Abstract
Administration of gonadotropin-releasing hormone (GnRH) induces a surge of luteinizing hormone and ovulation in a variety of species, including human beings. Our objectives were to determine the effect of follicle size at the time of ovulation on corpus luteum function and establishment and maintenance of pregnancy in cows in which ovulation was either spontaneous or induced with GnRH. GnRH-induced ovulation of follicles ≲11 mm in diameter resulted in decreased pregnancy rates and increased late embryonic mortality. This decrease in fertility was associated with lower circulating concentrations of estradiol on the day of insemination, a decreased rate of increase in progesterone after insemination, and, ultimately, decreased circulating concentrations of progesterone. In contrast, ovulatory follicle size had no apparent effect on fertility when ovulation occurred spontaneously. Follicles undergoing spontaneous ovulation do so at a wide range of sizes when they are physiologically mature. Therefore, administration of GnRH to induce ovulation likely initiates a preovulatory gonadotropin surge before some dominant follicles attain physiological maturity. GnRH-induced ovulation of follicles that are physiologically immature has a negative impact on pregnancy rates and late embryonic/fetal survival. These observations in cattle may have implications for assisted reproductive procedures in human beings.
Keywords: ovulation, cattle, artificial insemination, embryonic mortality, fertility
Procedures that control the timing of ovulation in human beings and other mammals are of enormous value in advancing the use of assisted reproductive technologies. In cattle, several protocols are effective at controlling the estrous cycle and reducing the time required to detect estrus (1–3), but the timing of ovulation is imprecise, which makes it difficult to inseminate cows at a fixed time. When fixed-time insemination protocols (protocols that synchronize ovulation) are attempted, gonadotropin-releasing hormone (GnRH) is used to induce ovulation. In some synchronization protocols, GnRH is administered 9 days before insemination to induce ovulation and corpus luteum (CL) formation and to initiate a new follicular wave. Two days before insemination, prostaglandin F2α is administered to induce luteolysis, and 48 h later, GnRH is administered to induce ovulation of the preovulatory follicle (4, 5). Insemination is performed at the time of the second GnRH injection (4) or 16–24 h after the second GnRH injection (5).
Bovine follicles achieve ovulatory capacity at ≈10 mm in diameter. However, a larger dose of luteinizing hormone is required to induce ovulation of a 10-mm follicle than to induce ovulation of larger follicles (6). In cattle, the efficiency of a single injection of GnRH to induce ovulation and thereby synchronize the initiation of the subsequent follicular wave is only 66% when evaluated across all stages of the estrous cycle (7). Because of this variation in ovulatory response, we hypothesized that considerable variation would exist in follicle size at the time of insemination/induction of ovulation by the second injection of GnRH. Accordingly, the objectives of these studies were to determine whether follicle size at GnRH-induced or spontaneous ovulation affected subsequent CL function, pregnancy rates, and embryonic/fetal mortality.
Materials and Methods
Experiment 1. Experimental design. Postpartum multiparous (3–13 years old) Angus-crossed beef cows (n = 40) at the University of Missouri–Columbia Beef Farm were divided equally into two estrous cycling-status (anestrous vs. cycling) groups according to age (4–13 years) and cow body condition score (1 = emaciated and 9 = obese; range 5–7). Blood samples were collected on days –23, –16, and –10 (day 0 = induction of ovulation and insemination). Cows were considered anestrous if serum concentrations of progesterone were <1 ng/ml in each of the preceding samples and were considered to be cycling if serum concentrations of progesterone were >1ng/ml on day –23, –16, or –10. Cows were injected with GnRH (100 μg as 2 ml of Cystorelin i.m., Merial, Athens, GA) on day –9 and prostaglandin F2α (PGF2α) (25 mg as 5 ml of Lutalyse i.m., Pharmacia Animal Health, Kalamazoo, MI) on day –2. Forty-eight hours after PGF2α injection (day 0), cows received GnRH (Cystorelin, 100 μg i.m.) and were artificially inseminated with semen from one of two bulls. All cows were inseminated by the same technician.
All cows in the experiment were maintained as a single group, and calves were allowed to suckle without restriction. Cows were observed twice daily for signs of estrous behavior. Four anestrous cows were removed from the study because they did not ovulate in response to the second GnRH injection. One cow in the cycling group died (unrelated to treatment) on day 30 and therefore does not appear in the final pregnancy data.
Blood sampling, RIA, and ELISA. Blood samples were collected by jugular venipuncture into 10-ml Vacutainer tubes (Fisher Scientific) on days –23, –16, and –10; daily from day –9 to 22; and weekly from day 25 to 60 postinsemination. Blood was allowed to coagulate at room temperature, stored at 4°C for 24 h, and centrifuged at 1,200 × g for 30 min. Serum was harvested and stored at –20°C until analysis was performed. Serum concentrations of progesterone were analyzed in all samples by RIA (8) (Diagnostic Products, Los Angeles). Intra- and interassay coefficients of variation for progesterone assays were 2.75% and 10%, respectively, and assay sensitivity was 0.5 ng/ml. Serum concentrations of estradiol-17β were analyzed in samples collected on days –3 to 0 by RIA (9). Intra- and interassay coefficients of variation for estradiol-17β assays were 3.3% and 9.8%, respectively, and assay sensitivity was 0.5 pg/ml. Serum concentrations of pregnancy-associated glycoproteins (PAGs) were analyzed in all serum samples by ELISA (10). Intra- and interassay coefficients of variation for PAG assays were 7.0% and 15.2%, respectively, and assay sensitivity was 1 ng/ml.
Ultrasonography. Ovaries of all cows were examined by transrectal ultrasonography to characterize follicular development (days –2, –1, and 0) and confirm ovulation (day 2) by using an Aloka 500V ultrasound with a 7.5-MHz linear probe (Aloka, Wallingford, CT). All follicles ≥8 mm in diameter were recorded. Follicle size was determined by averaging follicular diameter at the widest point and at a right angle to the first measurement by using the internal calipers on the Aloka 500V. Ovulation was defined as the disappearance of a large follicle from an ovary within 2 days after GnRH administration. Beginning on day 25, the uteri of all cows were examined weekly for 6 weeks by transrectal ultrasonography to determine pregnancy status and embryo viability (heartbeat) by using an Aloka 500V ultrasound with a 5-MHz linear probe.
Experiment 2. Experimental design. Postpartum (2–11 years old) beef cows (one-half Red Angus × one-fourth Charolais × one-fourth Tarentaise; n = 273) with suckling calves at the United States Department of Agriculture–Agricultural Research Service's Fort Keogh Livestock and Range Research Laboratory were treated according to the synchronization protocol described in experiment 1 and were artificially inseminated by one of two technicians with semen from 1 of 13 bulls at the time of the second GnRH injection (induced ovulation). Follicle size at time of insemination was used to determine the relationship between follicle size and pregnancy success among cows induced to ovulate. Subsequently, 179 cows were reinseminated after detection in estrus by the HeatWatch electronic estrous detection system (DDx, Denver) during a 37-day breeding season (spontaneous ovulation). Cows were considered to be in estrus when three mounts of 2 s or longer in duration were recorded within a 4-h period. Cows determined to be in estrus were artificially inseminated ≈12 h after initiation of standing estrus. Follicle size at time of insemination was also used to determine the relationship between follicle size and pregnancy success among cows that spontaneously ovulated. Blood samples were collected on days –16 and –9 to determine estrous cycling status as described in experiment 1. At the initiation of treatment (day –9), cows were divided into two replicate groups until day 1 (treatment of group 2 was initiated 24 h after group 1 to better handle the number of animals). Cows were maintained as a single group after insemination for the remainder of the experiment, and calves were allowed to suckle without restriction. Cows were removed from the induced ovulation group (n = 108) if they exhibited standing estrus before timed insemination (n = 49), did not ovulate in response to the second GnRH injection (n = 51), or exhibited a short luteal phase after insemination (n = 8).
Blood sampling, RIA, and ELISA. Blood samples were collected by means of puncture of a tail vessel into 10-ml Vacutainer tubes (Fisher Scientific) on days –16, –9, –2, and 0 to determine circulating concentrations of progesterone (days –16, –9, –2, and 0) and estradiol (days –2 and 0). Serum was collected as described in experiment 1 and stored at –20°C until analysis was performed. On days 13, 19, 27, 41, 55, and 68, blood samples were collected in 10-ml Vacutainer tubes (Fisher Scientific) containing EDTA and centrifuged at 1,200 × g for 30 min for the separation of plasma. Plasma was harvested and stored at –20°C until analysis was performed. Circulating concentrations of progesterone were determined in all samples collected by RIA (11) (Diagnostic Products). Intra- and interassay coefficients of variation were 1.7% and 11%, respectively, and assay sensitivity was 0.4 ng/ml. Concentrations of estradiol in serum were determined by RIA in samples collected on days –2 and 0 as previously described. Intra- and interassay coefficients of variation were 3.3% and 9.8%, respectively, and assay sensitivity was 0.5 pg/ml. Concentrations of PAGs were determined by ELISA in all plasma samples as previously described. Intra- and interassay coefficients of variation were 7.0% and 15.2%, respectively, and assay sensitivity was 1.0 ng/ml.
Ultrasonography. Ovaries were examined by transrectal ultrasonography to characterize follicular development and ovulation on days –2, 0, and 2 as described in experiment 1 (induced ovulation) and at time of insemination (spontaneous ovulation). Pregnancy status and embryo viability were determined as described in experiment 1 on days 27, 41, 55, and 68 after insemination. The first determination of pregnancy was performed in cows that spontaneously ovulated between days 25 and 39 after insemination, and the second pregnancy diagnosis was at >40 days after insemination.
Statistical analysis. In each experiment, logistical regression models were fit by the method of maximum likelihood by using sas (PROC LOGISTIC) (12) to determine the effect of follicle size at time of insemination on pregnancy rates at days 25–27 and days 60–68. First-order (linear) and second-order (quadratic) continuous effects of follicle size were modeled, because preliminary analyses indicated significant lack of fit to models that included only linear effects. Further, in experiment 1, it was determined whether the relationships between follicle size and pregnancy rate were homogenous for anestrous and cycling cows. Similarly, in experiment 2, it was determined whether the relationships between follicle size and pregnancy rate were homogenous for cows that were induced to ovulate and those that ovulated spontaneously. For each group of cows, the first derivative of pregnancy rate with respect to follicle size was solved for its root, in the range of observed follicle sizes, to obtain the follicle size resulting in maximum predicted pregnancy rate. A 90% confidence interval for the predicted maximum was calculated by back-transforming the corresponding critical values for their linear predictors and was used to establish the lower critical values where pregnancy rate was significantly reduced (P = 0.05) relative to the maximum. Follicle sizes for which pregnancy rate was less than the lower critical value of the confidence interval were thus concluded to be suboptimal.
Differences between anestrous and cycling cows (experiment 1) in growth of the dominant follicle, size of ovulatory follicle, and rate of progesterone rise were determined by analysis of variance in sas (PROC GLM) (12). When the F statistic was significant (P < 0.05), mean separation was performed by using least significant differences (means ± SEM) (13).
Circulating concentrations of progesterone, estradiol-17β, and PAGs were analyzed by analysis of variance for repeated measures in sas (PROC MIXED) (14). The statistical model consisted of the variable tested (follicle size, pregnancy status, or fetal mortality), day, and their interactions. The effects of follicle size, pregnancy status, or fetal mortality were analyzed by using animal within follicle size, pregnancy status, or fetal mortality as the error term, and effects of day and any interaction were analyzed by using the residual as the error term.
Results
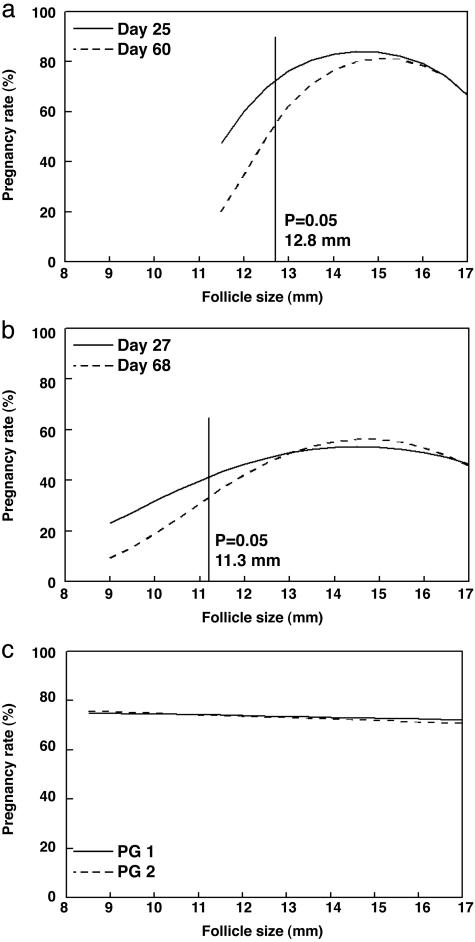
Experiment 1. Growth of the dominant follicle from day –2 to 0 (cycling animals, 1.2 ± 0.1 mm/day; anestrous animals, 0.9 ± 0.2 mm/day) and size of the ovulatory follicle at insemination (cycling animals, 13.9 ± 0.2 mm; anestrous animals, 14.8 ± 0.3 mm) did not differ (P > 0.20) between the cycling and anestrous groups. Furthermore, there was no difference in pregnancy rates (P > 0.05) between cycling and anestrous animals when follicles of a comparable size were ovulated. When animals from both of these groups were pooled, the log-odds relationship between follicle size at time of insemination and pregnancy rate tended to be curvilinear on day 25 (P = 0.13) (Fig. 1a). Regression analysis indicated a maximum pregnancy rate of 84.0 ± 16.6% at day 25 when ovulatory follicles were 14.7 mm. There was a tendency (P = 0.10) for cows that ovulated follicles <12.1 mm in diameter to be less likely to support pregnancy to day 25 after insemination compared with cows that ovulated 14.7-mm follicles (Fig. 1a). No cows ovulated follicles >17 mm or <10 mm in diameter.
Fig. 1.
Regression analysis of the effect of ovulatory follicle size on pregnancy rates. (a and b) Follicle sizes at which pregnancy rates were decreased (P < 0.05) below the maximal pregnancy rates are indicated with vertical lines. Results are shown for the effect of follicle size at time of GnRH injection on day 25 and day 60 pregnancy rates for experiment 1 (a) and day 27 and day 68 pregnancy rates for experiment 2 (b). (c) Effect of ovulatory follicle size at time of standing estrus on pregnancy rates at early (<39 days, PG 1) and late (>40 days, PG 2) diagnosis. The size of follicles that ovulated spontaneously had no effect on subsequent pregnancy rates. Results for experiment 2 are shown.
Thirteen percent of all cows experienced late embryonic mortality between days 25 and 39, which was reflected by loss of a heartbeat after pregnancy was initially confirmed by ultrasound on day 25. In fact, all late embryonic/fetal mortality occurred in cows that ovulated follicles ≤13.5 mm (33% of cows ovulating ≤13.5-mm follicles lost their pregnancies). The curvilinear relationship between follicle size and pregnancy rates at day 60 was more pronounced than at day 25 (P = 0.07), and regression analysis indicated a maximum pregnancy rate of 81.2 ± 17.6% at a follicle size of 15.2 mm (Fig. 1a). Again, cows that ovulated follicles <12.8 mm tended to be less likely (P = 0.10) to support pregnancy to day 60 after insemination compared with cows that ovulated 15.2-mm follicles.
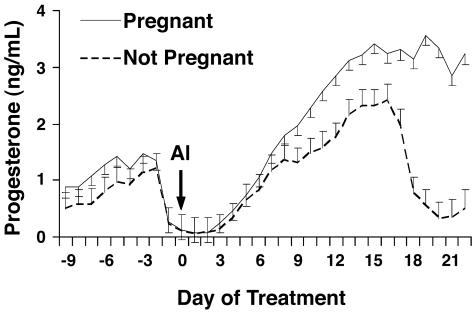
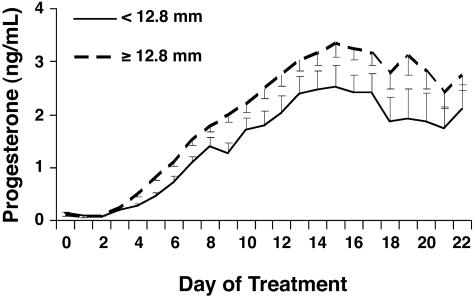
There was an effect of pregnancy status (P < 0.01) on serum concentrations of progesterone (Fig. 2) during the first 3 weeks of pregnancy. Concentrations of progesterone were significantly greater (P < 0.05) beginning on day 9, and the rate of increase in concentrations of progesterone from insemination until day 16 was greater (P < 0.01) in cows subsequently shown to be pregnant on day 25 compared with cows that were not pregnant. Ovulatory follicle size at time of insemination also influenced circulating concentrations of progesterone (Fig. 3). More specifically, concentrations of progesterone rose more slowly (P < 0.01) and tended to be lower (P = 0.11) after GnRH-induced ovulation of follicles <12.8 mm, compared with larger follicles (Fig. 3).
Fig. 2.
Serum concentrations of progesterone in cows in experiment 1 determined to be pregnant or not pregnant and rate of progesterone rise from day 0 to 16. AI, day of artificial insemination (day 0). Pregnancy status, P < 0.01; day, P < 0.01; pregnancy status by day, P < 0.01; pregnant vs. not pregnant, P < 0.01.
Fig. 3.
Effect of ovulatory follicle size on serum concentrations of progesterone in experiment 1 from day 0 (insemination) through 22 and the effect of ovulatory follicle size on rate of progesterone rise from day 0 through 16. Ovulatory follicle size, P = 0.04; day, P < 0.01; ovulatory follicle size by day, P = 0.12; rate of progesterone rise, P < 0.01.
The size of ovulatory follicles did not influence (P = 0.29) circulating concentrations of estradiol (5.69 ± 0.44 and 6.01 ± 0.27 for <12.8-mm and ≥12.8-mm follicles, respectively). Furthermore, the concentrations of estradiol were not different (P = 0.56) between anestrous (6.08 ± 0.36 pg/ml) and cycling (5.80 ± 0.32 pg/ml) cows or between pregnant (5.91 ± 0.28 pg/ml) and nonpregnant (5.97 ± 0.46 pg/ml; P = 0.90) cows.
The trophoblast secretory proteins known as PAGs were detectable between days 22 and 25 after insemination in cows that were pregnant. No differences (P = 0.57) were detected in the circulating concentrations of PAGs between cows that ovulated follicles ≥13.2 mm and cows that ovulated follicles <13.2 mm (data not shown).
Experiment 2. The log-odds relationship between follicle size and pregnancy rate on day 27 observed in experiment 2 depended curvilinearly on whether cows were induced to ovulate or ovulated spontaneously (P = 0.10). For those cows that were induced to ovulate, logistic regression indicated a maximum pregnancy rate on day 27 of 53.4 ± 10.7% at a follicle size of 14.5 mm. Cows that ovulated follicles <10.3 mm were less likely to support pregnancy to day 27 after insemination compared with cows that ovulated 14.5-mm follicles (P = 0.05; Fig. 1b). Ovulated follicles were ≤20 mm and >9 mm in all cows. Late embryonic/fetal mortality between days 27 and 68 occurred in 7% of all cows. Again, as in experiment 1, late embryonic loss was confined to those animals that ovulated small follicles. Thirty-nine percent of cows ovulating follicles ≤11 mm lost their pregnancy; no late embryonic/fetal mortality occurred in those animals ovulating larger follicles. In those cows that ovulated spontaneously, pregnancy rates at day 27 were essentially independent of follicle size (P > 0.40; Fig. 1c).
As at day 27, pregnancy rates at day 68 were essentially independent of follicle size (P > 0.40; Fig. 1b) in those cows that ovulated spontaneously. However, because of the loss of 39% of pregnancies among cows that were induced to ovulate and had follicles ≤11 mm, the relationship between follicle size at time of insemination and pregnancy rates on day 60 in this group was more markedly curvilinear than on day 27 (P < 0.02; Fig. 1b). When pregnancy rates were monitored at day 68, logistical regression analysis indicated a maximum pregnancy rate of 56.3 ± 10.8% at a follicle size of 14.6 mm. Cows that ovulated follicles <11.3 mm were less likely to support pregnancy to day 60 compared with cows that ovulated 14.6-mm follicles (Fig. 1b).
When comparing follicular size at the time of ovulation, a greater percentage of cows were induced to ovulate follicles ≤11 mm (26%; P < 0.02) than were induced to ovulate 12-, 14-, 15-, or ≥16-mm follicles (13%, 15%, 12%, and 12%, respectively). The percentage of animals that ovulated 13-mm follicles (22%) was intermediate to the two previous groups.
Twenty-three percent of cows exhibited standing estrus at timed insemination (± 24 h). Cows that exhibited estrus at insemination had greater (P < 0.01) pregnancy rates (90% and 88% on days 26 and 68, respectively) than those cows that did not exhibit estrus at insemination (29% and 26% on days 26 and 68, respectively). Serum concentrations of estradiol were greater (P < 0.01) in cows detected in estrus (6.1 ± 0.4 and 9.9 ± 0.6 pg/ml on days –2 and 0, respectively) compared with cows not detected in estrus (3.4 ± 0.2 and 8.1 ± 0.3 pg/ml, respectively).
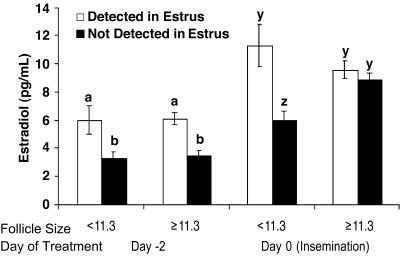
A noteworthy outcome from these results was that cows not detected in estrus and induced to ovulate follicles <11.3 mm had decreased (P < 0.01) concentrations of estradiol on day 0 compared with cows induced to ovulate larger follicles (6.04 ± 0.59 and 8.90 ± 0.38 pg/ml, respectively) (Fig. 4). In contrast, when cows were detected in standing estrus, follicle size did not influence serum concentrations of estradiol on day –2 or 0 (Fig. 4).
Fig. 4.
Effect of ovulatory follicle size on circulating concentration of estradiol among cows not detected or detected in estrus (24 h before or after timed insemination). a, b, y, and z indicate different mean concentrations of estradiol. (P < 0.02.)
No difference (P = 0.35) was detected in concentrations of PAGs between cows induced to ovulate follicles ≥11.3 mm compared with cows induced to ovulate follicles <11.3 mm. However, the concentration of PAGs did differ in those animals destined to undergo late embryonic loss. Compared with cows that successfully carried a pregnancy, cows that exhibited late embryonic mortality between days 27 and 41 tended (P = 0.11) to have decreased concentrations of PAGs on day 27, at the time that pregnancy was confirmed by ultrasound (9.94 ± 1.24 > 2.16 ± 4.63 ng/ml). On day 41, after embryonic/fetal loss had occurred, the PAG concentrations (18.11 ± 1.95 > 2.86 ± 7.3 ng/ml) had become significantly decreased (P = 0.05) compared with cows that maintained their pregnancy.
During the 37-day breeding season, more cows spontaneously ovulated follicles 13, 14, or ≥16 mm (21%, 24%, and 21%, respectively) after standing estrus compared with the percentage of cows that spontaneously ovulated ≤11-mm (8%), 12-mm (12%), or 15-mm (14%) follicles (P < 0.02). No cows ovulated follicles >23 mm or <9 mm, and follicle size of cows that spontaneously ovulated did not influence pregnancy rate (P > 0.40; Fig. 1c). Furthermore, follicle size in cows that spontaneously ovulated did not (P > 0.53) affect subsequent serum concentrations of progesterone (data not shown).
Discussion
Increasing the efficiency of timed-insemination protocols requires controlling the time of ovulation. The production of a viable embryo requires ovulation of a competent oocyte, adequate progesterone production by the CL, and an adequate uterine environment. In experiment 1, cows that ovulated follicles ≤13.2 mm had decreased concentrations of progesterone and a delayed rise in progesterone compared with cows that ovulated larger follicles. Dairy cows induced to ovulate small follicles (11.5 ± 0.2 mm) developed smaller CLs and secreted less progesterone compared with cows induced to ovulate larger follicles (14.47 ± 0.39 mm) (15). Similarly, ovine follicles induced to ovulate 12 h after luteal regression had fewer granulosa cells and formed smaller CLs that secreted less progesterone than follicles induced to ovulate 36 h after luteal regression (16). Granulosa cells differentiate into large luteal cells (17), and ≈80% of progesterone secreted by the ovine CL is reportedly secreted by large luteal cells (18). Furthermore, the number of ovine large luteal cells does not increase during the luteal phase (19). Consequently, decreased concentrations of progesterone after induced ovulation of smaller follicles might be the result of fewer granulosa cells present in the follicle before ovulation, resulting in fewer large luteal cells in the CL. McNatty et al. (20) suggested that development of a normal CL depends on preovulatory follicles having: (i) an adequate number of granulosa cells, (ii) an adequate number of luteinizing hormone receptors on granulosa and thecal cells, and (iii) granulosa cells capable of synthesizing adequate amounts of progesterone after luteinization. However, in experiment 2, follicle size did not influence subsequent concentrations of progesterone when cows spontaneously ovulated. Therefore, follicle size alone is not the distinguishing characteristic. Smaller follicles capable of spontaneous ovulation might possibly have reduced numbers of granulosa/large luteal cells, but this status does not preclude these follicular cells from secreting adequate amounts of progesterone after luteinization.
Intrafollicular concentrations of estradiol may play a role in preparation of follicular cells for luteal formation and function. Estradiol has the following actions within granulosa cells: (i) increased cellular proliferation (21–23), (ii) formation of gap junctions (24, 25), (iii) increased stimulatory action of follicle-stimulating hormone on aromatase activity (26–28), (iv) enhanced stimulation of progestin synthesis after gonadotropin stimulation (29, 30), and (v) enhanced acquisition of luteinizing hormone receptors (31–33). Furthermore, the ability of luteinized human granulosa cells to secrete progesterone was increased when cells were collected from follicles having increased follicular fluid concentrations of estradiol compared with granulosa cells collected from follicles with lower concentrations of estradiol (20), and secretion of progesterone was delayed in ewes given an aromatase inhibitor before induced ovulation (34). Therefore, cows that exhibited standing estrus may have attained concentrations of estradiol necessary to adequately prepare the follicular cells for luteinization regardless of follicular size.
Luteal progesterone secretion is required for maintenance of pregnancy (35) and stimulates endometrial secretions (36) and embryonic growth/development (37). Cows that were pregnant had greater serum concentrations of progesterone beginning on day 9 after insemination compared with nonpregnant cows. Similar differences in circulating concentrations of progesterone as early as day 6 postinsemination have been reported in cows that became pregnant compared with cows that did not become pregnant (38). Cows that underwent an earlier rise in progesterone had embryos that were further developed and produced more of the antiluteolytic protein IFN-τ by day 16 than cows that had a delayed rise in concentrations of progesterone (39). These data indicate that exposure to an earlier rise in progesterone may contribute to increased embryonic/fetal survival.
Embryonic/fetal mortality can greatly decrease reproductive efficiency. In dairy cows, the incidence of embryonic loss from day 25 to 98 after induced ovulation ranged from 14% to 40% (40–42), with the greatest proportion of the loss (17%) occurring between days 28 and 56.§ The late embryonic/fetal mortality that occurred in the present study was influenced by follicle size when ovulation was induced but not when spontaneous ovulation occurred. Causes of the late embryonic/fetal mortality after GnRH-induced ovulation of smaller follicles might be attributed to an improper uterine environment associated with decreased circulating concentrations of progesterone, a delayed rise in progesterone after ovulation, and/or decreased concentrations of estradiol on the day of insemination compared with cows induced to ovulate larger follicles. When an injection of estrogen preceding long-term progesterone treatment was omitted in ovariectomized ewes, embryo survival after embryo transfer,¶ uterine weight, uterine protein, RNA-to-DNA ratio, and the rate of protein synthesis were decreased (44). Therefore, decreased concentrations of estradiol before GnRH-induced ovulation could influence the uterus' ability to maintain a pregnancy.
Inadequate oocyte development is another possible explanation for embryonic/fetal mortality when small follicles were induced to ovulate. During oocyte growth and development, mRNA and proteins are produced and stored in the oocyte (45). Oocyte developmental competence continues to increase with increased follicular diameter in cattle (46), and low levels of RNA synthesis were still observed in large oocytes (47). Little is known about the variation that exists in oocyte quality among bovine preovulatory follicles. However, in human beings, oocytes collected from follicles <14 mm had decreased developmental rates compared with oocytes collected from follicles >14 mm (48). Furthermore, after conventional in vitro fertilization procedures, oocytes collected from human follicles >15 mm had higher fertilization and pregnancy rates compared with oocytes collected from smaller follicles (49). Thus, embryonic/fetal survival in several species may be impaired when oocytes are derived from follicles that have not completely matured. In the present study, when spontaneous ovulation occurred, no differences in pregnancy rates were detected among different follicle sizes. Consequently, when a follicle/oocyte has matured and is capable of initiating the cascade of events leading to ovulation, a viable embryo can develop regardless of follicular size.
Embryonic loss occurred in the present studies and others (40–42) around the time of attachment of the embryo to the uterus (days 21–42) (50). PAGs are expressed in the binucleate cells of the placenta around this time of gestation (51). Exocytosis of granules from binucleate cells toward the maternal capillary beds allows trophectoderm cell products (e.g., PAGs) to reach the maternal blood supply upon attachment of the trophectoderm to the uterus (43). The tendency for circulating concentrations of PAGs to be lower in cows experiencing fetal mortality likely indicated an insufficiency in placental development around the time of attachment, resulting from suboptimal embryo development, suboptimal uterine environment, or a combination of the two.
In summary, GnRH-induced ovulation of follicles ≤11 mm resulted in decreased pregnancy rates and increased late embryonic/fetal mortality. This observation is important because of the extensive use of GnRH in many synchronization regimens. The decrease in fertility was associated with decreased circulating concentrations of estradiol on the day of insemination, a lower rate of increase in progesterone after insemination, and decreased circulating concentrations of progesterone. However, ovulatory follicle size had no apparent effect on fertility when ovulation occurred spontaneously. Thus, follicles undergoing spontaneous ovulation do so at a wide range of sizes when they are physiologically mature. Therefore, administration of GnRH to induce ovulation likely initiates a preovulatory gonadotropin surge before a dominant follicle has attained physiological maturity, and GnRH-induced ovulation of follicles that are physiologically immature has a negative impact on pregnancy rates and late embryonic/fetal survival.
Acknowledgments
We gratefully acknowledge J. Bader, S. Bellows, B. McCormack, S. Reil, B. Shipp, E. Willis, and W. R. Lamberson for technical assistance.
Author contributions: G.A.P., M.F.S., M.C.L., and T.W.G. designed research; G.A.P., M.F.S., A.J.R., and T.W.G. performed research; J.A.G. and T.E.P. contributed new reagents/analytic tools; G.A.P. and M.D.M. analyzed data; and G.A.P. wrote the paper.
Abbreviations: GnRH, gonadotropin-releasing hormone; CL, corpus luteum; PAG, pregnancy-associated glycoprotein.
Footnotes
Vasconcelos, J., Silcox, R., Lacerda, J., Pursley, J. & Wiltbank, M. (1997) Biol. Reprod. 56, Suppl. 1, 140 (abstr.).
Miller, B. G. & Moore, N. W. (1976) Theriogenology 6, 636 (abstr.).
References
- 1.Odde, K. G. (1990) J. Anim. Sci. 68, 817–830. [DOI] [PubMed] [Google Scholar]
- 2.Lamb, G. C., Nix, D. W., Stevenson, J. S. & Corah, L. R. (2000) Theriogenology 53, 691–698. [DOI] [PubMed] [Google Scholar]
- 3.Lucy, M. C., Billings, H. J., Butler, W. R., Ehnis, L. R., Fields, M. J., Kesler, D. J., Kinder, J. E., Mattos, R. C., Short, R. E., Thatcher, W. W., et al. (2001) J. Anim. Sci. 79, 982–995. [DOI] [PubMed] [Google Scholar]
- 4.Geary, T. W. & Whittier, J. C. (1998) Prof. Anim. Sci. 14, 217–220. [Google Scholar]
- 5.Pursley, J. R., Silcox, R. W. & Wiltbank, M. C. (1998) J. Dairy Sci. 81, 2139–2144. [DOI] [PubMed] [Google Scholar]
- 6.Sartori, R., Fricke, P. M., Ferreira, J. C., Ginther, O. J. & Wiltbank, M. C. (2001) Biol. Reprod. 65, 1403–1409. [DOI] [PubMed] [Google Scholar]
- 7.Geary, T. W., Downing, E. R., Bruemmer, J. E. & Whittier, J. C. (2000) Prof. Anim. Sci. 16, 1–5. [Google Scholar]
- 8.Kirby, C. J., Smith, M. F., Keisler, D. H. & Lucy, M. C. (1997) J. Dairy Sci. 80, 273–285. [DOI] [PubMed] [Google Scholar]
- 9.Perry, G. A., Smith, M. F. & Geary, T. W. (2004) J. Anim. Sci. 82, 695–704. [DOI] [PubMed] [Google Scholar]
- 10.Green, J. A., Parks, T. E., Avalle, M. P., McLain, A. L., Peterson, A. J., McMillan, W., Mathialagan, N., Xie, S., Hook, R. R. & Roberts, R. M. (2005) Theriogenology 63, 1481–1503. [DOI] [PubMed] [Google Scholar]
- 11.Bellows, R. A., Staigmiller, R. B., Wilson, J. M., Phelps, D. A. & Darling, A. (1991) Theriogenology 35, 1069–1082. [Google Scholar]
- 12.sas (1992) SAS User's Guide: Statistics (SAS Intitute, Cary, NC), Release 6.03.
- 13.Snedecor, G. W. & Cochran, W. G. (1989) Statistical Methods (Iowa State Univ. Press, Ames).
- 14.Littell, R. C., Henry, P. R. & Ammerman, C. B. (1998) J. Anim. Sci. 76, 1216–1231. [DOI] [PubMed] [Google Scholar]
- 15.Vasconcelos, J. L., Sartori, R., Oliveira, H. N., Guenther, J. G. & Wiltbank, M. C. (2001) Theriogenology 56, 307–314. [DOI] [PubMed] [Google Scholar]
- 16.Murdoch, W. J. & Van Kirk, E. A. (1998) Endocrinology 139, 3480–3484. [DOI] [PubMed] [Google Scholar]
- 17.Smith, M. F., McIntush, E. W. & Smith, G. W. (1994) J. Anim. Sci. 72, 1857–1872. [DOI] [PubMed] [Google Scholar]
- 18.Niswender, G. D., Schwall, R. H., Fitz, T. A., Farin, C. E. & Sawyer, H. R. (1985) Recent Prog. Horm. Res. 41, 101–151. [DOI] [PubMed] [Google Scholar]
- 19.Farin, C. E., Moeller, C. L., Sawyer, H. R., Gamboni, F. & Niswender, G. D. (1986) Biol. Reprod. 35, 1299–1308. [DOI] [PubMed] [Google Scholar]
- 20.McNatty, K. P., Smith, D. M., Makris, A., Osathanondh, R. & Ryan, K. J. (1979) J. Clin. Endocrinol. Metab. 49, 851–860. [DOI] [PubMed] [Google Scholar]
- 21.Goldenberg, R. L., Bridson, W. E. & Kohler, P. O. (1972) Biochem. Biophys. Res. Commun. 48, 101–107. [DOI] [PubMed] [Google Scholar]
- 22.Dupont, S., Krust, A., Gansmuller, A., Dierich, A., Chambon, P. & Mark, M. (2000) Development (Cambridge, U.K.) 127, 4277–4291. [DOI] [PubMed] [Google Scholar]
- 23.Parrott, J. A. & Skinner, M. K. (1998) Endocrinology 139, 228–235. [DOI] [PubMed] [Google Scholar]
- 24.Merk, F. B., Botticelli, C. R. & Albright, J. T. (1972) Endocrinology 90, 992–1007. [DOI] [PubMed] [Google Scholar]
- 25.Burghardt, R. C. & Anderson, E. (1981) Cell Tissue Res. 214, 181–193. [DOI] [PubMed] [Google Scholar]
- 26.Zhuang, L. Z., Adashi, E. Y. & Hsuch, A. J. (1982) Endocrinology 110, 2219–2221. [DOI] [PubMed] [Google Scholar]
- 27.Adashi, E. Y. & Hsueh, A. J. (1982) J. Biol. Chem. 257, 6077–6083. [PubMed] [Google Scholar]
- 28.Reilly, C. M., Cannady, W. E., Mahesh, V. B., Stopper, V. S., De Sevilla, L. M. & Mills, T. M. (1996) Biol. Reprod. 54, 1336–1342. [DOI] [PubMed] [Google Scholar]
- 29.Welsh, T. H., Jr., Zhuang, L. Z. & Hsueh, A. J. (1983) Endocrinology 112, 1916–1924. [DOI] [PubMed] [Google Scholar]
- 30.Fanjul, L. F., Ruiz de Galarreta, C. M. & Hsueh, A. J. (1984) Biol. Reprod. 30, 903–912. [DOI] [PubMed] [Google Scholar]
- 31.Kessel, B., Liu, Y. X., Jia, X. C. & Hsueh, A. J. (1985) Biol. Reprod. 32, 1038–1050. [DOI] [PubMed] [Google Scholar]
- 32.Farookhi, R. & Desjardins, J. (1986) Mol. Cell. Endocrinol. 47, 13–24. [DOI] [PubMed] [Google Scholar]
- 33.Wang, X. N. & Greenwald, G. S. (1993) J. Reprod. Fertil. 99, 403–413. [DOI] [PubMed] [Google Scholar]
- 34.Benoit, A. M., Inskeep, E. K. & Dailey, R. A. (1992) Domest. Anim. Endocrinol. 9, 313–327. [DOI] [PubMed] [Google Scholar]
- 35.McDonald, L. E., Nichols, R. E. & McNutt, S. H. (1952) Am. J. Vet. Res. 13, 446–451. [PubMed] [Google Scholar]
- 36.Geisert, R. D., Morgan, G. L., Short, E. C., Jr. & Zavy, M. T. (1992) Reprod. Fertil. Dev. 4, 301–305. [DOI] [PubMed] [Google Scholar]
- 37.Garrett, J. E., Geisert, R. D., Zavy, M. T. & Morgan, G. L. (1988) J. Reprod. Fertil. 84, 437–446. [DOI] [PubMed] [Google Scholar]
- 38.Mann, G. E., Lamming, G. E., Robinson, R. S. & Wathes, D. C. (1999) J. Reprod. Fertil. 54, Suppl., 317–328. [PubMed] [Google Scholar]
- 39.Mann, G. E. & Lamming, G. E. (2001) Reproduction 121, 175–180. [DOI] [PubMed] [Google Scholar]
- 40.Vasconcelos, J. L., Silcox, R. W., Rosa, G. J., Pursley, J. R. & Wiltbank, M. C. (1999) Theriogenology 52, 1067–1078. [DOI] [PubMed] [Google Scholar]
- 41.Cartmill, J. A., El-Zarkouny, S. Z., Hensley, B. A., Lamb, G. C. & Stevenson, J. S. (2001) J. Dairy Sci. 84, 1051–1059. [DOI] [PubMed] [Google Scholar]
- 42.Moreira, F., Orlandi, C., Risco, C. A., Mattos, R., Lopes, F. & Thatcher, W. W. (2001) J. Dairy Sci. 84, 1646–1659. [DOI] [PubMed] [Google Scholar]
- 43.Wooding, F. B. (1992) Placenta 13, 101–113. [DOI] [PubMed] [Google Scholar]
- 44.Miller, B. G., Moore, N. W., Murphy, L. & Stone, G. M. (1977) Aust. J. Biol. Sci. 30, 279–288. [DOI] [PubMed] [Google Scholar]
- 45.Brevini-Gandolfi, T. A. & Gandolfi, F. (2001) Theriogenology 55, 1255–1276. [DOI] [PubMed] [Google Scholar]
- 46.Arlotto, T., Schwartz, J.-L., First, N. L. & Leibfried-Rutledge, M. L. (1996) Theriogenology 45, 943–956. [DOI] [PubMed] [Google Scholar]
- 47.Fair, T., Hyttel, P. & Greve, T. (1995) Mol. Reprod. Dev. 42, 437–442. [DOI] [PubMed] [Google Scholar]
- 48.Teissier, M. P., Chable, H., Paulhac, S. & Aubard, Y. (2000) Hum. Reprod. 15, 2471–2477. [DOI] [PubMed] [Google Scholar]
- 49.Bergh, C., Broden, H., Lundin, K. & Hamberger, L. (1998) Hum. Reprod. 13, 1912–1915. [DOI] [PubMed] [Google Scholar]
- 50.Peters, A. R. (1996) Anim. Breed. Abstr. 64, 587–598. [Google Scholar]
- 51.Green, J. A., Xie, S., Quan, X., Bao, B., Gan, X., Mathialagan, N., Beckers, J. F. & Roberts, R. M. (2000) Biol. Reprod. 62, 1624–1631. [DOI] [PubMed] [Google Scholar]