Abstract
The success of nanomedicines in the clinic depends on our comprehensive understanding of nano–bio interactions in tumor microenvironments, which are characterized by dense leaky microvasculature and acidic extracellular pH (pHe) values. Herein, we investigated the accumulation of ultrasmall renal-clearable gold NPs (AuNPs) with and without acidity targeting in xenograft mouse models of two prostate cancer types, PC-3 and LNCaP, with distinct microenvironments. Our results show that both sets of AuNPs could easily penetrate into the tumors but their uptake and retention were mainly dictated by the tumor microvasculature and the enhanced permeability and retention effect over the entire targeting process. On the other hand, increased tumor acidity indeed enhanced the uptake of AuNPs with acidity targeting, but only for a limited period of time. By making use of simple surface chemistry, these two effects can be synchronized in time for high tumor targeting, opening new possibilities to further improve the targeting efficiencies of nanomedicines.
Keywords: microvascular density, nanoparticles, renal clearance, tumor acidity, tumor targeting
The selective accumulation of nanomedicines in tumors over normal tissues and organs is key to minimizing their potential toxicity and expediting their clinical translation.[1] Distinct from normal tissues, solid tumors often exhibit unique microenvironments, such as dense, leaky microvasculature and acidic extracellular pH (pHe) values.[2] These two general features have been major targets for enhancing the delivery of nanomedicines.[2b] For example, by taking advantage of the dense, leaky tumor microvasculature and impaired lymphatic drainage, nanoparticles (NPs) can be selectively accumulated in cancerous tissues over normal tissues at high concentrations for much longer periods of time through the enhanced permeability and retention (EPR) effect.[3] By making use of the acidic tumor microenvironment (pHe 6.5–7.2), the tumor accumulation of NPs can be further enhanced.[4]
While selective accumulation in tumors has indeed been achieved with many inorganic NPs, they are often also severely accumulated in major organs such as the liver and spleen.[5] To address this long-standing roadblock to the clinical translation of non-degradable nanomedicines, renal-clearable inorganic NPs have started to emerge.[3a] For instance, Choi and co-workers developed renal-clearable, approximately 3 nm large cysteine-coated quantum dots that can escape reticuloendothelial system (RES) uptake and be rapidly excreted through the urinary system.[6] Bradbury and co-workers synthesized renal-clearable silica NPs (“C” dots) and demonstrated their application in cancer detection.[7] We also created a class of luminescent gold NPs (AuNPs) with a core size of 2–3 nm and high resistance to serum protein adsorption.[8] After intravenous injection, they are not only eliminated through the urinary system[8a,b] and report on the kidney function[9] but can also target a variety of tumor models through the EPR effect.[10] Over the past few years, many other renal-clearable nanostructures have also been developed.[11] More importantly, a recent clinical trial with “C” dots has clearly demonstrated the great promise of these renal-clearable NPs in cancer diagnosis in the clinic.[7a]
While these renal-clearable NPs successfully addressed a long-standing issue in the clinical translation of NPs, it remains very challenging to further enhance their targeting efficiencies, which requires fundamental understanding of their interactions with the tumor microenvironment.[3a] As these renal-clearable NPs (with hydrodynamic diameters < 6 nm) are much smaller than the pore sizes of most solid tumor vessels (300–1200 nm),[12] several fundamental questions naturally emerge. 1) Different types of solid tumors exhibit distinct microvasculature (e.g., different microvascular densities (MVDs), different vascular sizes, and different vascular endothelial areas),[13] but whether these differences will affect the tumor permeability of these ultrasmall NPs is unknown. 2) It has never been investigated how the MVD affects the retention of such ultrasmall NPs in tumors. 3) It has seldom been investigated how acidic tumor microenvironments will quantitatively impact the retention of renal-clearable NPs. Exploring these fundamental issues regarding the tumor targeting and retention of renal-clearable NPs will help advance our understanding of the nano–bio interactions in vivo in native tumor microenvironments.[1,14]
To answer these fundamental questions, we systematically studied the accumulation of two types of ultrasmall renal clearable AuNPs with and without acidity targeting in two prostate cancer models, namely PC-3 (pHe 6.9, high MVD, small vascular endothelial area) and LNCaP tumors (pHe 6.5, low MVD, large vascular endothelial area),[15] 1, 24, and 72 h after intravenous injection. We found that the tumor permeability of one set of AuNPs was independent of the tumor type or the tumor vasculature but the same type of tumor can exhibit distinct permeability to AuNPs with and without acidity targeting. In addition, the retention of ultrasmall AuNPs in the tumor is mainly governed by the tumor microvasculature: A high AuNP targeting efficiency was observed for the LNCaP tumors with a large endothelial area and low MVD. Moreover, the tumor acidity indeed significantly enhanced the accumulation of AuNPs with acidity targeting but this enhancement was temporary and only observed 24 h post injection (p.i.). At 72 h p.i., their tumor retention was mainly governed by the tumor vasculature.
Renal-clearable, 600 nm emitting, glutathione-coated AuNPs (GS-AuNPs) with a hydrodynamic diameter (HD) of 2.1 ± 0.4 nm were synthesized according to our reported approach.[8a,16] These GS-AuNPs do not interact with cell membranes at pH values from 7.4 to 5.5.[17] To obtain renal-clearable luminescent AuNPs with acidity targeting, we modified the surface of the luminescent GS-AuNPs by introducing a secondary ligand, namely cysteamine, in the presence of glutathione during the growth of the AuNPs (see the Supporting Information).[17] The core size of the AuNPs coated with glutathione and cysteamine (GC-AuNPs) was 2.3 ± 0.4 nm, and their hydrodynamic diameter was 2.9 ± 0.3 nm (Figure 1A). The obtained GC-AuNPs not only remained highly luminescent (Figure 1B) but also showed pH-dependent adsorption onto live cell membranes, as revealed by fluorescence microscopy imaging of NP–cell interactions at different pH values (Figure 1C,D). At pH 7.4, the GC-AuNPs were highly negatively charged (zeta potential: −22.1 ± 3.6 mV) and thus had little affinity to negatively charged cell membranes. As the pH value was decreased to 6.5, membrane adsorption became evident, leading to a 26.3-fold enhancement in the fluorescence intensity of the cell membrane because of cysteamine protonation (Figure 1C,D). As both the GS-AuNPs and GC-AuNPs are zwitterionic and highly resistant to serum protein adsorption at neutral and mildly acidic pH (see the Supporting Information, Figure S1), they retained their original sizes, which are smaller than the kidney filtration threshold (6–8 nm) during blood circulation, and 40–50% of the injected GC-AuNPs had been excreted into the urine 24 h p.i., which is comparable to the renal-clearance efficiency of GS-AuNPs (Figure S2).
Figure 1.
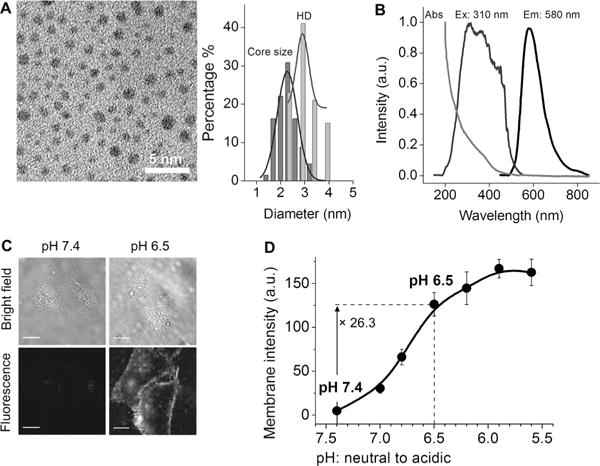
Renal-clearable AuNPs coated with both glutathione and cysteamine (GC-AuNPs) were designed for targeting tumor acidity. A) TEM image (scale bar: 5 nm) and core size and hydrodynamic diameter (HD) distributions of the GC-AuNPs. Core size: 2.3 ± 0.4 nm; HD: 2.9 ± 0.3 nm. B) Absorption, excitation, and emission spectra of GC-AuNPs in aqueous solution. C) Bright-field and fluorescence images of live HeLa cells incubated with GC-AuNPs at pH 7.4 and 6.5 in PBS at 25°C for 10 min (scale bar: 20 μm). D) Luminescence intensity of cell membranes incubated with GC-AuNPs at different pH values in PBS.
Xenograft mouse models of two well-known prostate cancers, PC-3 and LNCaP, were selected for our tumor-targeting study because they exhibit distinct features in terms of both their microvascular structure and tumor acidity.[15] To quantify the tumor vascular densities, we stained tumor blood vessels with CD31 antibodies to visualize the blood vessels. Our results show that PC-3 had a higher vascular density than LNCaP in both the tumor periphery and the tumor center (Figure 2A; see also Figures S3 and S4 and Table S1), but that LNCaP had dilated blood vessels with diameters of about 35.5 μm (in both the periphery and center), which are thus about three times wider than those in PC-3 (diameters of about 13.3 μm in the tumor periphery and 10.9 μm in the tumor center; Figure 2B and Table S2). These results are consistent with previous reports.[13b,15] On the other hand, the CD31 expression of LNCaP tumors was 42% higher than that of PC-3 tumors (Figure 2C), indicating that the total area of the tumor microvascular endothelium was still larger in LNCaP than in PC-3. In addition to these differences in vasculature, the two tumor models also exhibited distinct acidic microenvironments. By using in vivo magnetic resonance spectroscopy, we were able to measure the local pHe values based on the pH-dependent 31P shifts of 3-aminopropylphosphonate (3-APP) in tumor and normal tissues.[18] The pHe of LNCaP was determined to be 6.52 ± 0.30, which is thus more acidic than PC-3 (pHe 6.94 ± 0.28; Figures 2D and S5 and Table S3). These values are consistent with previous reports that LNCaP (pHe 6.78 ± 0.29) is more acidic than PC-3 (pHe 7.23 ± 0.10). [19] Using PC-3 and LNCaP model systems with these distinct features in terms of their tumor vasculature and acidity, we further investigated the accumulation of two AuNPs, GS-AuNPs and GC-AuNPs, at different time points p.i.
Figure 2.
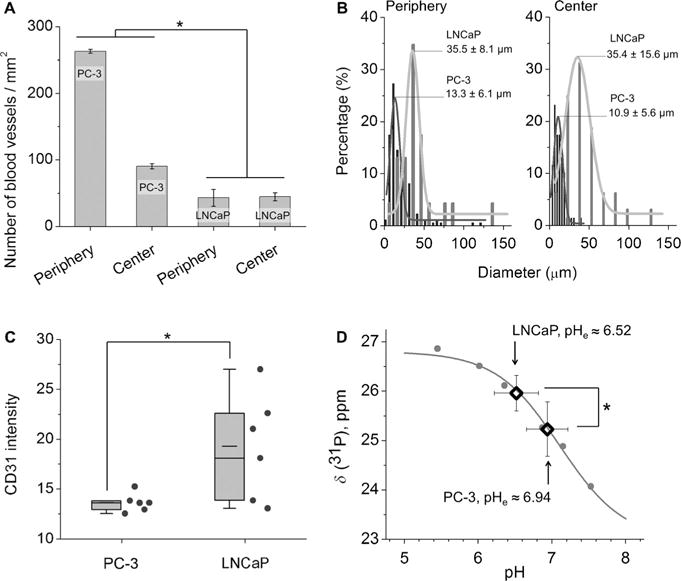
The two prostate cancer models PC-3 and LNCaP differ in both tumor vasculature and acidity. A) Densities and B) diameters of intratumoral vasculatures in the tumor periphery and tumor center quantified by analyzing CD31-antibody-stained tumor sections. C) Intensity of the CD31 expression measured in six fields that were selected from the tumor periphery and center. D) Extracellular pH (pHe) values of tumors determined by 31P magnetic resonance spectroscopy (MRS). The standard 31P MRS titration curve is shown in gray. N=4 for PC-3, N=8 for LNCaP. *P < 0.05.
Entering the tumor microenvironment is the first step for NPs to target tumors. Unraveling the effect of the microvasculature on the permeability of the NPs is thus key to a comprehensive understanding of the tumor targeting of these renal-clearable AuNPs. As the tumor permeability of a material can be defined as the ratio of its accumulation in the tumor and its amount in the blood,[20] by measuring the distribution of AuNPs in the blood and tumor at 1 h p.i. (Figure S6), we found that for the same AuNPs, LNCaP and PC-3 exhibited very similar targeting efficiencies and vascular permeability to NPs even though their microvasculatures are significantly different (tumor/blood ratios, P > 0.05, Figure 3A). This tumor-independent permeability is very likely due to the fact that the AuNPs are much smaller than the pore sizes of the tumor vasculature, unlike large, sub-100 nm polymeric NPs with particle-size-dependent permeability: Only 30 nm NPs penetrated poorly permeable pancreatic tumors BxPC3 although all of the NPs with diameters of 30, 50, 70, and 100 nm can penetrate highly permeable C26 tumors.[21] On the other hand, the same tumor model indeed revealed distinct permeabilities for different types of AuNPs. In general, the GS-AuNPs exhibited larger tumor-to-blood ratios (0.41 ± 0.10 and 0.51 ± 0.24) in both PC-3 and LNCaP than the GC-AuNPs (0.23 ± 0.02 and 0.23 ± 0.05) at 1 h p.i. (Figure 3A). The higher tumor permeability of the GS-AuNPs compared to the GC-AuNPs implies that the GS-AuNPs might be able to extravasate from leaky endothelium more readily than GC-AuNPs, which is consistent with their distribution half-lives. As shown in Figure 3B, the distribution half-life of the GS-AuNPs was nearly two times shorter than that of the GC-AuNPs (3.9 vs. 6.5 min; Table S4), further confirming that the GS-AuNPs indeed more easily extravasate from the blood stream than the GC-AuNPs. Considering that the GS-AuNPs and GC-AuNPs are both much smaller than the pore size of tumor leaky vasculature,[21] a possible reason for the differences in the distribution half-lives is that the GS-AuNPs (zeta potential: −48.0 ± 2.3 mV, pH 7.4) are much more negatively charged than the GC-AuNPs (zeta potential: −22.1 ± 3.6 mV, pH 7.4) and might marginate to the blood vessel wall more slowly than the GC-AuNPs.[10c] Such charge-dependent pharmacokinetics have been observed for molecular agents,[19] small, few-nanometer-sized antibodies,[22] as well as engineered NPs.[23]
Figure 3.
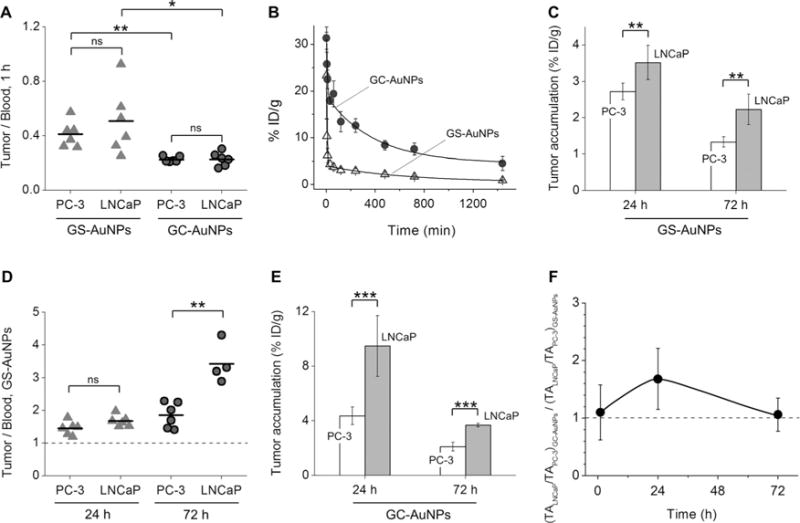
Analysis of the tumor targeting of GS-AuNPs and GC-AuNPs in PC-3 and LNCaP models at 1, 24, 72 h p.i. A) Tumor-to-blood ratios at 1 h p.i. Tumor accumulation of GS-AuNPs at 1 h p.i.: 2.48 ± 0.34%IDg−1 in PC-3, 2.43 ± 0.83%IDg−1 in LNCaP; GC-AuNPs at 1 h p.i.: 3.71 ± 0.43%IDg−1 in PC-3, 4.01 ± 0.83%IDg−1 in LNCaP. B) Blood pharmacokinetics of GS-AuNPs and GC-AuNPs. C) Tumor targeting efficiencies of GS-AuNPs at 24 and 72 h p.i. D) Tumor-to-blood ratios of GS-AuNPs at 24 and 72 h p.i. E) Tumor targeting efficiencies of GC-AuNPs at 24 and 72 h p.i. F) The (TALNCaP/TAPC-3)GC-AuNPs to (TALNCaP/TAPC-3)GS-AuNPs ratio at 1, 24, and 72 h p.i. TA =Tumor accumulation of the NPs. *P < 0.05, **P <0.01, ***P< 0.001, ns= no significant difference (P >0.05).
To unravel how the vascular structure impacts the tumor retention of these ultrasmall AuNPs, we quantified the biodistribution of GS-AuNPs in tumors, blood, and major organs at 24 and 72 h p.i. (Figures S7 and S8) and compared the tumor-to-blood ratios at these two time points in both PC-3 and LNCaP tumors. As shown in Figure 3C, at 24 and 72 h p.i., the targeting efficiency of the GS-AuNPs in LNCaP (3.52 ± 0.47%IDg−1 and 2.23 ± 0.42%IDg−1) was always greater than that in the PC-3 tumors (2.72 ± 0.23%IDg−1 and 1.35 ± 0.15%IDg−1, P < 0.01). Considering that the GS-AuNPs exhibit a comparable permeability in both LNCaP and PC-3, the high accumulation of GS-AuNPs in LNCaP implies that the GS-AuNPs have longer retention times in LNCaP than in PC-3. In addition, the tumor-to-blood ratios of the GS-AuNPs in both PC-3 and LNCaP tumors were all greater than one (Figure 3D), suggesting that the selective targeting of GS-AuNPs to PC-3 and LNCaP was due to the EPR effect, similar to non-renal-clearable proteins or NPs,[1,3b,24] but different from small molecular agents.[19] Although the tumor-to-blood ratios of GS-AuNPs in PC-3 and LNCaP were comparable at 1 and 24 h p.i. (P > 0.05; Figure 3A,D), the tumor-to-blood ratio of GS-AuNPs in the LNCaP tumor at 72 h p.i. is much larger than that in PC-3 (3.4 ± 0.6 vs. 1.8 ± 0.4, P < 0.01; Figure 3D), implying that the low vascular density of the LNCaP slows down diffusion of the GS-AuNPs back to the blood stream and prolongs the retention of GS-AuNPs in the tumor.
Similar vasculature effects on tumor targeting were also observed for the GC-AuNPs. As shown in Figure 3E, the uptake of GC-AuNPs was always higher in LNCaP tumors than in PC-3 tumors. However, unlike the GS-AuNPs, GC-AuNPs exhibited a high accumulation (9.48 ± 2.22%IDg−1) in LNCaP at 24 h p.i., which is nearly two times larger than that in PC-3 tumors (4.36 ± 0.65%IDg−1, P < 0.05; Figure 3E). This high tumor targeting of the GC-AuNPs not only resulted from the unique microvasculature of the LNCaP (low vasculature density, large vascular size, and large vascular endothelial area) but was also due to a synergistic effect between the acidic tumor microenvironment of LNCaP and the acidity targeting of the GC-AuNPs. To decouple EPR and acidity effects on the tumor targeting of GC-AuNPs and also avoid interference with NP pharmacokinetics (Figure S9) and tumor microvasculature, we first calculated the ratio between the tumor accumulation (TA) of one type of AuNPs in LNCaP to that in PC-3 (TALNCaP/TAPC-3). At 1, 24, and 72 h p.i., the TALNCaP/TAPC-3 ratios for the GS-AuNPs were 0.98 ± 0.36, 1.29 ± 0.20, and 1.65 ± 0.36, respectively. As the GS-AuNPs do not respond to tumor acidity, the TALNCaP/TAPC-3 values mainly reflected the vasculature effect on their retention in the tumors. On the other hand, the TALNCaP/TAPC-3 ratios of the GC-AuNPs were calculated to be 1.08 ± 0.26, 2.17 ± 0.60, and 1.75 ± 0.28, and were affected by both tumor vasculature and acidity. To exactly pinpoint the acidity effect on the tumor accumulation of GC-AuNPs, we compared GC-AuNPs with GS-AuNPs by calculating the ratios of (TALNCaP/TAPC-3)GC-AuNPs to (TALNCaP/TAPC-3)GS-AuNPs at 1, 24, and 72 h. The ratios at 1 and 72 h were very close to one (1.10 ± 0.48 and 1.06 ± 0.29, respectively; Figure 3F), indicating that the acidic tumor microenvironment was not really involved in the initial targeting and the very late retention stage of GC-AuNPs. On the other hand, this ratio reached 1.68 ± 0.53 at 24 h p.i., which is much larger than 1, indicating that the acidic tumor microenvironment indeed enhanced the tumor targeting of the GC-AuNPs in the early retention stage. These results clearly suggest that the tumor acidity enhances the retention of the AuNPs with acidity targeting in a time-dependent fashion, which is consistent with the previous observation that acidic tumor microenvironments mainly enhanced the tumor accumulation of small pH low insertion peptides (pHLIPs) at early time points.[19] Thus the high tumor targeting of the GC-AuNPs observed at 24 h p.i. indeed originated from the synergy between the EPR and acidity targeting effects.
Aside from the tumor vasculature and local acidic tumor microenvironment, the tumor size might be another factor that influences the accumulation of engineered NPs. For example, Chan and co-workers reported that the tumor size can impact the tumor uptake of large AuNPs (15–100 nm).[25] In our studies, we also observed that the targeting efficiency (%IDg−1) increased as the tumor size decreased from about 0.5 to 0.05 cm3 at 1 h p.i. when the NP tumor accumulation was mainly governed by the tumor permeability of the NPs (Figure S10A,B). This phenomenon could be attributed to elevated interstitial fluid pressures in large tumors,[26] which slow down the extravasation of NPs from blood vessels and the transport of NPs inside tumors. On the other hand, as no significant differences were found in the tumor sizes among the four groups (GS-AuNPs or GC-AuNPs in PC-3 or LNCaP tumors) used for studying tumor targeting at 1, 24, and 72 h p.i. (P > 0.05; Figure S11), the tumor size does not influence our conclusions on how tumor vasculature and the local acidic tumor microenvironment impact the targeting and clearance of these ultrasmall AuNPs.
In summary, using two prostate cancer models, namely PC-3 and LNCaP tumor-bearing mouse models, we have systematically investigated how the tumor vasculature and local acidity affect the targeting and retention of ultrasmall renal-clearable AuNPs that are much smaller than the pore size of the tumor vessels. Our results show that for the same type of AuNPs, differences in the tumor microvasculature have little impact on the tumor permeability of the AuNPs in early targeting stages (tumor-to-blood ratios at 5 min, 10 min, and 1 h p.i.; Figure S12 and Tables S5 and S6), but a large vascular endothelium and lower vasculature densities can increase the retention of the AuNPs in tumors. By slightly modulating the surface of the renal-clearable AuNPs, we obtained ultrasmall AuNPs with an acidity-targeting function, which allowed us to unravel the time dependence in the acidity targeting of the ultrasmall NPs. Our results further show that acidic tumor microenvironments only temporarily influence the accumulation of the AuNPs in acidic LNCaP tumor at 24 h p.i. while the tumor vasculature dictated the accumulation of renal-clearable AuNPs over the entire targeting process through the EPR effect. Thus to obtain renal-clearable NPs with high tumor targeting, factors such as size, shape, and charge that can enhance the EPR effect should always be taken into account first.[3b,20b,27] Moreover, other features such as acidic tumor microenvironments can also further enhance targeting but need to be synchronized in time. At 24 h p.i., the GC-AuNPs exhibited a tumor targeting efficiency of 9.48 ± 2.22%IDg−1, which is much higher than that of many other engineered NPs[3a] and six times higher than that (1.5%IDg−1) of 1.4 nm pH-responsive AuNPs conjugated with pHLIP.[4a] Our fundamental understanding of the in vivo nano–bio interactions of renal-clearable NPs in tumor microenvironments and simple surface chemistry for acidity targeting will provide a foundation for further enhancing the tumor targeting of renal-clearable NPs and enabling their translation to the clinic.
Supplementary Material
Acknowledgments
This study was supported by the NIH (R01DK103363), CPRIT (140544 and 160866), and the start-up fund from the University of Texas at Dallas.
Footnotes
Supporting information for this article can be found under: http://dx.doi.org/10.1002/anie.201612647.
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Prof. Dr. Mengxiao Yu, Department of Chemistry and Biochemistry, The University of Texas at Dallas, 800 W. Campbell Rd., Richardson, TX 75080 (USA)
Dr. Chen Zhou, Department of Chemistry and Biochemistry, The University of Texas at Dallas, 800 W. Campbell Rd., Richardson, TX 75080 (USA)
Prof. Dr. Li Liu, Department of Radiology, The University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd., Dallas, TX 75390 (USA)
Dr. Shanrong Zhang, Advanced Imaging Research Center, The University of Texas Southwestern Medical Center, Dallas, TX (USA)
Dr. Shasha Sun, Department of Chemistry and Biochemistry, The University of Texas at Dallas, 800 W. Campbell Rd., Richardson, TX 75080 (USA)
Julia D. Hankins, Department of Chemistry and Biochemistry, The University of Texas at Dallas, 800 W. Campbell Rd., Richardson, TX 75080 (USA)
Prof. Dr. Xiankai Sun, Department of Radiology, The University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd., Dallas, TX 75390 (USA)
Prof. Dr. Jie Zheng, Department of Chemistry and Biochemistry, The University of Texas at Dallas, 800 W. Campbell Rd., Richardson, TX 75080 (USA)
References
- 1.Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW. Nat Rev Mater. 2016;1:16014. [Google Scholar]
- 2.a) Kato Y, Ozawa S, Miyamoto C, Maehata Y, Suzuki A, Maeda T, Baba Y. Cancer Cell Int. 2013;13:89. doi: 10.1186/1475-2867-13-89. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Danhier F, Feron O, Preat V. J Controlled Release. 2010;148:135–146. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]; c) Fukumura D, Jain RK. Microvasc Res. 2007;74:72–84. doi: 10.1016/j.mvr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Brown JM, Giaccia AJ. Cancer Res. 1998;58:1408–1416. [PubMed] [Google Scholar]
- 3.a) Yu MX, Zheng J. ACS Nano. 2015;9:6655–6674. doi: 10.1021/acsnano.5b01320. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Albanese A, Tang PS, Chan WCW. Annu Rev Biomed Eng. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 4.a) Yao L, Daniels J, Moshnikova A, Kuznetsov S, Ahmed A, Engelman DM, Reshetnyak YK, Andreev OA. Proc Natl Acad Sci USA. 2013;110:465–470. doi: 10.1073/pnas.1219665110. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Du JZ, Sun TM, Song WJ, Wu J, Wang J. Angew Chem Int Ed. 2010;49:3621–3626. doi: 10.1002/anie.200907210. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2010;122:3703–3708. [Google Scholar]; c) Zhou KJ, Wang YG, Huang XN, Luby-Phelps K, Sumer BD, Gao JM. Angew Chem Int Ed. 2011;50:6109–6114. doi: 10.1002/anie.201100884. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem. 2011;123:6233–6238. [Google Scholar]; d) Crayton SH, Tsourkas A. ACS Nano. 2011;5:9592–9601. doi: 10.1021/nn202863x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Guo X, Huang L. Acc Chem Res. 2012;45:971–979. doi: 10.1021/ar200151m. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wang M, Thanou M. Pharmacol Res. 2010;62:90–99. doi: 10.1016/j.phrs.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 6.a) Choi HS, Liu WH, Liu FB, Nasr K, Misra P, Bawendi MG, Frangioni JV. Nat Nanotechnol. 2010;5:42–47. doi: 10.1038/nnano.2009.314. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Ipe BI, Bawendi MG, Frangioni JV. Nat Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a) Phillips E, Penate-Medina O, Zanzonico PB, Carvajal RD, Mohan P, Ye YP, Humm J, Gonen M, Kalaigian H, Schoder H, Strauss HW, Larson SM, Wiesner U, Bradbury MS. Sci Transl Med. 2014;6:260ra149. doi: 10.1126/scitranslmed.3009524. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Burns AA, Vider J, Ow H, Herz E, Penate-Medina O, Baumgart M, Larson SM, Wiesner U, Bradbury M. Nano Lett. 2009;9:442–448. doi: 10.1021/nl803405h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.a) Zhou C, Long M, Qin Y, Sun X, Zheng J. Angew Chem Int Ed. 2011;50:3168–3172. doi: 10.1002/anie.201007321. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem. 2011;123:3226–3230. [Google Scholar]; b) Zhou C, Hao GY, Thomas P, Liu JB, Yu MX, Sun SS, Oz OK, Sun XK, Zheng J. Angew Chem Int Ed. 2012;51:10118–10122. doi: 10.1002/anie.201203031. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2012;124:10265–10269. [Google Scholar]; c) Xu J, Peng C, Yu M, Zheng J. WIREs Nanomed Nanobiotechnol. 2017:e1453. doi: 10.1002/wnan.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a) Yu M, Liu J, Ning X, Zheng J. Angew Chem Int Ed. 2015;54:15434–15438. doi: 10.1002/anie.201507868. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem. 2015;127:15654–15658. [Google Scholar]; b) Yu M, Zhou J, Du B, Ning X, Authement C, Gandee L, Kapur P, Hsieh JT, Zheng J. Angew Chem Int Ed. 2016;55:2787–2791. doi: 10.1002/anie.201511148. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem. 2016;128:2837–2841. [Google Scholar]
- 10.a) Liu J, Yu M, Zhou C, Yang S, Ning X, Zheng J. J Am Chem Soc. 2013;135:4978–4981. doi: 10.1021/ja401612x. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Liu J, Yu M, Ning X, Zhou C, Yang S, Zheng J. Angew Chem Int Ed. 2013;52:12572–12576. doi: 10.1002/anie.201304465. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem. 2013;125:12804–12808. [Google Scholar]; c) Tang S, Peng C, Xu J, Du B, Wang Q, Vinluan RD, Yu M, Kim MJ, Zheng J. Angew Chem Int Ed. 2016;55:16039–16043. doi: 10.1002/anie.201609043. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem. 2016;128:16273–16277. [Google Scholar]; d) Peng C, Gao X, Xu J, Du B, Ning X, Tang S, Bachoo RM, Yu M, Ge WP, Zheng J. Nano Res. 2017 doi: 10.1007/s12274-017-1472-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.a) Chen HM, Wang GD, Sun XL, Todd T, Zhang F, Xie J, Shen BZ. Adv Funct Mater. 2016:26, 3973–3982. doi: 10.1002/adfm.201504803. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kang HM, Gravier J, Bao K, Wada H, Lee JH, Baek Y, El Fakhri G, Gioux S, Rubin BP, Coll JL, Choi HS. Adv Mater. 2016;28:8162–8168. doi: 10.1002/adma.201601101. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Huang XL, Zhang F, Zhu L, Choi KY, Guo N, Guo JX, Tackett K, Anilkumar P, Liu G, Quan QM, Choi HS, Niu G, Sun YP, Lee S, Chen XY. ACS Nano. 2013;7:5684–5693. doi: 10.1021/nn401911k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mei KC, Bai J, Lorrio S, Wang JTW, Al-Jamal KT. Biomaterials. 2016;106:276–285. doi: 10.1016/j.biomaterials.2016.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.a) Foekens JA, Peters HA, Grebenchtchikov N, Look MP, Meijer-van Gelder ME, Geurts-Moespot A, van der K-wast TH, Sweep CGJ, Klijn JGM. Cancer Res. 2001;61:5407–5414. [PubMed] [Google Scholar]; b) Eberhard A, Kahlert S, Goede V, Hemmerlein B, Plate KH, Augustin HG. Cancer Res. 2000;60:1388–1393. [PubMed] [Google Scholar]; c) Horak ER, Leek R, Klenk N, Lejeune S, Smith K, Stuart N, Greenall M, Stepniewska K, Harris AL. Lancet. 1992;340:1120–1124. doi: 10.1016/0140-6736(92)93150-l. [DOI] [PubMed] [Google Scholar]
- 14.Jiang W, Kim BYS, Rutka JT, Chan WCW. Nat Nanotechnol. 2008;3:145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 15.Wong CKE, Namdarian B, Chua J, Chin X, Speirs R, Nguyen T, Fankhauser M, Pedersen J, Costello AJ, Corcoran NM, Hovens CM. Br J Cancer. 2012;107:1564–1573. doi: 10.1038/bjc.2012.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou C, Sun C, Yu M, Qin Y, Wang J, Kim M, Zheng J. J Phys Chem C. 2010;114:7727–7732. doi: 10.1021/jp9122584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu MX, Zhou C, Liu JB, Hankins JD, Zheng J. J Am Chem Soc. 2011;133:11014–11017. doi: 10.1021/ja201930p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.a) Gillies RJ, Liu Z, Bhujwalla Z. Am J Physiol. 1994;267:C195–C203. doi: 10.1152/ajpcell.1994.267.1.C195. [DOI] [PubMed] [Google Scholar]; b) Zhang XM, Lin YX, Gillies RJ. J Nucl Med. 2010;51:1167–1170. doi: 10.2967/jnumed.109.068981. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Hugg JW, Matson GB, Twieg DB, Maudsley AA, Sappeymarinier D, Weiner MW. Magn Reson Imaging. 1992;10:227–243. doi: 10.1016/0730-725x(92)90483-g. [DOI] [PubMed] [Google Scholar]
- 19.Vavere AL, Biddlecombe GB, Spees WM, Garbow JR, Wijesinghe D, Andreev OA, Engelman DM, Reshetnyak YK, Lewis JS. Cancer Res. 2009;69:4510–4516. doi: 10.1158/0008-5472.CAN-08-3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.a) Maeda H, Nakamura H, Fang J. Adv Drug Delivery Rev. 2013;65:71–79. doi: 10.1016/j.addr.2012.10.002. [DOI] [PubMed] [Google Scholar]; b) Fang J, Nakamura H, Maeda H. Adv Drug Delivery Rev. 2011;63:136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]; c) Iyer AK, Khaled G, Fang J, Maeda H. Drug Discovery Today. 2006;11:812–818. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M, Terada Y, Kano MR, Miyazono K, Uesaka M, Nishiyama N, Kataoka K. Nat Nanotechnol. 2011;6:815–823. doi: 10.1038/nnano.2011.166. [DOI] [PubMed] [Google Scholar]
- 22.Boswell CA, Tesar DB, Mukhyala K, Theil FP, Fielder PJ, Khawli LA. Bioconjugate Chem. 2010;21:2153–2163. doi: 10.1021/bc100261d. [DOI] [PubMed] [Google Scholar]
- 23.Caliceti P, Veronese FM. Adv Drug Delivery Rev. 2003;55:1261–1277. doi: 10.1016/s0169-409x(03)00108-x. [DOI] [PubMed] [Google Scholar]
- 24.Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WCW. Nano Lett. 2009;9:1909–1915. doi: 10.1021/nl900031y. [DOI] [PubMed] [Google Scholar]
- 25.Sykes EA, Dai Q, Sarsons CD, Chen J, Rocheleau JV, Hwang DM, Zheng G, Cramb DT, Rinker KD, Chan WCW. Proc Natl Acad Sci USA. 2016;113:E1142–E1151. doi: 10.1073/pnas.1521265113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gutmann R, Leunig M, Feyh J, Goetz AE, Messmer K, Kastenbauer E, Jain RK. Cancer Res. 1992;52:1993–1995. [PubMed] [Google Scholar]
- 27.Ohta S, Glancy D, Chan WCW. Science. 2016;351:841–845. doi: 10.1126/science.aad4925. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


