Abstract
Hematopoietic stem cells (HSCs) have been proposed as a potential source of neural cells for use in repairing brain lesions, but previous studies indicate a low rate of neuronal differentiation and have not provided definite evidence of neuronal phenotype. To test the neurogenic potential of human HSCs, we implanted CD34+ HSCs from adult human bone marrow into lesions of the developing spinal cord in the chicken embryo and followed their differentiation by using immunohistochemistry, retrograde labeling, and electrophysiology. We find that human cells derived from the implanted population express the neuronal markers NeuN and MAP2 at substantially higher rates than previously reported. We also find that these cells exhibit neuronal cytoarchitecture, extend axons into the ventral roots or several segments in length within the spinal white matter, are decorated with synaptotagmin+ and GABA+ synaptic terminals, and exhibit active membrane properties and spontaneous synaptic potentials characteristic of functionally integrated neurons. Neuronal differentiation is accompanied by loss of CD34 expression. Careful examination with confocal microscopy reveals no signs of heterokaryons, and human cells never express a chicken-specific antigen, suggesting that fusion with host chicken cells is unlikely. We conclude that the microenvironment in the regenerating spinal cord of the chicken embryo stimulates substantial proportions of adult human HSCs to differentiate into full-fledged neurons. This may open new possibilities for a high-yield production of neurons from a patient's own bone marrow.
Keywords: bone marrow, neurogenesis, stem cell therapy
Many brain injuries are particularly refractory to self repair because of the limited regenerative potential of adult neural tissue. Stem cells may provide a means of surmounting this problem, because, in principle, they can generate unlimited numbers of cells for use in tissue replacement therapies (1–4). Several sources of adult and embryonic stem cells have been proposed for potential use in brain repair (5–8). Of these, hematopoietic stem cells (HSCs) are of special interest, because they are easily harvested, isolated, and purified, and they can be well characterized by using a repertoire of cell surface markers (9). Recent evidence suggests that subsets of adult human HSCs (hHSCs) can differentiate to neural cells, albeit at relatively low efficiency (3, 10–13). The potential of hHSCs to generate neural cells is poorly characterized, in terms of both the intrinsic genetic programs involved and the environmental signals that activate them. Several reports indicate an overlap in the molecular programs for hematopoiesis and neuropoiesis in mice (14, 15). Another report has shown that primary CD34+ hHSCs express mRNA for a number of proteins that are used by (among other cell types) neurons, including receptors for trophic factors and other mediators that are known to influence the development of neurons (16). Commonalities in the expression profiles of neural and HSCs provide molecular opportunities for across-tissue differentiation, but it remains to be seen how well these can be used in the organism or exploited in the clinic.
Mammalian HSCs have been observed to differentiate at low frequency into neural cells in several in vitro and in vivo systems. Adult HSCs from rodents and humans injected intravenously or intracerebrally into rodent hosts can settle in the brain and express neuronal markers, but the incidence of neuronal differentiation has never been reported to exceed 1–2% of those HSCs that integrate into the brain (1, 10, 17). Higher incidences have been reported for HSCs and other bone marrow stem cells in vitro under conditions designed to promote neuronal differentiation (2, 18). However, the characterization of neuronal phenotype in all these studies has been limited to the expression of selected molecular markers. Functional phenotypic features and integration into synaptic networks have not been demonstrated. Finding an in vivo system in which functional neuronal differentiation of hHSCs can be characterized and achieves high yields would be a major step toward understanding the biology of this type of differentiation.
Xenotypic grafting has been a powerful tool in studies of differentiation and embryogenesis for many years. The embryonic environment has little or no immunue response, obviating problems posed by tissue rejection and inflammatory responses. One of the traditional embryonic systems for such approaches is the chicken embryo. Recent reports have shown that both human ES cells, rat mesenchymal stem cells, and mouse neural stem cells can integrate into the chicken embryo and differentiate into various cell types with no apparent fusion to the host chicken cells (19–21).
Lesions to the developing brain and spinal cord of the chicken embryo repair themselves through a process called regulative regeneration. Neighboring neural stem cells proliferate to fill in the wound, producing neurons of the right types in the proper places (22). We surmised that the microenvironment within the regenerating neural tissue might stimulate multipotent stem cells in general to produce differentiated neurons. To test this idea, we implanted CD34+ HSCs from adult human donors into lesions of the developing spinal cord and followed their differentiation.
Methods
In Ovo Surgery and Cell Implantation. Chicken eggs were incubated to stage 15–16, at which time a one- to three-segment stretch of the lumbar spinal neural tube was excised unilaterally by microsurgery. Approximately 20,000 CD34+ hHSCs isolated from bone marrow were implanted into the lesion with a glass micropipette. The eggs were then incubated for 4–9 days before assessment of neuronal differentiation by the human cells.
Immunohistochemistry. At the end of incubation, CD34+ hHSCs were detected by using an antibody to human nuclear antigen (hNA). Embryos were collected from the eggs and the lumbar part of the embryo dissected out and fixed in buffered 1% glutaraldehyde/3% paraformaldehyde (for anti-GABA) or 4% paraformaldehyde (for all other antibodies), cryoprotected, and sectioned transversely at 10 μm. Immunohistochemistry was performed with conventional techniques.
Retrograde Axonal Tracing. Spinal motoneurons and interneuron populations were labeled retrogradely with 3 kDa of rhodamine dextran amine (Molecular Probes) in an in vitro preparation of the spinal cord, as described (23–25). The preparations were then fixed in 4% paraformaldehyde and sectioned and analyzed by immunohistochemistry.
Electrophysiology. Spinal cord slices from the segments containing human cells were cut manually (≈400 μm thick) and placed in an incubation chamber for a minimal recovery period of 30 min before they were transferred to a recording chamber. Patch–clamp recordings and intracellular labeling with biocytin were performed by using conventional techniques.
For detailed methods, see Supporting Text, which is published as supporting information on the PNAS web site.
Results
Expression of Neuronal Markers by hHSC-Derived Cells Integrated into Neural Tissue. In nearly 60% (87/154) of the embryos exhibiting full regulative regeneration, adult hHSCs had integrated into the regenerated spinal cord (Fig. 1B). There were no signs of rejection of the human cells or of inflammatory responses. hHSCs integrated into both the ventricular zone, where neuronal progenitors reside, and the developing mantle zone, where postmitotic neurons reside. In addition, many hHSCs ended up in peripheral tissue, including the dorsal root ganglion, which contains sensory neurons. Not all hHSCs, however, ended up in neural tissue. Indeed, they were most commonly found in blood vessels, suggesting that many hHSCs maintain their preimplantation hematopoietic fate despite being in a xenotypic environment (for more detailed information about the fate of the implanted hHSCs, see Supporting Text).
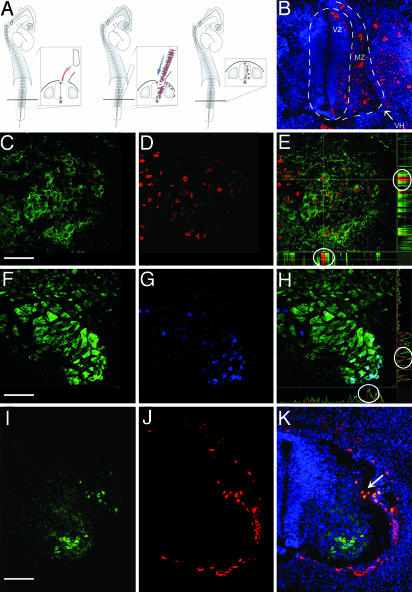
Fig. 1.
Expression of neuronal markers by human cells after integration into the regenerated embryonic spinal cord. (A) A one- to three-segment-long portion of the lumbar neural tube was excised unilaterally, and hHSCs were injected into the lesion with a micropipette. (B) PKH26-labeled (red) human cells integrated into the regenerated spinal cord. Nuclei counterstained blue with Hoechst 33342. (C–E) MAP2-(green) and hNA-(red) labeled human cells in ventral horn; colocalization demonstrated (circles) for one selected cell (at cross hairs) shown in side views of confocal stack (E). (F–H) NeuN-(green) and hNA-labeled (blue) human cells in ventral horn; colocalization demonstrated (circles) for one selected cell (at cross hairs) shown in side views of confocal stack (H). (I–K) Human cells (red, hNA) implanted outside the developing spinal cord do not invade neural tissue or express neuronal antigens (MAP2, green) except at small lesion site (arrow). Nuclei counterstained with Hoechst 33342 (blue). VZ, ventricular zone; MZ, mantle zone; VH, ventral horn. All images are from 10-μm transverse sections, dorsal up; B–H are of 0.5-μm optical sections. [Bars, 60 (B), 40 (C–H), and 80 (I–K) μm.]
Over the ensuing 4–9 days, a substantial fraction of hHSCs (Table 1) began to express the neuron-specific markers MAP2 and NeuN (Fig. 1 C–K). As was the case for the overall population of integrated hHSCs, these human neuron-like cells were more prevalent in the ventral horn of the regenerated spinal cord but were also found in the dorsal spinal cord and the spinal ganglia. The ventral region of the developing spinal cord of chicken embryos normally has a higher level of MAP2 and NeuN expression (see, for example, Fig. 1I). Thus, expression of MAP2 and NeuN by hHSCs seemed to obey a dorsal-to-ventral gradient normally found in the host neural tissue.
Table 1. Efficiency of neuronal differentiation by hHSCs (at the indicated days after implantation).
| Neuronal differentiation | n | Days after implantation | % of integrated hHSCs |
|---|---|---|---|
| Expressing neuronal markers* | 13 | 4-9 | 16 ± 4.8 (5-45) |
| With long axons in ventral funiculus† | 6 | 6 | 14 ± 11 (4-35) |
| With axons in peripheral nerve* | 9 | 6-9 | 9.6 ± 4.7 (5-18) |
| Receiving GABA+ synaptic terminals* | 5 | 9 | 10 ± 3.5 (6-15) |
| Receiving synaptotagmin+ synaptic terminals* | 8 | 9 | 12.7 ± 3.1 (9-15) |
Data in parentheses represent range.
Counted in 10 sections from each embryo.
Counted in five sections from each embryo.
Expression of Neuronal Markers by hHSC-Derived Cells Depends on the Regenerating Environment. Human cells found in surrounding nonneural tissue never expressed neuronal markers. In separate experiments, adult hHSCs implanted at sites outside but close to the intact developing spinal cord neither expressed NeuN or MAP2 nor invaded the spinal cord (n = 4). If a small cut was made in the developing spinal cord, however, a few human cells invaded the spinal cord through the cut (n = 2). In this case, both these and the few human cells situated immediately outside of the spinal cord near the cut expressed neuronal markers (Fig. 1 I–K). Evidently even a small lesion in the developing neural tissue is permissive for invasion and can stimulate neuronal differentiation at short range. We conclude that it is not developing neural tissue per se but rather the regenerative situation within the neural tissue that is particularly stimulatory for neuronal differentiation.
Neuronal Differentiation Is Paralleled by Loss of CD34 Expression with No Sign of Fusion to Host Chicken Cells. To assess cell type-specific gene expression occurring during neuronal differentiation of CD34+ hHSCs, we followed the expression of CD34, NeuN, and MAP2 at different time points (Fig. 2). All freshly isolated CD34+ hHSC tested expressed CD34, but none expressed NeuN or MAP2 (n = 1,500 cells from three different donors). We also tested for expression of the neural progenitor marker nestin in the freshly isolated CD34+ hHSCs, which was absent (not shown).
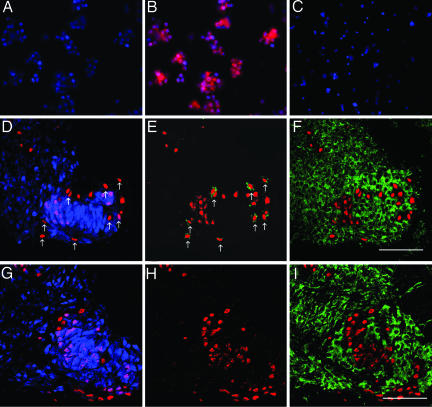
Fig. 2.
Neuronal differentiation by human cells involves loss of CD34 expression but never expression of a chicken-specific marker. (A–C) Freshly isolated CD34+ cells (B, red) do not express NeuN (A) or MAP2 (C). Nuclei counterstained with Hoechst 33342 (blue). (D–I) Expression of NeuN (blue), hNA (red), CD34 (green, E and H), and chicken-specific marker (green; F and I)3(D–F) and 5 days (G–I) after implantation of hHSCs. Arrows point to hNA+ cells that are NeuN-negative (D) and CD34-positive (E). F and I show the sections in D and G superimposed on neighboring sections. A–C are images of cytospins, and D–I are 0.5-μm optical sections from 10-μm transverse sections, dorsal up. [Bars, 40 μm(D–I).]
CD34+ hHSCs cultured in vitro lose CD34 expression over several days (26). We therefore tested for CD34 expression in vivo over a similar time frame. At 3 days after implantation, some of the human cells expressed CD34, and some expressed neuronal antigens, but none expressed both (Fig. 2 D and E). By 5 days after implantation, no human cells expressed CD34 (Fig. 2 G and H).
In earlier studies, some examples of apparent neuronal differentiation by hHSCs have been explained by fusion of hHSCs with endogenous neurons, creating hybrid cells of both donor and host origin with neuronal character (27, 28). Such fusion occurs at very low frequencies (≈0.01%) and produces neurons containing two or more nuclei (29). To assess whether this could explain the neuronal differentiation of CD34+ hHSCs, we compared the expression of hNA with that of a chicken specific marker (Fig. 2 F and I). We observed a complete segregation of human and chicken cells at both 3 and 5 days after implantation; no cell expressed both markers. We also searched for the presence of double nuclei using fluorescent nuclear stains and both conventional and confocal microscopy. Of 2,879 human cells in 10 embryos, none contained more than one nucleus, which was always larger than the nuclei of chicken cells and similar to the size of hHSC nuclei.
hHSC-Derived Cells Express Definitive Neuronal Characteristics. To determine whether human cells expressing neuronal markers were full-fledged neurons, we used retrograde axonal tracing to reveal long axonal projections and dendritic structure (Fig. 3) and electrophysiology to assess active membrane properties (Fig. 4).
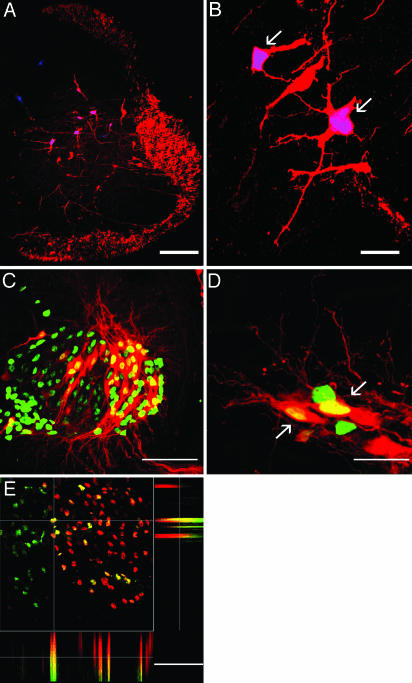
Fig. 3.
Neuronal morphology and expression of motoneuron-specific markers by human cells in the regenerated embryonic spinal cord. (A and B) Human cells retrogradely labeled from the ventral funiculus [arrows; red, rhodamine dextran amine (RDA); blue, hNA] are indistinguishable from spinal interneurons. (C and D) Human cells retrogradely labeled from peripheral nerve (arrows; red, RDA; green, hNA). (E) Colocalization of Islet-1 (red) and hNA (green), demonstrated for one selected cell (at cross hairs) in side views of confocal stack. A, B, and E are 0.5-μm optical sections, and C and D are confocal stacks from 10-μm transverse sections, dorsal up. [Bars, 150 (A), 15 (B), 40 (C), 10 (D), and 40 (E) μm.]
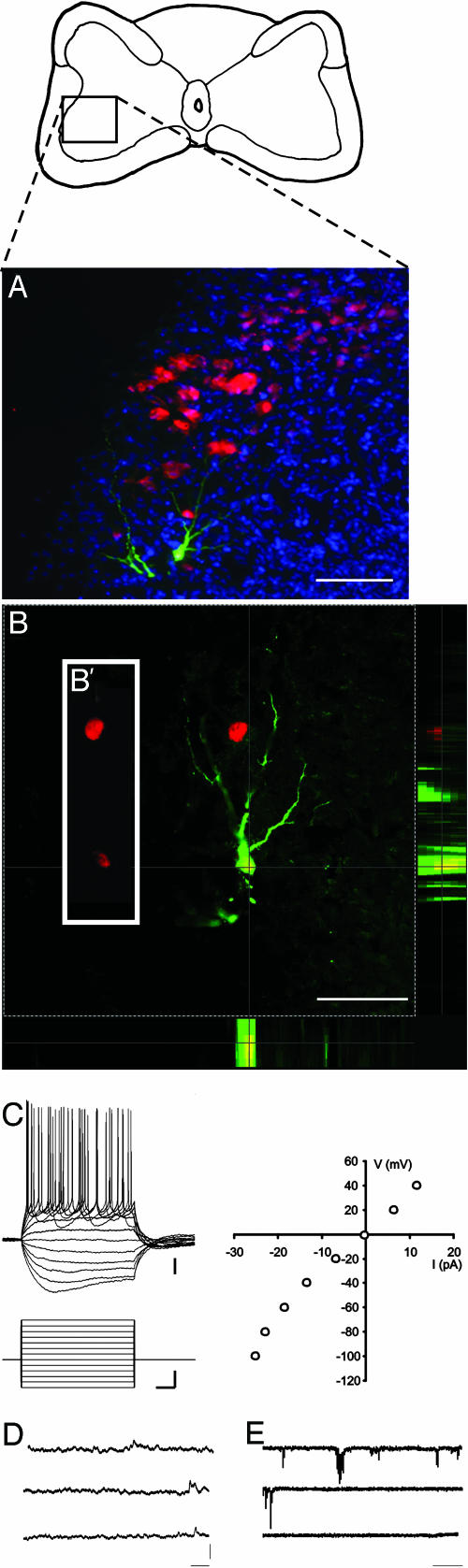
Fig. 4.
Electrophysiological properties of human neurons. (A) Transverse section through spinal cord 10 days after implantation of human cells (red, hNA), with one human cell labeled intracellularly with biocytin (green), counterstained with Hoechst 33324 (blue). Diagram above shows placement of the field shown within the cross-sectional area of the cord. (B) Optical section through this neuron, showing colocalization of biocytin (green) and hNA (red) in side views of confocal stack. B′ is a slice of the single optical section, showing the red channel only, displaced to the left to emphasize that the biocytin-labeled neuron expresses hNA. (C) Current steps (600 ms, incrementing + 20 from –120 pA) in current–clamp mode show that the human cell is excitable, fires overshooting action potentials in response to depolarization, and exhibits a voltage sag in response to hyperpolarizing pulses. I–V curve shown to the right. (D and E) Spontaneous synaptic events observed at resting membrane potential (–52 mV) in current–(D) and voltage–clamp (E) mode. The cell had an input resistance of 375 megaohms and a membrane time constant of 45 ms. [Bars, 100 μm(A); 60 μm(B); 10 mV, 60 pA, and 100 ms (C); 5 mV(D); 50 pA (E); and 1 sec (D and E).
To label interneurons with descending axons (24), we applied fluorescent dextran amines to the ventral funiculus three segments caudal to the regenerated segment. Retrogradely labeled human cells had morphologies indistinguishable from endogenous spinal interneurons, including well developed dendritic arbors (Fig. 3 A and B). The proportion of human cells with descending axons was similar to that of human cells expressing neuronal markers (Table 1). This is probably an underestimate, because the number of interneurons falls sharply as axon length increases (25). Because we restricted our attention to human cells with axons at least three segments long (a feature that would be expected only for neurons), the counts do not include larger numbers of retrogradely labeled cells at shorter distances.
To label motoneurons, we applied fluorescent dextran amines to a hindlimb nerve distal to the crural plexus (Fig. 3 C and D). This showed that ≈10% of human cells (Table 1) had long peripheral axons, a feature unique to motoneurons. To confirm differentiation to motoneurons, we tested for the expression of the motoneuron marker, islet-1. A substantial number of the human cells were islet-1+ (Fig. 3 E and F).
To assess the electrophysiological properties of human cells, we made whole-cell recordings from randomly selected neurons in the ventral horn of spinal cord slices. In five slices from three embryos, seven neurons were recorded and then labeled intracellularly with biocytin. Two of these were later confirmed to be human cells by immunohistochemistry for hNA. Both exhibited characteristics indistinguishable from genuine neurons, including the capacity to generate overshooting action potentials and a nonlinear current–voltage relationship indicative of voltage-sensitive conductances (Fig. 4).
hHSC-Derived Cells Receive Synaptic Connections. To assess the presence of synaptic connections onto human cells, we examined the expression of two presynaptic markers: synaptotagmin and the inhibitory neurotransmitter GABA. GABA+ and synaptotagmin+ terminals abutted human cells in patterns indistinguishable from those observed for endogenous chicken neurons (Fig. 5). The proportions of human cells with apposed synaptic terminals were similar to that of human cells expressing MAP2 or NeuN (Table 1), suggesting that all human cells that differentiate into neurons in the regenerated spinal cord are juxtaposed by synaptic terminals. In support of the presence of functional synapses, we observed spontaneous potentials in both of the human cells from which we recorded. These had opposite deflection in voltage and current clamp, as would be expected of synaptic potentials (Fig. 4 D and E).
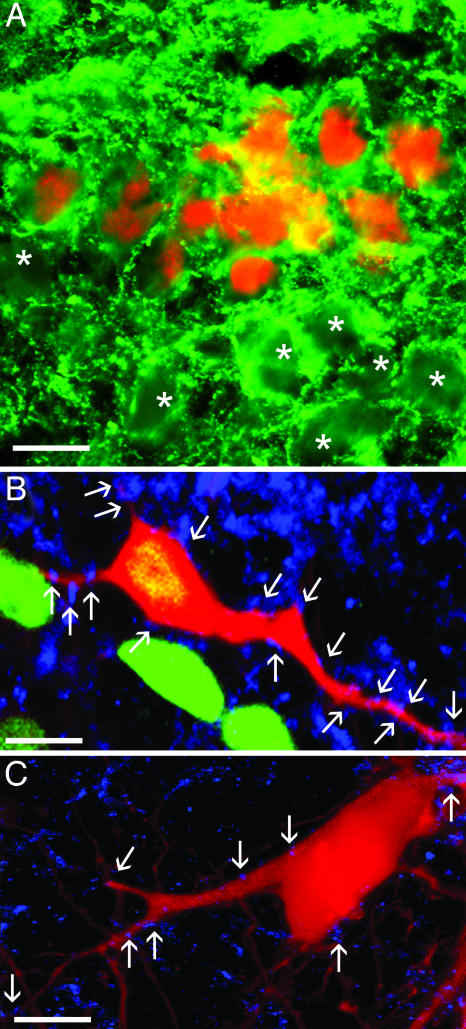
Fig. 5.
Morphological evidence of synaptic contacts onto human cells. (A) Human cells (red, hNA) are decorated with GABA+ synaptic terminals (green), as are nearby chicken neurons (asterisks). (B) Human (green, hNA) and (C) chicken neurons, retrogradely labeled from peripheral nerve (red, RDA), decorated with synaptotagmin+ terminals (blue). A–C are from 10-μm transverse sections, dorsal up. B and C are 0.5-μm optical sections. [Bars, 20 μm(A) and 10 μm(B and C).]
Discussion
There is now extensive evidence that hHSCs and other stem cells from bone marrow can differentiate into cells of other tissues, but the full differentiation potential is poorly understood (1–4, 10, 12, 13). Here we show that a substantial proportion of adult human CD34+ hHSCs that have integrated into the regenerating spinal cord of the chicken embryo differentiates into neurons. To our knowledge, definitive neuronal differentiation by hHSCs in vivo has not been described previously. We conclude that there is a population of HSCs within human bone marrow that has neurogenic potential and is capable of differentiating into neurons when placed into an appropriate environment.
We observed no neuronal differentiation when hHSCs were implanted next to the intact developing spinal cord, but hHSCs entering a small cut in the spinal cord did express neuronal markers. This suggests that it is not the embryonic environment per se but rather the specific microenvironment of the regenerating embryonic neural tissue that promotes neuronal differentiation by hHSCs. Regulative regeneration involves a controlled proliferation and differentiation of host chicken stem and progenitor cells into appropriate numbers and phenotypic arrangements of neurons (22), so it is not entirely surprising that the regenerative situation is particularly stimulatory for neuronal differentiation.
Earlier studies of neuronal differentiation by HSCs have been performed primarily in adult rodents, and most of these have used rodent HSCs, although a few have used hHSCs (3, 10–13). In these studies, HSCs were injected either intravenously or stereotactically into the brain of the recipient. Virtually none of these studies has assessed the proportion of injected HSCs that actually populate neural tissue. Typically, the proportions of HSCs that differentiate neuronal markers are very low, ranging from 0.01% to 4% (30). In our experiments, the proportion was much higher. It is therefore possible that the decisive difference between our results and those of earlier studies is the fact that the hHSCs are being exposed to an actively regenerating embryonic tissue, in which molecular factors that regulate proliferation and differentiation are expected to be expressed in profusion.
The CD34+ hHSCs represent a heterogeneous population of cells at many different stages of functional differentiation, from pluripotent stem cells to lineage-restricted progenitor cells. Most of the CD34+ cells are progenitors for myeloid and lymphoid lineages, but a subpopulation, defined by the expression of the c-kit receptor and lack of expression of CD38 and hematopoietic lineage markers, is considered to be composed of pluripotent HSCs that can give rise to all hematopoietic lineages (31). At some point during differentiation, these pluripotent HSCs become committed to particular lineages and lose their capacity to function as stem or progenitor cells. Several possibilities therefore exist for explaining the high degree of neuronal differentiation we observe in the regenerating embryonic spinal cord. At one extreme, we may be seeing 100% neuronal differentiation of a highly potent subpopulation of the injected HSCs, for example, the pluripotent subpopulation. At the other extreme, we may be seeing a lower efficiency of neuronal differentiation by all of the CD34+ HSCs, perhaps limited by extant environmental factors in the regenerating tissue. Whether our findings represent the behavior of a subpopulation of hHSCs with a preprogrammed propensity toward neuronal differentiation or a partial realization of a neurogenic potential in all hHSCs remains to be seen.
Our data suggest that fusion with host chicken cells is not a contributing factor to the neuronal differentiation of implanted human cells. Were it to occur, the hybrid cells would have to lose the expression of at least one chicken-specific marker and probably substantial numbers of chromosomes (to maintain the nuclear appearance of human cells). Moreover, the degree of fusion would necessarily have to be very much higher than ever observed without fusion-promoting manipulations. Cell fusion is a normal differentiation pathway for several cell types, including liver cells and skeletal and cardiac muscle cells. Nevertheless, using various methods, different research groups have shown that stem cells from the bone marrow either do not fuse with cells from these and other tissues (16, 32) or fuse at very low frequency (0.01–0.1%) (27, 28). In particular, a recent publication by Pochampally et al. (20) shows that rat bone marrow-derived mesenchymal stem cells implanted into the somites of chicken embryos do not fuse with chicken cells.
Our results suggest that hHSCs may have potential clinical benefits beyond the arena of hematopoiesis. The possibility of exposing hHSCs to an in vitro situation that mimics the key molecular signals present during regulative regeneration could provide a means of generating neurons for therapeutic purposes using a patient's own bone marrow as a source. In addition, implantation into the regenerating spinal cord of the chicken embryo under standardized conditions can now be used to test and compare the in vivo neurogenic potential of a variety of human and animal stem cells.
Supplementary Material
Acknowledgments
We thank Frode F. Jahnsen for invaluable assistance, Kobra Sultani for technical assistance, and Jean-Sebastian Renaud for help with graphics. This work was supported by the Norwegian Center for Stem Cell Research (Norwegian Research Council), European Union Grant QLG2-CT-2001-01467, the Memorial Fund of Helga Jonsdottir and Sigurlidi Kristjansson, the Human Frontier Science Program, and the Rikshospitalet Medinnova Fund.
Author contributions: O.E.S., M.-C.P., T.E., and J.C.G. designed research; O.E.S., M.-C.P., and J.C.G. performed research; O.E.S., M.-C.P., T.E., and J.C.G. analyzed data; and O.E.S., T.E., and J.C.G. wrote the paper.
Abbreviations: HSC, hematopoietic stem cell; hHSC, human HSC; hNA, human nuclear antigen.
References
- 1.Weimann, J. M., Charlton, C. A., Brazelton, T. R., Hackman, R. C. & Blau, H. M. (2003) Proc. Natl. Acad. Sci. USA 100, 2088–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanchez-Ramos, J., Song, S., Cardozo-Pelaez, F., Hazzi, C., Stedeford, T., Willing, A., Freeman, T. B., Saporta, S., Janssen, W., Patel, N., et al. (2000) Exp. Neurol. 164, 247–256. [DOI] [PubMed] [Google Scholar]
- 3.Mezey, E., Key, S., Vogelsang, G., Szalayova, I., Lange, G. D. & Crain, B. (2003) Proc. Natl. Acad. Sci. USA 100, 1364–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mezey, E., Chandross, K. J., Harta, G., Maki, R. A. & McKercher, S. R. (2000) Science 290, 1779–1782. [DOI] [PubMed] [Google Scholar]
- 5.Song, H. J., Stevens, C. F. & Gage, F. H. (2002) Nat. Neurosci. 5, 438–445. [DOI] [PubMed] [Google Scholar]
- 6.Liang, L. & Bickenbach, J. R. (2002) Stem Cells 20, 21–31. [DOI] [PubMed] [Google Scholar]
- 7.Li, Y., Chen J., Chen, X. G., Wang, L., Gautam, S. C., Xu, Y. X., Katakowski, M., Zhang, L. J., Lu, M., Janakiraman, N., et al. (2002) Neurology 59, 514–523. [DOI] [PubMed] [Google Scholar]
- 8.Kang, S. K., Lee, D. H., Bae, Y. C., Kim, H. K., Baik, S. Y. & Jung, J. S. (2003) Exp. Neurol. 183, 355–366. [DOI] [PubMed] [Google Scholar]
- 9.Steen, R., Tjonnfjord, G. E. & Egeland, T. (1994) J. Hematother. 3, 253–262. [DOI] [PubMed] [Google Scholar]
- 10.Wagers, A. J., Sherwood, R. I., Christensen, J. L. & Weissman, I. L. (2002) Science 297, 2256–2259. [DOI] [PubMed] [Google Scholar]
- 11.Brazelton, T. R., Rossi, F. M., Keshet, G. I. & Blau, H. M. (2000) Science 290, 1775–1779. [DOI] [PubMed] [Google Scholar]
- 12.Morshead, C. M., Benveniste, P., Iscove, N. N. & van der Kooy, D. (2002) Nat. Med. 8, 268–273. [DOI] [PubMed] [Google Scholar]
- 13.Cogle, C. R., Yachnis, A.T., Laywell, E. D., Zander, D. S., Wingard, J. R., Steindler, D. A. & Scott, E. W. (2004) Lancet 363, 1432–1437. [DOI] [PubMed] [Google Scholar]
- 14.Terskikh, A. V., Easterday, M. C., Li, L., Hood, L., Kornblum, H. I., Geschwind, D. H. & Weissman, I. L. (2001) Proc. Natl. Acad. Sci. USA 98, 7934–7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goolsby, J., Marty, M. C., Heletz, D., Chiappelli, J., Tashko, G., Yarnell, D., Fishman, P. S., Dhib-Jalbut, S., Bever, C. T., Jr., Pessac, B., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 14926–14931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steidl, U., Bork, S., Schaub, S., Selbach, O., Seres, J., Aivado, M., Schroeder, T., Rohr, R. Fenk, U. P., Kliszewski, S., et al. (2004) Blood 104, 81–88. [DOI] [PubMed] [Google Scholar]
- 17.Woodbury, D., Schwarz, E. J., Prockop, D. & Black, I. B. (2000) J. Neurosci. Res. 61, 364–370. [DOI] [PubMed] [Google Scholar]
- 18.Locatelli, F., Corti, S., Donadoni, C., Guglieri, F., Capra, F., Strazzer, S., Salani, S., Del Bo, R., Bordoni, A. & Comi, G. P. (2003) J. Hematother. Stem Cell Res. 12, 727–734. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein, R. S., Drukker, M., Reubinoff, B. E. & Benvenisty, N. (2002) Dev. Dyn. 225, 80–86. [DOI] [PubMed] [Google Scholar]
- 20.Pochampally, R. R., Neville, B. T., Schwarz, E. J., Li, M. M. & Prockop, D. J. (2004) Proc. Natl. Acad. Sci. USA 101, 9282–9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarke, D. L., Johansso, C. B., Wilbertz, J., Veress, B., Nilsson, E., Karlstrom, H., Lendahl, U. & Frisen, J. (2000) Science 288, 1660–1663. [DOI] [PubMed] [Google Scholar]
- 22.Diaz, C. & Glover, J. C. (1996) Development (Cambridge, U.K.) 122, 3095–3105. [DOI] [PubMed] [Google Scholar]
- 23.Glover, J. C. (1995) Neurosci. Protocols 30, 1–13. [Google Scholar]
- 24.Stokke, M. F., Nissen, U. V., Glover, J. C. & Kiehn, O. (2002) J. Comp. Neurol. 446, 349–359. [DOI] [PubMed] [Google Scholar]
- 25.Eide, A. L., Glover, J. C., Kjaerulff, O. & Kiehn, O. (1999) J. Comp. Neurol. 403, 332–345. [PubMed] [Google Scholar]
- 26.Sigurjonsson, O. E., Gudmundsson, K. O., Haraldsdottir, V., Rafnar, T. & Gudmundsson, S. J. (2002) Hematother. Stem Cell Res. 11, 389–400. [DOI] [PubMed] [Google Scholar]
- 27.Terada, N., Hamazaki, T., Oka, M., Hoki, M., Mastalerz, D. M., Nakano, Y., Meyer, E. M., Morell, L., Petersen, B. E. &. Scott, E. W. (2002) Nature 416, 542–545. [DOI] [PubMed] [Google Scholar]
- 28.Ying, Q. L., Nichols, J., Evans, E. P. & Smith, A. G. (2002) Nature 416, 545–548. [DOI] [PubMed] [Google Scholar]
- 29.Alvarez-Dolado, M., Pardal, R., Garcia-Verdugo, J. M., Fike, J. R., Lee, H. O., Pfeffer, K., Lois, C., Morrison, S. J. & Alvarez-Buylla, A. (2003) Nature 425, 968–973. [DOI] [PubMed] [Google Scholar]
- 30.Corti, S., Locatelli, F., Strazzer, S., Guglieri, M. & Comi, G. P. (2003) Curr. Gene Ther. 3, 247–272. [DOI] [PubMed] [Google Scholar]
- 31.Ikhara, S. (2000) Proc. Soc. Exp. Biol. Med. 223, 149–155. [DOI] [PubMed] [Google Scholar]
- 32.Jang, Y. Y., Collector, M. I., Baylin, S. B., Diehl, A. M. & Sharkis, S. J. (2004) Nat. Cell Biol. 6, 532–539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.