SUMMARY
Background
After brain metastasis resection, whole-brain radiation therapy (WBRT) decreases local recurrence but may cause cognitive decline. We performed this study to determine if stereotactic radiosurgery (SRS) to the surgical cavity improved local tumor tumor-free recurrence rates compared to surgical resection alone as an alternative to the need for immediate WBRT.
Methods
The main entry criteria for the study included patients >3 years of age, with a Karnofsky Performance Score ≥ 70, who were able to undergo an MRI scan and who had a complete resection of 1–3 brain metastases (the maximum diameter of the resection cavity had to be ≤4cm). Patients were assigned randomly to either SRS treatment of the resection cavity (within 30 days of surgery) or observation (OBS). Patients were stratified by histology, tumor size, and number of metastases. Patients were recruited at a single tertiary cancer center. The primary endpoint was time to local recurrence in the resection cavity assessed by blinded central review of brain MRI scans in the intention-to-treat population. The trial was registered at clinicaltrials.gov (Trial NCT00950001, status: closed to new participants).
Findings
Between 8/13/2009 and 2/16/2016, 132 patients were randomized to OBS (N=68) or SRS (N=64), with 128 patients available for analysis. We stratified by metastasis size (maximum diameter of ≥3 cm vs. <3 cm), histology (melanoma vs. other), and number of metastases (one vs. two or three). The 12-month local tumor recurrence-free rate was 43% (OBS) (95% CI 31%–59%) and 72% (SRS) (95% CI 60%–87%) (hazard ratio [HR] 0.46, 95% confidence interval [CI] 0.24–0.88, p=0.015).
Interpretation
This prospective randomized trial of patients undergoing surgical resection for 1–3 brain metastases indicates that SRS administered to the resection cavity significantly lowers local recurrence compared to observation alone. Thus, the use of SRS after brain metastasis resection is an alternative to WBRT.
INTRODUCTION
Brain metastases are a tremendous healthcare burden.1 Surgical resection is a mainstay of treatment for single metastases and has been shown to improve survival compared whole brain radiation (WBRT) alone.2 Independently, surgical resection has been thought to be insufficient to provide durable local control, and the addition of post-operative WBRT decreases the likelihood of recurrence within the resection cavity (local recurrence).3 Although WBRT is often considered the standard of care after surgical resection of brain metastases to improve local tumor-free recurrence rates, prospective studies have demonstrated its association with cognitive decline.4–6 Consequently, its routine use has been questioned and WBRT is now frequently withheld after resection, particularly for patients with a limited number of brain metastases7,8. Stereotactic radiosurgery (SRS) has been used to deliver targeted radiation to the resection cavity to minimize local recurrence as an alternative to WBRT. SRS can be performed postoperatively and delivers a high dose of radiation in one session to the margins of the resection cavity. Therefore, SRS should decrease local recurrence without the adverse effects of WBRT; however, only retrospective studies have been published demonstrating the feasibility of administering post-operative SRS to the resection cavity, and its efficacy remains unknown.9,10
Our primary aim was to determine whether administering post-operative SRS to the resection cavity improved the local tumor-free recurrence rate compared with surgical resection alone. Surgical techniques and adjuncts have improved significantly since the original studies performed by Patchell et al., with recent studies indicating that local control may be improved through more modern surgical techniques, particularly for smaller tumors.11 We compared surgical resection alone with surgical resection followed by SRS to determine the efficacy of post-operative SRS in improving local tumor control in the surgical bed.
METHODS
Study design and participants
We conducted a prospective randomized trial in patients with one to three brain metastases. The trial was performed at a single tertiary cancer institution in the USA. The study was approved by The University of Texas MD Anderson Cancer Center Clinical Research Committee and Institutional Review Board and was monitored by the institutional Data and Safety Monitoring Board. The authors vouch for the accuracy and completeness of the findings, wrote and take responsibility for the manuscript, and vouch for the fidelity of the study and of this report of the study protocol. We followed the CONSORT guidelines for reporting a randomized trial (www.consort-statement.org). After complete resection (verified by the study neuroradiologist (S.Rh)) of at least one metastasis, patients were randomized to SRS or observation (OBS) and treated within 30 days of surgery. Allocation was equal between the study arms. Patients gave written informed consent for inclusion in the study (if under the age of 18, consent was given by the parent or guardian). The unresected lesions, if present, were treated with SRS as clinically indicated. The exclusion criteria were age <3 years, prior radiotherapy administered to the brain, prior resection of any brain metastasis, evidence of leptomeningeal disease (LMD), small-cell lung cancer or hematologic malignancies, pregnancy, Karnofsky Performance Scale score <70, inability to undergo MR imaging, and a post-operative cavity of >4 cm (at the time of SRS) as determined by the study neuroradiologist. Systemic disease was assessed at baseline using RECIST criteria (primarily by CT scans). Patients were permitted to have active extracranial disease and may have been undergoing treatment with systemic treatments while on study. The treatment team (excluding the neuroradiologist) and patient were informed of the arm after randomization. The neuroradiologist was blinded with respect to the study arm after patient enrollment. The full protocol is supplied in the appendix (appendix pp 1–13)
Randomization and masking
Patients were enrolled by the study nurse and randomized (1:1) through an institutional, computerized patient registration system to receive either SRS or OBS. A block randomization schedule was used to generate the random allocation sequence. Allocation was done with stratification factors and a block size of four. To conceal the sequence, records were pre-allocated to each stratum. Patients were stratified by: 1) histologic type (melanoma vs. non-melanoma), 2) pre-operative size of brain metastases (<3 cm vs. ≥3 cm based on the largest cross-sectional diameter on contrast-enhanced T1-weighted magnetic resonance [MR] images), and 3) number of brain metastases (one vs. two or three). Patients and treating physicians (except for the study neuroradiologist) were not masked with respect to treatment group.
Procedures
All patients in the SRS arm underwent single session treatment. The SRS target volume was defined as the surgical cavity on the volumetric MR image with a 1-mm circumferential margin added. Prescription doses were 16, 14, and 12 Gy for target volumes of ≤10 cc, 10.1–15 cc, and >15 cc, respectively. Treatment with SRS was performed using the Elekta Perfexion Gamma Knife unit (Elekta, Stockholm, Sweden). Patients undergoing SRS had a Leksell (Elekta) stereotactic headframe applied on the day of the procedure. A volumetric MRI was performed on the morning of the procedure after headframe placement. The volumetric MRI is comprised of an Axial T1 weighed 3D-FSPGR (fast spoiled gradient echo) sequence performed at 1 mm slice thickness with a gap of 0 mm following the administration of gadobenate dimeglumine (multihance, Bracco, Milan, Italy),. Sagittal and coronal reconstructed images were routinely obtained for interpretation in all three planes. All studies were performed on 1.5 or 3.0 Tesla scanners. All radiation plans were reviewed by the treating radiation oncologist, neurosurgeon, and medical physicist to verify protocol-specific volume delineation and radiation dosimetry. If the lesion was close to the dura, a meningeal margin was included. The surgical tract (particularly for deep seated tumors) was not included in the planning. Dose constraints were as follows: brainstem: 1cc < 12Gy, and the optic nerve and tract <9Gy. Patients in both arms underwent surveillance brain MR imaging scan and clinical evaluation (including an evaluation for adverse events) within 5–8 weeks after the craniotomy and then every 6–9 weeks for the first year followed by brain MRI imaging every 9–12 weeks. All MRI scans were reviewed centrally by the study neuroradiologist. Local failures (in either arm) were treated at the discretion of the physician and treatments could include surgery if appropriate or SRS if the patient was in the OBS arm. New distant brain metastases (DBMs) distinct from the treated site(s) that did not require WBRT were noted and treated at the physician’s discretion. Patients with new DBMs remained in the study. Unscheduled follow-ups were also recorded to assess adverse events.
Outcomes
The local tumor-free recurrence rate was the primary endpoint. Local recurrences included radiographic evidence of a new contrast-enhancing lesion (specifically, any new, progressive, enhancing nodularity) contiguous with or within the resection cavity as confirmed by the study neuroradiologist. For any patient who had more than one lesion resected, local recurrence in any surgical cavity was considered a local failure. Equivocal areas of enhancement that were ultimately found to represent local recurrences were retroactively censored on the date of the first ambiguous MR imaging scan.
Secondary endpoints included development of DBMs and overall survival time (from time of randomization to death). DBMs were defined as the development of a new lesion separate from the surgical site. Overall survival was defined as the time from randomization to date of death. The type of death was categorized as neurologic if metastatic brain disease was the proximate cause of death or systemic if the patient died from extracranial disease.
Adverse events related to SRS were recorded at each clinical visit. They included complications related from stereotactic frame placement and radiation treatment. We also documented adverse events related to surgery (in both arms). This included 30-day surgical morbidity (complications, major and minor) and mortality. We had no radiographic evidence of necrosis in the SRS arm.
Statistical Analysis
Sample size calculations were based on the primary endpoint: Local tumor-free recurrence. On the basis of the available literature, local recurrence after surgical resection only was expected to occur in 50% of patients within 6 months, whereas local recurrence after treatment of the resection cavity with SRS was expected to occur in 25% of patients within 6 months3. On the basis of the exponential distribution, these values suggest a median time to local recurrence of 6 months in the OBS arm and 14.45 months in the SRS arm (HR 0.415). Under the alternative hypothesis for a log-rank test, a two-sided type I error of 0.05, and two interim futility looks, a total of 132 patients (61 to OBS and 61 to SRS) would have 99.6% power to detect differences based on a hazard ratio (HR) of 0.415 and approximately 80% power to detect differences based on a HR of 0.596. Thus, the maximum sample size to be accrued was 132 patients. Estimating an accrual rate of two to three patients per month, the projected time to complete the study was 44–66 months. For all time-to-event endpoints, a univariate test comparing treatment groups was conducted using a log-rank test along with Kaplan-Meier estimates. Multivariable analysis was conducted via the Cox proportional hazards model. The University of Texas MD Anderson Cancer Center Data Safety Monitoring Board monitored the study annually.
The primary and secondary analyses were a modified intent-to-treat that (1) Excluded ineligible patients from the analysis, (2) Preserved the original treatment assignment, and (3) Was based on the stratified log-rank test. For the primary and secondary endpoints, censoring occurred as follows: for recurrence, patients dying without evidence of central nervous system (CNS) recurrence were censored, and patients remaining free of the entity under study at the end of follow-up were censored. The HR comparing SRS with OBS was computed for each endpoint with and without adjustment for other covariates. Ninety-five percent confidence intervals (CIs) were computed for the HR estimates. We also compared overall survival times between treatment groups. We compared freedom from WBRT (defined as time to WBRT from randomization, patients who did not receive WBRT were censored) between arms. Finally, we performed subset analyses according to the three stratification factors (histologic type including melanoma versus non-melanoma; size of metastases; and number of metastases). The analysis of the common histologies (breast, lung and other) was performed post-hoc. The analysis of tumors stratified by size (≤2.5 cm, 2.5–3.5cm, and >3.5 cm) was performed post-hoc. We assessed the statistical significance of the subset differences by fitting Cox proportional hazards regression models with treatment-covariate interaction terms. Additional potential confounding factors included radiation dose, resection cavity volume, and systemic disease status at randomization (primarily assessed as stable vs. progressing using RECIST criteria12 and were analyzed post-hoc. A secondary analysis using competing risk proportional hazards regression analysis (Fine/Gray model) was performed to verify the results of the primary analysis. The competing risks included distant brain metastases and death without brain metastases. On March 22, 2016, the protocol was amended (after accrual had completed but before data were analyzed) to expand on and clarify analysis plans. This study was registered with www.clinicaltrials.gov, number NCT00950001).
Differences in local tumor-free recurrence were to be evaluated (1) after a total of 39 events occurred, (2) after 77 events occurred, and (3) after at least 115 events occurred. The test statistic used was based on a stratified log-rank test. The interim stopping rule consisted of a group sequential test based on a Gamma family type I error spending function. The stopping boundaries for the interim analysis was based on the stratified log rank test with p values 0.9866 (for first look) and 0.4692 (for second look). The statistical analysis was performed using the TIBCO Spotfire S+ for Windows software package (TIBCO Software, Inc., Palo Alto, USA).
Role of the funding source
Support for this study was provided by the National Institutes of Health Cancer Center Support Grant (Award: P30CA016672). The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
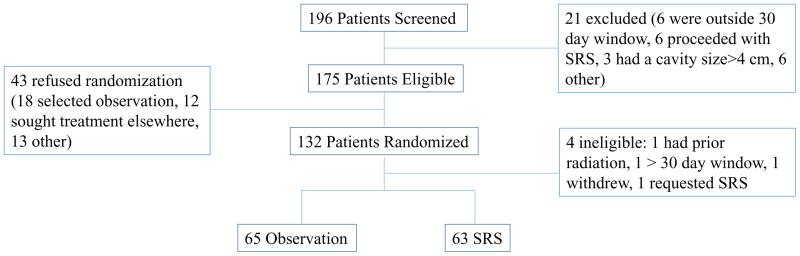
One hundred thirty-two patients were randomized to either the OBS or SRS study arm after undergoing resection of at least one brain metastasis from October 6, 2009, to September 1, 2015. The final analysis included 128 patients, with 65 in the OBS arm and 63 in the SRS arm (Figure 1). No significant demographic or baseline characteristic differences were noted between the SRS and OBS groups (Table 1). The median follow-up duration was 11.1 months (IQR=4.8–20.4). By the study’s conclusion, 85 patients had died. Four patients were declared ineligible after enrollment randomization and were excluded from the analysis: one patient had prior head and neck radiation extending into the brain, one patient’s MR imaging the day of SRS revealed residual tumor indicating incomplete resection, one patient withdrew from the study, and one patient underwent SRS after the 30-day window because of a pulmonary embolism.
Figure 1.
CONSORT diagram
Table 1.
Demographic and clinical characteristics
| Variable | OBS (N=65) | SRS (N=63) | |
|---|---|---|---|
| Sex | Female | 34 (52%) | 26 (41%) |
| Male | 31 (48%) | 37 (59%) | |
| Race/ethnicity | White | 49 (75%) | 45 (71%) |
| Other | 16(25%) | 18(29%) | |
| Age | <50 | 11 (17%) | 18 (29%) |
| 51–65 | 36 (55%) | 27 (43%) | |
| >65 | 18 (28%) | 18 (29%) | |
| Primary cancer | Melanoma | 13 (20%) | 14 (22%) |
| Lung | 13 (20%) | 13 (21%) | |
| Breast | 14 (22%) | 9 (14%) | |
| Other | 25 (38%) | 27 (43%) | |
| Systemic disease status | NED/PR | 21 (32%) | 21 (33%) |
| PD | 28 (43%) | 26 (41%) | |
| SD | 16 (25%) | 16 (25%) | |
| GPA | 1.0–2.0 | 29 (45%) | 25 (40%) |
| >2.0 – 3.0 | 23 (35%) | 29 (46%) | |
| >3.0 – 4.0 | 13 (20%) | 9 (14%) | |
| Number of metastases | 1 | 41 (63%) | 38 (60%) |
| 2 | 14 (22%) | 18 (29%) | |
| 3 | 10 (15%) | 7 (11%) | |
| Size | 0.5 – 2.5 cm | 19 (29%) | 21 (33%) |
| >2.5 to 3.5 cm | 19 (29%) | 21 (33%) | |
| >3.5 cm | 17 (26%) | 16 (25%) |
NED: No evidence of disease, PR: Partial Response, PD: Progressive Disease, SD; Stable Disease
There were 34 women and 31 men in the OBS arm and 26 women and 37 men in the SRS arm. The median age in the whole cohort was 59 years (range 20–80 years). The median age was 57 years (range 29–79 years) and 58 years (range 20–80 years) in the OBS and SRS cohorts respectively. The stratification factors were balanced between the cohorts.
The median dose of post-operative radiation was 16 Gy (range 12–18 Gy) to the 50% isodose line. The median pre-operative tumor maximal diameter was 3.0 cm (range 0.6–5.3 cm) in the SRS arm and 3.0 cm (range 0.7–5.7 cm) in the OBS arm. The median SRS-treated cavity volume was 8.9 cc (range 0.9–28.6 cc). In the SRS group, 66 lesions were treated (in 63 patients). Five deviations from the protocol occurred with respect to dosing: Three patients received 18 Gy to the 50% isodose line because of physician preference, and two received a lower dose (14 Gy instead of 16 Gy to the 50% isodose line) because of lesion proximity to the motor cortex.
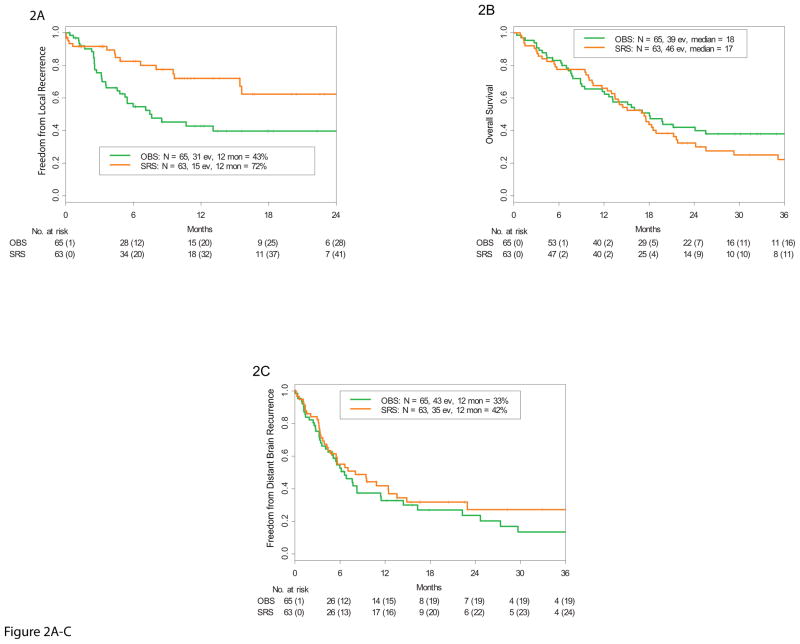
The 12-month local tumor-free recurrence rates were 43% (95% CI 31%–59%) and 72% (95% CI 60%–87%) in the OBS (31 events) and SRS (15 events) arms, respectively, (HR 0.46, 95% CI 0.24–0.88, stratified Cox model, p=0.015, stratified log-rank test) (Figure 2A). A competing risk analysis was performed and the results were very similar (HR = 0.41 (95% CI 0.21–0.80, p=0.0097)). The median time to local recurrence was 7.6 months (95% CI 5.3 months - not reached [NR]) in the OBS arm and was NR (95% CI 15.6 months - NR) in the SRS arm.
Figure 2.
A. Kaplan-Meier estimate of freedom from local recurrence rate demonstrating a significantly higher rate in the SRS study arm compared with the observation (OBS) arm. B. Kaplan-Meier estimate of overall survival demonstrating no difference between the SRS and OBS study arms. C. Kaplan-Meier estimate of freedom from distant brain metastasis demonstrating no difference between the SRS and OBS arms. (ev= events; mon= months).
In the OBS group, 31 patients developed local recurrence of their treated lesion. Of these patients, 13 underwent SRS alone, 9 underwent WBRT alone, three underwent surgery followed by WBRT, two underwent WBRT and SRS, one underwent surgery followed by SRS, one underwent surgery followed by fractionated external beam radiation, one underwent surgery alone, and one opted for non-treatment. In the SRS group, 15 patients developed local recurrence of the treated lesion. Of these patients, seven underwent WBRT, three underwent additional SRS, three underwent surgery (the pathological specimen confirmed metastatic cancer in all three cases), one underwent laser interstitial thermal therapy, and one opted for non-treatment.
The median overall survival time was 18 months (95% CI 13 months to NR) in the OBS arm (39 events) and 17 months (95% CI 13–22 months) in the SRS arm (46 events) (HR 1.29, 95% CI 0.84 –1.98, p=0.24) (Figure 2B). The cause of death was neurologic in 25/39 (64%) patients in the OBS arm and 22/46 (48%) in the SRS arm (the difference in proportions is 16% (95%C.I. [−5%, 37%], p=0.13). For the 38 patients who died of systemic disease progression, the main system involved at the time of death was lung (10), liver (4), skeletal (4) lymphatic involvement (2), multiple organ systems (5) and other (13). The probability of being free of DBM at 12 months was 33% (95% C.I., 22% –49%) in the OBS arm (43 events) and 42%(95% CI 30%–58%) in the SRS arm (35 events) (HR 0.81, 95% CI 0.51–1.27, p=0.35) (Figure 2C). WBRT was administered to 54 patients. The median freedom from WBRT was 15 months (95% CI 8.6 –42.5) in the OBS arm and 16 months (95% CI 10.1- N.R.) in the SRS arm (HR 0.8, 95% CI 0.47–1.37, p=0.42) (Appendix pp 14). Thirty patients (46%) eventually underwent WBRT in the OBS arm, and 24 (38%) had WBRT in the SRS arm. The median survival in these 54 patients after WBRT was 6.0 months (95% C.I. 5.1,9.2). Ultimately 78 patients in the entire cohort developed DBM, 43 in the OBS arm and 35 in the SRS arm which were managed in various ways (Appendix pp15). Twenty patients in the entire cohort developed LMD. The incidence of LMD did not differ between study arms; at 12 months, the estimated LMD incidence was 16% (95% CI 4%–26%) in the OBS arm and 28% (95% CI 12%–40%) in the SRS arm (HR 1.4, 95% CI 0.6–3.4, p=0.46). Nineteen of the 20 patients with LMD ultimately underwent treatment with WBRT.
The 12-month local tumor-free recurrence rate was 60% (95% CI 46% – 79%) in patients with GPA scores of 1.0–2.0 (N=54), 58% (95% CI 48% – 76%) in those with scores >2.0–3.0 (N=52), and 44% (95% CI 25% – 75%) in those with scores >3.0 – 4.0 (N=22). The difference in local tumor-free recurrence rates between these groups was not statistically significant (log-rank p=0.53) (Appendix pp16).
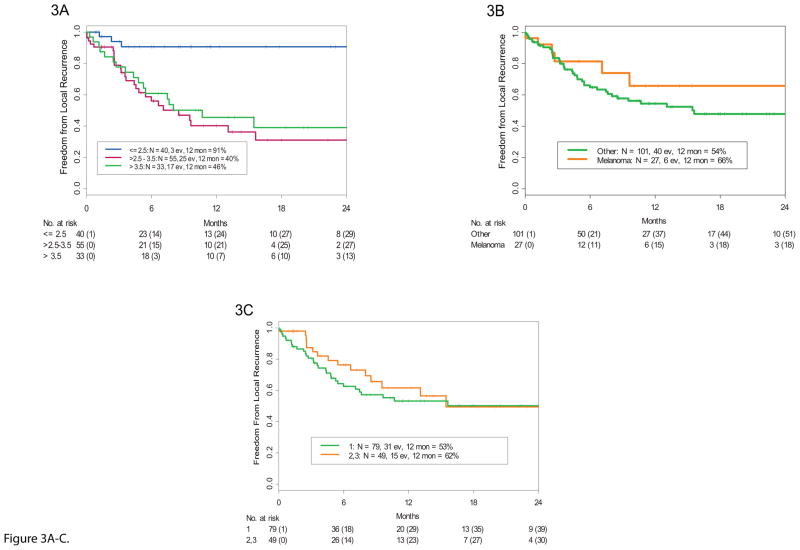
Patients with smaller tumors had a much lower likelihood of local recurrence. The 12-month local tumor-free recurrence rates were: 91% (95% CI 81% – 100%) for patients with tumors with a maximal diameter of ≤2.5 cm (N=40), 40% (95% CI 27% – 60%) for patients with tumors >2.5 to 3.5 cm (N=55) in diameter, and 46% (95% CI 31% – 68%) for patients with tumors >3.5 cm (N=33) in diameter. Smaller tumors (0–2.5 cm) showed significantly better local tumor-free recurrence rates than larger tumors (log-rank p=0.0002) (Figures 3A).
Figure 3.
A. Kaplan-Meier estimate of freedom from local recurrence stratified by brain metastasis size. Smaller tumors had a significantly higher local control rate than larger tumors for the whole cohort. (ev= events, mon = months) B. Kaplan-Meier estimate of freedom from local recurrence stratified by number of metastases (1 versus 2 or 3). C. Kaplan-Meier estimate of freedom from local recurrence stratified by melanoma and non-melanoma histologies.
The primary tumor histologic type did not appear to influence local tumor-free recurrence rates. We analyzed melanoma and non-melanoma cases (Figure 3B) as well as non-melanoma cases by their specific histology. The 12-month local tumor-free recurrence rate was 50% (95% CI 32%–78%) for patients with breast cancer (N=23), 73% (95% CI 56% – 94%) for patients with lung cancer (N=26), 66% (95% CI 46%–94%) for patients with melanoma (N=27), and 50% (95% CI 37 –68%) for patients with other cancers (N=52). Local tumor-free recurrence rates did not significantly differ according to histologic type (log-rank p=0.35) (Appendix pp17).
We compared the local tumor-free recurrence rate for patients with 1 metastasis to those with 2 or 3 (Figure 3C). For patients with one, two, and three brain metastases (N=79, N=32, and N=17, respectively), the 12-month local tumor-free recurrence rates were 53% (95% CI 42%–67%), 61% (95% CI 43%–86%, and 62% (95% CI 39% – 100%), respectively. The differences in rates between these groups was not statistically significant (log-rank p=0.67) (Appendix pp18).
The status of a patient’s systemic cancer did not influence the 12 month local tumor-free recurrence rate. There were 42 patients with no evidence of disease, 54 with progressive disease and 32 with stable disease at the time of SRS. The 12-month local tumor-free recurrence rates for these groups was 51% (95% CI 38%–70%), 65% (95% CI 50%–84%), and 48% (95%CI 30% –76%) respectively (log rank p=0.26)
Variables included in multivariable analyses for local tumor-free recurrence (Table 2), overall survival, and development of DBM were: type of treatment (SRS or OBS); primary cancer site, i.e., histologic type; systemic disease status (evidence of disease, stable, or progressing); GPA score; number of brain metastases; and size of brain metastases. The statistically significant predictors of local recurrence were SRS (HR 0.5, 95% CI 0.3–1.0, p=0.041) and a metastasis size of ≤2.5 cm compared with >2.5–3.5 cm (HR 6.7, 95% CI 2.0–23, p=0.0021) and >3.5 cm (HR 6.6, 95% CI 1.9–23, p=0.0032)). The only significant predictor of overall survival was stable disease compared with progressive disease (HR 3.6, 95% CI 2.0–6.6, p<0.0001). The only significant predictor of DBM development was the presence at presentation of 1 brain metastasis at presentation compared with 3 brain metastases (HR 3.1, 95% CI 1.5–6.4, p=0.0016).
Table 2.
Multivariable analysis for 12-month local tumor-free recurrence rate
Multivariable analysis for local control rate
| Variable | Comparators | HR (95% CI) | P Value |
|---|---|---|---|
| Treatment | SRS vs. OBS | 0.5 (0.3, 1.0) | 0.041 |
| Primary cancer histologic type | Lung vs. breast | 0.9 (0.3, 2.6) | 0.82 |
| Melanoma vs. breast | 0.7 (0.3, 2.1) | 0.56 | |
| Other vs. breast | 1.2 (0.5, 2.6) | 0.73 | |
| Systemic disease status | Progressing vs. NED/PR | 0.6 (0.3, 1.2) | 0.15 |
| Stable vs. NED/PR | 0.8 (0.4, 1.6) | 0.45 | |
| GPA score | 2.5–3.0 vs. 1.0–2.0 | 1.3 (0.6, 2.5) | 0.52 |
| 3.5–4.0 vs. 1.0–2.0 | 1.1 (0.5, 2.6) | 0.82 | |
| Number of metastases | 2 vs. 1 | 0.8 (0.4,1.8) | 0.59 |
| 3 vs. 1 | 0.9 (0.3, 2.6) | 0.90 | |
| Size of brain metastases | >2.5–3.5 cm vs. 0–2.5 cm | 6.7 (2.0, 23) | 0.0021 |
| >3.5 vs. 0–2.5 | 6.6 (1.9, 23) | 0.0032 |
NED: no evidence of disease, PR: Partial Response
The pre-specified subsets for analysis included histologic subtype (melanoma versus non-melanoma), number of metastases, and size of metastases. In each of these, the test for treatment-covariate interaction showed no significant difference in local tumor-free recurrence rates between groups (Appendix pp20). Given the significant correlation between small tumors and local tumor-free recurrence, we performed a post-hoc analysis to determine if SRS after resection of smaller lesions (≤2.5 cm) still provided a benefit. The tumor-free recurrence rate was 77% in the OBS arm and 100% in the SRS arm (Appendix pp19).
No patients experienced adverse events related to placement of a stereotactic frame or treatment with SRS. One patient experienced a pulmonary embolism after surgery. The treatment for this embolism resulted in subsequent SRS occurring outside of the 30-day treatment window which made the patient ineligible for the study. There were no treatment related deaths.
DISCUSSION
This trial of patients undergoing surgical resection for one to three brain metastases showed that local tumor-free recurrence rates are significantly lower after post-operative radiosurgery is administered to the resection cavity. We also confirmed that surgical resection of brain metastases is insufficient to provide durable local control. In prior studies, the benefit of surgical resection followed by WBRT has been well described, for both improved survival and for increased local tumor control.2,3 However, WBRT is associated with negative side effects.4–6 Treating the surgical cavity postoperatively with SRS is an appealing strategy to limit the neurocognitive insult while improving local tumor control. Indeed, several retrospective studies have reported an effective local tumor-free recurrence rates1,9; however, the efficacy of post-operative SRS has not yet been validated with level 1 evidence. In this study, we found that SRS after brain metastasis resection significantly lowers local tumor-free recurrence rates compared with OBS alone. This supports administering radiosurgery to the resection cavity after resection for one to three brain metastases.
In the entire cohort, metastasis size was inversely associated with better local control. Notably, patients with tumors ≤2.5 cm in maximal diameter had a >90% local tumor-free recurrence rate. Local tumor-free recurrence rates dropped to 46% for tumors between >2.5 and 3.5 cm and to 43% for tumors >3.5 cm. This suggests that small tumors that have been resected could merely be observed; however, in post-hoc analysis, SRS to lesions <2.5 cm demonstrated an increased local tumor-free recurrence rate suggesting that treatment of even these smaller tumors may benefit from SRS after resection. Conversely, larger tumors had worse tumor-free recurrence rates. Given the absence of toxicity in the SRS cohort, increasing the prescribed radiation dose for larger tumors is reasonable and may lead to improved local control for these tumors. There may be an opportunity to improve local control by escalating dose by at least 2Gy per size category. An alternative to single-fraction SRS, as described in the present study, may be hypofractionated stereotactic radiotherapy (HSRT13,14). In retrospective series, HSRT has been shown to have favorable local control rates and can provide higher cumulative radiation doses (e.g. 30 Gy in 5 fractions) to the resection cavity than single fraction SRS. This strategy may provide a higher tumor-free recurrence rate by delivering a higher dose of radiation provided there is an acceptable toxicity profile.
For the secondary endpoint of overall survival, there was no difference between the OBS and SRS arms. The median overall survival time was 18 months in the OBS arm and 17 months in the SRS arm. The cause of death (neurologic progression versus systemic progression) between the two arms was inconclusive but suggests a higher incidence of neurological deaths in the OBS arm.
No difference was identified in the development of parenchymal DBM between the two study arms, which is not surprising given the local nature of the initial treatment. Neither did we find a difference in the incidence of LMD between the study arms, but this study was underpowered to determine the effect of post-surgical SRS on LMD development. In a recent study, LMD adjacent to the surgical cavity after radiosurgical treatment was reported to be as high as 16.9%, a potential risk of local radiation that should be evaluated in future studies15. A predictor of freedom from DBM was an initial presentation with only one brain metastasis, suggesting that patients with multiple metastases on presentation were at higher risk for DBM. WBRT and/or SRS were used for DBM: SRS for a limited number of additional metastases or WBRT for numerous metastases or LMD. The median time to WBRT administration was 16 months in the OBS group and 15 months in the SRS group. Interestingly, over half of the entire cohort was able to avoid WBRT altogether.
Despite our finding that local control is improved after SRS, OS was similar for both groups. Notably, our finding of a median OS of >17 months for both cohorts demonstrates a higher survival rate relative to other recent reports4,6. These studies compared SRS treatment alone with WBRT in patients with one to three brain metastases and demonstrated significant cognitive decline and worse overall survival in the WBRT cohort. Our higher survival rate may be because this study was performed at a tertiary cancer center and may also reflect improvements in systemic therapies. The ability to deliver timely systemic treatments that prolong the patient’s life may be facilitated by local adjuvant treatments (i.e. SRS) instead of WBRT. Although neurocognitive outcomes were not specifically addressed in our study, it can be inferred that delaying WBRT until absolutely necessary may help patients maintain a higher quality of life and receive effective multidisciplinary care. Future studies evaluating local treatments such as SRS should include outcomes such as quality of life. A recently completed Phase III study (NCT00377156 at clinicaltrials.gov) will address the value of WBRT compared to SRS to a surgical resection cavity. Although slightly different in design, as the study included incompletely resected metastases, and resection cavities without an upper size limit, the preliminary results of this study show, similar to our study that there is no survival benefit for WBRT compared to SRS after resection of 1–3 metastases. Further, they showed a poorer cognitive outcome associated with WBRT. Their preliminary conclusion (presented at the 2016 Annual meeting of the American Society for Therapeutic Radiation Oncology) is that SRS after surgical resection is superior to WBRT primarily owing to less toxicity.
Our study is subject to the biases of a single-institutional study. As a specialized cancer center, the patient population being drawn from may be eligible for specialized care and clinical trials not widely available. Also, these patients may have the resources to undergo increased surveillance clinical and imaging examinations. We note that our overall survival was several months longer than the survival reported in NCT00377156 (approximately 17 months in our study compared to approximately 11.5 months in their study). Although the study was performed at a single, high-volume, institution, the study took over six years to complete. During that time systemic treatments have evolved and can influence survival and possibly local control. We also used the same SRS unit for the entire study. The treating physicians (except the study neuroradiologist) were aware of which treatment arm each patient was on, potentially introducing some element of bias. On the other hand, over 15 different neurosurgeons and 9 different radiation oncologists treated the patients perhaps making the study more generalizable.
Surgical techniques have evolved since the original studies that demonstrated the utility of surgical resection in managing metastatic brain disease2,16. Stereotactic navigation and cortical mapping are used ubiquitously in surgically managing of brain metastases. In this study, we evaluated whether modern surgery, without SRS, was sufficient to provide satisfactory local tumor control. The 12-month local tumor-free recurrence rate was only 45% for the surgery-alone group, lower than the local tumor-free recurrence rate of 54% for surgery alone described by Patchell et al. in 1998.3 Despite improvements in surgical techniques and adjuncts our results confirm that surgery alone is insufficient to provide durable local control. Our lower local tumor-free recurrence rate compared to Patchell et al. may be due to our more frequent surveillance which was every 2 months post-surgery, versus every 3 months in their study. Ultimately, we suspect, as Patchell et al. did, that after a gross total resection, residual microscopic tumor can locally recur. Unlike other excisional procedures which can be extended to include negative tumor margins, the continued resection of surrounding normal brain parenchyma to achieve negative tumor margins is generally not feasible. Thus additional modalities are necessary to address microscopic tumor cells at the edge of the resection cavity. In a future report we intend to report the patterns of local failure which should further clarify the dosing and margins to be used for post-operative SRS to maximize local control. Here, we show that patients who have undergone resection of between 1–3 brain metastases will benefit from the administration of SRS to the resection cavity to decrease local recurrence while avoiding the toxicities associated with WBRT.
Supplementary Material
Page 14: Kaplan-Meier estimate of freedom from Whole brain radiation therapy by study arm (observation [OBS) and SRS)
Page 15: Diagram depicting the subsequent management of patients undergoing treatment for distant brain metastases.
Page 16: Kaplan-Meier estimate of local recurrence stratified by graded prognostic assessment (GPA) status
Page 17: Kaplan-Meier estimate of local recurrence stratified by primary cancer
Page 18: Kaplan-Meier estimate of local recurrence stratified by number of brain metastases
Page 19: Kaplan-Meier estimate of local recurrence according to study arm (observation [OBS] and SRS) for metastases sized (A) ≤2.5 cm, (B) 2.5–3.5 cm and (C) >3cm in maximal diameter.
Page 20: Variables influencing local control rate by study arm with treatment-covariate interaction.
Research in context.
Evidence before this study
Until now, there have been no completed randomized controlled trials demonstrating the efficacy of SRS to improve local control after surgical resection of brain metastases. WBRT after surgical resection has been the standard of care but is associated with cognitive deficits. Many clinicians have advocated the use of SRS after surgical resection to improve local control to avoid cognitive side effects of WBRT. Numerous retrospective studies have been reported but these are subject to various limitations. We searched PubMed for articles published prior to the writing of the manuscript (from 1/1/1980 to 12/31/2016) reporting on the use of radiation to improve local tumor control after surgical resection of brain metastases. Search terms included brain, local control, metastasis, neoplasm, radiation, surgery, survival and we limited the search to English language articles. We filtered for randomized controlled trials and identified 49 articles. We then limited our results to studies that specifically addressed the use of radiation to increase local tumor control after surgical resection of brain metastases. This search yielded 3 studies. All three studies evaluated the utility of whole brain radiotherapy (WBRT) in the context of surgical resection of brain metastases. No study evaluated the use of stereotactic radiosurgery (SRS) after surgical resection. Therefore, level 1 evidence supporting the use of SRS to improve local control after surgical resection of brain metastases is lacking. Further, the most recent study evaluating the use of radiation after surgical resection (albeit using WBRT) was in 1998. Since that time surgical techniques have evolved significantly and no recent studies have evaluated local control rates after surgical resection alone.
Added value of this study
The results of this prospective randomized trial add to the existing evidence for the management of brain metastases by demonstrating a significant improvement in local control when SRS is used after resection of 1–3 brain metastases compared to resection alone. The results also reinforce that surgical resection alone is insufficient to provide durable local control.
Implications of all the available evidence
This randomized controlled trial is, to our knowledge, the first to show a significant improvement in local control after surgical resection of 1–3 brain metastases when SRS is administered to the surgical cavity compared to surgical resection alone. Our results support a departure from the current practice of administering WBRT for patients after surgical resection of 1–3 brain metastases. Future trials should explore increased radiation doses to improve local control rates and report outcomes with respect to quality of life.
Acknowledgments
Funding: This work was supported by the Cancer Center Support Grant Award: P30CA016672
Footnotes
Author Contributions:
Data Collection: (A.M., M.F.M., J.L., P.B., S.S., S.M., E.S., A.G., J.S.W., S.S.P., F.F.L., N.L., I.E.M., S.Az., D.C., C.T., A.B.H., S.F., F.D., S.R., R.S., G.R.)
Data Analysis: (A.M., S.Ah., K.H., J.Y., G.R.)
Data Interpretation: (A.M., S.Ah., M.F.M., P.B., S.M., F.F.L., D.C., N.G-T., R.S., G.R.)
Writing: (A.M., K.H., G.R.)
The authors declared no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Owonikoko TK, Arbiser J, Zelnak A, et al. Current approaches to the treatment of metastatic brain tumours. Nat Rev Clin Oncol. 2014;11(4):203–22. doi: 10.1038/nrclinonc.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322(8):494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 3.Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. Jama. 1998;280(17):1485–9. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 4.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. The Lancet Oncology. 2009;10(11):1037–44. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 5.Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro-oncology. 2013;15(10):1429–37. doi: 10.1093/neuonc/not114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown PD, Jaeckle K, Ballman KV, et al. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. Jama. 2016;316(4):401–9. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McPherson CM, Suki D, Feiz-Erfan I, et al. Adjuvant whole-brain radiation therapy after surgical resection of single brain metastases. Neuro-oncology. 2010;12(7):711–9. doi: 10.1093/neuonc/noq005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Brown PD. The Diminishing Role of Whole-Brain Radiation Therapy in the Treatment of Brain Metastases. JAMA oncology. 2017 doi: 10.1001/jamaoncol.2016.5411. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed Z, Balagamwala E, Murphy E, et al. Postoperative stereotactic radiosurgery for resected brain metastasis. CNS Oncol. 2014;3(3):199–207. doi: 10.2217/cns.14.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419–25. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel AJ, Suki D, Hatiboglu MA, et al. Factors influencing the risk of local recurrence after resection of a single brain metastasis. J Neurosurg. 2010;113(2):181–9. doi: 10.3171/2009.11.JNS09659. [DOI] [PubMed] [Google Scholar]
- 12.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1. 1) European journal of cancer. 2009;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Al-Omair A, Soliman H, Xu W, et al. Hypofractionated stereotactic radiotherapy in five daily fractions for post-operative surgical cavities in brain metastases patients with and without prior whole brain radiation. Technology in cancer research & treatment. 2013;12(6):493–9. doi: 10.7785/tcrt.2012.500336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manning MA, Cardinale RM, Benedict SH, et al. Hypofractionated stereotactic radiotherapy as an alternative to radiosurgery for the treatment of patients with brain metastases. International journal of radiation oncology, biology, physics. 2000;47(3):603–8. doi: 10.1016/s0360-3016(00)00475-2. [DOI] [PubMed] [Google Scholar]
- 15.Johnson MD, Avkshtol V, Baschnagel AM, et al. Surgical Resection of Brain Metastases and the Risk of Leptomeningeal Recurrence in Patients Treated With Stereotactic Radiosurgery. International journal of radiation oncology, biology, physics. 2016;94(3):537–43. doi: 10.1016/j.ijrobp.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 16.Bindal RK, Sawaya R, Leavens ME, Lee JJ. Surgical treatment of multiple brain metastases. J Neurosurg. 1993;79(2):210–6. doi: 10.3171/jns.1993.79.2.0210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Page 14: Kaplan-Meier estimate of freedom from Whole brain radiation therapy by study arm (observation [OBS) and SRS)
Page 15: Diagram depicting the subsequent management of patients undergoing treatment for distant brain metastases.
Page 16: Kaplan-Meier estimate of local recurrence stratified by graded prognostic assessment (GPA) status
Page 17: Kaplan-Meier estimate of local recurrence stratified by primary cancer
Page 18: Kaplan-Meier estimate of local recurrence stratified by number of brain metastases
Page 19: Kaplan-Meier estimate of local recurrence according to study arm (observation [OBS] and SRS) for metastases sized (A) ≤2.5 cm, (B) 2.5–3.5 cm and (C) >3cm in maximal diameter.
Page 20: Variables influencing local control rate by study arm with treatment-covariate interaction.