Abstract
Objective
BMSCs create a special microenvironment for hematopoiesis and immunity and also display robust immunomodulatory properties which are impaired in SLE. This study was undertaken to define the mechanisms of defects in human SLE BMSCs.
Methods
Patients fulfilling SLE classification criteria and healthy controls were recruited under an Institutional Review Board approved protocol (n=6 each). BMSCs were isolated with low density Ficoll/Hypaque. BMSCs were verified by flow cytometry and studied using immunocytochemistry, real-time PCR, western blotting, comet assay, beta-galactosidase assay, and RNA interference.
Results
SLE BMSCs have a senescent phenotype characterized by reduced proliferation rate, increased production of reactive oxygen species (ROS), increased DNA damage and repair, increased expression of p53 and p16 which block the cell cycle, and altered cytokine production (increased pro-inflammatory cytokine and decreased immunomodulatory cytokine production). Moreover, SLE BMSCs have a 5 fold increase in IFNβ (p<0.05) and increased IFNβ-induced mRNAs including mRNA for the intracellular nucleic acid sensing adaptor protein MAVS whose expression was highly correlated with IFNβ levels (r > 0.9, p < 0.01). Since MAVS is known to induce IFNβ production, we hypothesized a positive feedback loop between MAVS and IFNβ. Strikingly, silencing MAVS markedly decreased IFNβ, p53, and p16 protein levels and expression of mRNAs for pro-inflammatory cytokines.
Conclusions
This study demonstrates a novel pathway for elevated IFNβ signaling in SLE that is not dependent on stimulation by immune complexes but rather is cell-intrinsic and critically mediated by IFNβ and MAVS, implicating new pathways as potential therapeutic targets.
Introduction
SLE is a multisystem autoimmune disease with substantial morbidity and mortality disproportionately targeting young women. SLE pathogenesis is characterized by autoantibody production, immune complex formation, and systemic or organ-specific inflammation (1). Bone marrow mesenchymal stem cells (BMSCs) provide a supportive microenvironment for maintenance of hematopoietic stem cells (HSCs) and for hematopoiesis. BMSCs are also capable of differentiating into various cell types such as bone, fat and cartilage. In recent years, human MSC transplantation has become an interesting but controversial approach to treating SLE and other human autoimmune diseases (2). MSC transplantation in murine models of lupus has been promising (3), and the immunomodulatory potential of human MSCs have been clearly demonstrated in these murine models (4). Open trials of MSC transplantation in humans have also shown promise, but well-controlled clinical trials are lacking (5). Interestingly, MSCs from humans with lupus are abnormal and even the earliest studies have speculated these abnormalities might be related to the pathogenesis of lupus (6). BMSCs from humans with lupus and from murine models of lupus do not grow well, show evidence of senescence, and have poor immunomodulatory capacity compared to health controls (6, 7). The present study was aimed at determining the mechanism of the BMSC abnormalities in SLE and in particular the role of type I interferon.
A predominant paradigm in SLE research is that self-nucleic acids, particularly when oxidized or complexed with auto-antibodies, are able to stimulate leukocytes via Fc receptor (FcR)-mediated uptake and ligation of TLR7/8 and TLR9 in endocytic vesicles (8). Chronic TLR stimulation is presumed to drive ongoing elevation in type I interferon production, particularly IFNα, driving elevated levels of type I IFN biological activity in serum and a type I IFN signature in peripheral blood leukocytes (9, 10). However, human clinical trials blocking IFNα have proved only limited inhibition of the IFN signature and no or limited clinical efficacy (11, 12). Trials blocking the type I IFN receptor have been more successful in blocking the IFN signature and preliminary studies suggest clinical efficacy (13). The type I IFN system consists of multiple isotypes (13 IFNα, 1 IFNβ, 1 IFNé, 1 IFNκ, and 1 IFNω) which all bind to the same IFN-I receptor (14). IFNα and IFNβ are the best studied of the type I IFN. They both induce anti-viral responses, but IFNβ binds with a much higher affinity to the IFN-I receptor and induces unique anti-proliferative and immunomodulatory effects in a cell-type specific and ‘tunable’ fashion (15). For example, IFNβ has been shown to induce reactive oxygen species, DNA damage, and p53-dependent cellular senescence, properties not shared by IFNα (16). Constitutive production of IFNβ also plays an important role in priming the immune system (17), e.g. regulating response to TLR4 ligands by macrophages, TLR7 ligands by B cells, and IFNα production by fibroblasts (18, 19). IFNβ, originally known as fibroblast IFN, is the major IFN produced by mesenchymal cells. Thus, it seemed possible that some of the defects in SLE MSC could be related to IFNβ. Of note, modular transcriptome repertoire analysis provided evidence of IFNβ production in some patients with SLE (20, 21). For example, module 3.4 is increased in peripheral blood cells from a subset of SLE patients (20, 21). Module 3.4 is not upregulated by IFNα in patients with hepatitis C but is upregulated in multiple sclerosis patients receiving IFNβ (21).
Although Fc receptors and endosomal TLRs (especially TLR7 andTLR9) are important in triggering IFN production by leukocytes, different mechanisms are involving in triggering IFN production by non-leukocytes such as MSCs. MAVS, also known as Interferon Beta Promoter Stimulator Protein 1, is a strong stimulator of IFNβ in a variety of cell types. MAVS is an adaptor protein linking cytoplasmic sensors of nucleic acid such as RIG-1 and MDA5 with production of IFN and activation of NFκB. Overexpression of MAVS constitutively stimulates type I IFN and type I IFN stimulated genes (ISGs) (22). MAVS is critical for host defenses and IFN production in responses to RNA viruses. In addition, IFNβ can be triggered in response to cytosolic DNA by signaling involving RNA polymerase III, RIG-1 and MAVS (23). A gain of function mutation of MDA5 has been associated with risk of SLE (24), and MAVS polymorphisms have been linked to manifestations of SLE in a Chinese population (25).
The present study focuses on the intrinsic BMSC defects in SLE and the underlying mechanisms of these defects. We describe striking abnormalities in mesenchymal stem cells isolated from SLE bone marrow, with several features of cellular senescence including decreased replication, evidence of DNA damage, increased reactive oxygen species (ROS), high levels of cell cycle arrest proteins p53 and p16, and increased senescence-associated pro-inflammatory cytokines. Further, IFNβ and MAVS both mediate these defects via a positive feedback loop as silencing MAVS completely reverses the senescent phenotype.
Patients and Methods
Human BMSC isolation and culture
Detailed written informed consent was obtained from all patients and healthy donors, in accordance with protocols approved by the Human Subjects Institutional Review Board (IRB) of the University of Rochester Medical Center. SLE patients fulfilled American College of Rheumatology classification criteria (26). Clinical data included a comprehensive medical history, medications profile, clinical laboratory tests, and assessment of SLE disease activity by SLEDAI (SLE Disease Activity Index) (27) (Table 1). Research bone marrow (BM) aspirates were drawn from 6 SLE patients and over 6 healthy controls (HC) as we have previously described (28). Genomic samples from SLE patients were genotyped at rs11905552 site in the MAVS gene for C79F MAVS SNP (29). BMSCs were isolated from the BM of healthy donors and SLE patients using Ficoll PREMIUM (1.073g/ml): 10 ml of bone marrow samples were diluted with 2 parts of PBS and then 12 ml of Ficoll PREMIUM (1.073g/ml) was placed underneath. The mixture was centrifuged at room temperature 400 RCF/g for 30 min. The BMSC layer was removed, washed and plated in 6-well plate and cultured at 37°C, in 5% oxygen. Medium was changed every other day until the cells reach 80% confluence.
Table 1.
SLE patient characteristics and relative IFNβ mRNA level
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
|---|---|---|---|---|---|---|
| Ethnicity | AA | C | C | C | AA | AA |
| Age | 33 | 57 | 50 | 46 | 64 | 33 |
| Age of paired healthy donor | 31 | 58 | 51 | 44 | 59 | 36 |
| Sex | F | F | F | F | F | F |
| Passage | 2 | 2 | 2 | 2 | 2 | 2 |
| Total SLEDAI Score | 6 | 6 | 2 | 7 | 0 | 2 |
| anti-RNP (nl 0.0–0.9) | >7.3 | 3.3 | N.D.* | 1.2 | <0.2 | >8.0 |
| anti-Sm (nl 0.0–0.9) | >8.0 | 0.2 | N.D.* | >8.0 | <0.2 | <0.2 |
| anti-SM/RNP (nl 0.0–0.9) | >8.0 | >8.0 | N.D.* | >8.0 | <0.2 | 1.2 |
| anti-Ro (nl 0.0–0.9) | >8.0 | <0.2 | 7.4 | >8.0 | <0.2 | >8.0 |
| anti-La (nl 0.0–0.9) | 1.5 | <0.2 | 2 | 1.6 | <0.2 | 0.5 |
| anti-DNA (nl 0–4 U/mL) | 17 | 14 | 194 | 24 | 3 | <1 |
| C3 (nl 90–180 mg/dL) | 84 | 129 | 120 | 80 | 97 | 151 |
| C4 (nl 10–40 mg/dL) | 15 | 21 | 31 | 9 | 31 | 31 |
| Relative IFNβ mRNA level compared to healthy controls (fold change) | 3.4 | 3.1 | 2.7 | 4.3 | 1.7 | 2.9 |
Not determined.
Western blotting
Western blotting was performed as previously described (30). The antibodies used included IFNβ, MAVS, p53, p16, 53BP1, Actin (Thermo Fisher Scientific).
Quantitative real-time RT-PCR
Total RNA from cultured cells was isolated using the RNeasy kit (Qiagen, Valencia, CA, USA). Total RNA (1 µg) from cells or articular tissues was reverse transcribed into cDNA using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). Real-time PCR was performed on a Rotor-Gene 6000 real-time DNA amplification system (Qiagen, Valencia, CA, USA) using the PerfeCTa SYBR Green SuperMix (Quanta BioSciences, Inc., Gaithersburg, MD, USA) according to the manufacturer's instructions. Table S1 includes a list of primers used.
Immunofluorescent labeling
Human BMSCs were cultured in chamber slides for 24 hours. After washing with PBS, the cells were fixed with 4% paraformaldehyde at 4°C for 1 hour. The cells were incubated with antibodies against different antibodies γH2AX and pATM (Fisher Scientific, Pittsburgh, PA, USA) diluted in PBS containing 5% BSA, 0.5% Tween-20, and incubated at 4°C for 12 hours. This was followed by incubation with a fluorophore-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) at room temperature for 1 hour. The chamber slides were rinsed with water, air-dried, and mounted with Vectashield (Vector Laboratories, Burlingame, CA).
Comet assay
Comet assay was performed as previously described (31). Briefly, MSCs from healthy controls and SLE patient were isolated from bone marrow and subjected to neutral lysis and electrophoresis for DNA double-strand breaks detection. Slides were stained with DAPI before analysis.
Beta-galactosidase assay
Beta-galactosidase assay was performed using Beta-galactosidase assay kit (Thermo Fisher Scientific) following manufacture’s protocol.
Flow cytometry
Single cell suspensions of BMSCs (106/sample) were labeled at 4°C with predetermined optimal concentrations of fluorophore-conjugated mAbs against CD34, CD45, CD73, CD90, CD105, CD31, CD19, CD11b, HLA-ABC, CD44, CD29, and HLA-DR surface markers (ebioscience, San Diego, CA, USA). Pair-matched isotype controls were also used. ROS was detected using CellRox (Thermo Fisher Scientific). Ki67 was used for proliferation analysis following manufacturer’s protocol (ebioscience, San Diego, CA, USA).
Cytokine ELISA assay
IL-6, IL-8 and GM-CSF in the supernatant of BMSCs were evaluated with human IL-6, IL-8, and GM-CSF immunoassay kit following manufacture’s protocol (R&D systems, Minneapolis, MN, USA).
Statistics
Data are presented as the means ± s.e.m. Statistical significance was determined by Student’s t-tests or as indicated in the figure legends; P-values of less than 0.05 were considered significant.
Results
SLE BMSCs display mesenchymal stem cell markers and behavior
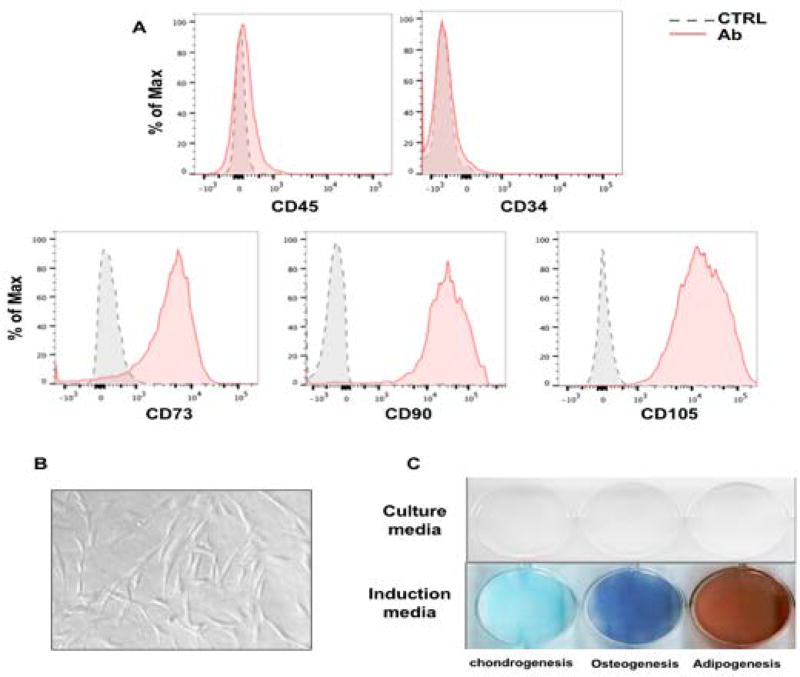
BMSCs were isolated with Ficoll from the bone marrow of healthy donors and SLE patients. It has been reported that C79F MAVS SNP leads to MAVS loss of function mutation by impairing MAVS-TRAF3 interaction, and this mutation is more frequent in individuals of African origin (32). Therefore, the genomic samples from SLE patients were genotyped at rs 11905552 site in the MAVS gene for C79F MAVS SNP (29). No C79F MAVS SNP was found in the SLE patients in this study (data not shown), ruling out the possibility that the MAVS loss of function mutation could have contributed to the phenotypes observed in the present work. To identify the cell types isolated, the cells were phenotyped with cell surface markers using flow cytometry. More than 95% of the isolated cells of second passage were positive for CD73, CD90 and CD105 and negative for CD34 and CD45 (Fig.1A). In addition, further analysis revealed that the isolated cells were also positive for CD29, CD44, HLA-ABC and negative for CD31, CD11b, CD19 and HLA-DR (Fig. S1). No difference in the MSC surface markers was observed between healthy donors and SLE patients. When the isolated cells were plated on cell culture discs, they adhered to the plastic surface in spindle shapes (Fig. 1B). To assess the differentiation potential of these cells, conditional medium for osteogenesis, chondrogenesis or adipogenesis were applied. At the end of the induction, the isolated cells were positively stained with alkaline phosphatase staining, alcian blue staining or oil red to identify differentiation to these respective cell lineages (Fig. 1C). Taken together, these findings indicate that the isolated cells are MSCs with MSC cell surface markers and three-lineage differentiation potentials.
Fig. 1. Characterizing human BMSCs.
(A) Flow cytometry analysis using CD45, CD34, CD73, CD90 and CD105 antibody. (B) BMSC morphology when attached to plastic surface. (C) Left to right: Alcian blue staining for chondrogenesis, alkaline phosphatase staining for osteogenesis, and oil red staining for adipogenesis.
DNA double strand breaks activate DNA damage and repair pathways in SLE BMSCs
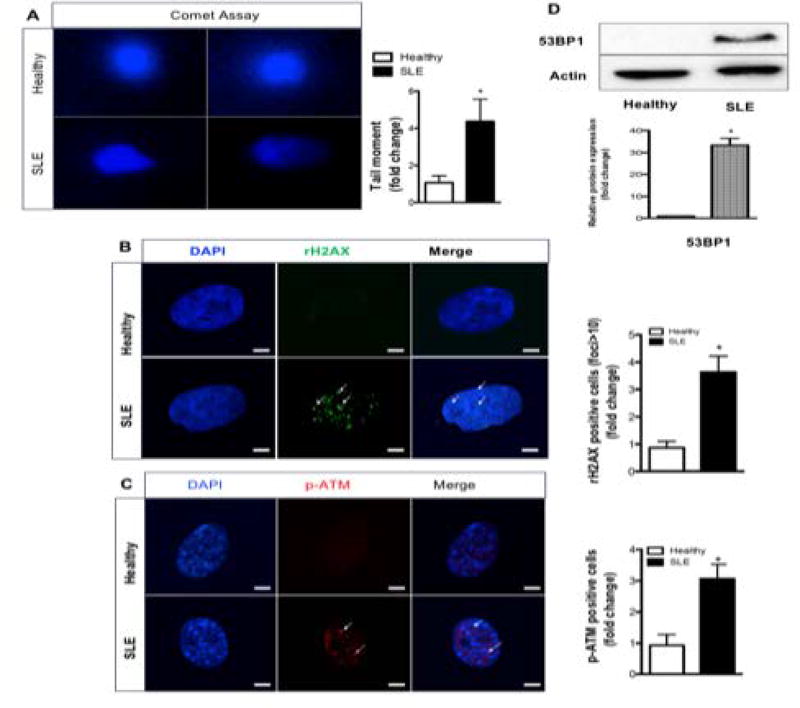
Given that DNA damage and aberrant DNA products have been found in the serum of SLE patients (33), we next evaluated DNA double breaks in BMSCs using comet assay (Fig. 2A). SLE BMSCs have 5 fold more comet tail moments than healthy controls (Fig. 2A), suggesting that SLE BMSCs have elevated DNA double strand breaks. We then investigated the cell response to the DNA double strand breaks - the DNA damage and repair (DDR) pathways. The immunofluorescence images demonstrate that the phosphorylation of the histone variant H2AX, an early and specific DNA damage marker, was strongly activated in SLE BMSCs (Fig. 2B). ATM is a key mediator in DDR becoming phosphorylated upon activation. The p-ATM immunofluorescence staining demonstrated that ATM was activated in SLE BMSCs (Fig. 2C). 53BP1, a DDR protein found in persistent DNA damage foci (34), was increased in SLE BMSCs (Fig. 2D). These findings demonstrate that persistent DNA double strand breaks activate DDR pathway in SLE BMSCs.
Fig. 2. Increased DNA double-strand breaks and activated DDR in human SLE BMSCs.
(A) Comet assay for DNA double strand breaks. (B–C) Confocal images for rH2AX and p-ATM. (D) Western blotting against 53BP1 antibody. Scale bars, 10 µm. *p<0.05, Student’s t-test.
SLE BMSCs undergo SASP and have reduced immunomodulatory function
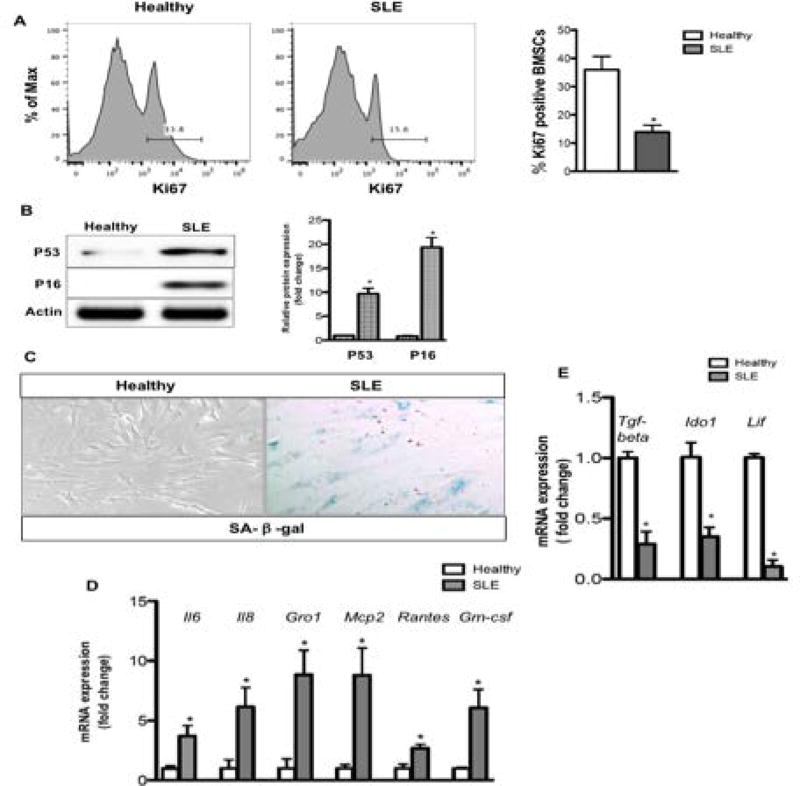
Given that DNA damage accumulation can result in senescence and senescence-related secretory phenotypes, we next evaluated SLE BMSC senescence and SASP. As growth arrest is a hallmark of senescence, proliferation was assessed with Ki67 expression with flow cytometry (Fig. 3A). SLE BMSCs have a significant reduced percentage of proliferating cells when compared to healthy controls, suggesting a growth arrest in SLE BMSCs (Fig. 3A). A number of proteins compatible with senescence induction including p53 and p16 were markedly increased in SLE BMSCs (Fig. 3B). Senescence is also associated with SA-β-gal expression (35). In the healthy controls, SA-β-gal positive cells were barely detected, as compared to the markedly elevated SA-β-gal activity in SLE BMSCs (Fig. 3C). SASP is a phenomenon whereby senescent cells increase the expression and secretion of certain cytokines, chemokines and other proteins (36). Notably, the SASP related gene expression (Il6, Il8, Gro1, Mcp2, Rantes, Gm-csf) was significantly higher in SLE BMSCs than in healthy controls (Fig. 3D). When IL6, IL8 and GM-CSF in the supernatant were evaluated with ELISA, increased concentrations of these cytokines were observed in the supernatant of SLE BMSCs than in healthy controls (Fig. S3). In contrast, the immunoregulatory genes (Tgf-β, Ido1, Lif) were down-regulated in SLE BMSCs (Fig. 3E). Together, these findings demonstrate that SLE BMSCs undergo SASP and have impaired immunomodulatory function.
Fig. 3. SLE BMSCs undergo SASP and have reduced immunomodulatory factor expression.
(A) Flow cytometry using Ki67 antibody. (B) Western blotting against p53 and p16 antibodies. (C) SA-β-gal staining. (D) RT-PCR analysis for SASP associated factors. (E) RT-PCR analysis of immunomodulatory factors. *p<0.05, Student’s t-test.
Nucleic acid sensing protein MAVS and its target IFNβ are activated in SLE BMSCs
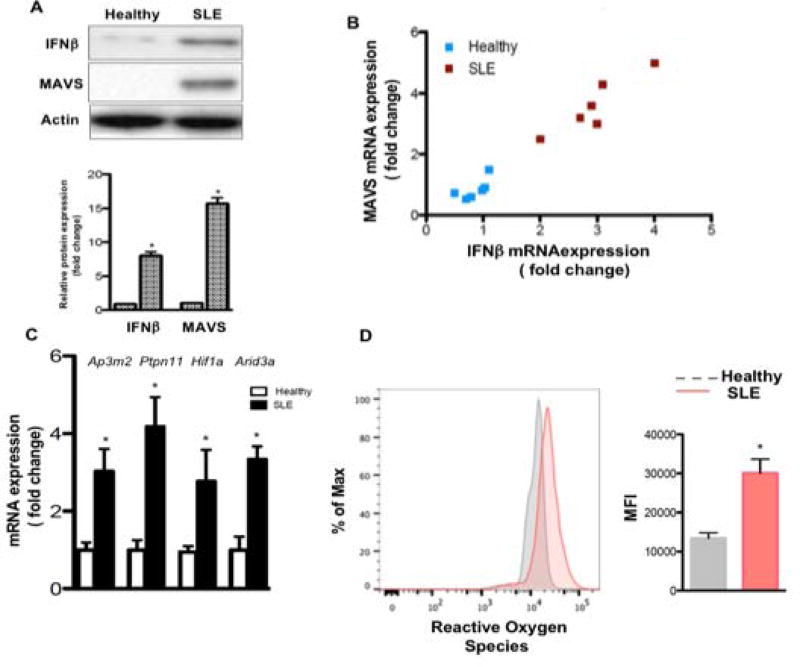
It has been reported that MAVS plays an important role in SASP in contexts other than SLE. When MAVS was silenced in senescent HUVECs, the IL-6 and IL-8 expression was suppressed (37). Therefore, we evaluated MAVS in SLE BMSCs. MAVS expression was significantly elevated in SLE BMSCs (Fig. 4A). As an IFNβ stimulator, MAVS protein activates IFNβ production (38). The RT-PCR and western blot results revealed that IFNβ expression was dramatically upregulated in SLE BMSCs (Fig. 4A). Notably, the expression level of MAVS and IFNβ were highly correlated, r > 0.9, p < 0.01 (Fig. 4B). IFNβ and IFNα are both type I interferons that comprise the IFN-I signature in SLE. We next evaluated IFNβ specific transcripts in BMSCs by stimulating BMSCs with either IFNα or IFNβ. Similar to human fibrosarcoma cells (16), the genes Arid3a, Ptpn11, Hif1a and Ap3m2 only respond to IFNβ stimulation not IFNα in human BMSCs (Fig. S2). Moreover, IFNβ activates Mavs (Fig. S2). Next, we compared the IFNβ specific genes in healthy vs SLE BMSCs. The results demonstrate that these IFNβ specific targets are markedly upregulated in SLE BMSCs (Fig. 4C). Because the activation of both MAVS and IFNβ are linked to ROS production and DNA damage (16, 39), we also evaluated ROS level in SLE BMSCs. SLE BMSCs have 2 fold more ROS than healthy controls (Fig. 4D). These findings suggest that the nucleic acid sensor MAVS is activated in SLE BMSCs and MAVS together with IFNβ and ROS may be key regulators of DNA damage and senescence in SLE BMSCs.
Fig.4. Elevated MAVS, IFNβ and ROS level in SLE BMSCs.
(A) Western blotting against MAVS and IFNβ antibody. (B) mRNA correlation analysis between MAVS and IFNβ. (C) RT-PCR analysis of IFNβ specific genes. (D) Flow cytometry analysis for ROS using CellROX. *p<0.05, Student’s t-test.
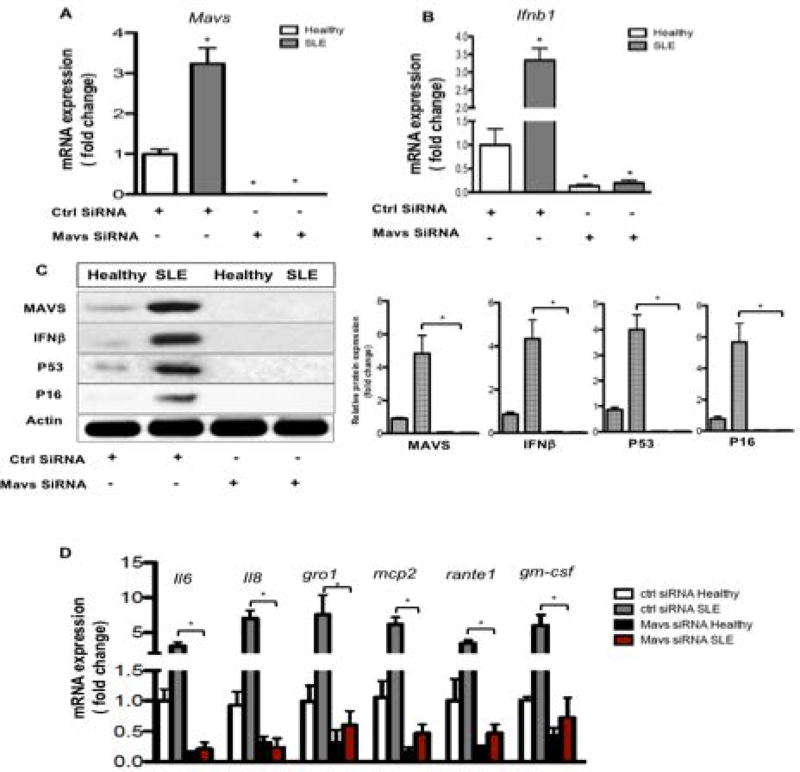
Silencing MAVS blocks IFNβ expression, reduces SASP related cytokine production and improves immunomodulatory factor expression in SLE BMSCs
To further explore the role of MAVS in SLE BMSC SASP, MAVS siRNA was introduced in both healthy and SLE BMSCs. MAVS expression was significantly silenced following siRNA application (Fig. 5A&C). In addition, silencing MAVS led to inhibited IFNβ expression (Fig. 5B&C). More surprisingly, senescence compatible proteins p53 and p16 were blocked following MAVS siRNA (Fig. 5C). SASP related cytokine production was also inhibited (Fig. 5D). When immunomodulatory factors were evaluated, silencing MAVS alleviated the inhibition of Tgf-β, Ido1 and Lif in SLE BMSCs (Fig. S4). These findings further establish the key role of MAVS together with IFNβ in regulating SLE BMSC SASP and immunomodulatory function (Fig. S5).
Fig. 5. Silencing MAVS rescues SASP in SLE BMSCs.
(A–B) RT-PCR analysis of MAVS and IFNβ genes. (C) Western blotting analysis against MAVS, IFNβ, p53 and p16 antibody. (D) RT-PCR analysis for SASP associated factors. *p<0.05, Student’s t-test.
Discussion
The present work demonstrates for the first time that SLE BMSCs have a pro-inflammatory and senescence-associated phenotype which is mediated by a MAVS and IFNβ feedback loop. Compared to healthy controls, SLE BMSCs produced increased amounts of IFNβ and had increased mRNA for genes induced specifically by IFNβ rather than IFNα. They also had decreased proliferation, increased ROS, increased DNA damage and repair (DDR), a senescence-associated secretory phenotype, and increased senescence-associated β-galactosidase. MAVS mRNA was induced by IFNβ, and there was a very strong correlation between levels of MAVS and IFNβ mRNA. MAVS was originally characterized based on its ability to induce IFNβ, setting up the potential for an IFNβ-MAVS mediated positive feedback loop promoting cellular senescence. Silencing MAVS disrupts the IFNβ positive loop by downregulating IFNβ, p53, and p16 proteins levels, and inhibits the expression of pro-inflammatory cytokines in SLE BMSCs. These results establish the critical role of an IFNβ-MAVS positive feedback loop in SLE and provide strong rationale for targeting this feedback loop in SLE and potentially other autoimmune diseases.
A distinguishing function of IFNβ is induction of ROS, double strand breaks, DNA repair mechanism, and cellular senescence (16, 40). Exogenously added IFNβ has been found to induce double stranded breaks and senescence in cell lines (16). Similarly, endogenously produced IFNβ can play an important role in senescence as well, e.g. anti-human IFNβ antibodies rescued normal fibroblasts as well as fibroblasts from patients with Werner Syndrome (a type of adult onset progeria) from replicative senescence, decreased protein markers of DNA damage and senescence (p53 and p16), reduced expression of the senescence-associated markers β galactosidase (40). Conversely, double-strand DNA breaks from radiation, genotoxic agents, or genetic manipulation stimulate IFNβ secretion (40). Thus, a DNA damage-IFNβ feedback loop with the potential to promote and perpetuate inflammation and cellular dysfunction has been previously described in several other systems but as far as we know not in SLE. Here we provide evidence for similar senescence-associated processes and IFNβ production in bone marrow resident MSC from SLE patients strongly suggesting that targeting IFNβ may reverse some of these abnormalities. Indeed, silencing MAVS, the primary inducer of IFNβ in many cell types, blocked IFNβ production and reversed several of the molecular markers of senescence.
The molecular epidemiology of SLE suggests a role for cytoplasmic sensory of nucleic acids in pathogenesis. A gain of function mutation of MDA5 has been associated with increased risk of SLE (24), and MAVS polymorphisms have been linked to different manifestations of SLE in Chinese (25). Moreover, prion-like MAVS aggregation was found in peripheral blood of a subset of lupus patients with increased interferon-I levels (41). A MAVS loss of function mutation has been reported in 27.6% of a sub-Saharan African population, 1.7% of Europeans, and 9.4% of African Americans (32). This mutation was present in 12.6% of African American patients with SLE. These African American SLE patients had lower levels of IFN-I (p = 0.0032) and were enriched in patients who lacked autoantibodies against RNA binding proteins (OR = 2.6, p = 0.00084). In animal studies MAVS and IFNβ were found to be essential for expression and function of TLR-7 in B cells and TLR-7 has been shown to be essential for generation of antibodies against RNA binding proteins (18, 42). Thus, lack of autoantibodies against RNA binding proteins in African-American lupus patients with the loss of function of MAVS may be secondary to a defect in expression of TLR-7 in B cells. A role for intracellular nucleic acid sensing in idiopathic lupus is also suggested by the association of SLE with a mutations in the 3’ repair DNA exonuclease Trex1 (43). Mutations in Trex1 lead to increased cytosolic DNA and activation of the STING pathway. Moreover, activating mutations of STING, another molecule involved in cytoplasmic sensing of nucleic acids, has been associated with an inflammatory syndrome having some lupus-like features (44). Thus, there are hints from the literature that cytoplasmic sensors for nucleic acids may be important in the pathogenesis of SLE. Here we have directly demonstrated a critical role for MAVS in activation of and IFNβ production by SLE BMSC.
BMSCs play an important role in bone and cartilage metabolism by differentiating into osteoblasts and chondrocytes and are critical for the development of hematopoietic stem cells into blood and lymphoid cells. Thus, BMSC abnormalities in SLE patients may be directly related to an increased risk of osteoporosis and avascular necrosis of bone (45). In addition, SLE BMSCs may have cell-non-autonomous effects on the microenvironment due to their reduced immunomodulatory capacity as suggested by the down-regulation of immunomodulatory factors (Tgf-β, Ido1 and Lif). However, in the paper published by Opitz et al.(46), they proposed a novel finding that TLR enhances immunosuppressive function of MSCs by activating IDO1 via IFNβ. They also noted that this finding contradicted the previous publication from Liotta et al.(47), possibly due to a shorter exposure time of MSCs to TLR ligand. In our studies, BMSCs from lupus patient would have been chronically stimulated in vivo. Thus, it is not surprising that our results are similar to Liotta et al., that long term stimulation suppressed IDO1 expression in lupus BMSCs.
Thus, alterations in BMSC function in SLE may jeopardize the bone marrow microenvironment, potentially skewing hematopoiesis and altering immune responses. Indeed, there are published reports of increased ARID3a, a protein induced by IFNβ but not IFNα, in B cells from a subset of patients with SLE (48). ARID3a has been linked to development of B1 B cells in mice, and hematopoietic stem cells from SLE patients with high levels of ARID3a in B cells when transferred to mice produced increased levels of autoantibodies (48). Moreover, innate and type I IFN pathways are markedly upregulated in ARID3a high B cells (49). We have found ARID3a upregulated in BMSC and ARID3a mRNA was downregulated also with mRNA for IFNβ when MAVS was silenced. We speculate that IFNβ production in the bone marrow microenvironment is also responsible for the induction of ARID3a in hematopoietic stem cells and B cells and that BMSC are an important source of IFNβ.
Collectively, our work has found increased IFNβ production in SLE BMSCs and established MAVS as a critical player by regulating IFNβ production, with an IFNβ-MAVS positive feedback loop (Fig. S5). We propose that IFNβ and its target gene products contribute to chronic inflammation and ROS damaging DNA which is then sensed by MAVS. In turn, MAVS stimulates the IFNβ promoter and increases IFNβ expression and secretion, which further contributes to the chronic inflammation and ROS generation. Under the influence of this IFNβ positive feedback loop, cells eventually exit cell cycle and undergo senescence but continue to release IFNβ and other inflammatory factors altering adjacent cells and the bone marrow microenvironment. This IFNβ-MAVS feedback loop in BMSCs has the potential to alter development of immune cells in the bone marrow and contribute to SLE pathogenesis. In ongoing work we will explore where other cell types in SLE are similarly activated and potentially contribute to accelerated development of inflammation, degenerative disease and organ dysfunction in SLE patients (50).
Supplementary Material
Flow cytometry analysis using CD44, CD29, HLA-ABC, CD31, CD11b, CD19 and HLA-DR antibody.
RT-PCR analysis for IFNβ specific genes in BMSCs. *p<0.05, Student’s t-test.
Elisa analysis for SASP associated factors in BMSCs supernatant. *p<0.05, Student’s t-test.
RT-PCR analysis of immunomodulatory factors. *p<0.05, Student’s t-test.
Acknowledgments
Thanks to Deborah Campbell for her help with the regulatory documents and logistics for this study.
Supported by a grant from the Alliance for Lupus Research and Pfizer, Inc. Dr. Anolik has been supported by R01-AI-077674, NIAMS Accelerated Medicines Partnership (1UH2AR067690) and the Bertha and Louis Weinstein research fund.
Footnotes
Author contributions: L.G. performed all experiments, data analysis and wrote the manuscript; A.K.B., N.M. K.D., and J.L. recruited patients processed BMSCs and revised the manuscript; J.A. contributed to patient recruitment, sample collection and manuscript revision; R.J.L. designed and supervised all experiments and wrote the manuscript.
References
- 1.Mok CC, Lau CS. Pathogenesis of systemic lupus erythematosus. J Clin Pathol. 2003;56(7):481–90. doi: 10.1136/jcp.56.7.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cras A, Farge D, Carmoi T, Lataillade JJ, Wang DD, Sun L. Update on mesenchymal stem cell-based therapy in lupus and scleroderma. Arthritis research & therapy. 2015;17:301. doi: 10.1186/s13075-015-0819-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou K, Zhang H, Jin O, Feng X, Yao G, Hou Y, et al. Transplantation of human bone marrow mesenchymal stem cell ameliorates the autoimmune pathogenesis in MRL/lpr mice. Cell Mol Immunol. 2008;5(6):417–24. doi: 10.1038/cmi.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins E, Gu F, Qi M, Molano I, Ruiz P, Sun L, et al. Differential efficacy of human mesenchymal stem cells based on source of origin. Journal of immunology. 2014;193(9):4381–90. doi: 10.4049/jimmunol.1401636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, Li J, Zhang Y, Zhang M, Chen J, Li X, et al. Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: a multicenter clinical study. Arthritis research & therapy. 2014;16(2):R79. doi: 10.1186/ar4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun LY, Zhang HY, Feng XB, Hou YY, Lu LW, Fan LM. Abnormality of bone marrow-derived mesenchymal stem cells in patients with systemic lupus erythematosus. Lupus. 2007;16(2):121–8. doi: 10.1177/0961203306075793. [DOI] [PubMed] [Google Scholar]
- 7.Gu Z, Tan W, Feng G, Meng Y, Shen B, Liu H, et al. Wnt/beta-catenin signaling mediates the senescence of bone marrow-mesenchymal stem cells from systemic lupus erythematosus patients through the p53/p21 pathway. Mol Cell Biochem. 2014;387(1–2):27–37. doi: 10.1007/s11010-013-1866-5. [DOI] [PubMed] [Google Scholar]
- 8.Ronnblom L. Potential role of IFNalpha in adult lupus. Arthritis research & therapy. 2010;12(Suppl 1):S3. doi: 10.1186/ar2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crow MK, Kirou KA, Wohlgemuth J. Microarray analysis of interferon-regulated genes in SLE. Autoimmunity. 2003;36(8):481–90. doi: 10.1080/08916930310001625952. [DOI] [PubMed] [Google Scholar]
- 10.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197(6):711–23. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morehouse CCLWL, Brohawn P. Target modulation of a type I interferon (IFN) gene signature with sifalimumab or anifrolumab in systemic lupus erythematosus (SLE) patients in two open label phase 2 Japanese trials. Arthritis and rheumatism. 2014;66(suppl 10) abstract 719. [Google Scholar]
- 12.Kalunian KC, Merrill JT, Maciuca R, McBride JM, Townsend MJ, Wei X, et al. A Phase II study of the efficacy and safety of rontalizumab (rhuMAb interferon-alpha) in patients with systemic lupus erythematosus (ROSE. Annals of the rheumatic diseases. 2016;75(1):196–202. doi: 10.1136/annrheumdis-2014-206090. [DOI] [PubMed] [Google Scholar]
- 13.Furie RMJ, Werth V, Khamashta M, Kalunian K, Brohawn P, Illei G, Drappa J, Wang L, Yoo Anifrolumab, an Anti-Interferon Alpha Receptor Monoclonal Antibody, in Moderate to Severe Systemic Lupus Erythematosus (SLE) Arthritis & rheumatology. 2015;67(suppl 10) doi: 10.1002/art.39962. abstract 3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez de Padilla CM, Niewold TB. The type I interferons: Basic concepts and clinical relevance in immune-mediated inflammatory diseases. Gene. 2016;576(1 Pt 1):14–21. doi: 10.1016/j.gene.2015.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schreiber G, Piehler J. The molecular basis for functional plasticity in type I interferon signaling. Trends Immunol. 2015;36(3):139–49. doi: 10.1016/j.it.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Moiseeva O, Mallette FA, Mukhopadhyay UK, Moores A, Ferbeyre G. DNA damage signaling and p53-dependent senescence after prolonged beta-interferon stimulation. Mol Biol Cell. 2006;17(4):1583–92. doi: 10.1091/mbc.E05-09-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gough DJ, Messina NL, Clarke CJ, Johnstone RW, Levy DE. Constitutive type I interferon modulates homeostatic balance through tonic signaling. Immunity. 2012;36(2):166–74. doi: 10.1016/j.immuni.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green NM, Laws A, Kiefer K, Busconi L, Kim YM, Brinkmann MM, et al. Murine B cell response to TLR7 ligands depends on an IFN-beta feedback loop. Journal of immunology. 2009;183(3):1569–76. doi: 10.4049/jimmunol.0803899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheikh F, Dickensheets H, Gamero AM, Vogel SN, Donnelly RP. An essential role for IFN-beta in the induction of IFN-stimulated gene expression by LPS in macrophages. Journal of leukocyte biology. 2014;96(4):591–600. doi: 10.1189/jlb.2A0414-191R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaussabel D, Quinn C, Shen J, Patel P, Glaser C, Baldwin N, et al. A modular analysis framework for blood genomics studies: application to systemic lupus erythematosus. Immunity. 2008;29(1):150–64. doi: 10.1016/j.immuni.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiche L, Jourde-Chiche N, Whalen E, Presnell S, Gersuk V, Dang K, et al. Modular transcriptional repertoire analyses of adults with systemic lupus erythematosus reveal distinct type I and type II interferon signatures. Arthritis Rheumatol. 2014;66(6):1583–95. doi: 10.1002/art.38628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biacchesi S, LeBerre M, Lamoureux A, Louise Y, Lauret E, Boudinot P, et al. Mitochondrial antiviral signaling protein plays a major role in induction of the fish innate immune response against RNA and DNA viruses. J Virol. 2009;83(16):7815–27. doi: 10.1128/JVI.00404-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiu YH, Macmillan JB, Chen ZJ. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell. 2009;138(3):576–91. doi: 10.1016/j.cell.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliveira L, Sinicato NA, Postal M, Appenzeller S, Niewold TB. Dysregulation of antiviral helicase pathways in systemic lupus erythematosus. Front Genet. 2014;5:418. doi: 10.3389/fgene.2014.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Jiao Y, Wen X, Wang L, Ma C, Gao X, et al. Possible association of VISA gene polymorphisms with susceptibility to systemic lupus erythematosus in Chinese population. Mol Biol Rep. 2011;38(7):4583–8. doi: 10.1007/s11033-010-0590-4. [DOI] [PubMed] [Google Scholar]
- 26.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis and rheumatism. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 27.Touma Z, Urowitz MB, Ibanez D, Gladman DD. SLEDAI-2K 10 days versus SLEDAI-2K 30 days in a longitudinal evaluation. Lupus. 2011;20(1):67–70. doi: 10.1177/0961203310385163. [DOI] [PubMed] [Google Scholar]
- 28.Palanichamy A, Bauer JW, Yalavarthi S, Meednu N, Barnard J, Owen T, et al. Neutrophil-mediated IFN activation in the bone marrow alters B cell development in human and murine systemic lupus erythematosus. Journal of immunology. 2014;192(3):906–18. doi: 10.4049/jimmunol.1302112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xing F, Matsumiya T, Hayakari R, Yoshida H, Kawaguchi S, Takahashi I, et al. Alteration of Antiviral Signalling by Single Nucleotide Polymorphisms (SNPs) of Mitochondrial Antiviral Signalling Protein (MAVS) PLoS One. 2016;11(3):e0151173. doi: 10.1371/journal.pone.0151173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao L, Sheu TJ, Dong Y, Hoak DM, Zuscik MJ, Schwarz EM, et al. TAK1 regulates SOX9 expression in chondrocytes and is essential for postnatal development of the growth plate and articular cartilages. J Cell Sci. 2013;126(Pt 24):5704–13. doi: 10.1242/jcs.135483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olive PL, Banath JP. The comet assay: a method to measure DNA damage in individual cells. Nat Protoc. 2006;1(1):23–9. doi: 10.1038/nprot.2006.5. [DOI] [PubMed] [Google Scholar]
- 32.Pothlichet J, Niewold TB, Vitour D, Solhonne B, Crow MK, Si-Tahar M. A loss-of-function variant of the antiviral molecule MAVS is associated with a subset of systemic lupus patients. EMBO Mol Med. 2011;3(3):142–52. doi: 10.1002/emmm.201000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blount S, Griffiths HR, Lunec J. Reactive oxygen species damage to DNA and its role in systemic lupus erythematosus. Mol Aspects Med. 1991;12(2):93–105. doi: 10.1016/0098-2997(91)90005-7. [DOI] [PubMed] [Google Scholar]
- 34.Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11(8):973–9. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92(20):9363–7. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuilman T, Peeper DS. Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer. 2009;9(2):81–94. doi: 10.1038/nrc2560. [DOI] [PubMed] [Google Scholar]
- 37.Liu F, Wu S, Ren H, Gu J. Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat Cell Biol. 2011;13(3):254–62. doi: 10.1038/ncb2167. [DOI] [PubMed] [Google Scholar]
- 38.Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122(5):669–82. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 39.West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nat Rev Immunol. 2011;11(6):389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu Q, Katlinskaya YV, Carbone CJ, Zhao B, Katlinski KV, Zheng H, et al. DNA-damage-induced type I interferon promotes senescence and inhibits stem cell function. Cell reports. 2015;11(5):785–97. doi: 10.1016/j.celrep.2015.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shao WH, Shu DH, Zhen Y, Hilliard B, Priest SO, Cesaroni M, et al. Prion-like MAVS aggregation in lupus patients associates with increased interferon-I. Arthritis Rheumatol. 2016 doi: 10.1002/art.39733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. The Journal of experimental medicine. 2005;202(9):1171–7. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee-Kirsch MA, Gong M, Chowdhury D, Senenko L, Engel K, Lee YA, et al. Mutations in the gene encoding the 3'–5' DNA exonuclease TREX1 are associated with systemic lupus erythematosus. Nature genetics. 2007;39(9):1065–7. doi: 10.1038/ng2091. [DOI] [PubMed] [Google Scholar]
- 44.Jeremiah N, Neven B, Gentili M, Callebaut I, Maschalidi S, Stolzenberg MC, et al. Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. The Journal of clinical investigation. 2014;124(12):5516–20. doi: 10.1172/JCI79100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia-Carrasco M, Mendoza-Pinto C, Escarcega RO, Jimenez-Hernandez M, Etchegaray Morales I, Munguia Realpozo P, et al. Osteoporosis in patients with systemic lupus erythematosus. Isr Med Assoc J. 2009;11(8):486–91. [PubMed] [Google Scholar]
- 46.Opitz CA, Litzenburger UM, Lutz C, Lanz TV, Tritschler I, Koppel A, et al. Toll-like receptor engagement enhances the immunosuppressive properties of human bone marrow-derived mesenchymal stem cells by inducing indoleamine-2,3-dioxygenase-1 via interferon-beta and protein kinase R. Stem Cells. 2009;27(4):909–19. doi: 10.1002/stem.7. [DOI] [PubMed] [Google Scholar]
- 47.Liotta F, Angeli R, Cosmi L, Fili L, Manuelli C, Frosali F, et al. Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling. Stem Cells. 2008;26(1):279–89. doi: 10.1634/stemcells.2007-0454. [DOI] [PubMed] [Google Scholar]
- 48.Ward JM, Rose K, Montgomery C, Adrianto I, James JA, Merrill JT, et al. Disease activity in systemic lupus erythematosus correlates with expression of the transcription factor AT-rich-interactive domain 3A. Arthritis Rheumatol. 2014;66(12):3404–12. doi: 10.1002/art.38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ward JM, Ratliff ML, Dozmorov MG, Wiley G, Guthridge JM, Gaffney PM, et al. Human effector B lymphocytes express ARID3a and secrete interferon alpha. Journal of autoimmunity. 2016 doi: 10.1016/j.jaut.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow cytometry analysis using CD44, CD29, HLA-ABC, CD31, CD11b, CD19 and HLA-DR antibody.
RT-PCR analysis for IFNβ specific genes in BMSCs. *p<0.05, Student’s t-test.
Elisa analysis for SASP associated factors in BMSCs supernatant. *p<0.05, Student’s t-test.
RT-PCR analysis of immunomodulatory factors. *p<0.05, Student’s t-test.