resolution and myocardial infarct size reduction by 33% on day 5 compared to placebo-treated controls24. Our results raise the intriguing possibility that these beneficial effects of NAC are mediated by its thrombolytic effects.
Our data support that the observed thrombolytic effect of NAC administration is mainly mediated by cleavage of the VWF that crosslinks platelets inside arterial thrombi. Indeed, we demonstrated that NAC is able to disaggregate platelets crosslinked by VWF. Moreover, we demonstrated that NAC does not act solely through platelet inhibition, coagulation inhibition or fibrinolysis since antiplatelet agents, anticoagulants and fibrinolytic agents all failed to restore vessel patency in the FeCl3 model. Moreover, NAC did not improve outcome in a permanent model of cerebral ischemia, suggesting that its other described neuroprotective properties are not sufficient to significantly improve stroke outcome in the present experimental conditions25. It should however be mentioned that NAC can influence hemostasis by other pathways in vivo, including anticoagulation and antiplatelet effects26, that may have participated in the observed thrombolytic effect. Whether NAC acts exclusively through direct VWF multimer cleavage or also through thiol-containing intermediates remains to be investigated. It raises the intriguing possibility that naturally occurring thiol-containing compound (such as glutathione) could actively participate to the endogenous thrombolytic process27. Overall, our results suggest that disulfide bond reducing agents constitute a new large family of thrombolytics. Accordingly, further studies may aim at developing an ideal thrombolytic agent with maximal VWF cleaving abilities and minimal side effects. Notably, our results are consistent with the multistep model of occlusive thrombus formation that we described previously4. According to this model, in situ formed occlusive thrombi are made of a base, which constitutes at low shear rates, an inner core, which constitutes at medium-high shear rates and an outer part, which constitutes at very high shear rates. Our present results suggest that NAC destabilizes the outer part of the thrombus since it is predominantly made of platelet crosslinked by VWF, thereby exposing the thrombus core which is mainly made of platelet crosslinked by GpIIb/IIIa-fibrinogen interactions. Consequently, co-administration of a GpIIb/IIIa inhibitor with NAC destabilizes this inner part of the thrombus by competing with endogenous GpIIb/IIIa ligands and leads to complete recanalization.
This study has however some limitations. First, human thrombi may have a different inner structure compared to FeCl3 induced thrombi in mice and thrombus composition may change according to the occlusion site and the delay between occlusion and start of thrombolytic treatment. Interestingly, a recent study showed that thrombi retrieved from intracranial arteries in acute ischemic stroke patients contain on average 20.3 ± 10.1% VWF7. Therefore, the target of NAC is present in a significant proportion in thrombi from human stroke patients. Besides, even if we demonstrated that NAC+anti-GpIIb/IIIa treatment improved stroke outcome when injected up to 90 minutes after stroke onset in the large vessel thromboembolic model, the influence of thrombus age on NAC efficacy deserves further investigations. Similarly, whether NAC induces downstream embolization of microthrombi due to destabilization of the thrombus structure after VWF multimers reduction remains unknown and could explain the observed cyclic flow reduction pattern in the FeCl3 model (thrombus embolization followed by regrowth and re-occlusion). Second, since there is no spontaneous hemorrhagic transformation in the ischemic stroke models performed in the present study, we investigated the pro-hemorrhagic effect of NAC in a dedicated intracranial hemorrhage model which may be less relevant to the human condition. Therefore, our safety data should be interpreted with caution. Regarding anti-GpIIb/IIIa treatments that enhance the thrombolytic efficacy of NAC, tirofiban has been shown to be safe in ischemic stroke patients regarding the risk of hemorrhagic transformation in phase IIb studies20, 28. The fact that tirofiban was safe in human stroke and that we did not observe hemorrhagic transformation in two ischemic stroke models in mice after treatment with NAC and a tirofiban analog is reassuring for further investigation of this drug combination for human use. Third, NAC may influence the post-stroke immune system. Indeed, low doses of NAC have been shown to reduce inflammatory biomarkers in post-menoposal women29, to regulate the function of macrophages in endotoxic shock30 and to have a weakly strengthening effect on the innate immune system31. Regarding higher doses of NAC, data from clinical trials, in which NAC was administered to prevent the toxicity of acetaminophen poisoning (at 300 mg/kg versus 400 mg/kg in the present study), show that only 1–2% of the patients experienced infectious complications32. However, since the immune system is already weakened in the first days following ischemic stroke33, the effect of NAC on immune parameters should be monitored to ensure safe translational development of this treatment.
Overall, the superiority of NAC compared to clinically available antithrombotic treatments combined with its favorable safety profile in a hemorrhagic stroke model, makes it a promising drug for the treatment of acute arterial thrombosis such as stroke, myocardial infarction or acute limb ischemia. Intravenous NAC administration could provide an effective, cheap and safe thrombolytic treatment, especially in low-income areas with limited access to expensive recombinant proteins and in the presence of platelet-rich thrombi. Clinical trials are now needed to provide definitive proof of NAC thrombolytic efficacy, for instance by comparing NAC to a placebo for facilitated percutaneous coronary intervention in acute coronary syndrome patients or to tPA in ischemic stroke patients.
Supplementary Material
Clinical Perspectives.
What Is New?
This study shows that N-Acetylcysteine has thrombolytic effects in the arterial circulation by reducing the size of von Willebrand Factor (VWF) multimers, a key constituent of platelet-rich thrombus.
N-Acetylcysteine exerts its thrombolytic effect essentially by reducing the disulfide bridges in large VWF multimers, leading to their fragmentation and subsequent platelet disaggregation.
In three different experimental models of acute ischemic stroke, intravenous injection of N-Acetylcysteine induces arterial recanalization and improves ischemic lesion size and neurological deficits.
Co-administration of N-Acetylcysteine and an antagonist of platelet GpIIb/IIIa further increases its thrombolytic efficiency by preventing and possibly reversing platelet re-aggregation.
What Are the Clinical Implications?
N-Acetylcysteine may constitute an alternative to currently available thrombolytic agents (such as tissue-type plasminogen activator and its derivatives), with potential applications in ischemic stroke, acute myocardial infarction and acute limb ischemia.
The large availability, low cost and apparent safety of N-Acetylcysteine may improve the access to thrombolytic therapy in low-income countries with possible dramatic improvement in the management of acute thrombotic disorders at a global level.
The mechanistical interpretation of the effects of high-dose N-Acetylcysteine administration in clinical trials deserves to be re-evaluated in light of its thrombolytic properties.
Acknowledgments
Funding: This work was supported by the “Institut National de la Santé Et de la Recherche Médicale” (INSERM), the French Ministry of Research and Technology, the Conseil Régional de Basse-Normandie, the Eurostroke-Arise Program (FP7/2007-2013-201024) and the Fondation pour la Recherche sur les AVC (FR-AVC-002).
Footnotes
Conflict of interest: The authors declare no conflict of interest related to this work.
Author contributions: S.M.D.L. and M.G. designed the study, analyzed the data and wrote the manuscript; S.M.D.L., B.A.H, C.G., Y.R., S.L.D and M.G. performed the experiments; C.V.D. and P.J.L. provided critical reagents and performed in vitro VWF experiments; C.A., E.T. and D.V. analyzed the data, secured the funding and critically revised the manuscript.
References
- 1.Vivien D, Gauberti M, Montagne A, Defer G, Touze E. Impact of tissue plasminogen activator on the neurovascular unit: from clinical data to experimental evidence. J Cereb Blood Flow Metab. 2011;31:2119–2134. doi: 10.1038/jcbfm.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatia R, Hill MD, Shobha N, Menon B, Bal S, Kochar P, Watson T, Goyal M, Demchuk AM. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke. 2010;41:2254–2258. doi: 10.1161/STROKEAHA.110.592535. [DOI] [PubMed] [Google Scholar]
- 3.Kim EY, Heo JH, Lee SK, Kim DJ, Suh SH, Kim J, Kim DI. Prediction of thrombolytic efficacy in acute ischemic stroke using thin-section noncontrast CT. Neurology. 2006;67:1846–1848. doi: 10.1212/01.wnl.0000244492.99737.a8. [DOI] [PubMed] [Google Scholar]
- 4.Le Behot A, Gauberti M, Martinez De Lizarrondo S, Montagne A, Lemarchand E, Repesse Y, Guillou S, Denis CV, Maubert E, Orset C, Vivien D. GpIbalpha-VWF blockade restores vessel patency by dissolving platelet aggregates formed under very high shear rate in mice. Blood. 2014;123:3354–3363. doi: 10.1182/blood-2013-12-543074. [DOI] [PubMed] [Google Scholar]
- 5.Nesbitt WS, Westein E, Tovar-Lopez FJ, Tolouei E, Mitchell A, Fu J, Carberry J, Fouras A, Jackson SP. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat Med. 2009;15:665–673. doi: 10.1038/nm.1955. [DOI] [PubMed] [Google Scholar]
- 6.Ruggeri ZM, Orje JN, Habermann R, Federici AB, Reininger AJ. Activation-independent platelet adhesion and aggregation under elevated shear stress. Blood. 2006;108:1903–1910. doi: 10.1182/blood-2006-04-011551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denorme F, Langhauser F, Desender L, Vandenbulcke A, Rottensteiner H, Plaimauer B, Francois O, Andersson T, Deckmyn H, Scheiflinger F, Kleinschnitz C, Vanhoorelbeke K, De Meyer SF. ADAMTS13-mediated thrombolysis of t-PA-resistant occlusions in ischemic stroke in mice. Blood. 2016;127:2337–2345. doi: 10.1182/blood-2015-08-662650. [DOI] [PubMed] [Google Scholar]
- 8.Crescente M, Thomas GM, Demers M, Voorhees JR, Wong SL, Ho-Tin-Noe B, Wagner DD. ADAMTS13 exerts a thrombolytic effect in microcirculation. Thromb Haemost. 2012;108:527–532. doi: 10.1160/TH12-01-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Reheman A, Gushiken FC, Nolasco L, Fu X, Moake JL, Ni H, Lopez JA. N-acetylcysteine reduces the size and activity of von Willebrand factor in human plasma and mice. J Clin Invest. 2011;121:593–603. doi: 10.1172/JCI41062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denis C, Methia N, Frenette PS, Rayburn H, Ullman-Cullere M, Hynes RO, Wagner DD. A mouse model of severe von Willebrand disease: defects in hemostasis and thrombosis. Proc Natl Acad Sci U S A. 1998;95:9524–9529. doi: 10.1073/pnas.95.16.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karatas H, Erdener SE, Gursoy-Ozdemir Y, Gurer G, Soylemezoglu F, Dunn AK, Dalkara T. Thrombotic distal middle cerebral artery occlusion produced by topical FeCl(3) application: a novel model suitable for intravital microscopy and thrombolysis studies. J Cereb Blood Flow Metab. 2011;31:1452–1460. doi: 10.1038/jcbfm.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orset C, Macrez R, Young AR, Panthou D, Angles-Cano E, Maubert E, Agin V, Vivien D. Mouse model of in situ thromboembolic stroke and reperfusion. Stroke. 2007;38:2771–2778. doi: 10.1161/STROKEAHA.107.487520. [DOI] [PubMed] [Google Scholar]
- 13.Gauberti M, Montagne A, Marcos-Contreras OA, Le Behot A, Maubert E, Vivien D. Ultra-sensitive molecular MRI of vascular cell adhesion molecule-1 reveals a dynamic inflammatory penumbra after strokes. Stroke. 2013;44:1988–1996. doi: 10.1161/STROKEAHA.111.000544. [DOI] [PubMed] [Google Scholar]
- 14.Gaberel T, Gakuba C, Hebert M, Montagne A, Agin V, Rubio M, Emery E, Vivien D, Gauberti M. Intracerebral hematomas disappear on T2*-weighted images during normobaric oxygen therapy. Stroke. 2013;44:3482–3489. doi: 10.1161/STROKEAHA.113.002045. [DOI] [PubMed] [Google Scholar]
- 15.Gakuba C, Gauberti M, Mazighi M, Defer G, Hanouz JL, Vivien D. Preclinical evidence toward the use of ketamine for recombinant tissue-type plasminogen activator-mediated thrombolysis under anesthesia or sedation. Stroke. 2011;42:2947–2949. doi: 10.1161/STROKEAHA.111.620468. [DOI] [PubMed] [Google Scholar]
- 16.Herbig BA, Diamond SL. Pathological von Willebrand factor fibers resist tissue plasminogen activator and ADAMTS13 while promoting the contact pathway and shear-induced platelet activation. J Thromb Haemost. 2015;13:1699–1708. doi: 10.1111/jth.13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colace TV, Diamond SL. Direct observation of von Willebrand factor elongation and fiber formation on collagen during acute whole blood exposure to pathological flow. Arterioscler Thromb Vasc Biol. 2013;33:105–113. doi: 10.1161/ATVBAHA.112.300522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marcos-Contreras OA, Martinez de Lizarrondo S, Bardou I, Orset C, Pruvost M, Anfray A, Frigout Y, Hommet Y, Lebouvier L, Montaner J, Vivien D, Gauberti M. Hyperfibrinolysis increases blood-brain barrier permeability by a plasmin- and bradykinin-dependent mechanism. Blood. 2016;128:2423–2434. doi: 10.1182/blood-2016-03-705384. [DOI] [PubMed] [Google Scholar]
- 19.Adam F, Casari C, Prevost N, Kauskot A, Loubiere C, Legendre P, Reperant C, Baruch D, Rosa JP, Bryckaert M, de Groot PG, Christophe OD, Lenting PJ, Denis CV. A genetically-engineered von Willebrand disease type 2B mouse model displays defects in hemostasis and inflammation. Sci Rep. 2016;6:26306. doi: 10.1038/srep26306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W, Lin L, Zhang M, Wu Y, Liu C, Li X, Huang S, Liang C, Wang Y, Chen J, Feng W. Safety and Preliminary Efficacy of Early Tirofiban Treatment After Alteplase in Acute Ischemic Stroke Patients. Stroke. 2016;47:2649–2651. doi: 10.1161/STROKEAHA.116.014413. [DOI] [PubMed] [Google Scholar]
- 21.Cabanillas G, Popescu-Martinez A. N-Acetylcysteine for Relapsing Thrombotic Thrombocytopenic Purpura: More Evidence of a Promising Drug. Am J Ther. 2016;23:e1277–e1279. doi: 10.1097/MJT.0000000000000386. [DOI] [PubMed] [Google Scholar]
- 22.George JN, Lopez JA, Konkle BA. N-Acetylcysteine: an old drug, a new insight, a potentially effective treatment for thrombotic thrombocytopenic purpura. Transfusion (Paris) 2014;54:1205–1207. doi: 10.1111/trf.12561. [DOI] [PubMed] [Google Scholar]
- 23.Arstall MA, Yang J, Stafford I, Betts WH, Horowitz JD. N-acetylcysteine in combination with nitroglycerin and streptokinase for the treatment of evolving acute myocardial infarction. Safety and biochemical effects. Circulation. 1995;92:2855–2862. doi: 10.1161/01.cir.92.10.2855. [DOI] [PubMed] [Google Scholar]
- 24.Alexander W. European Society of Cardiology Congress. P & T. 2016;41:645–649. [PMC free article] [PubMed] [Google Scholar]
- 25.Bavarsad Shahripour R, Harrigan MR, Alexandrov AV. N-acetylcysteine (NAC) in neurological disorders: mechanisms of action and therapeutic opportunities. Brain and behav. 2014;4:108–122. doi: 10.1002/brb3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niemi TT, Munsterhjelm E, Poyhia R, Hynninen MS, Salmenpera MT. The effect of N-acetylcysteine on blood coagulation and platelet function in patients undergoing open repair of abdominal aortic aneurysm. Blood Coagul Fibrinolysis. 2006;17:29–34. doi: 10.1097/01.mbc.0000195922.26950.89. [DOI] [PubMed] [Google Scholar]
- 27.Blankenberg S, Rupprecht HJ, Bickel C, Torzewski M, Hafner G, Tiret L, Smieja M, Cambien F, Meyer J, Lackner KJ. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Engl J Med. 2003;349:1605–1613. doi: 10.1056/NEJMoa030535. [DOI] [PubMed] [Google Scholar]
- 28.Siebler M, Hennerici MG, Schneider D, von Reutern GM, Seitz RJ, Rother J, Witte OW, Hamann G, Junghans U, Villringer A, Fiebach JB. Safety of Tirofiban in acute Ischemic Stroke: the SaTIS trial. Stroke. 2011;42:2388–2392. doi: 10.1161/STROKEAHA.110.599662. [DOI] [PubMed] [Google Scholar]
- 29.Arranz L, Fernandez C, Rodriguez A, Ribera JM, De la Fuente M. The glutathione precursor N-acetylcysteine improves immune function in postmenopausal women. Free Radic Biol Med. 2008;45:1252–1262. doi: 10.1016/j.freeradbiomed.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Victor VM, Rocha M, De la Fuente M. Regulation of macrophage function by the antioxidant N-acetylcysteine in mouse-oxidative stress by endotoxin. Int Immunopharmacol. 2003;3:97–106. doi: 10.1016/s1567-5769(02)00232-1. [DOI] [PubMed] [Google Scholar]
- 31.Hussain S, Varelogianni G, Sarndahl E, Roomans GM. N-acetylcysteine and azithromycin affect the innate immune response in cystic fibrosis bronchial epithelial cells in vitro. Exp Lung Res. 2015;41:251–260. doi: 10.3109/01902148.2014.934411. [DOI] [PubMed] [Google Scholar]
- 32.Kerr F, Dawson A, Whyte IM, Buckley N, Murray L, Graudins A, Chan B, Trudinger B. The Australasian Clinical Toxicology Investigators Collaboration randomized trial of different loading infusion rates of N-acetylcysteine. Ann Emerg Med. 2005;45:402–408. doi: 10.1016/j.annemergmed.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 33.Dirnagl U, Klehmet J, Braun JS, Harms H, Meisel C, Ziemssen T, Prass K, Meisel A. Stroke-induced immunodepression: experimental evidence and clinical relevance. Stroke. 2007;38:770–773. doi: 10.1161/01.STR.0000251441.89665.bc. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.