Abstract
Podocalyxin (Podxl) is a CD34 orthologue and cell surface sialomucin with reported roles in renal podocyte diaphragm slit development, vascular cell integrity, and the progression of blood, breast, and prostate cancers. Roles for Podxl during non-malignant hematopoiesis, however, are largely undefined. Presently we have developed a Vav-Cre Podxl knockout mouse model, and report on novel roles for Podxl in governing stress myelopoiesis. At steady-state, Podxl expression among hematopoietic progenitor cells was low-level but was induced by GCSF (granulocyte colony stimulating factor) in myeloid progenitors, and by TPO (thrombopoietin) in HSCs. In keeping with low level Podxl expression at steady-state, Vav-Cre deletion of Podxl did not markedly alter peripheral blood cell levels. G-CSF challenge in Podxl-KO mice, in contrast, hyper-elevated peripheral blood neutrophil and monocyte levels. Podxl-KO also substantially heightened neutrophil levels following 5-fluorouracil myeloablation. These LOF phenotypes were selective, and Podxl-KO did not alter lymphocyte, basophil or eosinophil levels. Within bone marrow (and following G-CSF challenge), Podxl deletion moderately decreased CFU-GEMM and CD16/32posCD11bpos progenitors but did not affect Gr-1pos cell populations. Notably, Podxl-KO did significantly heighten peripheral blood neutrophil migration capacities. To interrogate Podxl’s action mechanisms, a co-immunoprecipitation plus LC-MS/MS (liquid chromatography – mass spectrometry) approach was applied using hematopoietic progenitors from G-CSF-challenged mice. Rap1a, a Ras-related small GTPase, was a predominant co-retrieved Podxl partner. In bone marrow HPC’s, Podxl-KO led to heightened GCSF activation of Rap1aGTP, and Rap1aGTP inhibition attenuated Podxl-KO neutrophil migration. Studies reveal novel roles for Podxl as an important modulator of neutrophil and monocyte formation, and of Rap1a activation, during stress hematopoiesis.
Keywords: Podocalyxin, stress myelopoiesis, G-CSF, 5-fluorouracil, Rap1a
INTRODUCTION
Podocalyxin (Podxl) is a transmembrane sialomucin, and an orthologue of CD34 and Endoglycan [1] [2]. Investigations of Podxl’s actions have predominantly focused on renal podocytes, in which Podxl is important for podocyte foot extension and glomerular diaphragm slit formation [3]. Podxl has also been implicated in supporting vascular integrity [4], and its co-expression with Flk1 distinguishes embryonic Flk1posPodxlneg endothelial precursor mesodermal cells from definitive Flk1posPodxlpos hematopoietic progenitors [5]. Podxl additionally can mark sub-populations of embryonic CD34pos hematopoietic stem cells [6], and Podxl/Pclp1 has been defined as functional marker of hematopoiesis within AGM regions [7]. Recent studies have further linked elevated Podxl expression with B-ALL and AML leukemogenesis [8] [9, 10] as well as invasive breast carcinoma [11], and tumor angiogenesis [12].
Roles for Podxl during non-malignant adult hematopoiesis are less well understood. Presently, we have investigated actions of Podxl during blood cell development by generating Podxlflox/flox and conditional Vav-Cre Podxl-KO mice. In keeping with observed low-level expression of Podxl at steady-state, Podxl-KO did not substantially alter peripheral blood cell levels. However, under conditions of 5-fluorouracil (5-FU) myeloablation or GCSF challenge, Podxl-KO proved to substantially and selectively up-regulate the formation of peripheral blood neutrophils and monocytes. Mechanistically (and as studied in primary hematopoietic progenitor cells) Podxl further proved to associate with Rap1a, to regulate Rap1aGTP formation, and to enhance the migration properties of peripheral blood neutrophils. Studies point to Podxl as a novel hematopoietic growth factor (HGF) target, and regulator of stress granulomyelopoiesis.
MATERIALS AND METHODS
For all approaches, method details are defined in Supplemental Text.
RESULTS AND DISCUSSION
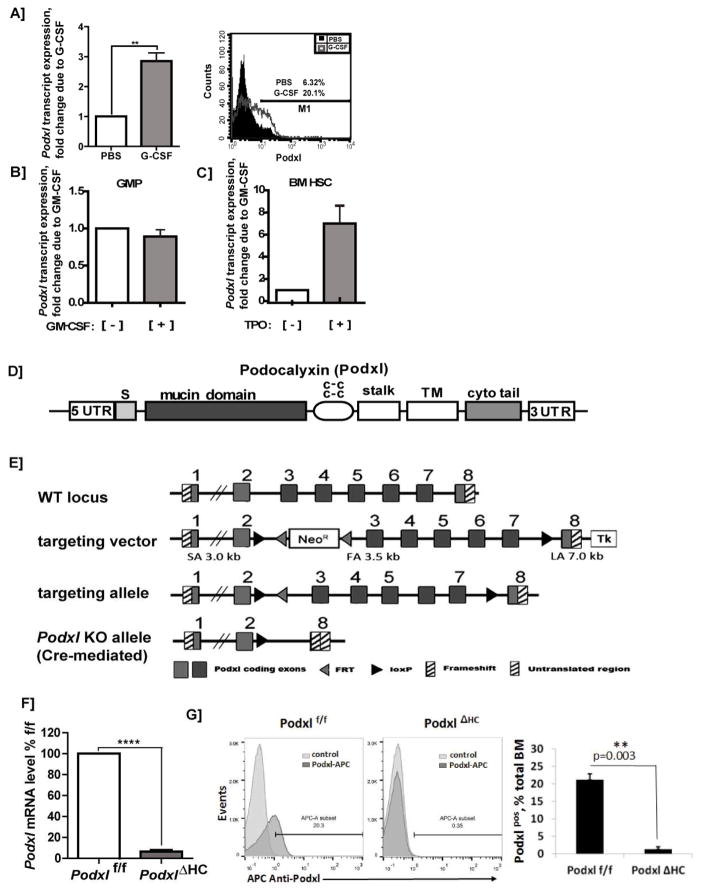
As a basic but informative analysis, Podxl transcript expression profiles among hematopoietic cells first were assessed. In silico analyses defined generally low level expression of Podxl at steady-state (as contrasted with CD34) (Fig-S1). Previously, we described the induced expression of Podxl by EPO in primary bone marrow erythroid progenitors [13, 14]. Here, we extended these studies to assess possible Podxl regulation by three additional hematopoietic growth factors (HGFs). Based additionally on indicated roles for Podxl in myeloid leukemia [9, 15], we first investigated possible G-CSF modulation of Podxl. G-CSF challenge in vivo significantly heightened Podxl transcript levels in isolated bone marrow myeloid HPCs (2.85 +/− 0.47-fold). Such increases in Podxl also were observed at the protein level via flow cytometry (Fig-1A). For GM-CSF, effects on Podxl expression were assessed using isolated bone marrow GMPs, with possible Tpo effects investigated in bone marrow HSCs. In GMP, Podxl levels were unchanged due to GM-CSF challenge (Fig-1B). In HSCs, however, Tpo induced Podxl 7.18 +/− 2.09-fold (Fig-1C). Select HGFs therefore can significantly heighten Podxl levels in HPCs.
Figure 1. Hematopoietic growth factor induction of Podxl, locus targeting, and hematopoietic deletion of Podxl.
A–C] GCSF, and TPO induction of Podxl expression: A] Up-modulation of Podxl transcript expression in bone marrow granulomyeloid progenitors in vivo: Wild-type C57Bl/6 mice were administered G-CSF (125μg/kg/IP) or PBS. On d6, granulomyeloid progenitor populations were isolated from bone marrow via the retrieval of CD11b, Gr-1, Ly6G positive cells. These hematopoietic progenitor cells (HPCs) were then analyzed for Podxl expression by RT-PCR (left sub-panel)(mean values +/− SE, n=3), and by flow cytometry (right sub-panel). B] Possible effects of GM-CSF on Podxl expression were assessed in isolated bone marrow GM progenitors (GMPs) as lineage negative, CD117+, Sca-1−, CD34+, CD16/32+ cells. These HPCs were challenged ex vivo [+/− GMCSF (granulocyte macrophage colony stimulating factor)], and isolated total RNA was used to determine Podxl levels. C] In HSC, possible effects of TPO on Podxl expression were assessed. HPCs were isolated from bone marrow as Linneg cells (using biotinylated antibodies to CD5, CD11b, CD19, CD45R, Ly6G/C, Ter119). Linneg cells were then labeled with fluorescent antibodies to c-Kit and Sca1, and HSCs were purified via FACS. HSCs were then challenged [+] vs [−] TPO. Total RNA was purified, and used to determine Podxl expression levels. D] Podxl’s structural subdomains are diagrammed including its signal peptide (S), mucin domain, cysteine-rich region (c-c), stalk plus transmembrane (TM) domains, and cytoplasmic tail (cyto tail). E] Targeting (floxing) of the Podxl gene: Details are diagrammed for exon floxing, and Cre-mediated deletion. F] RT-qPCR analysis of Podxl transcripts in hematopoietic progenitor cells from wild-type and PodxlΔHC bone marrow: Hematopoietic progenitor cells (HPCs) were prepared as Linneg populations. Podxl levels were then determined as normalized to beta-Actin (means +/− SE, n=3). G] Representative flow cytometric analysis of Podxl expression in bone marrow cell preparations from wild-type and PodxlΔHC-KO mice (left panel). In the right panel, results for triplicate samples are illustrated (mean % positive +/− SE).
To investigate functional roles for Podxl in regulating hematopoietic cell formation, we generated Podxl+/flox ES cells, and Podxlf/f mice. Figs-1D,E outline Podxl’s protein and gene structures, together with the constructs used to flox (and delete) Podxl gene exons 3–7. Podxl+/flox mice were generated via ES cell blastocyst fusion, and mice with high chimerism were interbred to yield Podxl flox/flox mice. Crosses with Vav-Cre mice were then performed to generate conditionally deleted PodxlΔHC (hematopoietic cell deleted) mice. Flow cytometry analysis using bone marrow cells from wild type vs knockout mice further demonstrated a clear loss of signal due to Podxl-KO (Fig-1G). Podxl protein levels additionally were assessed by western blotting (Supplemental Figure S2).
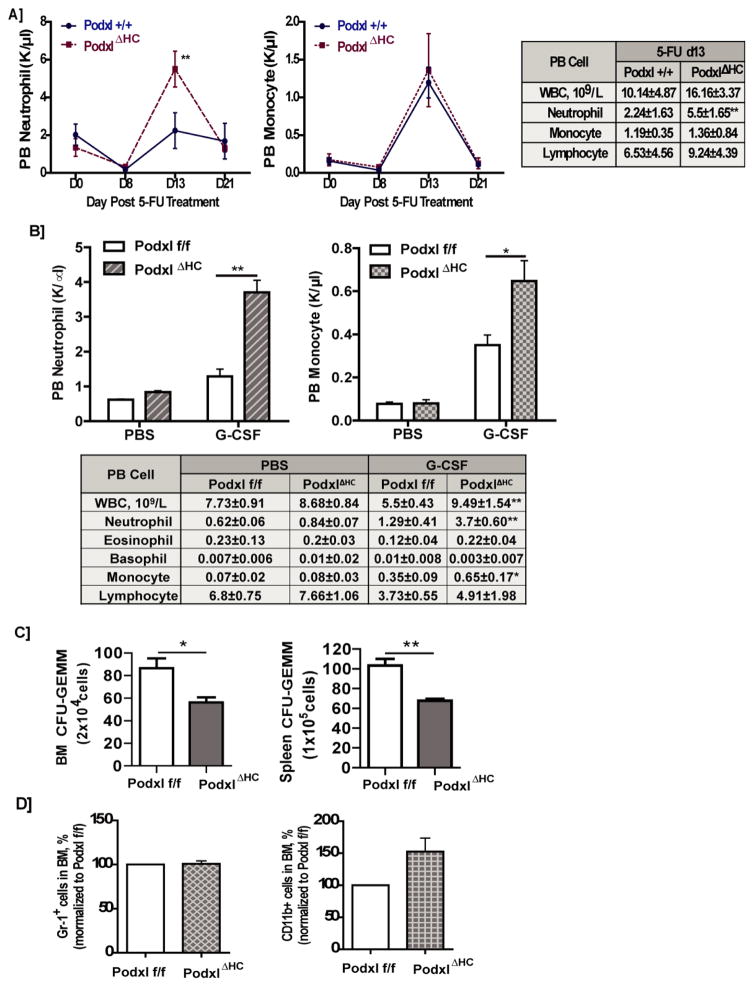
At steady-state, Podxl’s hematopoietic deletion (PodxlΔHC mice) did not significantly alter peripheral blood cell levels (with the exception of a limited ~2.8% increase in hematocrits) (Supplemental Table-1). Following 5-FU challenge, however, neutrophil levels in PodxlΔHC mice were heightened up to 2.5-fold over wild-type congenic control mice (Fig-2A). When PodxlΔHC mice were challenged with G-CSF, peripheral blood neutrophil levels similarly were hyper-elevated, with significant increases in monocyte production also observed (Fig-2B). These effects were selective, with no such effects of Podxl-KO observed for other blood cell types. When bone marrow progenitors were analyzed (following G-CSF challenge), CFU-GEMM progenitor levels were moderately decreased (Fig-2C). Podxl-KO therefore may compromise levels of select myeloid progenitor pools. For bone marrow CD11b cells and Gr-1pos HPCs, however, levels were not significantly affected by Podxl-KO (absolute numbers for CD11b: wt=117250±8504/mL, Podxl-KO=137583±4666/mL; absolute numbers for Gr1: wt=298083±927/mL, Podxl-KO=301066±1760/mL) (Fig-2D).
Figure 2. Governing of stress myelopoiesis by Podxl.
A] Podxl+/+ and PodxlΔHC mice were administered 5-FU (150 mg/kg). On the days indicated, peripheral blood cell populations were assayed. Left and center panels report on numbers of peripheral blood (PB) neutrophils and monocytes among Podxl+/+ and PodxlΔHC mice (means +/− SE, n=4). The right panel reports on total white blood cell (WBC), neutrophil, monocyte and lymphocyte counts in PB from Podxl+/+ and PodxlΔHC mice at d13 post 5-FU dosing (means +/− SE, n=4). B] Podxl deletion dysregulates G-CSF induced neutrophil and monocyte formation: Podxlf/f and PodxlΔHC mice (n=4) were dosed on d1–5 with G-CSF (125μg/kg) or PBS. Levels of peripheral blood cells then were determined. Upper panels define neutrophil and monocyte numbers at d6 (means +/− SE). In the lower panel, overall levels of peripheral blood neutrophils, eosinophils, basophils, monocytes and lymphocytes are reported. C] Following G-CSF administration (125μg/kg, d1–5), bone marrow and splenic cells were prepared from Podxlf/f and PodxlΔHC mice. HPCs were then prepared as lineage negative bone marrow populations (depleted using antibodies to CD5, CD11b, CD19, CD45R, Ly6G/C and Ter119) and were used in CFU assays (means +/− SE< n=3). Findings for CFU-GEMM are illustrated. For CFU-GM no significant effects of Podxl-KO were observed (data not shown) D] For granulomyeloid progenitors prepared from Podxlf/f or PodxlΔHC mouse bone marrow (at steady-state) populations of FITC-anti-Gr-1 or FITC-anti-CD11b positive HPCs were assayed by flow cytometry. Here, granulomyeloid progenitors were prepared as CD11b, Gr-1, Ly6G positive populations. Data are mean frequencies of positive cells (means +/− SE, n=3).
To begin to understand how peripheral blood neutrophil levels become elevated due to Podxl-KO, we further analyzed bone marrow cells from GCSF- dosed wt vs. KO mice using three markers: CD16 (early myeloid/monocytic progenitor marker), CD11b/Mac1 (myeloid/granulocytic progenitor marker), and Ly6G (granulocytic progenitor/granulocyte marker). In Podxl-KO bone marrow, a modest decrease in CD16posCD11bpos progenitors was observed (Supplemental Figure S3A, B), and may relate to modest decreases observed for CFU-GEMM (Fig-2C). Otherwise, Podxl-KO effects on these progenitors were limited (i.e., not significant). These findings tend to discount hyper-expansion of pro-neutrophil progenitors within bone marrow as a possible explanation for elevated production of neutrophils due to Podxl-KO.
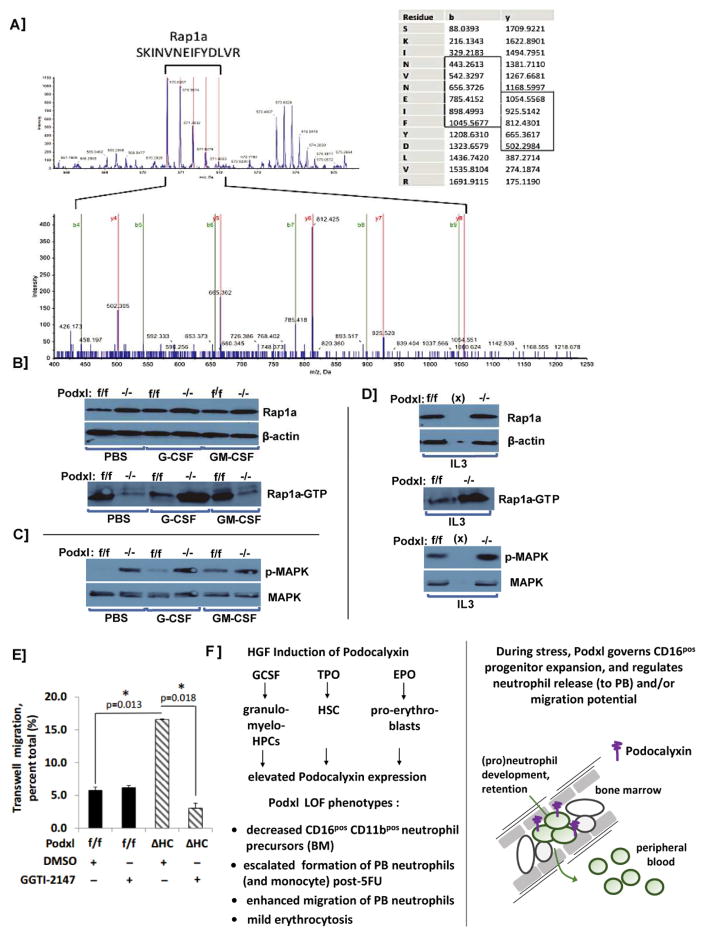
To initially seek candidate molecular mechanisms associated with Podxl’s discovered effects on stress myelopoiesis, a co-immunoprecipitation plus LC-MS/MS approach was employed. Specifically, wild-type C57BL/6 mice were dosed with G-CSF (125μg/kg, d1–d5). Bone marrow HPCs were then isolated (d6), and cell lysates were prepared. Podxl together with associated partner proteins were then immunoprecipitated, reduced and alkylated. Tryptic peptides were generated and analyzed by LC-MS/MS, essentially as previously described [16]. One prime Podxl co-immunoprecipitated protein was the Ras family small GTPase, Rap1a (Fig-3A). Follow-up western blot experiments revealed interesting effects of Podxl deletion on activated Rap1aGTP. As analyzed in primary bone marrow HPCs, levels of total Rap1a were unaffected by Podxl-KO (Figs 3B–D). In the absence of G-CSF challenge, Podxl-KO led to modestly lowered baseline levels of activated Rap1aGTP. Upon G-CSF (but not GM-CSF) challenge, levels of activated Rap1aGTP became markedly elevated (Figs-3B,D). Analyses of activated p-MAPK indicated only limited consequences of Podxl-KO on this candidate target of Rap1aGTP (Figs-3C,D). Results similar to those observed for G-CSF also were exhibited when HPCs were challenged with IL3 (Fig-3D). To additionally assess possible pre-association of Podxl with Rap1a in the absence of GCSF challenge, co-immunoprecipitation experiments were performed using lineage negative bone marrow HPCs from wild-type mice, and detectable co-IP of Rap1a with Podxl was observed (AK and PS, data not shown). This latter observation may help to explain how, in the absence of GCSF challenge, Podxl-KO resets baseline activation of Rap1a-GTP. Podxl therefore may be prepositioned to regulate Rap1a.
Figure 3. Podxl interacts with Rap-1A in HPCs, and regulates Rap1aGTP levels.
A] Following G-CSF dosing of wild-type mice, hematopoietic progenitor cells (HPCs) were isolated from bone marrow cell preparations as Linneg populations (depleted for CD5, CD11b, CD19, CD45R, Ly6G/C and Ter119). From cell lysates, Podxl (co-)immunoprecipitates were then prepared, reduced, alkylated and used to generate tryptic peptides. Peptides were analyzed by LC-MS/MS. For one predominant Podxl partner, Rap1a, representative time of flight mass spectra (MS) are shown (e.g., SKINVNEIFYDLVR). The left panel identifies precursor peptide ion mass 1708.9148, z=3 and delta mass= −0.0001 (99% confidence). The right panel summarizes collision-induced decay fragment spectra for sequence determination of SKINVNEIFYDLVR. B] Podxl regulates levels of activated Rap1a-GTP: Hematopoietic progenitor cells were prepared from the bone marrow of Podxlf/f and PodxlΔHC mice, and plated in IMDM (1x106cells/mL). Here, Linneg bone marrow HPCs from wild type and Podxl-KO mice were used. Cells were then exposed to PBS, G-CSF, or GM-CSF. At 30 minutes, lysates were generated and analyzed by western blotting for total Rap-1A levels, and levels of GTP-bound Rap-1A (lower panel). C] For the above samples (4B), levels of p-MAPK and total MAPK also were assessed. D] Podxl-KO dysregulates IL3- induced Rap1aGTP formation: HPCs isolated from Podxl+/+ and PodxlΔHC mouse bone marrow. Following HGF withdrawal, cells were stimulated with IL3 (10ng/mL, 30 minutes). Cell lysates were then prepared, and levels of Rap1a and Rap1aGTP were determined (as in 4B above). E] Podxl deletion enhances neutrophil migration: Peripheral blood neutrophils were prepared via density gradient centrifugation using Histopaque-1077 and Histopaque-1119. Trans-well migration of peripheral blood neutrophils from wild type and Podxl-KO mice was then assessed +/− the Rap1a inhibitor GGTI-2147. Graphed results are mean values +/− SE (n=3) for trans-membrane migrating cells. F] Initial model for Podxl governing of stress myelopoiesis: Left panel: HGF effects on Podxl expression are outlined (upper sub-panel), together with Vav1-conditional Podxl-KO phenotypes. Right panel: A working model is outlined at a cellular level for proposed Podocalyxin governing of peripheral blood neutrophil (e)migrations.
In a final set of experiments, migration properties of Podxl-KO peripheral blood neutrophils were analyzed. Migration rates (as assayed using trans-well chambers) interestingly were significantly enhanced over Podxl+/+ neutrophils (Fig-3E). To initially assess roles for Rap1a in Podxl-KO potentiated neutrophil migration, we utilized a specific Rap1a inhibitor, GGTI-2147. GGTI-2147 treatment attenuated Podxl KO induced neutrophil migration ≥3 fold (Fig-3E). It is notable, however GGTI-2147 did not significantly affect the migration of Podxl wild-type peripheral blood neutrophils. Heightened sensitivity of Podxl-KO neutrophils to GGTI-2147 therefore may relate to decreased levels of Rap1aGTP observed in bone marrow granulomonocytes (see Fig-3B). In Figure 3G, working models are outlined for HGF regulation of Podxl, and for Podxl governing of neutrophil migration from bone marrow and/or (e)migration potential.
In summary of the present findings, using a floxed allele plus Vav1-Cre approach, we have generated a blood cell conditional knockout mouse model to investigate Podocalyxin’s roles during adult hematopoiesis (and prospectively, leukemogenesis). In keeping with low Podxl expression among HPCs at steady-state (see Fig-S1), Podxl-KO did not significantly alter peripheral blood populations (with the exception of mild polycythemia) (see Supplemental Table-S1). G-CSF dosing, however, proved to induce Podxl expression in bone marrow myeloid progenitors, and this focused our investigations on stress hematopoiesis. Following G-CSF or 5-FU treatment, Podxl-KO substantially heightened peripheral blood neutrophil levels, with G-CSF additionally boosting Podxl-KO monocyte levels. In relating these phenotypes to bone marrow HPC pools, CFU-GEMM were diminished compared to Podxlf/f controls as were bone marrow CD16/32posCD11bpos granulomyeloid progenitors. Gr1pos cell levels, however, were not significantly affected. Podxl-KO effects on heightened blood granulocytes (and monocytes) therefore likely involve mechanisms beyond a simple over-production of myeloid progenitors. Our initial data further indicate roles for Podxl in neutrophil migration (Fig-3E). Notably, as a complex cell surface sialomucin, Podxl has been demonstrated to interact with select integrins and selectins [1, 2] and via intracellular domains can also interact with Ezrin and actin as cytoskeletal components [1, 2].
Co-immunoprecipitation LC-MS/MS experiments provided initial molecular insight into Podxl’s effects on granulomyelocytic cells, with Rap1a identified as a novel Podxl partner. One major effect of Podxl-KO was to increase Rap1aGTP levels in G-CSF challenged hematopoietic progenitors. As Ras-related GTPases, Raps are tightly regulated by select GEFs and GAPs, and by extracellular cues [17]. Their engagement in diverse tissues and cell types in addition can modulate cell migration, and adhesion [18]. This is consistent with our observed effects of Podxl-KO on enhancing neutrophil migration (see Fig-3E).
Taken together, the present investigations have uncovered novel roles for Podxl in governing stress myelopoiesis. We have also begun to define correlations between Podxl deletion, Rap1aGTP dysregulation, and heightened neutrophil migration due to Podxl-KO. This points to a Podxl plus Rap1a circuit that may regulate peripheral blood neutrophil levels during stress hematopoiesis (Fig-3F). Consistent with our findings, the knockout of the positive Rap1aGTP regulator, Radil, recently has been reported to compromise neutrophil chemotaxis [19]. Rap1 additionally has been characterized as a mediator of TLR4 effects on neutrophil beta-2 Integrin activation and leukocyte rolling [20]. To further understand Podxl plus Rap1 connections and actions during myelopoiesis, future studies involving the manipulation of GEF, GAP and Rap1 factors will be required. Such investigations may extend to hematopoietic malignancies, and dysregulation of Podxl, and of Rap1, recently has been associated with T-cell acute lymphoblastic leukemia [21].
HIGHLIGHTS.
Podxl is induced in hematopoietic progenitors by GCSF and TPO
Podxl-KO increases PB granulocyte and monocyte levels following GCSF or 5FU dosing
Podxl-KO neutrophils exhibit heightened migration capacities
Rap1a is a Podxl partner that modulates Podxl’s effects on neutrophil migration
Acknowledgments
Support for investigations includes a Hyundai Hope on Wheels Schlolar Award grant to PS; National Natural Sciences Foundation of China (No.81270637) to KX; and NIH R01 DK089439 to DMW. Additional support was provided by MMCRI core facilities in Progenitor Cell Analysis, Physiology, and Molecular Phenotyping as supported by NIH/NIGMS P30 GM106391 (DM Wojchowski, PI) and by the Protein, Nucleic Acid and Cell Imaging core (supported by NIH/NIGMS P30GM103392, R Friesel, PI). The authors also thank Dr. L Hennighausen and Dr. Daisuke Yamaji for their generous provision of gene profiling data for GMCSF-challenged GMP, and for TPO- challenged HSCs.
Footnotes
Disclaimers: None
Authorship contributions
All authors contributed in substantial ways to experimental designs, study execution, data acquisition plus analysis, and manuscript construction.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Furness SG, McNagny K. Beyond mere markers: functions for CD34 family of sialomucins in hematopoiesis. Immunologic research. 2006;34:13–32. doi: 10.1385/IR:34:1:13. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen JS, McNagny KM. Novel functions of the CD34 family. Journal of cell science. 2008;121:3683–3692. doi: 10.1242/jcs.037507. [DOI] [PubMed] [Google Scholar]
- 3.Ichimura K, Powell R, Nakamura T, Kurihara H, Sakai T, Obara T. Podocalyxin regulates pronephric glomerular development in zebrafish. Physiol Rep. 2013:1. doi: 10.1002/phy2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Debruin EJ, Hughes MR, Sina C, et al. Podocalyxin regulates murine lung vascular permeability by altering endothelial cell adhesion. PloS one. 2014;9:e108881. doi: 10.1371/journal.pone.0108881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H, Nieves JL, Fraser ST, et al. Expression of podocalyxin separates the hematopoietic and vascular potentials of mouse embryonic stem cell-derived mesoderm. Stem cells. 2014;32:191–203. doi: 10.1002/stem.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doyonnas R, Nielsen JS, Chelliah S, et al. Podocalyxin is a CD34-related marker of murine hematopoietic stem cells and embryonic erythroid cells. Blood. 2005;105:4170–4178. doi: 10.1182/blood-2004-10-4077. [DOI] [PubMed] [Google Scholar]
- 7.Minehata K, Mukouyama YS, Sekiguchi T, Hara T, Miyajima A. Macrophage colony stimulating factor modulates the development of hematopoiesis by stimulating the differentiation of endothelial cells in the AGM region. Blood. 2002;99:2360–2368. doi: 10.1182/blood.v99.7.2360. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen JS, McNagny KM. The role of podocalyxin in health and disease. Journal of the American Society of Nephrology: JASN. 2009;20:1669–1676. doi: 10.1681/ASN.2008070782. [DOI] [PubMed] [Google Scholar]
- 9.Favreau AJ, Cross EL, Sathyanarayana P. miR-199b-5p directly targets PODXL and DDR1 and decreased levels of miR-199b-5p correlate with elevated expressions of PODXL and DDR1 in acute myeloid leukemia. American journal of hematology. 2012;87:442–446. doi: 10.1002/ajh.23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riccioni R, Calzolari A, Biffoni M, et al. Podocalyxin is expressed in normal and leukemic monocytes. Blood cells, molecules & diseases. 2006;37:218–225. doi: 10.1016/j.bcmd.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Forse CL, Yilmaz YE, Pinnaduwage D, et al. Elevated expression of podocalyxin is associated with lymphatic invasion, basal-like phenotype, and clinical outcome in axillary lymph node-negative breast cancer. Breast cancer research and treatment. 2013;137:709–719. doi: 10.1007/s10549-012-2392-y. [DOI] [PubMed] [Google Scholar]
- 12.Amo L, Tamayo-Orbegozo E, Maruri N, et al. Involvement of platelet-tumor cell interaction in immune evasion. Potential role of podocalyxin-like protein 1. Frontiers in oncology. 2014;4:245. doi: 10.3389/fonc.2014.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sathyanarayana P, Menon MP, Bogacheva O, et al. Erythropoietin modulation of podocalyxin and a proposed erythroblast niche. Blood. 2007;110:509–518. doi: 10.1182/blood-2006-11-056465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wojchowski DM, Sathyanarayana P, Dev A. Erythropoietin receptor response circuits. Current opinion in hematology. 2010;17:169–176. doi: 10.1097/MOH.0b013e328338008b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Favreau AJ, McGlauflin RE, Duarte CW, Sathyanarayana P. miR-199b, a novel tumor suppressor miRNA in acute myeloid leukemia with prognostic implications. Exp Hematol Oncol. 2015;5:4. doi: 10.1186/s40164-016-0033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirov A, Kacer D, Conley BA, Vary CP, Prudovsky I. AHNAK2 Participates in the Stress-Induced Nonclassical FGF1 Secretion Pathway. J Cell Biochem. 116:1522–1531. doi: 10.1002/jcb.25047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gloerich M, Bos JL. Regulating Rap small G-proteins in time and space. Trends Cell Biol. 2011;21:615–623. doi: 10.1016/j.tcb.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Minato N. Rap G protein signal in normal and disordered lymphohematopoiesis. Experimental cell research. 2013;319:2323–2328. doi: 10.1016/j.yexcr.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Liu L, Aerbajinai W, Ahmed SM, Rodgers GP, Angers S, Parent CA. Radil controls neutrophil adhesion and motility through beta2-integrin activation. Mol Biol Cell. 2012;23:4751–4765. doi: 10.1091/mbc.E12-05-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pruenster M, Kurz AR, Chung KJ, et al. Extracellular MRP8/14 is a regulator of beta2 integrin-dependent neutrophil slow rolling and adhesion. Nature communications. 2015;6:6915. doi: 10.1038/ncomms7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doi K, Imai T, Kressler C, et al. Crucial role of the Rap G protein signal in Notch activation and leukemogenicity of T-cell acute lymphoblastic leukemia. Scientific reports. 2015;5:7978. doi: 10.1038/srep07978. [DOI] [PMC free article] [PubMed] [Google Scholar]