Abstract
Purpose
We assessed whether circulating bone formation and resorption markers (BTM) were individual predictors for trabecular and cortical bone loss, periosteal expansion, and fracture risk in older adults aged 66 to 93 years from the AGES-Reykjavik study.
Methods
The sample for the quantitative computed tomography (QCT)-derived cortical and trabecular BMD and periosteal expansion analysis consisted of 1069 participants (474 men and 595 women) who had complete baseline (2002 to 2006) and follow-up (2007 to 2011) hip QCT scans and serum baseline BTM. During the median follow up of 11.7 years (range 5.4-12.5), 54 (11.4%) men and 182 (30.6%) women sustained at least one fracture of any type.
Results
Increase in BTM levels was associated with faster cortical and trabecular bone loss at the femoral neck and proximal femur in men and women. Higher BTM levels were positively related with periosteal expansion rate at the femoral neck in men. Markers were not associated with fracture risk.
Conclusion
This data corroborates the notion from few previous studies that both envelopes are metabolically active and that BTM levels may moderately reflect the cellular events at the endosteal and periosteal surfaces. However, our results do not support the routine use of BTM to assess fracture risk in older men and women. In light of these findings, further studies are justified to examine whether systemic markers of bone turnover might prove useful in monitoring skeletal remodeling events and the effects of current osteoporosis drugs at the periosteum.
Keywords: bone turnover, endosteal bone loss, periosteal apposition, fracture risk, QCT, aging
Introduction
During aging, the cellular activity on the mineralized skeleton periosteal and endosteal surfaces is unbalanced and the remodeling rate is higher, resulting in external size and shape, internal architecture, and total mass changes [1]. However, the age-related changes in the two bone surfaces have opposite consequences for bone fragility. While increased endosteal remodeling will impose structural damage due to the trabecular thinning, disappearance and loss of connectivity, cortical thinning and increased intracortical porosity, the concurrent periosteal bone formation increases the cross-sectional area (CSA) of bone partly offsetting endosteal bone loss [2,1]. This cellular activity can be aggregated for the whole skeleton and estimated by biochemical bone turnover markers (BTM), and BTM can also mirror changes in histomorphometric measurements of bone formation and resorption. Consequently, biochemical evidence has been of high value to investigate the complex pathways of bone metabolism in several male and female cohorts for the last 20 years [3].
A large body of literature has shown that BTM modestly predict bone loss at various skeletal sites in untreated individuals [4,5]. Thus, it is currently believed that BTM should only be used to supplement bone mineral density (BMD) values and not measured in isolation. These conclusions were based on several large population-based studies measuring areal BMD by DXA [6-9], and this two-dimensional bone measure does not differentiate between the cortical and trabecular components of the endosteal surface nor specific changes in CSA (periosteal formation). Thus, the extent to which systemic BTM reflect trabecular versus cortical bone remodeling, or their changes with aging, is also poorly understood. It has often been asserted that trabecular bone has higher turnover than cortical bone, but this is not always true, because bone turnover depends on both the bone formation rate and the surface-to-volume ratio [10]. Although for the same bone region turnover in trabecular bone is substantially higher than cortical bone, the overall volume of cortical is higher than trabecular (80% vs 20%), thus cortical bone loss over time is greater than trabecular bone loss. Altogether, it is expected that different bones and regions within bones have very large differences in turnover [10] and their impact on circulating levels of BTM may differ as well.
Another factor that may play a largely unrecognized role in serum concentrations of BTM is the periosteum bone remodeling. Despite the importance of periosteal apposition in maintaining structural strength during aging, research into the pathogenesis of bone fragility has been dominated by the study of menopause and age-related (endosteal) bone loss. Recently, increased BTM levels have been associated with greater bone size [11,12]. However, it remains uncertain whether high bone turnover is associated with the rate of periosteal apposition, as previous research has been limited to cross-sectional data.
Previous cohort studies have also documented the ability of BTM to predict future fracture risk in untreated postmenopausal and elderly women, and the evidence has been recently reviewed [3,13,5]. Fewer studies examined the prediction of fractures by BTM in men with discordant results [6,14,15,9]. In our study we further explored this analysis by including a large sample of older men and women, over a long-term follow-up and accounting for the possible effect of several important confounders.
In the present study we aimed to determine whether markers of bone formation and resorption were more predictive of the loss of trabecular or cortical BMD, and whether they are positively associated with bone size change, as reflected by CSA, at the proximal femur. In addition, we aimed to examine whether BTM levels are associated with long-term incidence of fracture in older women and men. Although there are no prospective data using QCT data, we hypothesized that baseline level of BTM would be modestly associated with trabecular and cortical bone loss and with higher periosteal apposition. Furthermore we hypothesized that higher levels of BTM (both bone resorption and formation markers) would be moderately associated with fracture risk, independently of volumetric BMD and other potential confounders.
Methods
Study Population
The present study was based on longitudinal data from the Age, Gene/Environment Susceptibility (AGES) –Reykjavik Study, a single-center prospective population study of Icelandic men and women. Design and recruitment have been described in detail [16]. From a total of 2,300 subjects who formed an interim cohort that underwent an interview and clinical examinations and also received a set of serum biochemical markers of bone remodeling measurements at baseline (from 2002 to 2006) data was complete for 1,916 participants. From 2007 to 2011, 1,185 surviving participants were examined. Reasons for not attending the follow-up examination included death following the baseline study (n=227), refusal (n=446), and lost to follow-up (n=58). Of the 1,185 subjects who took part in the baseline and follow-up examinations, QCT data were missing for 116 individuals. Therefore, 1,069 people (474 men and 595 women) who had complete baseline and follow-up QCT scans and serum baseline BTM provided data in the analyses. Compared with the excluded participants, included participants were younger, more physically active, heavier, and had lower prevalence of mobility disability and diabetes. In addition, the analytical sample had higher BMD and serum vitamin D, and lower parathyroid hormone (PTH) and osteocalcin (OC) levels than the excluded participants. No differences were observed for current smoking habits, history of previous fractures and osteoporosis, bone medication and baseline total bone cross-sectional areas.
Written informed consent was obtained from all participants, and the study was approved by the Icelandic National Bioethics Committee (VSN: 00-063) and the Institutional Review Board of the Intramural Research Program of the National Institute of Aging.
BTM measurement
At baseline, fasting venous blood samples were collected from 8.00 to 11.30 am and samples were aliquoted and stored at -80°C on-site in the Icelandic Heart Association biorepository until analysis. Bone formation was assessed by serum procollagen type I N propeptide (PINP) and OC, and bone resorption was assessed by C-terminal cross-linking telopeptide of type I collagen (CTX). BTM were determined using a sandwich immunoassay on an Elecsys 2010 analyzer (Roche Diagnostics, Mannheim) at the Icelandic Heart Association (Kopavogur, Iceland) according to the manufacturers' protocols. The interassay coefficients of variation were <3.0% for all BTM.
QCT Volumetric BMD measurement
Left hip was scanned and analyzed using the same four-row detector CT system (Sensation; Siemens Medical Systems, Erlangen, Germany), using a standardized protocol and encompassed the proximal femur from a level 1 cm superior to the acetabulum to a level 3–5 mm inferior to the lesser trochanter at settings of 120 kVp, 140 mAs, 1-mm slice thickness, pitch=1, pixel size of 0.977 mm. Scans were performed at baseline and repeated after an average follow-up of 5.2 years (range 2.7–8.7).
Proximal femoral vQCT images were processed to extract measures of BMD and size in the total femur region and femoral neck. vBMD measurements (in mg/cm3) were derived for each segment. Cortical vBMD was calculated from the voxels classified as being within the cortex and trabecular vBMD from the remaining pixels interior to the endosteal cortical boundary. Bone size estimates were based on measurements of two cross-sections through the proximal femur transverse to the femoral neck axis, one positioned at the minimum CSA of the femoral neck and one positioned at the maximum CSA through intertrochanteric plane.
To assess the in vivo reproducibility of our measurements, 8 participants underwent a repeated CT scan after they got off the table and were then repositioned. The coefficients of variation (CVs) were 1.4 for femoral neck trabecular vBMD, 1.8% for femoral neck cortical vBMD, 1.7% for total hip trabecular vBMD, 0.8% for total hip cortical vBMD.
Other measurements
Information on education (dichotomized as primary/secondary vs college/university), age at menopause, current smoking (yes/no), current alcohol use (converted into grams per week using 14 g of alcohol as a standard drink), health history (including medical conditions, previous fractures, and falls in the last 12 months), and physical activity was gathered at baseline by questionnaire. Use of medications known to affect bone density was ascertained based on medications brought to the clinic at the time of the examination. The anti-bone loss drugs included estrogens, progestogens, SERM, anti-parathyroid agents (ACT code H05B, such as calcitonin preparations), bisphosphonates, parathyroid hormones and analogues (such as teriparatide), indapamide, and thiazide diuretics. Drugs that may induce bone damage included use of oral glucocorticoid or antiepileptic medications. Participants were categorized as moderate/high physically active or occasionally physically active at most, as previously described [17].
Self-reported mobility disability was defined as having much difficulty or unable to walk 500 m and/or climb 10 steps.
Chronic health conditions were determined using self-report with confirmation by treatment and medications. These conditions included diabetes mellitus, kidney disease, thyroid diseases, rheumatoid arthritis, Parkinson disease, cancer, chronic obstructive pulmonary disease, myocardial infarction, hypertension, congestive heart failure, or stroke.
Information on protein intake (recoded as g/day for the sum of meat, fish, and bread ingestion), coffee consumption (recoded as “yes” for consumption ≥3 cups per day; otherwise, no), and milk intake as a teenager (recoded as “yes” for consumption ≥3 times a week; otherwise, no) was gathered using a validated food frequency questionnaire [18]. The questionnaires were administered in the clinic by a trained interviewer.
Height (m) and weight (kg) were measured using a Seca stadiometer and a digital scale (Marel, Reykjavik, Iceland) at the clinical assessment, and body mass index (BMI) was calculated in kg/m2. The maximal isometric knee extension strength (N) was measured using an adjustable dynamometer chair with the knee flexed at 60°. Subjects were instructed to exert maximal muscular force and three attempts were recorded, with 30 seconds of rest in between. The highest value was used for further analyses.
Total 25-hydroxyvitamin D (25OHD) was determined using the Liaison chemiluminescence immunoassay (DiaSorin Inc, Stillwater, Minnesota). The inter-assay coefficient of variation was <6.5%, using a previously frozen serum pool as the control sample and was <12.7% when using Liaison quality controls. Intact PTH was assayed using electrochemiluminescence technology from Cobas-Roche (West Sussex, United Kingdom) on a 2-site immunoassay. The interassay coefficient of variation was <0.5% when using a frozen serum pool as the control sample and was <2.8% when using Cobas-Roche quality controls.
Serum creatinine was measured using the Roche-Hitachi 912 instrument with Roche Creatinine Jaffé compensated method; Roche Diagnostics, Mannheim, Germany. The coefficient of variation for the creatinine assay was 2.5%.
Fracture assessment
Fracture data were recorded, verified and confirmed from medical and radiological records as previously described [19]. Incident clinical fractures were recorded from participants' enrollment into the study until 15th of March 2015. Type of fractures was defined according to ICD10 diagnostic codes. Fractures not typically associated with BMD (hand, foot, skull, and face) were excluded.
The date of first hip (S72.0, S72.1, S72.2), nonvertebral (fractures with codes S22.0 and S32.0 were excluded) and any type of fracture were used as endpoints for follow-up in the prediction of fracture risk (maximum 12.5 years of follow-up time, and median (Q1, Q3) of 8.6 (7.4, 10.2), 6.1 (2.8, 8.2), and 5.7 (2.4, 8.1) years, respectively for fractures cases) to determine whether the magnitude of the association between bone markers and BMD with fractures differed by fracture type. Each subject contributed only one record in each analysis, regardless of the number of fractures they may have had. Prediction of fractures was analyzed in two ways. First, we used the entire follow-up data until 2015. Second, different lengths of follow-up were used to evaluate the influence of follow-up time on the prediction of fracture by BTM. Since the shortest follow-up time (data available for all 1069 participants) was nearly 5 years, we used 5 years as the maximum follow-up time for the second approach.
Statistical analysis
To be consistent with previous reports and because of the known differences in bone mineral and bone markers distribution, all analyses were performed separately for women and men. Mean ± SD or percentages for categorical variables were used to summarize subject characteristics. Variables with non-Gaussian distribution were normal-scored transformed using Van der Waerden's Formula. To estimate annual percentage (%) change in each bone parameter we divided the inter-visit difference relative to absolute baseline, divided by the number of years between the visits, as follows: [(follow-up value – baseline value)/ baseline value * time between CT scans] × 100. Higher negative values correspond to faster rates of bone loss.
Prediction of accelerated bone loss (quartile (Q) with highest negative values) and periosteal apposition (Q4 - highest positive values) was assessed by logistic regression. Odds ratios (OR) and 95% confidence interval (CI) were calculated for BTM levels dichotomized as fourth quartile (Q4–high bone turnover) vs three lowest quartiles (Q1-Q3).
Adjustments were made for age (first model) and then age at menopause, BMI, current smoking status (yes/no), alcohol consumption (g/week), physical activity level (low/high), mobility disability (yes/no), serum 25OHD, PTH, follow-up duration, DM (yes/no), oral glucocorticoid or antiepileptic drugs (yes/no), and anti-bone loss drugs (yes/no) were included in the full-adjusted models.
As an additional (sensitivity) analysis, we incorporated several other potential confounders (baseline education level, weight, handgrip strength, protein, coffee and milk (as teenager) consumption, kidney disease, stroke, cancer, rheumatoid arthritis, thyroid diseases, and renal function (estimated by serum creatinine).
We used Cox proportional hazards regression models, age- and multivariable-adjusted to determine the hazard ratios (HR) and 95% CI of first fracture following baseline per SD increase in each marker level. Follow-up time represents the time from baseline visit to either (1) occurrence of first fracture (all types, nonvertebral, and hip) or (2) to the end of follow-up period or (3) to death if no fracture had occurred. Participants without any fracture formed the reference (control) group. The multivariate model was adjusted for age, BMI, current smoking, serum 25OHD, physical activity, previous fracture, fall in the past 12 months, total number of 10 chronic health conditions, use of oral glucocorticoid or antiepileptic medications, and maximal isometric knee extension strength. In addition, the predictive ability was tested with or without correction for femoral neck integral vBMD. In order to estimate influence of follow-up time, Cox regression analyses were performed for three different maximum lengths of follow-up for nonvertebral and all types of fractures, namely, 2.5 and 5 years after baseline, and from 2.5 to 5 years after baseline visit. Hip fracture prediction was not performed because no fractures occurred before 5 years after baseline. Thus, using a different follow-up time (e.g. 5 to 10 years) would imply a smaller sample size and survival bias.
In sensitivity analyses we examined risk of fracture by threshold using the highest BTM quartile vs the three lowest quartiles. We also explored a different definition for control group, where subjects were considered controls if they had never experienced the specific fracture (e.g. first hip fracture vs never had a hip fracture).
Statistical analyses were conducted using SPSS software, version 22.0 (IBM, USA).
Results
Subjects and baseline and follow-up measurements
The mean age of the study population at baseline was 74.2 years and 55.7% were women. Mean BMI was in the overweight range (27 kg/m2). More women reported osteoporosis (13.3%) compared with men (3%), and in total 11.8% of the subjects reported they currently smoke (Table 1). Mean age at menopause was 48.4 years. As expected, the three BTM were significantly inter-correlated (r = 0.8, p<0.01). Mean time between the baseline and follow-up QCT examinations was 5.2 years (range 2.7–8.7). More women reported previous fractures (55.1%) compared with men (45.1%).
Table 1. Descriptive analysis of the analytic sample (n= 1,069).
| Women (n=595) | Men (n=474) | p-value | |
|---|---|---|---|
| Age, years (mean (SD)) | 73.99 (4.99) | 74.44 (4.88) | 0.100 |
| Weight, kg (mean (SD)) | 71.17 (12.00) | 82.90 (12.55) | <0.001 |
| Height, cm (mean (SD)) | 161.56 (5.48) | 175.89 (5.97) | <0.001 |
| BMI, kg/m2 (mean (SD)) | 27.25 (4.30) | 26.75 (3.51) | 0.194 |
| Current Smoking, n (%) | 66 (11.1) | 60 (12.7) | 0.436 |
| Alcohol intake, g/week (mean (SD)) | 10.62 (21.33) | 25.07 (44.77) | <0.001 |
| High physical activity level, n (%) | 119 (20.4) | 112 (23.8) | 0.178 |
| Mobility disability, n (%) | 33 (5.5) | 18 (3.8) | 0.185 |
| Previous fracture, n (%) | 328 (55.1) | 214 (45.1) | 0.001 |
| History of osteoporosis, n (%) | 79 (13.3) | 11 (2.3) | <0.001 |
| History of Diabetes, n (%) | 47 (7.9) | 58 (12.2) | 0.018 |
| Oral glucocorticoid or antiepileptic drugs, n (%) | 27 (4.5) | 24 (5.1) | 0.689 |
| Anti-bone loss drugs, n (%) | 162 (27.2) | 28 (5.9) | <0.001 |
| Number of medical conditions (mean (SD)) | 1.44 (1.02) | 1.56 (1.04) | 0.025 |
| Fall last 12 months, n (%) | 178 (29.9) | 51 (10.8) | 0.529 |
| 25OHD, nmol/L (mean (SD)) | 51.17 (21.94) | 55.97 (23.00) | 0.001 |
| PTH, pmol/L (mean (SD)) | 44.87 (16.89) | 43.15 (15.35) | 0.056 |
| CTX, ng/mL (mean (SD)) | 0.38 (0.21) | 0.38 (0.22) | 0.378 |
| OC, ng/mL (mean (SD)) | 26.04 (10.93) | 23.08 (9.74) | <0.001 |
| PINP, ng/mL (mean (SD)) | 37.35 (19.16) | 34.74 (18.98) | 0.003 |
| Longitudinal changes in measures of bone density, mg/cm3/year (mean (SD))b/ %/year (mean (SD)): | |||
| Femoral neck | |||
| Trabecular BMD | -2.23 (3.39)/ -1.98 (3.29) | -1.70 (2.98)/ -1.42 (2.88) | <0.001 |
| Cortical BMD | -0.13 (4.76)/ -0.01 (1.06) | 0.13 (4.54)/ 0.04 (0.97) | 0.163 |
| Total hip | |||
| Trabecular BMD | -2.21 (1.92)/ -1.66 (1.37) | -1.53 (2.05)/ -1.02 (1.33) | <0.001 |
| Cortical BMD | -0.20 (2.46)/ -0.04 (0.54) | 0.19 (3.10)/ 0.05 (0.70) | 0.005 |
| Longitudinal changes in measures of bone size, mm2/year (mean (SD))/ %/year (mean (SD)): | |||
| Minimum CSAc | -0.92 (22.30)/ -0.05 (1.70) | 0.69 (23.76)/ 0.05 (1.41) | 0.630 |
| Maximum CSAd | -2.90 (30.00)/ -0.08 (0.95) | -0.99 (27.73)/ -0.02 (0.84) | 0.057 |
| First Incident Fracture, n (%) | |||
| Any type | 182 (30.6) | 54 (11.4) | <0.001 |
| Nonvertebral | 178 (29.9) | 51 (10.8) | <0.001 |
| Hip | 55 (9.2) | 22 (4.6) | 0.004 |
Abbreviations: BMI, body mass index; CSA, cross-sectional area; CTX, C-terminal cross-linking telopeptide of type I collagen; FN, femoral neck; OC, osteocalcin; PINP, procollagen type I N propeptide; PTH, parathyroid hormone
Areal density units are g/cm2;
Absolute annual change = final value – baseline value/ number of years between CT scans;
cross-section positioned at the minimal cross-sectional area of the femoral neck;
cross-section positioned at the plane of maximal cross-sectional area between the trochanters
During the median follow-up period of 11.7 years (Q1=11.2, Q3=12.1), 236 of the 1,069 participants (22.1%) experienced a fracture of any type. The incidence of hip fracture (intertrochanteric, subtrochanteric, and femoral neck fractures), nonvertebral fracture and all types of fracture was higher in women (8.3/1,000, 31.2/1,000, and 32.1/1,000 person-year, respectively) than in men (4.3/1,000, 10.2/1,000, and 10.9/1,000 person-year).
BTM and volumetric bone loss
Logistic regression models (Table 2) showed that at the femoral neck only women with OC in the upper quartile were 2.3 times (full-adjusted model) more likely to be in the upper quartile of annual % trabecular bone loss. Associations were more consistent for cortical bone loss. Thus, women with CTX, OC and PINP in the upper quartiles were ∼2 times more likely to be in the upper quartile of annual % cortical bone loss at the total hip and femoral neck, compared to those with BTM in the lower quartiles. Only higher OC levels (upper quartile) were associated with higher annual % bone loss at both skeletal sites and across compartments (trabecular and cortical) in both regression-adjusted models. In general, the prediction of accelerated loss of cortical BMD at both skeletal sites was stronger than for faster trabecular bone loss.
Table 2. Prediction of accelerated vBMD loss (defined as being in the upper quartile of annual % bone loss) of femoral neck and total hip by BTM in women.
| vBMD loss | Model | CTX | OC | PINP | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Femoral neck | |||||||
| Trabecular (-14.3 to 3.4 %) | 1 | 1.44 | 0.94-2.20 | 1.94 | 1.27-2.96 | 1.35 | 0.89-2.06 |
| 2 | 1.49 | 0.94-2.34 | 2.30 | 1.44-3.70 | 1.45 | 0.92-2.28 | |
| Cortical (-6.5 to -0.4 %) | 1 | 1.98 | 1.31-2.99 | 2.44 | 1.61-3.70 | 2.08 | 1.38-3.13 |
| 2 | 1.76 | 1.13-2.75 | 2.20 | 1.39-3.48 | 1.89 | 1.22-2.92 | |
| Total hip | |||||||
| Trabecular (-7.6 to -2.5 %) | 1 | 1.16 | 0.75-1.80 | 1.73 | 1.13-2.66 | 1.52 | 1.00-2.32 |
| 2 | 1.23 | 0.77-1.97 | 2.02 | 1.25-3.27 | 1.71 | 1.09-2.69 | |
| Cortical (-3.6 to -0.4 %) | 1 | 1.70 | 1.12-2.59 | 2.00 | 1.31-3.04 | 1.63 | 1.08-2.47 |
| 2 | 1.57 | 1.01-2.47 | 1.94 | 1.22-3.08 | 1.48 | 0.95-2.30 | |
Data are presented as odds ratios (95% CI) and are bold if p< 0.05 for the highest quartile of BTM levels (CTX ≥0.50, OC ≥31.80, and PINP ≥47.95) calculated by logistic regression; Model 1 - adjusted for age; Model 2 - adjusted for age, BMI, smoking, alcohol consumption, physical activity level, mobility disability, serum 25OHD, PTH, follow-up duration, DM, Oral glucocorticoid or antiepileptic drugs, anti-bone loss drugs, and age at menopause.
In men, results consistently showed that elevated BTM levels (Q4) had a significant association with accelerated bone loss at the total hip and across bone compartments, compared to those with BTM in the lower quartiles (Table 3). Results were less consistent between elevated levels of BTM and accelerated bone loss at the femoral neck. No significant associations were found between increased BTM levels and accelerated trabecular bone loss at the femoral neck. Men with high bone formation markers (Q4) were more likely to be in the upper quartile of annual % cortical bone loss at the femoral neck.
Table 3. Prediction of accelerated vBMD loss (defined as being in the upper quartile of annual % bone loss) of femoral neck and total hip by BTM in men.
| vBMD loss | Model | CTX | OC | PINP | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Femoral neck | |||||||
| Trabecular (-12.1 to -2.6 %) | 1 | 1.42 | 0.89-2.28 | 1.25 | 0.78-2.02 | 1.47 | 0.92-2.35 |
| 2 | 1.43 | 0.88-2.32 | 1.25 | 0.76-2.05 | 1.44 | 0.89-2.34 | |
| Cortical (-6.4 to -0.3 %) | 1 | 1.34 | 0.83-2.15 | 1.88 | 1.18-2.98 | 1.75 | 1.10-2.78 |
| 2 | 1.29 | 0.79-2.10 | 1.84 | 1.13-2.99 | 1.68 | 1.04-2.72 | |
| Total hip | |||||||
| Trabecular (-7.7 to -1.7 %) | 1 | 2.32 | 1.47-3.67 | 2.70 | 1.71-4.28 | 2.26 | 1.43-3.58 |
| 2 | 2.41 | 1.50-3.89 | 2.84 | 1.75-4.61 | 2.26 | 1.40-3.63 | |
| Cortical (-3.4 to -0.3 %) | 1 | 2.17 | 1.37-3.44 | 2.43 | 1.53-3.86 | 2.52 | 1.59-3.99 |
| 2 | 2.24 | 1.38-3.63 | 2.45 | 1.51-3.99 | 2.50 | 1.55-4.03 | |
Data are presented as odds ratios (95% CI) and are bold if p< 0.05 for the highest quartile of BTM levels (CTX ≥0.472, OC ≥26.94, and PINP ≥41.90) calculated by logistic regression; Model 1 - adjusted for age; Model 2 - adjusted for age, BMI, smoking, alcohol consumption, physical activity level, mobility disability, serum 25OHD, PTH, follow-up duration, DM, Oral glucocorticoid or antiepileptic drugs, and anti-bone loss drugs.
BTM and changes in bone size
Elevated BTM levels did not predict periosteal expansion rate of femoral neck (minimum CSA) and intertrochanteric (maximum CSA) in women (data not shown).
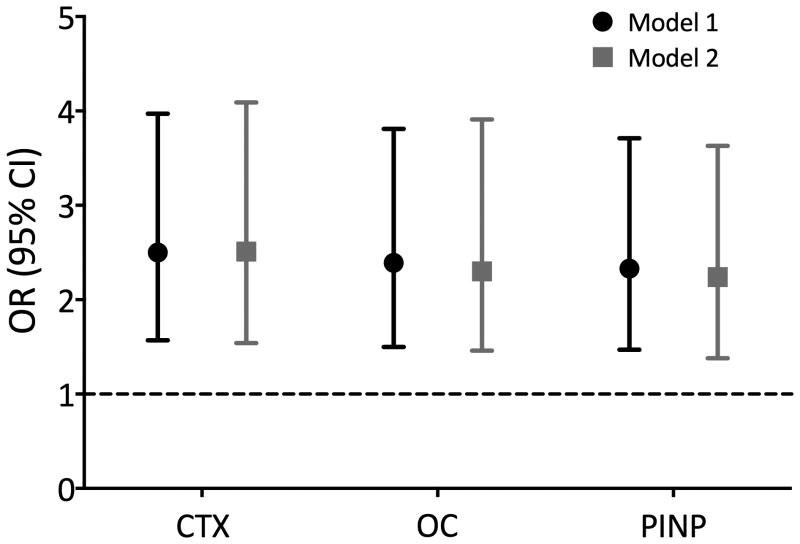
In men, elevated BTM levels (upper quartile) in men predicted higher femoral neck (minimum CSA) periosteal expansion (OR ∼2.4, see Figure 1). Further adjustment for several baseline confounders (model 2) did not changed these results. Results for the prediction of higher periosteal apposition at the intertrochanteric CSA were mainly non-significant. Only men with OC in the upper quartile were 1.65 (95% CI 1.02-2.68) and 1.89 (95% CI 1.13-3.16) times more likely to be in the upper quartile of periosteal apposition at the intertrochanteric CSA, for the age-adjusted and full-adjusted models, respectively (data not shown).
Fig. 1. Association of high bone turnover (Q4) with greater periosteal expansion at the femoral neck minimum CSA (Q4=0.12 to 9.66%) in men.
OR (95% CI) are adjusted for age (Model 1), and then for BMI, smoking, alcohol consumption, physical activity level, mobility disability, serum 25OHD, PTH, follow-up duration, DM, Oral glucocorticoid or antiepileptic drugs, and anti-bone loss drug (Model 2).
Bars indicate 95% confidence intervals. Dotted line, null value (OR = 1.0).
BTM and first incident fracture risk
BTM were not associated with risk for hip fracture, nonvertebral fractures and any fractures (neither per SD increase or highest quartile) during the entire follow-up time (median 11.7 years). Markers did not predict nonvertebral and any fractures when shorter follow-up periods were examined (0-2.5 years, 0-5 years, 2.5-5 years). Results were similar for the multivariable-adjusted models. Calculating the HR by comparing subjects with hip fracture, nonvertebral fractures any type of fractures with the control group without the same type of fracture rather than without any fracture did not change the results.
Additional analyses
To determine the robustness of our results we ran additional analyses. Using weight instead of BMI and handgrip strength instead self-reported mobility disability did not change the results. Because education, dietary factors, renal function and medical conditions might differentially affect the associations between BTM and bone loss, we further adjusted our multivariate results for these variables, but the results were unchanged.
Discussion
We sought to determine whether analyses of systemic BTM could provide insights into geometry, trabecular and cortical BMD changes with aging and fracture risk in a large cohort of older men and women. Higher bone turnover was associated with faster mineral loss of the femoral neck and total hip. Serum catabolic and anabolic biomarkers correlated most strongly with the rate of vBMD loss than with the periosteal expansion rate. Higher levels of BTM showed a stronger association with changes in cortical BMD compared to trabecular bone loss, particularly at the femoral neck. In men, higher levels of BTM were more strongly associated with faster endosteal vBMD loss at the hip compared to femoral neck, and the opposite was found in women. Elevated BTM levels only predicted periosteal expansion rate in men. BTM did not predict fractures in both men and women.
We confirmed the significant but weak relationship between BTM and bone loss in women [7,20,21] and men [6,22,9]. In women the association was stronger between BTM and bone loss at femoral neck compared to total hip, and was stronger with cortical bone loss at both site locations. In contrast, in men the association was stronger between BTM and bone loss at total hip compared to femoral neck. Surprisingly, in the present study BTM reflected gender and site specific associations. Together, these results do not corroborate the notion from previous studies that the association between BTM and bone loss is weaker in men than women [9]. However, previous studies used DXA-derived variables and in shorter follow-ups. Such findings need to be corroborated by additional studies with 3D imaging techniques, long-term follow-up and both sexes.
We observed that, in general, higher BTM levels were more predictive of faster cortical bone loss than trabecular. These data confirm that later in life endocortical and intracortical remodeling increase and bone loss comes primarily from cortical bone due to increased intracortical porosity (number and size) that increases bone surface [23]. Our finding is in agreement with previous cross-sectional reports of an association between BTM and intracortical surface area and intracortical porosity at the distal tibia in women aged 27 to 98 years [24], and with increased cortical porosity and thinner cortices of the femoral subtrochanter in postmenopausal women [12]. In contrast, microCT of male mice suggested that the decline in BTM significantly correlated with the age-related reduction in trabecular bone volume and surface [25].
The periosteal surface displays evidence of bone turnover due to bone remodeling although much slower than endosteal turnover, and its overall surface is very low compared with endosteal surfaces [26]. The observed modest increase in periosteal surface at the femoral neck in men was associated with both increased serum bone formation and resorption markers, which lends further support to the idea that this surface enjoys both osteoblastic and osteoclastic activity [2]. The existence of periosteum at the femoral neck has been debated but the extent of periosteal covering at this clinically relevant site has been recently demonstrated [27]. The femoral neck surface has a significant amount of mineralizing tissue and a significant lower periosteal cellularity than femoral diaphysis [27,28]. In women, baseline levels of BTM were not associated with the rate of periosteal apposition. The reason for the difference between men and women is not clear, but it can be related to the higher rate of periosteal expansion in men, which may lead to a more accurate estimation of its aging-related changes. Sex hormones may also cause an interaction between mechanical loading, systemic bone markers and cellular events at the periosteal surface. As we did not measure sex steroids, we cannot exclude such effect.
There is discordance in the literature regarding the relationship between BTM and periosteal apposition in older adults. In two previous studies baseline bone formation and resorption markers were not significantly associated with cross-sectional data of bone size [29] or with the periosteal expansion rate at any skeletal site [9]. Previous cross-sectional studies in our laboratory and others have reported a positive association between BTM and proximal femur size [17,12].
Serum BTM did not predict long-term fracture risk in both elderly men and women. Although several previous reports have found similar results [30,31,9], recent reviews concluded that BTM levels (mainly bone resorption markers) provide information on fracture risk that is independent of bone density [3,13,5]. In a retrospective case-control study of 211 postmenopausal women (aged 54-94 years) elevated BTM levels were associated with increased fracture risk partially independent of cortical porosity, thickness and areal BMD [12]. Nonetheless, due to the inconsistent results between studies (depending on markers, fracture anatomical location, clinical populations and follow-up length) and the fact that BTM have high biological variability, they are not being currently used for the management of bone fragility in clinical practice. Our data also showed that increased BTM level did not predict fractures regardless of follow-up duration, i.e., entire follow-up time (12.5 years), or shorter periods (0-2.5 years, 0-5 years, 2.5-5 years). In contrast, the prediction of any fracture was higher in the beginning of the follow-up period (2.5 years from baseline) compared to the entire follow-up period (range 7.4-10.9 years) in a prospective cohort study of elderly women [30]. Nevertheless, biochemical evidence for higher resorption and formation marker in fracture cases than in controls is conflicting [6,32,30,12]. In addition to turnover, other factors may also contribute to bone fragility during aging, including changes in matrix composition (such as accumulation of advanced glycation end products and increased cross-linking of collagen) [33], osteocyte deficiency [34], and increased number of fatigue damage [35].
The strengths of this study included its prospective design, large community-based population, the long follow-up time of up to 12.5 years, CT scans made at a single-center, relatively large number of hip and nonvertebral fracture outcomes, and use of state-of-the-art resorption and formation markers measured in fasting conditions. This analysis includes a comprehensive number of covariates with a potential effect on bone loss and fracture risk. We also evaluated baseline levels of serum 25OHD and PTH. In women, fracture cases had lower serum 25OHD than controls, and therefore it was included in our COX regression models. Currently, no other studies have considered the potential confounding effect of these factors to assess the relation of biological BTM with accelerating bone loss and rate of periosteal growth with aging.
There are, however, a number of limitations to be considered when interpreting the results. An important technical limitation in our study is the effect of partial volume averaging on our cortical bone measurements, as previously described by our group [36]. Briefly, as reported previously for a smaller sample from the AGES-REYKJAVIK study, the relative bone loss in the mid-femoral neck superior region is substantially (about threefold) and significantly greater compared to those estimated in the inferior region in both sexes [37]. Since the geometry is determined by a threshold based method, with the threshold held constant, the thinning of the superior cortex leads to larger errors in apparent thickness and volume, resulting in a decrease in apparent CSA. Thus, the small overall change in our measured cortical BMD, in line with the data published in an earlier study from our group [36], is likely to be due to the large decline in the superior cortical thickness, which contributes relatively little to the overall apparent cortical BMD. Another potential weakness concerns the measurement of bone tissue activity. Despite the use of well-established biochemical markers of bone metabolism, they have some limitations in accuracy because they are not bone-tissue specific, they reflect mainly the activities of osteoblasts or osteoclasts and not the activity of osteocytes, they may have limited sensitivity to mirror the nature of periosteal remodeling events (which remains little investigated and poorly understood), and their levels reflect the aggregated remodeling intensity for the whole skeleton (circulating concentrations). We also considered the possibility that bone turnover is influenced by many factors and a single measurement of BTM may not correspond to the average bone turnover rate over a long period of time. Serial assessment of bone turnover was more strongly correlated with bone loss then baseline levels of BTM [8]. We did not investigate the prediction of vertebral fracture alone, due to the small number of cases (7 cases in men and 23 cases in women) and consequent limited statistical power. Because all participants were older than 65 years and Caucasian, our results may not be generalizable to other populations and ethnic groups. Endogenous sex steroids may influence BTM concentrations in men and women, and provide some clues to the distinct gender differences in BTM levels. However, there were no measurements of sex hormones in the participants.
The underlying biological reasons through which bone fragility could result from elevated bone turnover might involve the age-related cellular machinery defects of the BMU, as each BMU forms less bone and increases the volume of bone resorbed [1]. Other relevant mechanism may be qualitative abnormalities in bone precipitated by increased perforative resorption by individual BMUs causing trabecular plate perforation, and loss of connectivity [38]. More detailed studies combining biochemical, biomechanical, and micro-architectural analysis on both inner and outer bone surfaces may help to shed some light on this complex and not well understood question.
In summary, from the pathophysiological point of view, there is a moderate relationship between baseline BTM levels and the two processes linked to the age-related bone mass and geometry changes: endosteal bone loss and periosteal apposition. However, these associations are variable, depending on bone site. Associations of BTM levels with fracture risk were not significant. From a clinical point of view, the dual source of BTM weaken the appropriate interpretation of their levels to identify patients with higher risk of fracture, as they reflect simultaneously bone fragility due to accelerated bone loss and a possible increase in bone strength because the resistance of the bone to bending or torsional forces is related exponentially to its diameter. Additional studies are needed to clarify the relationship of biological markers of bone metabolism and periosteal apposition, since our results demonstrated weak associations with increased CSA at different locations compared to our previous cross-sectional report [17]. At present periosteal bone biology is insufficiently understood, and should be the focus of research to assist the development of prevention and treatment options (including mechanical loading and osteoporosis drugs) of bone fragility.
Acknowledgments
This study was funded by National Institutes of Health contract N01-AG-012100, the National Institute on Aging Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament). EAM and TBH were supported in part by and the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
Footnotes
Elisa A Marques, Vilmundur Gudnason, Thomas Lang, Gunnar Sigurdsson, Sigurdur Sigurdsson, Thor Aspelund, Kristin Siggeirsdottir, Lenore Launer, Gudny Eiriksdottir and Tamara B Harris declare that they have no disclosures.
References
- 1.Seeman E. Bone modeling and remodeling. Critical reviews in eukaryotic gene expression. 2009;19(3):219–233. doi: 10.1615/critreveukargeneexpr.v19.i3.40. [DOI] [PubMed] [Google Scholar]
- 2.Orwoll ES. Toward an expanded understanding of the role of the periosteum in skeletal health. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2003;18(6):949–954. doi: 10.1359/jbmr.2003.18.6.949. [DOI] [PubMed] [Google Scholar]
- 3.Chopin F, Biver E, Funck-Brentano T, Bouvard B, Coiffier G, Garnero P, Thomas T. Prognostic interest of bone turnover markers in the management of postmenopausal osteoporosis. Joint, bone, spine : revue du rhumatisme. 2012;79(1):26–31. doi: 10.1016/j.jbspin.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Naylor K, Eastell R. Bone turnover markers: use in osteoporosis. Nature reviews Rheumatology. 2012;8(7):379–389. doi: 10.1038/nrrheum.2012.86. [DOI] [PubMed] [Google Scholar]
- 5.Vasikaran S, Eastell R, Bruyere O, Foldes AJ, Garnero P, Griesmacher A, McClung M, Morris HA, Silverman S, Trenti T, Wahl DA, Cooper C, Kanis JA Group I-IBMSW. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2011;22(2):391–420. doi: 10.1007/s00198-010-1501-1. [DOI] [PubMed] [Google Scholar]
- 6.Bauer DC, Garnero P, Harrison SL, Cauley JA, Eastell R, Ensrud KE, Orwoll E Osteoporotic Fractures in Men Research G. Biochemical markers of bone turnover, hip bone loss, and fracture in older men: the MrOS study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2009;24(12):2032–2038. doi: 10.1359/jbmr.090526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garnero P, Sornay-Rendu E, Duboeuf F, Delmas PD. Markers of bone turnover predict postmenopausal forearm bone loss over 4 years: the OFELY study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1999;14(9):1614–1621. doi: 10.1359/jbmr.1999.14.9.1614. [DOI] [PubMed] [Google Scholar]
- 8.Ivaska KK, Lenora J, Gerdhem P, Akesson K, Vaananen HK, Obrant KJ. Serial assessment of serum bone metabolism markers identifies women with the highest rate of bone loss and osteoporosis risk. The Journal of clinical endocrinology and metabolism. 2008;93(7):2622–2632. doi: 10.1210/jc.2007-1508. [DOI] [PubMed] [Google Scholar]
- 9.Szulc P, Montella A, Delmas PD. High bone turnover is associated with accelerated bone loss but not with increased fracture risk in men aged 50 and over: the prospective MINOS study. Annals of the rheumatic diseases. 2008;67(9):1249–1255. doi: 10.1136/ard.2007.077941. [DOI] [PubMed] [Google Scholar]
- 10.Parfitt AM. Misconceptions (2): turnover is always higher in cancellous than in cortical bone. Bone. 2002;30(6):807–809. doi: 10.1016/s8756-3282(02)00735-4. [DOI] [PubMed] [Google Scholar]
- 11.Kemp JP, Sayers A, Paternoster L, Evans DM, Deere K, St Pourcain B, Timpson NJ, Ring SM, Lorentzon M, Lehtimaki T, Eriksson J, Kahonen M, Raitakari O, Laaksonen M, Sievanen H, Viikari J, Lyytikainen LP, Smith GD, Fraser WD, Vandenput L, Ohlsson C, Tobias JH. Does bone resorption stimulate periosteal expansion? A cross-sectional analysis of beta-C-telopeptides of type I collagen (CTX), genetic markers of the RANKL pathway, and periosteal circumference as measured by pQCT. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2014;29(4):1015–1024. doi: 10.1002/jbmr.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shigdel R, Osima M, Ahmed LA, Joakimsen RM, Eriksen EF, Zebaze R, Bjornerem A. Bone turnover markers are associated with higher cortical porosity, thinner cortices, and larger size of the proximal femur and non-vertebral fractures. Bone. 2015;81:1–6. doi: 10.1016/j.bone.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 13.Johansson H, Oden A, Kanis JA, McCloskey EV, Morris HA, Cooper C, Vasikaran S Turnover I-IJWGoSoBMoB. A meta-analysis of reference markers of bone turnover for prediction of fracture. Calcified tissue international. 2014;94(5):560–567. doi: 10.1007/s00223-014-9842-y. [DOI] [PubMed] [Google Scholar]
- 14.Luukinen H, Kakonen SM, Pettersson K, Koski K, Laippala P, Lovgren T, Kivela SL, Vaananen HK. Strong prediction of fractures among older adults by the ratio of carboxylated to total serum osteocalcin. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2000;15(12):2473–2478. doi: 10.1359/jbmr.2000.15.12.2473. [DOI] [PubMed] [Google Scholar]
- 15.Meier C, Nguyen TV, Center JR, Seibel MJ, Eisman JA. Bone resorption and osteoporotic fractures in elderly men: the dubbo osteoporosis epidemiology study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2005;20(4):579–587. doi: 10.1359/JBMR.041207. [DOI] [PubMed] [Google Scholar]
- 16.Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, Thorgeirsson G, Aspelund T, Garcia ME, Cotch MF, Hoffman HJ, Gudnason V. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. American journal of epidemiology. 2007;165(9):1076–1087. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marques EA, Gudnason V, Sigurdsson G, Lang T, Johannesdottir F, Siggeirsdottir K, Launer L, Eiriksdottir G, Harris TB. Are bone turnover markers associated with volumetric bone density, size, and strength in older men and women? The AGES-Reykjavik study. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2015 doi: 10.1007/s00198-015-3442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eysteinsdottir T, Thorsdottir I, Gunnarsdottir I, Steingrimsdottir L. Assessing validity of a short food frequency questionnaire on present dietary intake of elderly Icelanders. Nutrition journal. 2012;11:12. doi: 10.1186/1475-2891-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siggeirsdottir K, Aspelund T, Sigurdsson G, Mogensen B, Chang M, Jonsdottir B, Eiriksdottir G, Launer LJ, Harris TB, Jonsson BY, Gudnason V. Inaccuracy in self-report of fractures may underestimate association with health outcomes when compared with medical record based fracture registry. European journal of epidemiology. 2007;22(9):631–639. doi: 10.1007/s10654-007-9163-9. [DOI] [PubMed] [Google Scholar]
- 20.Lofman O, Magnusson P, Toss G, Larsson L. Common biochemical markers of bone turnover predict future bone loss: a 5-year follow-up study. Clinica chimica acta; international journal of clinical chemistry. 2005;356(1-2):67–75. doi: 10.1016/j.cccn.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Ross PD, Knowlton W. Rapid bone loss is associated with increased levels of biochemical markers. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1998;13(2):297–302. doi: 10.1359/jbmr.1998.13.2.297. [DOI] [PubMed] [Google Scholar]
- 22.Gielen E, O'Neill T, Pye S, Adams J, Ward K, Wu F, Laurent M, Claessens F, Boonen S, Vanderschueren D, Verschueren S. Bone turnover markers predict hip bone loss in elderly European men: results of the European Male Ageing Study (EMAS) Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2015;26(2):617–627. doi: 10.1007/s00198-014-2884-1. [DOI] [PubMed] [Google Scholar]
- 23.Zebaze RM, Ghasem-Zadeh A, Bohte A, Iuliano-Burns S, Mirams M, Price RI, Mackie EJ, Seeman E. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet. 2010;375(9727):1729–1736. doi: 10.1016/S0140-6736(10)60320-0. [DOI] [PubMed] [Google Scholar]
- 24.Bjornerem A, Ghasem-Zadeh A, Bui M, Wang X, Rantzau C, Nguyen TV, Hopper JL, Zebaze R, Seeman E. Remodeling markers are associated with larger intracortical surface area but smaller trabecular surface area: a twin study. Bone. 2011;49(6):1125–1130. doi: 10.1016/j.bone.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Shahnazari M, Dwyer D, Chu V, Asuncion F, Stolina M, Ominsky M, Kostenuik P, Halloran B. Bone turnover markers in peripheral blood and marrow plasma reflect trabecular bone loss but not endocortical expansion in aging mice. Bone. 2012;50(3):628–637. doi: 10.1016/j.bone.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Balena R, Shih MS, Parfitt AM. Bone resorption and formation on the periosteal envelope of the ilium: a histomorphometric study in healthy women. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1992;7(12):1475–1482. doi: 10.1002/jbmr.5650071216. [DOI] [PubMed] [Google Scholar]
- 27.Allen MR, Burr DB. Human femoral neck has less cellular periosteum, and more mineralized periosteum, than femoral diaphyseal bone. Bone. 2005;36(2):311–316. doi: 10.1016/j.bone.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Power J, Loveridge N, Rushton N, Parker M, Reeve J. Evidence for bone formation on the external “periosteal” surface of the femoral neck: a comparison of intracapsular hip fracture cases and controls. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2003;14(2):141–145. doi: 10.1007/s00198-002-1333-8. [DOI] [PubMed] [Google Scholar]
- 29.Szulc P, Garnero P, Marchand F, Duboeuf F, Delmas PD. Biochemical markers of bone formation reflect endosteal bone loss in elderly men--MINOS study. Bone. 2005;36(1):13–21. doi: 10.1016/j.bone.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Ivaska KK, Gerdhem P, Vaananen HK, Akesson K, Obrant KJ. Bone turnover markers and prediction of fracture: a prospective follow-up study of 1040 elderly women for a mean of 9 years. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010;25(2):393–403. doi: 10.1359/jbmr.091006. [DOI] [PubMed] [Google Scholar]
- 31.Melton LJ, 3rd, Crowson CS, O'Fallon WM, Wahner HW, Riggs BL. Relative contributions of bone density, bone turnover, and clinical risk factors to long-term fracture prediction. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2003;18(2):312–318. doi: 10.1359/jbmr.2003.18.2.312. [DOI] [PubMed] [Google Scholar]
- 32.Gerdhem P, Ivaska KK, Alatalo SL, Halleen JM, Hellman J, Isaksson A, Pettersson K, Vaananen HK, Akesson K, Obrant KJ. Biochemical markers of bone metabolism and prediction of fracture in elderly women. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2004;19(3):386–393. doi: 10.1359/JBMR.0301244. [DOI] [PubMed] [Google Scholar]
- 33.Paschalis EP, Tatakis DN, Robins S, Fratzl P, Manjubala I, Zoehrer R, Gamsjaeger S, Buchinger B, Roschger A, Phipps R, Boskey AL, Dall'Ara E, Varga P, Zysset P, Klaushofer K, Roschger P. Lathyrism-induced alterations in collagen cross-links influence the mechanical properties of bone material without affecting the mineral. Bone. 2011;49(6):1232–1241. doi: 10.1016/j.bone.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiu S, Rao DS, Palnitkar S, Parfitt AM. Age and distance from the surface but not menopause reduce osteocyte density in human cancellous bone. Bone. 2002;31(2):313–318. doi: 10.1016/s8756-3282(02)00819-0. [DOI] [PubMed] [Google Scholar]
- 35.Donahue SW, Galley SA. Microdamage in bone: implications for fracture, repair, remodeling, and adaptation. Critical reviews in biomedical engineering. 2006;34(3):215–271. doi: 10.1615/critrevbiomedeng.v34.i3.20. [DOI] [PubMed] [Google Scholar]
- 36.Sigurdsson G, Aspelund T, Chang M, Jonsdottir B, Sigurdsson S, Eiriksdottir G, Gudmundsson A, Harris TB, Gudnason V, Lang TF. Increasing sex difference in bone strength in old age: The Age, Gene/Environment Susceptibility-Reykjavik study (AGES-REYKJAVIK) Bone. 2006;39(3):644–651. doi: 10.1016/j.bone.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 37.Johannesdottir F, Aspelund T, Reeve J, Poole KE, Sigurdsson S, Harris TB, Gudnason VG, Sigurdsson G. Similarities and differences between sexes in regional loss of cortical and trabecular bone in the mid-femoral neck: the AGES-Reykjavik longitudinal study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2013;28(10):2165–2176. doi: 10.1002/jbmr.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seeman E. Reduced bone formation and increased bone resorption: rational targets for the treatment of osteoporosis. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 14 Suppl. 2003;3:S2–8. doi: 10.1007/s00198-002-1340-9. [DOI] [PubMed] [Google Scholar]