Abstract
Cyclin-dependent kinase 6 (CDK6) binds to and is activated by cyclin D1 and thereby enhances the transition of cells through the G1 phase of the cell cycle. The present study indicates that, in human prostate cancer cells, CDK6 can also bind to the androgen receptor (AR) and stimulate its transcriptional activity in the presence of dihydrotestosterone. This effect of CDK6 does not require its kinase activity and is inhibited by cyclin D1 and p16INK4a. The T877A mutant of the AR frequently found in advanced cases of prostate cancer displays an exaggerated stimulation of transcriptional activity by CDK6. Androgen-sensitive LNCaP prostate cancer cells engineered to stably overexpress CDK6 display increased expression of the prostate-specific antigen and enhanced growth in the presence of dihydrotestosterone. CDK6 is present in the chromatin structure of these cells in association with the AR and the promoter region of the prostate-specific antigen gene. These findings suggest that CDK6 may play an important role in the development and/or progression of a subset of human prostate cancers by stimulating the activity of the AR.
Keywords: transcription, cyclin D1
Prostate cancer is the most frequently diagnosed cancer and the second leading cause of cancer deaths in men in the United States. American men have a one-in-six lifetime risk for developing prostate cancer (1). Various abnormalities in function of the androgen receptor (AR) and in AR-related signaling pathways have been implicated in the development and progression of human prostate cancer (2, 3). Although the precise mechanism by which androgens affect the development of prostate cancer is not known, it appears that the disease develops only in the presence of androgens (4).
Most prostate cancers initially depend on androgens for their growth. Therefore, they can often be controlled by various therapies that block the synthesis of androgens, their conversion to the active form dihydrotestosterone (DHT) or the function of the AR. However, the major barrier to therapy is the eventual progression of the disease to what appears to be an androgen-independent stage. Two mechanisms have been proposed for the development of androgen-independent prostate cancers. The first mechanism is based on increased androgen receptor signaling caused by mutations in the AR that allow it to be activated by ligands other than DHT or by signaling pathways induced by a tyrosine kinase receptor, such as Her/2neu (5, 6). The second mechanism is based on activation of growth-enhancing pathways that function independent of the AR, thus overcoming the growth inhibition caused by antiandrogen therapies (5).
Cyclin D1 enhances the transition of cells through the G1 phase of the cell cycle by binding to and activating cyclin-dependent kinase (CDK)4 or CDK6. It is often overexpressed in human breast (7) and colon (8) carcinomas. It is of interest that it is rarely overexpressed in human prostate carcinoma (9, 10). In addition to its role in cell cycle control, cyclin D1 also binds to and activates the estrogen receptor (11) but binds to and inhibits the activity of the AR (12, 13). Because of these findings (and unpublished data), we decided to examine the possible effects of CDKs on the function of the AR. In this study, we demonstrate that CDK6, but not other CDKs, binds to and activates the transcriptional activity of the AR. We also show that this effect is independent of cyclin D1 and CDK6 kinase activity. Furthermore, LNCaP prostate cancer cells engineered to stably overexpress CDK6 displayed increased expression of the endogenous prostate-specific antigen (PSA) protein and mRNA when compared with vector control cells. Our studies suggest that overexpression of CDK6 can contribute to the progression of prostate cancers to an androgen-independent stage.
Materials and Methods
Reagents, Antibodies, and Cells. DHT was purchased from Sigma. The primary antibodies and ratios used were as follows: PSA (DAKO), 1:1,000; AR (Santa Cruz Biotechnology), 1:2,000; CDK6 (Santa Cruz Biotechnology), 1:500; hemagglutinin (HA; Covance), 1:2,000; β-actin (Sigma), 1:500; and insulin-like growth factor binding protein-2 (Upstate Biotechnology, Lake Placid, NY), 1:2,000. HA epitope-tagged mutants of CDK6 were constructed by using the overlapping PCR technique with appropriate primers (14). The PCR samples were thermocycled for 30 s at 95°C, at 55°C, and at 72°C. The accuracy of the PCR-generated fragments was confirmed by dideoxysequencing using 3100 capillary sequencers (Applied Biosystems) in forward and reverse directions. LNCaP, PC3, and 293T cell lines were purchased from the American Type Culture Collection. LNCaP and PC3 cells were routinely cultured in RPMI medium 1640 (GIBCO) with 10% FBS at 37°C with 5% CO2. The 293T cells were cultured in DMEM medium (GIBCO) with 10% FBS. The HA-tagged CDK6 plasmid was stably transfected into LNCaP cells by using a liposome protocol (GIBCO) as described in ref. 15. Colony-forming assays and growth curves were performed as described in refs. 16–18.
Transient Transfection Reporter Assays. These assays were conducted as described in ref. 16. Briefly, 104 cells were plated and transiently transfected with 2 μg of the luciferase reporter and 1 μg of the pCMV–β-gal plasmids. The cells were also cotransfected with additional plasmid constructs as described in individual experiments. The cells were cultured in RPMI medium 1640 supplemented with 10% charcoal-stripped FBS and in the absence or presence of DHT. After 20 h, whole-cell extracts were prepared. Luciferase and β-gal assays were performed according to the manufacturer's instructions (Promega), and β-gal values were used to correct for efficiency of transfection (16). Results are presented as “relative luciferase activity” with mean values of triplicate assays ± SD. Statistical analysis of the data were performed by using Student's t test (sigmaplot 8.0l, SPSS, Surrey, U.K.). A P value of <0.05 was considered statistically significant (n = 3 per group).
Immunoprecipitation and Immunoblotting. 293T cells were transfected by using a liposome procedure (GIBCO) with the AR and/or HA-tagged CDK6 WT or mutant constructs. After 48 h, sonicated total cell lysates were prepared in 100 μl of M2 buffer (17). Lysates were precleared and incubated with either 2 μg of AR (BD Biosciences) or 2 μg of HA (Covance) antibodies, and then protein Sepharose beads were added. After 3 h at 4°C, the protein complex bound to the beads was washed with M2 buffer, and the beads were resuspended in 20 μl of sample buffer (16). The protein samples were then separated by 10% SDS/PAGE.
Western blot analysis was performed as described in ref. 10 with the following modifications. Cells (107) were sonicated in 200 μl of lysis buffer (10). Whole-cell extracts, immunoprecipitation samples, or 50 μl of tissue culture media [for secreted PSA expression studies (19)] were mixed with sample buffer (16), subjected to 10% SDS/PAGE, and immunoblotted with the indicated antibodies as described in ref. 16.
RT-PCR. Total RNA was isolated from cells by using TRIzol reagent and the methods described in ref. 16. The primers used for amplification were as follows: AR, forward 5′-AGCTACTCCGGACCTTACG-3′ and reverse 5′-AGGTGCCATGGGAGGGTTAG-3′; CDK6, forward 5′-CGGGATCCACCATGGAGAAGGACGGCCTG-3′ and reverse 5′-CGGATCCATTGCTCAGGCTGTATTCAGCTCCGA-3′; PSA, forward 5′-TTGTGGCCTCTCGTGGCAGGGCAGT-3′ and reverse 5′-TGGTCACCT TCTGAGGGTGA ACT TGC-3′; GA PDH, forward 5′-GCCACATCGCTCAGACACCA-3′ and reverse 5′-GATGACCCTTTTGGCTCCCC-3′. Negative controls consisted of omission of RNA from the reaction mixture. PCR products were separated by using a 1% agarose gel and identified by ethidium bromide staining.
Chromatin Immunoprecipitation Assay. Chromatin immunoprecipitation assays were performed as described in ref. 20 with minor modifications. The cells were grown in the standard RPMI medium 1640 containing 10% FBS and harvested. Then 107 cells were treated with 1% formaldehyde and lysed; and then the chromatin was sheared. The cell extracts were precleared with salmon sperm DNA/protein A agarose beads (Upstate Biotechnology). Primary antibodies (10 μg) and 60 μl of salmon sperm DNA/protein A agarose beads (Upstate Biotechnology) were added. The protein–DNA complexes were immunoprecipitated for 4 h at 4°C. The beads were washed with buffer containing increasing concentrations of NaCl, and the complexes were eluted from the beads as described in ref. 20. The RT-PCR primers for the PSA promoter sequence were position –149 forward 5′-CCCTCCCCTTCCACAGCTCTGGGT-3′ and position –48 reverse 5′-CCGCCCCTGCCCTGCTGGCACCC-3′, which amplifies a 101-bp fragment. The DNA samples were separated on a 1% agarose/3% NuSieve–agarose gel and detected with ethidium bromide.
Results
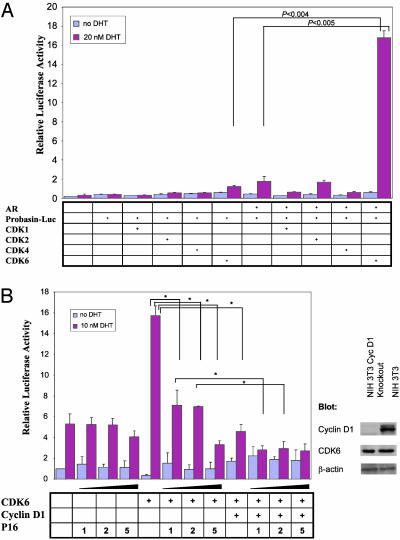
CDK6 Activates the AR Pathway Independent of Cyclin D1 or CDK Activity. PC3 human prostate cancer cells that lack expression of the AR were cotransfected with an androgen-responsive probasin luciferase reporter construct together with an AR expression plasmid and plasmids that encode CDKs 1, 2, 4, or 6. We found that expression of CDK6 markedly enhanced activation of the probasin luciferase reporter in the presence of the AR and 20 nM DHT. No significant effects were seen with CDKs 1, 2, or 4. This effect of CDK6 depended on the presence of the AR and DHT (Fig. 1A). To extend our findings, we studied PSA luciferase reporter activity in AR-positive human prostate cancer LNCaP cells. CDK6 also markedly enhanced reporter activity in these cells and the stimulation by DHT was dose-dependent, with a detectable effect at 1 nM and near maximal effect at 10–20 nM. Similar studies with PC3 cells indicated that when the cells were cotransfected with the AR plasmid, CDK6 also stimulated PSA luciferase reporter activity in the presence of 1, 10, or 20 nM DHT (results not shown). Because CDK6 associates with cyclin D1 during the G1 phase of the cell cycle to form a functionally active kinase complex, we examined whether CDK6 activation of the PSA promoter is cyclin D1-dependent by using NIH 3T3 fibroblasts that are deficient in the cyclin D1 gene (21). When these cells were transfected with the plasmids that express the AR and the PSA luciferase reporter and treated with DHT, cotransfection with CDK6 markedly enhanced activation of the PSA luciferase reporter despite the absence of cyclin D1. Indeed, cotransfection with exogenous cyclin D1 actually suppressed CDK6 activation of this reporter. (Fig. 1B). Activation by CDK6 of the PSA luciferase reporter was also suppressed when the cells were cotransfected with increasing amounts of a plasmid that encodes the CDK4/6 inhibitor p16INK4a. Furthermore, transfection with p16INK4a increased the extent of cyclin D1 suppression (Fig. 1B).
Fig. 1.
CDK6 activates the AR signaling pathway. (A) The effect of various CDKs on activation of the probasin promoter. AR-negative PC3 cells were cotransfected with plasmids that encode the probasin luciferase reporter, the CMV–β-gal reporter, the AR, and various CDKs as indicated. Relative luciferase reporter activity was measured after cells were then incubated in the absence or presence of 20 nM DHT for 20 h. (B) Dose-dependent inhibition of CDK6-mediated activation of the AR by cyclin D1 and p16INK4a. NIH 3T3 cells deficient in the cyclin D1 gene were cotransfected with the indicated amounts (in micrograms) of p16INK4a, 2 μg of cyclin D1, and 3 μg of CDK6. The cells were then incubated in the absence or presence of 10 nM DHT for 20 h; extracts were assayed for relative luciferase reporter activity. Extracts of nontransfected cyclin D1-deficient and WT NIH 3T3 cells were also immunoblotted for cyclin D1, CDK6, and β-actin with the respective antibodies. Error bars designate standard deviations for triplicate assays. *, P < 0.05.
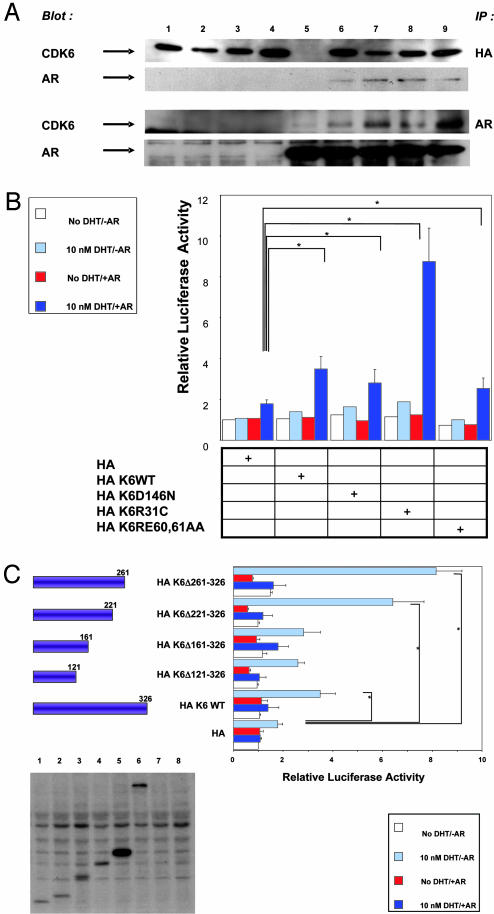
Next, we examined whether CDK6 associates with the AR as a complex in vivo. We used 293T cells because they express relatively low levels of endogenous cyclin D1 (12). The cells were cotransfected with the AR, HA-tagged WT CDK6, or a series of HA-tagged point-mutated constructs of CDK6 (see legend to Fig. 2A). Coimmunoprecipitation studies of these cell extracts indicated that CDK6 binds to the AR protein in vivo. Furthermore, it appears that point mutations in the p16INK4a-binding (CDK6R31C), cyclin D1-binding (CDK6R60A,E61A) or ATP transfer (CDK6D146N) regions of CDK6 are not essential for binding to the AR (Fig. 2 A). We also found that none of these point mutations interfered with the stimulatory effect of CDK6 in the above-described PSA–luciferase reporter activity in PC3 cells (Fig. 2B). Indeed, the results obtained with HAK6R31C indicate that a mutation in CDK6 at residue 31, previously shown to relieve p16INK4a inhibition (24), caused a marked increase in activity (Fig. 2B). This finding suggests that the endogenous p16INK4a in PC3 cells exerts an inhibitory effect on CDK6 activation of the AR. To further examine the roles of specific domains of CDK6, we constructed a series of truncations of the CDK6 cDNA sequence and examined their effects on PSA luciferase reporter activity in PC3 cells (Fig. 2C). We found that a truncation of the CDK6 cDNA at the C terminus (up to amino acid 121) did not interfere with CDK6 stimulation of this activity. These results together with our findings with a point mutation in codon 146 (Fig. 2B), a region required for the kinase activity of CDK6, indicate that the kinase activity of CDK6 is not required for CDK6 activation of the AR pathway. The region between residues 261–326 appears to contain an inhibitory site, because when this region of CDK6 was deleted there was greater stimulation of reporter activity than that obtained with WT CDK6 (Fig. 2C).
Fig. 2.
Effects of mutations in CDK6 and the AR on the association between the two proteins and activation of the PSA promoter. (A) CDK6 associates with the AR. 293T cells were transfected with HA-tagged WT or point-mutated CDK6 plasmids plus or minus the AR plasmid. Lanes: 1, HA K6WT; 2, HA K6R31C; 3, HA K6D146N; 4, HA K6R60A,E61A; 5, AR; 6, AR and HA K6WT; 7, AR and HA K6R31C; 8, AR and HA K6D146N; and 9, AR and HA K6RE60,61AA. Whole-cell extracts were immunoprecipitated with the HA (Upper) or the AR (Lower) antibody and immunoblotted with the HA or AR antibody. (B) The effect of CDK6 point mutations on activation of the PSA promoter. PC3 cells were transfected with the PSA–luciferase reporter and CDK6 WT or the CDK6 mutant constructs used in Fig. 2 A. As indicated, the cells were also transfected with the AR expression plasmid. Relative luciferase reporter activity was measured after the cells were grown in the absence or presence of DHT for 20 h. (C) The effects of CDK6 truncation mutants on activation of the PSA promoter. PC3 cells were transfected with the PSA–luciferase reporter and full-length CDK6 or the indicated CDK6 truncation constructs. The AR expression plasmid was also cotransfected as indicated. Relative luciferase activity was measured after the cells were grown in the absence or presence of DHT for 20 h. (Lower) Extracts were also examined for CDK6 expression by using the HA-specific IgG antibody. Lanes: 1, HA K6Δ121–326; 2, HA K6Δ161–326; 3, HA K6Δ221–326; 4, HA K6Δ261–326; 5, HA K6WT; 6, HA Lac-Z; 7, HA; and 8, no DNA. *, P < 0.05.
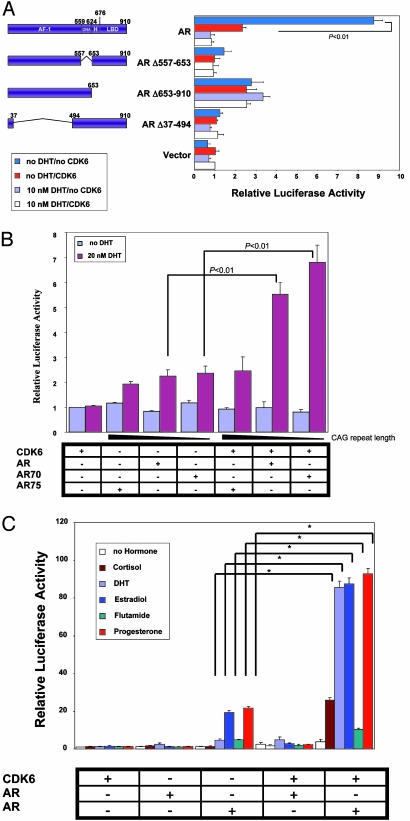
Shorter CAG Repeats and a T877A Mutation in the AR Enhance Activation by CDK6. We also explored the roles of specific functional domains of the AR in the above reporter assays by using a series of plasmids encoding the simian virus 40 promoter and WT or mutant forms of the AR (23). We found that deletions in the transactivation (ARΔ37–494) or the DNA-binding (ARΔ557–653) domains of the AR markedly impaired the ability of CDK6 to stimulate PSA–luciferase reporter activity (Fig. 3A). A mutant in the ligand-binding domain of the AR (ARΔ653–910) that displays constitutive activity in the absence of DHT (23) was still active but not as active as the WT AR activity with respect to CDK6 stimulation of reporter activity (Fig. 3A). Thus, it appears that the ability of CDK6 to stimulate the activity of the AR requires multiple domains in the AR.
Fig. 3.
Effects of CDK6 on various mutations in the AR and with respect to activation of the PSA promoter. (A) The effects of AR deletion mutants on CDK6 activation of the PSA promoter. PC3 cells were transfected with the PSA–luciferase and β-gal plasmids. As indicated, the cells were also cotransfected with one of the AR deletion constructs (AR Δ37–494, AR Δ653–910, AR Δ557–653, or the full-length AR) and the full-length CDK6 expression plasmid. Relative luciferase activity was then measured after the cells were grown in the absence or presence of 10 nM DHT for 20 h. (B) The effects of CAG polymorphism in the AR on CDK6 activation of the PSA promoter. PC3 cells were transfected with the PSA–luciferase reporter and β-gal plasmids. As indicated, the cells were also cotransfected with an AR plasmid encoding 48 (AR75), 20 (AR), or no (AR 70) CAG repeats and CDK6. (C) The effects of an AR mutant found in human prostate cancer on CDK6 activation of the PSA promoter in the presence of various steroids. PC3 cells were transfected with the PSA–luciferase reporter and β-gal plasmids. As indicated, the cells were also cotransfected with WT AR or the AR mutant AR T877A and CDK6 and grown in the absence or presence of 10 nM cortisol, DHT, β-estradiol, flutamide, or progesterone. *, P < 0.05.
The transcriptional activation domain in exon 1 of the AR gene contains a polymorphic CAG repeat sequence (24). Previous reports suggest that African-American men with shorter CAG repeat length are at greater risk for prostate cancer (24, 25). Therefore, we examined the effects of CDK6 activation on AR constructs that vary in CAG repeat lengths by using the above described PSA–luciferase reporter assays. In the presence of DHT but absence of CDK6, AR proteins containing 48 (AR75), 20 (AR), or no (AR70) CAG repeats displayed approximately equal activities, although the protein with 48 repeats had somewhat lower activity. In the presence of DHT and CDK6, the AR protein with 48 repeats displayed negligible stimulation by CDK6, but the AR protein with no repeats gave an even higher stimulation by CDK6 than that obtained with the WT AR (20 CAG repeats) (Fig. 3B). These results suggest that the stimulation by CDK6 is enhanced with ARs that contain shorter CAG repeats.
The ligand-binding domain of the AR is frequently mutated in human prostate cancer (23, 26). Mutations that convert leucine to histidine in codon 701 (AR L701H) and threonine to alanine in codon 877 (AR T877A) of the AR are of particular interest because these mutant ARs bind and are activated by steroids other than androgens. Thus, they may lead to the development of an androgen-independent state (27). Therefore, we examined the ability of CDK6 to enhance the transcriptional activities of these two mutant ARs in PSA promoter–luciferase reporter assays. In the presence of DHT, the activity of the mutant AR L701H was stimulated by CDK6 to about the same extent as that obtained with the WT AR (results not shown). However, in the presence of DHT, CDK6 produced a dramatic stimulation of the activity of the AR T877A mutant. Indeed, this activity was ≈9-fold higher than the comparable activity obtained with the WT AR (Fig. 3C). We then examined the effects of various steroid nuclear receptor ligands in similar PSA promoter reporter assays by using cortisol, DHT, β-estradiol, flutamide, and progesterone (Fig. 3C). When AR T877A was tested in the absence of CDK6, β-estradiol and progesterone caused a stimulation that was even greater than that obtained with DHT. When AR T877A was tested in the presence of CDK6, β-estradiol and progesterone caused a dramatic stimulation equal to that obtained with DHT, and significant stimulation was also seen with cortisol and flutamide (Fig. 3C). These results suggest that in the subset of prostate tumors that express the T877A mutant form of the AR, CDK6 expression may enhance the transition of an androgen-dependent prostate tumor to an androgen-independent tumor.
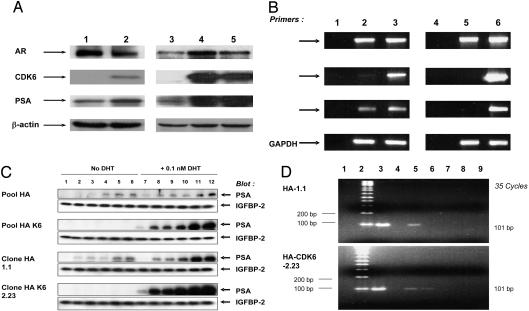
Effects of Stable Overexpression of CDK6 in LNCaP Prostate Cancer Cells. To demonstrate that the ability of CDK6 to stimulate the transcriptional activity of the AR is not confined to transient transfection reporter assays, we engineered LNCaP cells to stably overexpress CDK6 (as described in Materials and Methods). Western blot analysis of a pool of these derivatives (Fig. 4A, lane 2) and, especially, two clonal derivatives (Fig. 4A, lanes 4 and 5) of LNCaP cells indicated that they stably overexpressed higher levels of CDK6 than parallel vector control cells. It is of interest that these derivatives also showed increased expression of the PSA protein (Fig. 4A) when compared with the vector control cells. The increased expression of PSA was especially striking in the two clonal derivatives, which is consistent with the fact that they express higher levels of CDK6 than the pooled cells (Fig. 4A, compare lanes 4 and 5 to lane 2). Because expression of the AR gene is itself stimulated by the AR it is also of interest that the two clonal derivatives that expressed high levels of CDK6 also expressed increased levels of the AR (Fig. 4A, lanes 4 and 5). However, this was not seen with the pool of CDK6 cells (Fig. 4A, lane 2), perhaps because a higher level of CDK6 is required to stimulate transcription of the AR gene than the PSA gene. RT-PCR analysis indicated that these derivatives also expressed increased levels of PSA mRNA when compared with the vector control cells (Fig. 4B). Therefore, increased expression of CDK6 can markedly increase the expression of the endogenous AR-responsive gene PSA in human prostate cancer cells. Analysis of the media indicated that, when incubated in a medium containing charcoal-stripped FBS to reduce basal levels of androgens, the LNCaP cells that overexpress CDK6 secreted more PSA into the medium in response to the addition of 0.1 nM DHT than the vector control cells (Fig. 4C).
Fig. 4.
Overexpression of CDK6 in LNCaP cells and in prostate tumors. (A) Stable overexpression of CDK6 in LNCaP cells increases expression of the endogenous PSA protein. Levels of expression of CDK6, the AR, and PSA were determined by using Western blot analysis, with the respective antibodies, of extracts of the following derivatives of LNCaP cells: a pool of vector control cells, lane 1; a pool of CDK6 overexpressing cells, lane 2; a clone (HA 1.1) of vector control cells, lane 3; a clone (HAK62.23) of CDK6-overexpressing cells, lane 4; and a second clone (HAK62.24) of CDK6-overexpressing cells, lane 5. (B) CDK6 overexpression up-regulates expression of PSA mRNA. RT-PCR analysis of total cellular RNA. Lanes: 1, no RNA; 2, pooled vector control cells; 3, pooled CDK6-overexpressing cells; 4, no RNA; 5, clone HA 1.1 cells; and 6, clone HAK62.23 cells. (C) CDK6 overexpression confers increased sensitivity to induction of secreted PSA by DHT. The indicated derivatives of LNCaP cells were incubated in the presence or absence of 0.1 nM DHT in charcoal-stripped 10% FBS medium for 6 (lanes 1 and 7), 20 (lanes 2 and 8), 24 (lanes 3, 9), 30 (lanes 4 and 10), 48 (lanes 5 and 11), or 54 (lanes 6 and 12) h. The media were collected and assayed for secreted PSA by Western blot analysis. Insulin growth factor-binding protein-2 (IGFBP-2) was used as a loading control for secreted PSA (18). (D) CDK6 associates with the AR transcription complex. Chromatin immunoprecipitation assays were done on extracts of the vector control clone (HA 1.1) (Upper) or the CDK6-overexpressing clone (HAK62.23) (Lower). Lanes: 1, no DNA; 2, molecular weight marker; 3, total DNA; 4, no antibody; 5, AR immunoprecipitation (IP); 6, CDK6 IP; 7, normal IgG2a immunoprecipitation; 8, no crosslink; and 9, CDK6 immunoprecipitation. PSA primers were used for lanes 1 and 3–8 and GAPDH primers for lane 9.
We also assessed the effects of stable overexpression of CDK6 on the growth of LNCaP cells by determining their ability to form colonies when plated at a low density (18) in medium containing charcoal-stripped FBS. Without the addition of DHT, the vector control HA clone 1.1 cells and the CDK6 overexpressor HA CDK6 clone 2.23 cells yielded 72 ± 4 and 74 ± 4 colonies per plate. When grown in the presence of 0.1 nM DHT, the respective vales were 107 ± 4 and 159 ± 9 colonies per plate. Thus, overexpression of CDK6 in LNCaP cells enhances the growth stimulatory effects of DHT in LNCaP cells (P < 0.02). We also examined the effects of stable overexpression of CDK6 on the growth of monolayer cultures of LNCaP cells in medium containing 10% charcoal-stripped serum, plus 0.1 nM DHT. Under these conditions, the exponential doubling times of the vector control clone HA 1.1 and the CDK6 overexpresor clone HAK62.23 were 32 and 17 h, respectively. These results provide further evidence that overexpression of CDK6 stimulates the growth of LNCaP cells.
We then used the chromatin immunoprecipitation assay (20) to determine whether CDK6 can physically associate with a transcriptional complex that contains the AR and the promoter sequence of the endogenous PSA gene (Fig. 4D). As expected, immunoprecipitation with the AR antibody pulled down the –149 to –48 DNA region of the promoter sequence of the PSA gene (Fig. 4D, lane 5). Immunoprecipitation of CDK6 in the chromatin isolated from LNCaP cells that overexpress CDK6 also pulled down the same promoter sequence (Fig. 4D, lane 6). Control studies confirmed the specificity of these results (Fig. 4D, lanes 4 and 7–9). These findings provide strong evidence that CDK6 is part of the AR transcriptional machinery that binds to the PSA promoter sequence, at least in LNCaP cells that overexpress CDK6.
Discussion
The present studies provide the first evidence that, in addition to its known role of enhancing the progression of cells through the G1 phase of the cell cycle, in human prostate cancer cells CDK6 can bind to and markedly enhance the transcriptional activity of the AR in a ligand-dependent fashion (Figs. 1 A and C). Although cyclin D1 binds to and activates the serine/threonine kinase activity of CDK6 (28), we found that cyclin D1 actually inhibited the ability of CDK6 to stimulate the activity of the AR (Fig. 1B). This finding may explain why cyclin D1 is only rarely overexpressed in human prostate cancer (9, 10). Furthermore, our studies with mutant forms of CDK6 indicated that the kinase activity of CDK6 is not required for its ability to stimulate the AR (Fig. 2B). Presumably CDK6 exerts its effect on the AR by forming an association with it and other transcriptional control proteins. Indeed, we obtained evidence that in vivo CDK6 is associated with a transcriptional complex that contains the AR and the promoter sequence for the AR gene PSA (Fig. 4D). Several studies suggest that African-American men who are at higher risk for prostate cancer than Caucasian or Asian men more frequently display shorter CAG repeats in exon 1 of the AR (24, 25). We found that a decrease in CAG repeats in the AR enhances stimulation of its transcriptional activity by CDK6 (Fig. 3B). This finding suggests that overexpression of CDK6 could be especially effective in stimulating the activity of the AR in men whose AR has shorter CAG repeats, thereby further enhancing the development of prostate cancer in these individuals. Our evidence that CDK6 can stimulate the transcriptional activity of the AR is not confined to transient transfection reporter assays, because derivatives of LNCaP cells that stably overexpress CDK6 display increased expression of PSA mRNA and protein and increased secretion of the PSA protein (Figs. 4 A–C). In addition, the cells that overexpress CDK6 display enhanced growth in the presence of DHT.
The molecular and cellular mechanisms responsible for the progression of prostate cancer from the androgen-dependent to the androgen-independent state are currently poorly understood. Contrary to previous belief, recent results indicate that the AR is often expressed and active during all stages of prostate carcinogenesis, including the “androgen-independent” stage (29). Furthermore, it appears that during the initial treatment of prostate cancer with androgen deprivation or antiandrogens, there is a selective pressure for the development of mutant forms of the AR, some of which can be activated by ligands other than DHT, such as other steroids or the drug flutamide (6, 30). We found that a point-mutated AR originally identified in human prostate cancers, designated AR T877A, displayed a dramatic stimulation of transcriptional activity by CDK6 in the presence of DHT, β-estradiol, or progesterone (Fig. 3C). These findings suggest that in some cases of “androgen-independent” prostate cancers, AR-mediated pathways of gene expression that enhance growth are maintained, even when there are low levels of DHT or other steroids, by the stimulatory effects of CDK6 on mutant forms of the AR.
We should emphasize that all of the above described results on the stimulatory effects of CDK6 on the transcriptional activity of the AR were obtained in cell culture systems. We believe that they may be relevant to human prostate cancer because a preliminary study indicates that CDK6 is overexpressed in 44% of a series of 34 cases of prostate cancer (our unpublished studies). It will be of interest to examine a larger series of cases for possible correlations between overexpression of CDK6 and specific clinical and pathologic parameters. There is evidence that decreased expression of p16INK4a in high-grade prostate cancers can predict early relapses and that increased expression of p16INK4a in prostate cancer cells leads to senescence (31). Because p16INK4a can inhibit CDK6 stimulation of the transcriptional activity of the AR (Fig. 1C), an examination of the relationship between overexpression of CDK6 and the expression of p16INK4a in cases of prostate cancer will also be of interest. Hopefully, these clinical studies and further mechanistic studies will indicate whether CDK6 may provide a useful prognostic marker or a target for the therapy of prostate cancer.
Acknowledgments
We thank Drs. Robert Matusik (Vanderbilt University, Nashville, TN), Scott Cramer (Wake Forest University, Winston-Salem, NC), Phil Hinds (Harvard University, Cambridge, MA), David Feldman (Stanford University, Stanford, CA) and A. O. Brinkmann (Erasmus University, Rotterdam, The Netherlands) for the (ARR)3–luciferase, PSA–luciferase, CDK6, AR point mutants, and AR plasmids, respectively. We also thank Dr. Jiang Wei (The Burnham Institute, La Jolla, CA) for the CDK1, CDK2, and CDK4 constructs and Dr. Richard Pestell (Georgetown University Medical Center, Washington, D.C.) for generously providing the NIH 3T3 cells deficient in cyclin D1. This study was supported by U.S. Department of Defense Medical Research Grant PC010375 (to J.T.E.L. and I.B.W.), the T. J. Martell Foundation (I.B.W.), and the National Foundation for Cancer Research (I.B.W.).
Author contributions: J.T.E.L. and I.B.W. designed research; J.T.E.L. and M.M. performed research; J.T.E.L., M.M., and I.B.W. analyzed data; and J.T.E.L. and I.B.W. wrote the paper.
Abbreviations: CDK, cyclin-dependent kinase; HA, hemagglutinin; PSA, prostate-specific antigen; DHT, dihydrotestosterone; AR, androgen receptor.
References
- 1.Jemal, A., Murray, T., Ward, E., Samuels, A., Tiwari, R. C., Ghafoor, A., Feuer, E. J., Thun, M. J. (2005) CA Cancer J. Clin. 55, 10–30. [DOI] [PubMed] [Google Scholar]
- 2.Culig, Z., Hobisch, A., Bartsch, G. & Klocker, H. (2000) Urol. Res. 28, 211–219. [DOI] [PubMed] [Google Scholar]
- 3.Ross, R. K., Bernstein, L., Lobo, R. A., Shimizu, H., Stanczyk, F. Z., Pike, M. C. & Henderson, B. E. (1992) Lancet 339, 887–889. [DOI] [PubMed] [Google Scholar]
- 4.Huang, H. & Tindall, D. J. (2002) Crit. Rev. Eukaryotic Gene Expression 12, 193–207. [DOI] [PubMed] [Google Scholar]
- 5.Feldman, B. J. & Feldman, D. (2001) Nat. Rev. Cancer 1, 34–45. [DOI] [PubMed] [Google Scholar]
- 6.Craft, N., Shostak, Y., Carey, M. & Sawyers, C. L. (1999) Nat. Med. 5, 280–285. [DOI] [PubMed] [Google Scholar]
- 7.Buckley, M. F., Sweeney, K. J., Hamilton, J. A., Sini, R. L., Manning, D. L., Nicholson, R. I., deFazio, A., Watts, C. K., Musgrove, E. A. & Sutherland, R. L. (1993) Oncogene 8, 2127–2133. [PubMed] [Google Scholar]
- 8.Sutter, T., Doi, S., Carnevale, K. A., Arber, N. & Weinstein, I. B. (1997).J. Med. 28, 285–309. [PubMed] [Google Scholar]
- 9.Gumbiner, L. M., Gumerlock, P. H., Mack, P. C., Chi, S. G., DeVere White, R. W., Mohler, J. L., Pretlow, T. G. & Tricoli, J. V. (1999) Prostate 38, 40–45. [DOI] [PubMed] [Google Scholar]
- 10.Han, E. K., Lim, J. T., Arber, N., Rubin, M. A., Xing, W. Q. & Weinstein, I. B. (1998) Prostate 35, 95–101. [DOI] [PubMed] [Google Scholar]
- 11.Zwijsen, R. M., Wientjens, E., Klompmaker, R., van der Sman, J., Bernards, R. & Michalides, R. J. (1997) Cell 88, 405–415. [DOI] [PubMed] [Google Scholar]
- 12.Reutens, A. T., Fu, M., Wang, C., Albanese, C., McPhaul, M. J., Sun, Z., Balk, S. P., Janne, O. A., Palvimo, J. J. & Pestell, R. G. (2001) Mol. Endocrinol. 15, 797–811. [DOI] [PubMed] [Google Scholar]
- 13.Knudsen, K. E., Cavenee, W. K. & Arden, K. C. (1999) Cancer Res. 59, 2297–2301. [PubMed] [Google Scholar]
- 14.Yan, H., Krishnan, K., Lim, J. T., Contillo, L. G. & Krolewski, J. J. (1996) Mol. Cell. Biol. 16, 2074–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou, P., Jiang, W., Weghorst, C. M. & Weinstein, I. B. (1996) Cancer Res. 56, 36–39. [PubMed] [Google Scholar]
- 16.Suzui, M., Masuda, M., Lim, J. T., Albanese, C., Pestell, R. G. & Weinstein, I. B. (2002) Cancer Res. 62, 3997–4006. [PubMed] [Google Scholar]
- 17.Shirin, H., Pinto, J. T., Kawabata, Y., Soh, J. W., Delohery, T., Moss, S. F., Murty, V., Rivlin, R. S., Holt, P. R. & Weinstein, I. B. (2001) Cancer Res. 61, 725–731. [PubMed] [Google Scholar]
- 18.Masuda, M., Suzui, M. & Weinstein, I. B. (2001) Clin. Cancer Res. 7, 4220–4229. [PubMed] [Google Scholar]
- 19.Wang, L. G., Liu, X. M., Kreis, W. & Budman, D. R. (1997) Cancer Res. 57, 714–719. [PubMed] [Google Scholar]
- 20.Luo, R. X., Postigo, A. A. & Dean, D. C. (1998) Cell 92, 463–473. [DOI] [PubMed] [Google Scholar]
- 21.Albanese, C., Wu, K., D'Amico, M., Jarrett, C., Joyce, D., Hughes, J., Hulit, J., Sakamaki, T., Fu, M., Ben-Ze'ev, A., et al. (2003) Mol. Biol. Cell 14, 585–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grossel, M. J., Baker, G. L. & Hinds, P. W. (1999) J. Biol. Chem. 274, 29960–29967. [DOI] [PubMed] [Google Scholar]
- 23.Jenster, G., van der Korput, H. A., van Vroonhoven, C., van der Kwast, T. H., Trapman, J. & Brinkmann, A. O. (1991) Mol. Endocrinol. 5, 1396–1404. [DOI] [PubMed] [Google Scholar]
- 24.Stanford, J. L., Just, J. J., Gibbs, M., Wicklund, K. G., Neal, C. L., Blumenstein, B. A. & Ostrander, E. A. (1997) Cancer Res. 57, 1194–1198. [PubMed] [Google Scholar]
- 25.Kantoff, P., Giovannucci, E. & Brown, M. (1998) Biochim. Biophys. Acta 1378, C1–C5. [DOI] [PubMed] [Google Scholar]
- 26.Taplin, M. E., Bubley, G. J., Shuster, T. D., Frantz, M. E., Spooner, A. E., Ogata, G. K., Keer, H. N. & Balk, S. P. (1995) N. Engl. J. Med. 332, 1393–1398. [DOI] [PubMed] [Google Scholar]
- 27.Gaddipati, J. P., McLeod, D. G., Heidenberg, H. B., Sesterhenn, I. A., Finger, M. J., Moul, J. W. & Srivastava, S. (1994) Cancer Res. 54, 2861–2864. [PubMed] [Google Scholar]
- 28.Meyerson, M. & Harlow, E. (1994) Mol. Cell. Biol. 14, 2077–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trapman, J. & Brinkmann, A. O. (1996) Pathol. Res. Pract. 192, 752–760. [DOI] [PubMed] [Google Scholar]
- 30.Zhao, X. Y., Malloy, P. J., Krishnan, A. V., Swami, S., Navone, N. M., Peehl, D. M. & Feldman, D. (2000) Nat. Med. 6, 703–706. [DOI] [PubMed] [Google Scholar]
- 31.Sandhu, C., Peehl, D. M. & Slingerland, J. (2000) Cancer Res. 60, 2616–2622. [PubMed] [Google Scholar]