Abstract
Systemic sclerosis (SSc) is a multi-organ fibrotic disease with few treatment options. Activated fibroblasts are the key effector cells in SSc responsible for the excessive production of collagen and the development of fibrosis. PDGF, a potent mitogen for cells of mesenchymal origin, has been implicated in the activation of SSc fibroblasts. Our aim was to examine the therapeutic potential of crenolanib, an inhibitor of PDGF receptor signaling, in cultured fibroblasts and in angiotensin II (Ang II)-induced skin and heart fibrosis. Crenolanib effectively inhibited proliferation and migration of SSc and healthy control (HC) fibroblasts, and attenuated basal and TGF-β-induced expression of CCN2/CTGF and periostin. In contrast to HC fibroblasts, SSc fibroblasts proliferated in response to PDGFAA, while a combination of PDGFAA and CCN2 was required to elicit a similar response in HC fibroblasts. Importantly, PDGFRα mRNA correlated with CCN2 and other fibrotic markers in the skin of SSc patients. In mice challenged with Ang II, PDGFRα-positive cells were increased in the skin and heart. These PDGFRα-positive cells co-localized with PDGFRβ, procollagen and periostin. Treatment with crenolanib attenuated the skin and heart fibrosis. Our data indicate that inhibition of PDGF signaling presents an attractive therapeutic approach for SSc.
INTRODUCTION
Systemic sclerosis (SSc) is a devastating multiorgan disease with few treatment options. Prominent skin and organ fibrosis is a hallmark feature of SSc and is accompanied by fibroproliferative vasculopathy and immune dysfunction (Allanore et al., 2015). Activated fibroblasts are the key effector cells in SSc responsible for the excessive production of collagen and a subsequent development of fibrosis. A large number of soluble paracrine mediators have been implicated in fibrosis; in particular, the TGF-β signaling pathway plays a central role in inducing pro-fibrogenic cellular programs (Lafyatis, 2014).
In addition to soluble mediators, several matricellular proteins have also been shown to contribute to the development of SSc. For example, CCN2 (also known as connective tissue growth factor, CTGF), which is present at the elevated levels in SSc serum and fibrotic skin, has been associated with fibrosis in multiple organs (Igarashi et al., 1995, Leask and Abraham, 2006, Sato et al., 2000). CCN2 has been shown to cooperate with TGF-β to induce persistent fibrosis (Mori et al., 1999). Furthermore, fibroblast specific ablation of CCN2 prevented development of dermal fibrosis in the bleomycin injection model (Liu et al., 2011). It has also been suggested that CCN2 may contribute to myofibroblast recruitment during bleomycin-induced skin fibrosis (Liu et al., 2011). Recent studies have also implicated periostin in the process of fibrosis (Huang et al., 2015, Lorts et al., 2012). Elevated levels of periostin are present in the lesional skin and serum of SSc patients (Yamaguchi et al., 2013). Periostin was shown to induce fibroblast proliferation and a myofibroblast phenotype in hypertrophic scarring (Crawford et al., 2015). Periostin-deficient mice were protected from the bleomycin-induced dermal fibrosis (Yang et al., 2012).
Platelet-derived growth factors (PDGFs), the primary mitogens for cells of mesenchymal origin, mediate their biological effects through the activation of two structurally related tyrosine kinase receptors, PDGFRα and PDGFRβ (Heldin and Lennartsson, 2013). Although PDGF-A is a relatively weak mitogen for dermal fibroblasts in comparison with PDGF-B, recent studies have provided evidence for the important role of activated PDGFRα signaling in the development of organ fibrosis, including skin fibrosis (Olson and Soriano, 2009). On the other hand, activation of PDGFRβ leads to increased immune activation, but not fibrosis (Olson and Soriano, 2011). Furthermore, administration of PDGF-A promotes atrial fibrosis, while neutralizing PDGFRα suppresses atrial fibrosis (Liao et al., 2010). Analyses of SSc skin biopsies also support the involvement of PDGF-A/PDGFRα in SSc. PDGF-A is overexpressed in SSc lesions (Yamakage et al., 1992) and increased expression of PDGFRα and PDGFRβ on spindle cells correlates with collagen deposition in SSc biopsies (Daoussis et al., 2012). Increased PDGF-A has also been reported in the dermal interstitial blister fluid of SSc patients (Clark et al., 2015). In addition, agonistic anti-PDGFRα autoantibodies have been detected in the serum of SSc patients (Moroncini et al., 2015).
Crenolanib besylate is an orally bioavailable, well tolerated, selective inhibitor of type III tyrosine kinases (PDGFRα, PDGFRβ, and FMS-like tyrosine kinase 3) (Galanis et al., 2014). Crenolanib has higher sensitivity for PDGFRα (IC50 0.4 ng/ml) vs PDGFRβ (IC50 0.8 ng/ml) and preferentially binds to phosphorylated active kinases. Crenolanib has been clinically evaluated in Phase I and -II settings for treatment of solid tumors, including glioma and gastrointestinal stromal tumors (Heinrich et al., 2012). Given the key role of PDGFR signaling in the development of SSc fibrosis, the goal of this study was to evaluate the potential efficacy of crenolanib as a potential therapeutic agent for SSc using patient derived dermal fibroblasts and a murine model of angiotensin II (Ang II)-induced skin and heart fibrosis.
RESULTS
PDGFR signaling contributes to expression of periostin and CCN2 in healthy control and SSc dermal fibroblasts
In an initial experiment, we examined the effect of crenolanib on the activation of PDGF receptors in human skin fibroblasts stimulated with PDGFAA or PDGFBB. Crenolanib (25–100 nM) dose-dependently blocked phosphorylation of PDGFRα upon stimulation with PDGFAA or PDGFBB (Supplementary Figure S1a and b) and PDGFRβ upon stimulation with PDGFBB (Supplementary Figure S1b). Crenolanib did not affect cell viability.
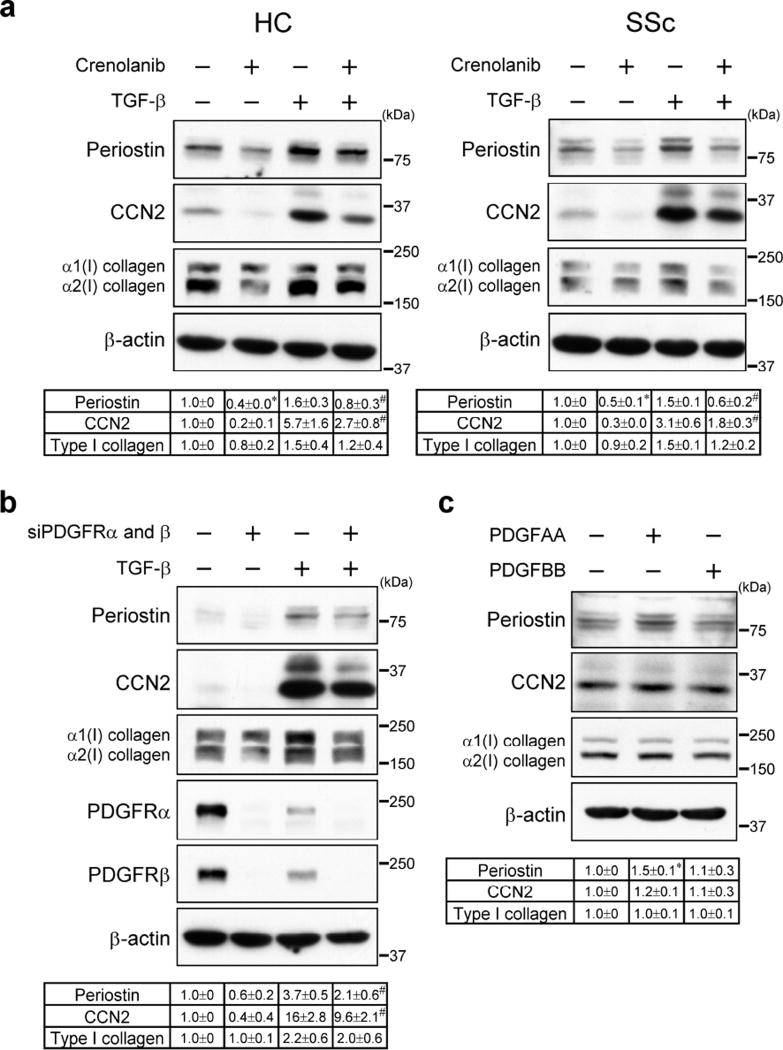
While TGF-β is the principal inducer of the ECM proteins, PDGF has also been shown to contribute to matrix production (Horikawa et al., 2015). Therefore, we examined the effect of crenolanib on type I collagen and other selected ECM proteins in healthy dermal (HC) and SSc fibroblasts at the basal level and after TGF-β stimulation. Crenolanib (100 nM) significantly reduced basal and TGF-β-induced periostin and CCN2 protein expression, while only modest inhibition was observed for type I collagen (Figure 1a). This dose of crenolanib is comparable with the dose used in human preclinical trials (Lewis et al., 2009). Crenolanib also partially reduced basal and TGF-β-induced mRNA levels of type I collagen, periostin and CCN2 in HC and SSc fibroblasts (Supplementary Figure S2 and S3). Depletion of PDGFRα and PDGFRβ in HC fibroblasts reproduced the inhibitory effects of crenolanib on periostin, CCN2 and type I collagen protein levels (Figure 1b). We next assessed whether PDGF ligands induce expression of periostin, CCN2, and type I collagen in HC fibroblasts. Interestingly, PDGFAA, but not PDGFBB, up-regulated periostin protein levels; however, CCN2 or type I collagen were not affected (Figure 1c). Together, these results suggest that PDGFR signaling contributes to the expression of selected matrix proteins in dermal fibroblasts.
Figure 1. Crenolanib inhibits periostin, CCN2, and collagen protein levels in HC and SSc skin fibroblasts.
(a) HC and SSc fibroblasts were pre-treated for 1 h with crenolanib (100 nM), followed by 24 h treatment with TGF-β (2.5 ng/ml). (b) HC fibroblasts were transfected with siRNA targeting both PDGFRα and PDGFRβ for 24 h followed by 24 h treatment with TGF-β. (c) HC fibroblasts were stimulated with PDGFAA (10 ng/ml) or PDGFBB (10 ng/ml). (a, b, c) Cell lysates were subjected to immunoblotting. The values below each blot showed mean ± SD of the relative protein levels by densitometry. Values are normalized relative to control. n = 3 per each group. *P versus control; #P versus TGF-β. *P < 0.05, #P < 0.05.
Crenolanib inhibits proliferation and migration of fibroblasts
PDGF is a potent mitogenic and chemotactic agent for fibroblasts, therefore we evaluated the effect of crenolanib on proliferation and migration in HC and SSc fibroblasts using the IncuCyte® system. Crenolanib was very effective in inhibiting HC and SSc fibroblast proliferation in response to 10% FBS or PDGFBB (Figure 2a). PDGFAA upregulated proliferation only in SSc fibroblasts (Figure 2a) and crenolanib blocked this response. We also examined the mitogenic effect of CCN2, which has previously been reported to indirectly induce fibroblast proliferation (Xu et al., 2015). Interestingly, while CCN2 alone had no effect, addition of PDGFAA together with CCN2 induced proliferation of HC fibroblasts with similar potency to that of PDGFBB (Figure 2b). The co-stimulatory effect of CCN2 on mitogenic effects of PDGFAA was not present in SSc fibroblasts (Figure 2b). The addition of CCN2 did not influence stimulatory effects of PDGFBB.
Figure 2. Crenolanib inhibits proliferation of HC and SSc fibroblasts.
(a, b) Proliferation of HC or SSc fibroblasts was measured by IncuCyte system. Fibroblasts were cultured with 10% FBS or 1% FBS media containing PDGFAA, PDGFBB, crenolanib or CCN2 as shown in the right panels. The line graphs represent mean (n = 3) confluence measurements (%). The bar graphs represent mean ± SD (n = 3) of area under the curve (AUC). Values are normalized relative to control. *P < 0.05. n.s., not significant. (c) HC fibroblasts were pre-treated for 1 h with crenolanib (100 nM), followed by 30 min stimulation with PDGFAA (10 ng/ml) or PDGFBB (10 ng/ml). Cell Lysates were subjected to immunoblotting. The values below each blot showed mean ± SD of the relative protein levels. Values are normalized relative to control, n = 3 per each group. *P versus control; #P versus PDGFAA or BB. *P < 0.05, #P < 0.05. (d) HC and SSc fibroblasts were stimulated with PDGFAA (10 ng/ml) and/or CCN2 (25 ng/ml) for 30 min. Cell Lysates were subjected to immunoblotting. The values below each blot showed mean ± SD of the relative protein levels. Values are normalized relative to control. n = 3 per each group. *P versus PDGFAA. *P < 0.05.
We also investigated the effects of crenolanib on HC and SSc fibroblast migration in response to 10% FBS or PDGF ligands. Cell migration was measured as relative wound density over time. 10% FBS and both PDGF ligands stimulated migration of HC and SSc fibroblast and crenolanib blocked these responses (Supplementary Figure S4a). CCN2 did not induce cell migration and did not cooperate with either PDGFAA or -BB in inducing cell migration (Supplementary Figure S4b). The effects of crenolanib on cell proliferation and migration in response to 10% FBS may also include the inhibitory effects on other agonists, which signal via PDGFRs such as VEGF (Ball et al., 2007). We also cannot exclude off target effects of crenolanib.
To further investigate the cooperation between PDGFAA and CCN2 in HC fibroblast proliferation, we focused on ERK1/2 and Akt, the main intracellular signaling pathways activated by PDGFRs. Both, PDGFAA and -BB induced phosphorylation of ERK1/2 and Akt with PDGFBB eliciting a stronger response (Figure 2c) and, as expected, crenolanib blocked these responses. CCN2 had no effect on ERK1/2 and Akt phosphorylation, however, addition of CCN2 together with PDGFAA, significantly increased ERK1/2, but not Akt, phosphorylation as compared to PDGFAA alone in HC fibroblasts, (Figure 2d). The cooperation between PDGFAA and CCN2 did not occur in SSc fibroblasts. These results are consistent with the proliferation assay and suggest a cross-talk between PDGFAA and CCN2 in HC fibroblasts.
PDGFRα and CCN2 mRNAs correlate in SSc skin biopsies
The association between PDGFAA and CCN2 prompted us to investigate the relationship between PDGFRα and CCN2 in HC and SSc skin biopsies. Skin biopsy specimens obtained from 33 diffuse cutaneous SSc (dcSSc) and 15 HC subjects were analyzed by microarrays. The mRNA expressions of PDGFRα, PDGFRβ and periostin were comparable in SSc and HC skin and did not correlate with mRSS (Figure 3a and d). CCN2 mRNA was significantly increased in SSc skin and correlated with mRSS (Figure 3a and d). Interestingly, there was a significant correlation between PDGFRα and CCN2 and PDGFRα and periostin mRNAs in SSc skin, which was not observed in HC skin (Figure 3b and c). PDGFRβ did not correlate with CCN2 or periostin in either SSc or HC skin (Supplementary Figure S5a and b). Analysis of additional profibrotic genes associated with SSc (Rice et al., 2015b), including MS4A4A, THBS1, COMP, ACTA2, COL1A1 and COL1A2, revealed a significant association between PDGFRα and MS4A4A, THBS1, COMP, PDGFRβ, COL1A1 and COL1A2 (Figure 3d). PDGFRβ also correlated with COL1A1 and COL1A2 (Figure 3d).
Figure 3. Relationship between PDGFRα/β and CCN2 in HC and SSc skin.
RNA of skin biopsies from HC or SSc patients was analyzed by microarray. Modified Rodnan skin score (mRSS) is also added in microarray analysis. (a) Gene expressions between HC and SSc skin are shown in log2 scale. (b, c) Correlations were assessed by Pearson’s correlation coefficient. Correlation coefficient r and P values are shown in each panel. Gene expressions are shown in log2 scale. (b) PDGFRα vs CCN2. (d) PDGFRα vs Periostin. (d) The intersection of each marker represents r and P value. Blue and red color indicates high or low r-value. The circle size correlates with an absolute value of r value. The number in each box shows P value. No number in each box means that the P value is less than 0.05.
Crenolanib mitigates skin fibrosis in the Ang II-challenged mice
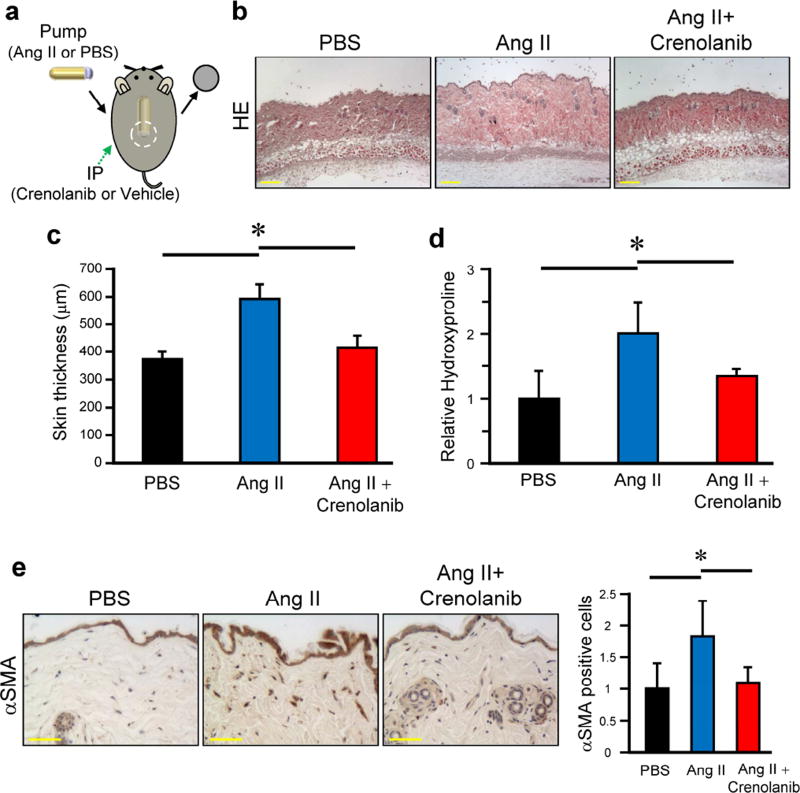
Ang II-induced skin and heart fibrosis recapitulates many fibrotic and vascular features of SSc (Stawski et al., 2012) and we used this model to evaluate therapeutic effects of crenolanib. Ang II was administered by subcutaneous osmotic pump, and the skin surrounding the pump outlet was collected on day 14 (Figure 4a). Crenolanib 15 mg/kg was administered intraperitoneally to C57BL/6J mice once daily for 2 weeks. Treatment with crenolanib significantly reduced dermal thickness and collagen content in the back skin of Ang II-challenged mice (Figure 4b–d). Crenolanib also significantly reduced the number of αSMA-positive cells in the upper dermis (Figure 4e).
Figure 4. Crenolanib ameliorates skin fibrosis in Ang II-induced SSc mice model.
(a) Skin fibrosis in C57BL/6J mice was induced by Ang II administered via subcutaneously installed osmotic pumps. Mice were treated daily for 2 weeks with 15 mg/kg crenolanib or vehicle. (b) Mice skin sections were stained with hematoxylin and eosin (HE). Scale bar 200 µm. (c) Dermal thickness is summarized as a graph. (d) Collagen contents were measured by hydroxyproline assay. Values are normalized relative to PBS control group. (e) Skin sections were stained with anti-αSMA antibody. Representative images are shown. The αSMA positive cells were counted in all experimental mice in five random high-power fields per mouse using a light microscope. The mean score was used for analysis. Values are normalized relative to PBS control group. Each graph represents mean ± SD of the indicated parameters. n = 5 per group. *P < 0.05.
Characterization of PDGFR positive cells in the Ang II-challenged mice
We used PDGFRα-GFP mice to monitor PDGFRα-positive cells in relation to the expression of other profibrotic mediators. In mice challenged with Ang II, PDGFRα-positive cells were increased in the upper dermis on day 7 and persisted until day 14 (Figure 5). Immunofluorescence revealed that the majority of the PDGFRα-positive cells were co-localized with PDGFRβ, procollagen and periostin (Figure 5a–c), suggesting that PDGFR-positive cells directly contribute to fibrosis in this model.
Figure 5. PDGFR positive cells contribute to fibrosis in Ang II-induced SSc mice model.
Skin fibrosis in PDGFRα-GFP mice was induced by Ang II administered via subcutaneously installed osmotic pumps. Skin was extracted 7 days after pump installed. Immunofluorescent staining was performed on cryosections using antibodies to (a) PDGFRβ, (b) procollagen, and (c) periostin and counterstained with DAPI. The green fluorescence represents the PDGFRα positive cells. Arrows indicate the PDGFRα positive cells stained with each antibody. Epidermis and hair follicle are stained nonspecifically by secondary antibody. Scale bar 10 µm.
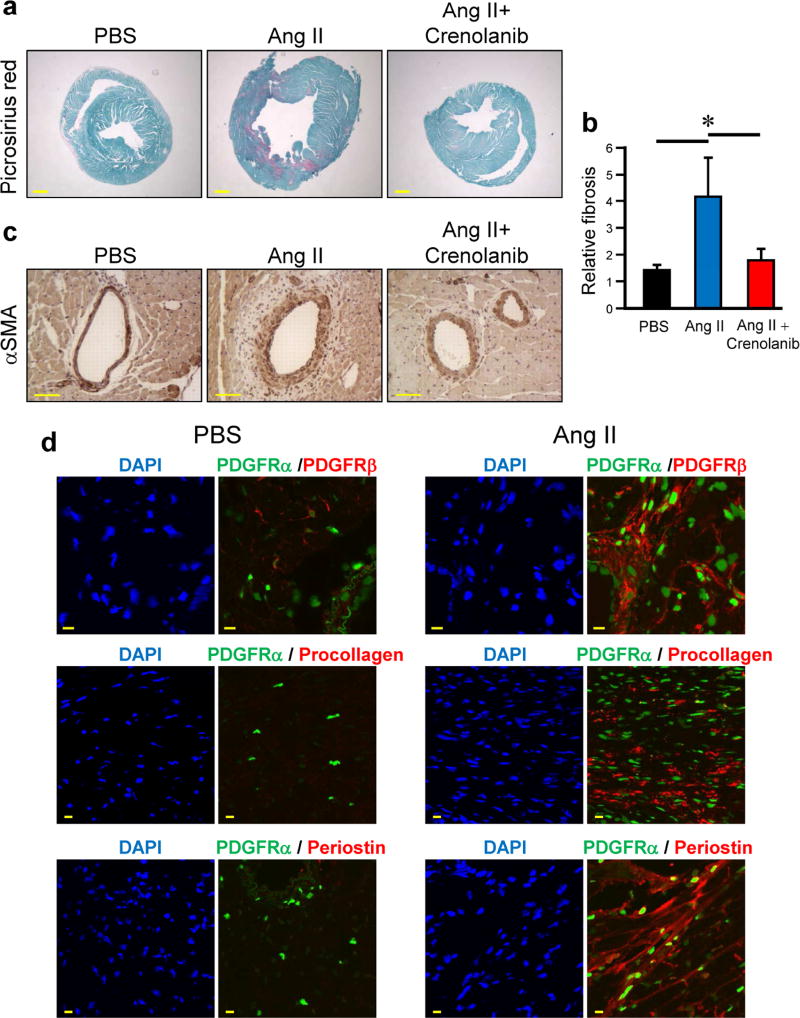
Crenolanib attenuates heart fibrosis in the Ang II-challenged mice
As reported before, mice challenged with Ang II develop heart fibrosis (Choi et al., 2016). Crenolanib treatment significantly reduced heart fibrosis as determined by picrosirius red-positive area per total area (Figure 6a and b). Crenolanib also lowered the number of αSMA-positive cells in perivascular lesions (Figure 6c). To confirm the contribution of PDGFR-positive cell in the fibrotic regions of the heart, we analyzed the presence of PDGFRα using PDGFRα-GFP mice. Ang II-challenged mice showed an increased number of PDGFRα-positive cells in the perivascular regions compared to PBS control mice (Figure 6d). As observed in the skin, PDGFRα-positive cells co-localized with PDGFRβ, procollagen and periostin (Figure 6d). Collectively, these data indicate that PDGFRα/β positive cells promote fibrosis in the Ang II mice model.
Figure 6. Crenolanib prevents Ang II-induced heart fibrosis.
(a) Heart fibrosis in C57BL/6J mice was induced by Ang II pump. Mice were treated daily for 2 weeks with 15 mg/kg crenolanib or vehicle. Representative heart images of picrosirius red staining are shown. Scale bar 500 µm. (b) Collagen content in the heart was quantified by measuring the ratio of red area to the total area in the picrosirius red staining. (c) Heart sections were stained with anti-αSMA antibody. (d) Heart fibrosis in PDGFRα-GFP mice was induced by Ang II pumps. The heart was extracted 14 days after pump installed. Immunofluorescent staining was performed on cryosections using antibodies to PDGFRβ, procollagen, and periostin and counterstained with DAPI. The green fluorescence represents the PDGFRα positive cells. Scale bar 10 µm.
DISCUSSION
At present, there are only limited treatment options for patients with SSc, but increasing understanding of the biological processes involved in SSc pathogenesis has a potential to promote the development of new, more effective therapies (Baron, 2016). Based on extensive research linking aberrant activation of tyrosine kinase (TK) receptors to fibrosis, several small molecule TK inhibitors (TKIs) initially developed for cancer treatment, have now been adopted for the treatment of fibrotic diseases (Baron, 2016). Although numerous evidence supports contribution of PDGFR signaling to the pathogenesis of SSc and other fibrotic diseases, only a handful of studies have evaluated the therapeutic potential of specifically targeting this pathway (Ieronimakis et al., 2016). In comparison to other broad spectrum TKIs such as imatinib, nilotinib, or nintedanib, crenolanib more specifically targets PDGFRs, and may offer beneficial effects with fewer side effects (Shah et al., 2013). To our knowledge our study provides previously unreported demonstration of the beneficial effects of crenolanib in SSc.
Previous studies using cancer cell lines have demonstrated that crenolanib was significantly more potent than other TKIs in inhibiting PDGFR signaling (Heinrich et al., 2012). In our study, we also observed inhibition of PDGFRα/β phosphorylation in dermal fibroblasts starting at low doses of crenolanib (25 nM). Moreover, crenolanib effectively inhibited proliferation and migration of cultured SSc and HC fibroblasts. Notably, while SSc and HC fibroblasts responded similarly to the mitogenic stimulation with PDGFBB, only SSc fibroblasts proliferated in response to PDGFAA, albeit the magnitude of response was smaller than that observed with PDGFBB. Notably, in the presence of CCN2, PDGFAA elicited a potent mitogenic response in HC fibroblasts. The response of SSc fibroblasts to PDGFAA was not affected by the addition of CCN2. These different proliferative responses of SSc and HC fibroblasts correlated with the effects of PDGFAA and CCN2 on ERK1/2 phosphorylation. PDGFAA stimulated ERK1/2 phosphorylation more potently in SSc fibroblasts, while CCN2 and PDGFAA cooperated in ERK1/2 activation in HC fibroblasts. It is conceivable that the constitutively higher endogenous levels of CCN2 in SSc fibroblasts facilitated the mitogenic response to PDGFAA (Holmes et al., 2003). Consistent with this notion, significant correlation between PDGFRα and CCN2 mRNAs was observed in SSc skin biopsies, suggesting a mechanistic link between these two pathways. To our knowledge, the co-stimulatory effect between PDGFAA and CCN2 has not been reported before.
The pro-fibrotic function of PDGF is mainly associated with its role as an inducer of proliferation and migration of mesenchymal cells, however PDGF could also contribute to the deposition of ECM proteins (Gabrielli et al., 2007, Horikawa et al., 2015). Herein, we found that PDGFR signaling was required for the basal and TGF-β-induced expression of periostin and CCN2. Previous studies in smooth muscle cells have shown that growth factors stimulate periostin expression through a PI-3-kinase dependent pathway (Li et al., 2006). Given the important role of periostin and CCN2 in the development of fibrosis (Liu et al., 2011, Yang et al., 2012), blockade of PDGF signaling by crenolanib, would not only limit the expansion of PDGFR-positive ECM producing cells, but also would suppress production of ECM proteins by those cells.
To evaluate therapeutic effects of crenolanib in an in vivo model of SSc, we used a mouse model of Ang II-induced skin and heart fibrosis (Stawski et al., 2014). Ang II-induced fibrosis is accompanied by diverse pathogenic mechanisms, including collagen accumulation, CCN2 upregulation, myofibroblast accumulation, endothelial cell injury, and inflammation (Stawski et al., 2014). These pathogenic features observed in the Ang II model have many similarities with human SSc. Elevated serum levels of Ang II have been reported in SSc patients in the early stage of the disease (Kawaguchi et al., 2004). Moreover, functional, pathogenic antibodies directed against AT1R and ETAR were identified in patients with SSc and linked to increased prevalence of SSc-related vascular and fibrotic complications and higher risk for SSc-induced mortality (Kill et al., 2014, Riemekasten et al., 2011). Ang II induced fibrosis correlated with accumulation of the PDGFRα positive cells in the skin and heart. Majority of PDGFRα positive cells also expressed PDGFRβ, procollagen and periostin. Crenolanib was very effective in preventing dermal and heart fibrosis in this model. These results are consistent with the notion that PDGFR-positive cells directly contribute to the ECM deposition (Chen et al., 2011, Gallini et al., 2016, Ieronimakis et al., 2016). This is further supported by the analysis of SSc skin biopsies that showed significant correlation between PDGFRα, PDGFRβ, COL1A1 and COL1A2. The limitation of this study was that it only assessed the preventive effect of crenolanib and further studies are needed to evaluate whether crenolanib is effective in reversing the established fibrosis.
In summary, we demonstrated that crenolanib is effective in reducing skin and heart fibrosis in vivo by inhibiting accumulation of PDGFR-positive collagen producing fibroblasts. Because crenolanib is already used in clinical trials with good safety records, this study strongly supports the testing of crenolanib as a therapy for patients with SSc.
MATERIALS AND METHODS
Cell culture
Upon informed written consent and in compliance with the Institutional Review Board for Human Studies, skin fibroblasts were obtained by skin biopsy from the affected areas (dorsal forearm) of patients with diffuse cutaneous SSc (dcSSc). All patients fulfilled the criteria of the American College of Rheumatology for SSc. Control fibroblasts were obtained by skin biopsy of a healthy donor; these were matched with each SSc patient for age, race, gender, and biopsy site and were processed in parallel. Fibroblasts were grown in DMEM with 10% FBS. Fibroblasts between the third and eight subpassages were used for experiments.
Microarray
Skin biopsy samples collected from 8 healthy controls and data from 33 dcSSc and 7 healthy controls from previous studies using the same U133A 2.0 chip (Affymetrix, Santa Clara, CA) were included in the microarray analysis (Rice et al., 2015a, Rice et al., 2015b). Characteristics of study subjects are described in Supplemental Table S1. Microarray data were normalized using MAS5.0 and adjusted for batch effects using the ComBat algorithm (Hubbell et al., 2002, Johnson et al., 2007). Microarray probesets were mapped using Brainarray (version 19.0.0) Entrez Gene mapping (http://brainarray.mbni.med.umich.edu/Brainarray/Database/CustomCDF). Pearson’s correlations were calculated to examine the expression relationship between the selected genes. All microarray data used for analysis are deposited in the GEO Database, accession numbers GSE55036 and GSE95065. All samples and clinical data were collected under a protocol approved by the Boston University Medical Campus Institutional Review Board, informed consent was obtained from all patients and healthy controls.
Reagents
Recombinant PDGFAA and PDGFBB were from PeproTech (Rocky Hill, NJ). Crenolanib and CCN2 were from Selleckchem (Houston, TX) and EMP Genetech (Ingolstadt, Germany), respectively. Recombinant human TGF-β1 was obtained from PeproTech.
RNA isolation and quantitative real-time PCR
Gene expression levels were determined by quantitative real-time PCR, as described previously (Hattori et al., 2011). mRNA levels were normalized to those of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene. Relative expression was calculated using the ΔΔCt method. The primer sequences used for qPCR are available upon request.
Immunoblotting
Human dermal fibroblasts were lysed and subjected to immunoblotting, as described previously (Hattori et al., 2011). Primary antibodies used (1:1000): phospho-PDGFRα, PDGFRα, phospho-PDGFRβ, PDGFRβ, phospho-ERK1/2, ERK1/2, phospho-Akt, Akt from Cell Signaling (Danvers, MA), Type I collagen from Southern Biotech (Birmingham, AL), CCN2 from Santa Cruz Biotechnology (Dallas, TX), periostin from Abcam (Cambridge, MA) and β-actin from Sigma (St. Louis, MO). Densitometric quantification was done using Image J.
siRNA Transient Transfections
Human dermal fibroblasts were transfected with siRNA specific to both human PDGFRα and PDGFRβ (ON-TARGETplus SMART pool, GE Dharmacon, Lafayette, CO) or scrambled siRNA for the duration of 24 h at the concentration of 20 nM using Lipofectamine RNAiMAX Transfection Reagent (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer's protocol.
Proliferation assays
Fibroblasts proliferation and migration assays were carried out using Incucyte live cell imaging system (Essen BioScience, Ann Arbor, MI). For the proliferation assay, fibroblasts (1.5 × 103 cells/well) were seeded in 96-well ImageLock plates (Essen Bioscience) on the day before proliferation assay, followed by incubation in 1% FBS medium overnight. Each well was then replaced with 1% or 10% FBS medium containing: PDGFAA (10 ng/ml), PDGFBB (10 ng/ml), crenolanib (100 nM) and CCN2 (25 ng/ml). Images were taken every 3 h for 60 h. The cell proliferation is expressed as a percentage of confluence.
Mice
B6. 129S4-Pdgfratm11(EGFP)Sor/J (PDGFRα-GFP) and Wild-type C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME). All mice experiments were performed in accordance with the National Institutes of Health and institutional guidelines for animal care, and approved by the Committee on the Ethics of Animal Experiments of the Boston University.
Ang II-induced fibrosis mice model
Alzet osmotic miniature pumps (Model 1002, DURECT, Cupertino, CA) delivering Ang II (EMD Millipore, Billerica, MA) at a rate of 1000 ng/kg/min (pressor dose) or PBS, were implanted subcutaneously on the back of 8-week old mice, as described previously (Stawski et al., 2012). Crenolanib was dissolved in 5% glycerol formal at the concentration of 1 mg/ml. Crenolanib 15 mg/kg via intraperitoneal injection was carried out daily for 1 or 2 weeks. At the end of experiment mice were euthanized and the heart and skin surrounding the pump outlet were collected.
Histologic assessment
5 µm thick skin samples were stained with haematoxylin and eosin (HE). Dermal thickness was evaluated by measuring the distance between the epidermal-dermal junction and the dermal-fat junction in HE sections. Heart samples were stained with picrosirius red to detect collagen deposition, as described previously (Stawski et al., 2014). Collagen content was determined by picrosirius red-positive area per total area. Densitometric quantification was done using Image J.
Hydroxyproline assay
Collagen deposition was determined by measuring total hydroxyproline content in 8 mm skin punch biopsies obtained from PBS and Ang II infusion sites as described previously (Stawski et al., 2014).
Immunohistochemistry
Primary antibodies used: anti-αSMA (1:100, Novus Biologicals, Littleton, CO).
Immunofluorescence staining on frozen sections
The 5 µm cryosections were incubated at 4°C overnight with primary antibodies: PDGFRβ (1:50, Cell signaling), procollagen (COL1A1 propeptide, 1:50, Thermo Fisher Scientific), periostin (1:50, Abcam). Secondary antibodies conjugated with Alexa 594 (Thermo Fisher Scientific) were used. Coverslips were mounted by using Vectashield with DAPI (Vector Laboratories, Burlingame, CA). Fluorescence images were recorded with FV10i fluorescence microscope (Olympus, Tokyo, Japan).
Statistical analysis
Values are presented as means ± standard deviation (SD). One-way analysis of variance with Tukey-Kramer test was used to determine significance between more than two groups. Correlations were assessed by Pearson’s correlation coefficient using Graphpad Prism Software (GraphPad software, La Jolla, CA). Analyses other than correlations were performed with Statcel software (OMS, Tokorozawa, Japan). Significance was defined as P <0.05.
Supplementary Material
Acknowledgments
This work was supported in part by the NIH NIAMS grant R01 AR44883 (MT), and the P50 AR 060780 (RL, RS, MT).
ABBREVIATIONS
- Ang II
angiotensin II
- ECM
extracellular matrix
- HC
healthy control
- mRSS
modified Rodnan skin score
- PDGF
platelet-derived growth factor
- PDGFR
platelet-derived growth factor receptor
- siRNA
small interfering RNA
- SSc
systemic sclerosis
- TGF-β
transforming growth factor-β
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- Allanore Y, Simms R, Distler O, Trojanowska M, Pope J, Denton CP, et al. Systemic sclerosis. Nat Rev Dis Primers. 2015;1:15002. doi: 10.1038/nrdp.2015.2. [DOI] [PubMed] [Google Scholar]
- Ball SG, Shuttleworth CA, Kielty CM. Vascular endothelial growth factor can signal through platelet-derived growth factor receptors. J Cell Biol. 2007;177(3):489–500. doi: 10.1083/jcb.200608093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron M. Targeted Therapy in Systemic Sclerosis. Rambam Maimonides Med J. 2016;7(4) doi: 10.5041/RMMJ.10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YT, Chang FC, Wu CF, Chou YH, Hsu HL, Chiang WC, et al. Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int. 2011;80(11):1170–81. doi: 10.1038/ki.2011.208. [DOI] [PubMed] [Google Scholar]
- Choi YS, de Mattos AB, Shao D, Li T, Nabben M, Kim M, et al. Preservation of myocardial fatty acid oxidation prevents diastolic dysfunction in mice subjected to angiotensin II infusion. J Mol Cell Cardiol. 2016;100:64–71. doi: 10.1016/j.yjmcc.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KE, Lopez H, Abdi BA, Guerra SG, Shiwen X, Khan K, et al. Multiplex cytokine analysis of dermal interstitial blister fluid defines local disease mechanisms in systemic sclerosis. Arthritis Res Ther. 2015;17:73. doi: 10.1186/s13075-015-0575-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford J, Nygard K, Gan BS, O'Gorman DB. Periostin induces fibroblast proliferation and myofibroblast persistence in hypertrophic scarring. Exp Dermatol. 2015;24(2):120–6. doi: 10.1111/exd.12601. [DOI] [PubMed] [Google Scholar]
- Daoussis D, Tsamandas AC, Liossis SN, Antonopoulos I, Karatza E, Yiannopoulos G, et al. B-cell depletion therapy in patients with diffuse systemic sclerosis associates with a significant decrease in PDGFR expression and activation in spindle-like cells in the skin. Arthritis Res Ther. 2012;14(3):R145. doi: 10.1186/ar3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielli A, Svegliati S, Moroncini G, Luchetti M, Tonnini C, Avvedimento EV. Stimulatory autoantibodies to the PDGF receptor: a link to fibrosis in scleroderma and a pathway for novel therapeutic targets. Autoimmun Rev. 2007;7(2):121–6. doi: 10.1016/j.autrev.2007.02.020. [DOI] [PubMed] [Google Scholar]
- Galanis A, Ma H, Rajkhowa T, Ramachandran A, Small D, Cortes J, et al. Crenolanib is a potent inhibitor of FLT3 with activity against resistance-conferring point mutants. Blood. 2014;123(1):94–100. doi: 10.1182/blood-2013-10-529313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallini R, Lindblom P, Bondjers C, Betsholtz C, Andrae J. PDGF-A and PDGF-B induces cardiac fibrosis in transgenic mice. Exp Cell Res. 2016;349(2):282–90. doi: 10.1016/j.yexcr.2016.10.022. [DOI] [PubMed] [Google Scholar]
- Hattori T, Stawski L, Nakerakanti SS, Trojanowska M. Fli1 is a negative regulator of estrogen receptor α in dermal fibroblasts. J Invest Dermatol. 2011;131(7):1469–76. doi: 10.1038/jid.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich MC, Griffith D, McKinley A, Patterson J, Presnell A, Ramachandran A, et al. Crenolanib inhibits the drug-resistant PDGFRA D842V mutation associated with imatinib-resistant gastrointestinal stromal tumors. Clin Cancer Res. 2012;18(16):4375–84. doi: 10.1158/1078-0432.CCR-12-0625. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Lennartsson J. Structural and functional properties of platelet-derived growth factor and stem cell factor receptors. Cold Spring Harb Perspect Biol. 2013;5(8):a009100. doi: 10.1101/cshperspect.a009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Abraham DJ, Chen Y, Denton C, Shi-wen X, Black CM, et al. Constitutive connective tissue growth factor expression in scleroderma fibroblasts is dependent on Sp1. J Biol Chem. 2003;278(43):41728–33. doi: 10.1074/jbc.M305019200. [DOI] [PubMed] [Google Scholar]
- Horikawa S, Ishii Y, Hamashima T, Yamamoto S, Mori H, Fujimori T, et al. PDGFRα plays a crucial role in connective tissue remodeling. Sci Rep. 2015;5:17948. doi: 10.1038/srep17948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Liu W, Xiao H, Maitikabili A, Lin Q, Wu T, et al. Matricellular protein periostin contributes to hepatic inflammation and fibrosis. Am J Pathol. 2015;185(3):786–97. doi: 10.1016/j.ajpath.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Hubbell E, Liu WM, Mei R. Robust estimators for expression analysis. Bioinformatics. 2002;18(12):1585–92. doi: 10.1093/bioinformatics/18.12.1585. [DOI] [PubMed] [Google Scholar]
- Ieronimakis N, Hays A, Prasad A, Janebodin K, Duffield JS, Reyes M. PDGFRα signalling promotes fibrogenic responses in collagen-producing cells in Duchenne muscular dystrophy. J Pathol. 2016;240(4):410–24. doi: 10.1002/path.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi A, Nashiro K, Kikuchi K, Sato S, Ihn H, Grotendorst GR, et al. Significant correlation between connective tissue growth factor gene expression and skin sclerosis in tissue sections from patients with systemic sclerosis. J Invest Dermatol. 1995;105(2):280–4. doi: 10.1111/1523-1747.ep12318465. [DOI] [PubMed] [Google Scholar]
- Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8(1):118–27. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Takagi K, Hara M, Fukasawa C, Sugiura T, Nishimagi E, et al. Angiotensin II in the lesional skin of systemic sclerosis patients contributes to tissue fibrosis via angiotensin II type 1 receptors. Arthritis Rheum. 2004;50(1):216–26. doi: 10.1002/art.11364. [DOI] [PubMed] [Google Scholar]
- Kill A, Tabeling C, Undeutsch R, Kuhl AA, Gunther J, Radic M, et al. Autoantibodies to angiotensin and endothelin receptors in systemic sclerosis induce cellular and systemic events associated with disease pathogenesis. Arthritis Res Ther. 2014;16(1):R29. doi: 10.1186/ar4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafyatis R. Transforming growth factor β--at the centre of systemic sclerosis. Nat Rev Rheumatol. 2014;10(12):706–19. doi: 10.1038/nrrheum.2014.137. [DOI] [PubMed] [Google Scholar]
- Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119(Pt 23):4803–10. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- Lewis NL, Lewis LD, Eder JP, Reddy NJ, Guo F, Pierce KJ, et al. Phase I study of the safety, tolerability, and pharmacokinetics of oral CP-868,596, a highly specific platelet-derived growth factor receptor tyrosine kinase inhibitor in patients with advanced cancers. J Clin Oncol. 2009;27(31):5262–9. doi: 10.1200/JCO.2009.21.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Oparil S, Sanders JM, Zhang L, Dai M, Chen LB, et al. Phosphatidylinositol-3-kinase signaling mediates vascular smooth muscle cell expression of periostin in vivo and in vitro. Atherosclerosis. 2006;188(2):292–300. doi: 10.1016/j.atherosclerosis.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CH, Akazawa H, Tamagawa M, Ito K, Yasuda N, Kudo Y, et al. Cardiac mast cells cause atrial fibrillation through PDGF-A-mediated fibrosis in pressure-overloaded mouse hearts. J Clin Invest. 2010;120(1):242–53. doi: 10.1172/JCI39942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Shi-wen X, Abraham DJ, Leask A. CCN2 is required for bleomycin-induced skin fibrosis in mice. Arthritis Rheum. 2011;63(1):239–46. doi: 10.1002/art.30074. [DOI] [PubMed] [Google Scholar]
- Lorts A, Schwanekamp JA, Baudino TA, McNally EM, Molkentin JD. Deletion of periostin reduces muscular dystrophy and fibrosis in mice by modulating the transforming growth factor-β pathway. Proc Natl Acad Sci U S A. 2012;109(27):10978–83. doi: 10.1073/pnas.1204708109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Kawara S, Shinozaki M, Hayashi N, Kakinuma T, Igarashi A, et al. Role and interaction of connective tissue growth factor with transforming growth factor-β in persistent fibrosis: A mouse fibrosis model. J Cell Physiol. 1999;181(1):153–9. doi: 10.1002/(SICI)1097-4652(199910)181:1<153::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Moroncini G, Grieco A, Nacci G, Paolini C, Tonnini C, Pozniak KN, et al. Epitope Specificity Determines Pathogenicity and Detectability of Anti-Platelet-Derived Growth Factor Receptor α Autoantibodies in Systemic Sclerosis. Arthritis Rheumatol. 2015;67(7):1891–903. doi: 10.1002/art.39125. [DOI] [PubMed] [Google Scholar]
- Olson LE, Soriano P. Increased PDGFRα activation disrupts connective tissue development and drives systemic fibrosis. Dev Cell. 2009;16(2):303–13. doi: 10.1016/j.devcel.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson LE, Soriano P. PDGFRβ signaling regulates mural cell plasticity and inhibits fat development. Dev Cell. 2011;20(6):815–26. doi: 10.1016/j.devcel.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice LM, Padilla CM, McLaughlin SR, Mathes A, Ziemek J, Goummih S, et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J Clin Invest. 2015a;125(7):2795–807. doi: 10.1172/JCI77958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice LM, Ziemek J, Stratton EA, McLaughlin SR, Padilla CM, Mathes AL, et al. A longitudinal biomarker for the extent of skin disease in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheumatol. 2015b;67(11):3004–15. doi: 10.1002/art.39287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemekasten G, Philippe A, Nather M, Slowinski T, Muller DN, Heidecke H, et al. Involvement of functional autoantibodies against vascular receptors in systemic sclerosis. Ann Rheum Dis. 2011;70(3):530–6. doi: 10.1136/ard.2010.135772. [DOI] [PubMed] [Google Scholar]
- Sato S, Nagaoka T, Hasegawa M, Tamatani T, Nakanishi T, Takigawa M, et al. Serum levels of connective tissue growth factor are elevated in patients with systemic sclerosis: association with extent of skin sclerosis and severity of pulmonary fibrosis. J Rheumatol. 2000;27(1):149–54. [PubMed] [Google Scholar]
- Shah DR, Shah RR, Morganroth J. Tyrosine kinase inhibitors: their on-target toxicities as potential indicators of efficacy. Drug Saf. 2013;36(6):413–26. doi: 10.1007/s40264-013-0050-x. [DOI] [PubMed] [Google Scholar]
- Stawski L, Haines P, Fine A, Rudnicka L, Trojanowska M. MMP-12 deficiency attenuates angiotensin II-induced vascular injury, M2 macrophage accumulation, and skin and heart fibrosis. PloS one. 2014;9(10):e109763. doi: 10.1371/journal.pone.0109763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawski L, Han R, Bujor AM, Trojanowska M. Angiotensin II induces skin fibrosis: a novel mouse model of dermal fibrosis. Arthritis Res Ther. 2012;14(4):R194. doi: 10.1186/ar4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Li P, Liu M, Liu C, Sun Z, Guo X, et al. CCN2 and CCN5 exerts opposing effect on fibroblast proliferation and transdifferentiation induced by TGF-β. Clin Exp Pharmacol Physiol. 2015;42(11):1207–19. doi: 10.1111/1440-1681.12470. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Ono J, Masuoka M, Ohta S, Izuhara K, Ikezawa Z, et al. Serum periostin levels are correlated with progressive skin sclerosis in patients with systemic sclerosis. Br J Dermatol. 2013;168(4):717–25. doi: 10.1111/bjd.12117. [DOI] [PubMed] [Google Scholar]
- Yamakage A, Kikuchi K, Smith EA, LeRoy EC, Trojanowska M. Selective upregulation of platelet-derived growth factor α receptors by transforming growth factor β in scleroderma fibroblasts. J Exp Med. 1992;175(5):1227–34. doi: 10.1084/jem.175.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Serada S, Fujimoto M, Terao M, Kotobuki Y, Kitaba S, et al. Periostin facilitates skin sclerosis via PI3K/Akt dependent mechanism in a mouse model of scleroderma. PloS one. 2012;7(7):e41994. doi: 10.1371/journal.pone.0041994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.