Abstract
The Anterior-Posterior (AP) axis is the most ancient of the embryonic axes, and exists in most metazoans. Different animals use a wide variety of mechanisms to create this axis in the early embryo. Here we focus on three animals, including two insects (Drosophila and Tribolium) and a vertebrate (zebrafish) to examine different strategies used to form the AP axis. While Drosophila forms the entire axis within a syncytial blastoderm using transcription factors as morphogens, zebrafish uses signaling factors in a cellularized embryo, progressively forming the AP axis over the course of a day. Tribolium uses an intermediate strategy that has commonalities with both Drosophila and zebrafish. We discuss the specific molecular mechanisms used to create the AP axis, and identify conserved features.
Introduction
The Anterior-Posterior (AP) axis was the first embryonic axis to arise in evolution since it allowed animals to move unidirectionally. In modern bilaterians, the AP axis corresponds to the head-tail axis (Sidebar 1). Since this issue has fascinated embryologists for over a century, there is a vast literature associated with it, and it remains a very active area of research. We have chosen to focus on three organisms to elucidate different types of AP patterning: a long germ-band insect, Drosophila, a short germ-band insect, Tribolium, and a vertebrate, zebrafish. These are certainly not the only organisms that could have been chosen, and in many cases information about these systems derived from work on other organisms. Nonetheless, they provide a good chance to examine different strategies in organizing the AP axis.
Sidebar 1. Anterior-Posterior.
We use anterior-posterior throughout this review to refer to the rostral-caudal (head-tail) axis. It is interesting to consider that for a walking human, the anterior-posterior axis is the ventral-dorsal axis, whereas for a swimming human, and most other animals, anterior-posterior corresponds to the rostral-caudal axis. While in some animals it is possible to locate an anterior-posterior axis in the egg or cleavage stage embryo, in many cases the anterior-posterior axis can only really be correctly defined at the end of gastrulation when cell movements have positioned cells along this axis of the body.
INVERTEBRATES
Long vs. short germ-band insect development
We will be discussing the AP specification of two different insects, the long germ-band Drosophila melanogaster (a fly), and the short germ-band Tribolium castaneum (a beetle). The long germ-band mode of insect development refers to the simultaneous establishment of the anterior and posterior body, including all of the intervening body segments, during the blastoderm stage (Figure 1A). This mode differs from the majority of insects, which undergo short or intermediate germ-band development, where the anterior body segments are initially specified at the blastoderm stage, but the remaining posterior segments are formed sequentially in a process of posterior growth (Figure 1B). Long germ-band development is a highly derived developmental mechanism, and is only observed in a group of dipterans (flies and mosquitoes)1.
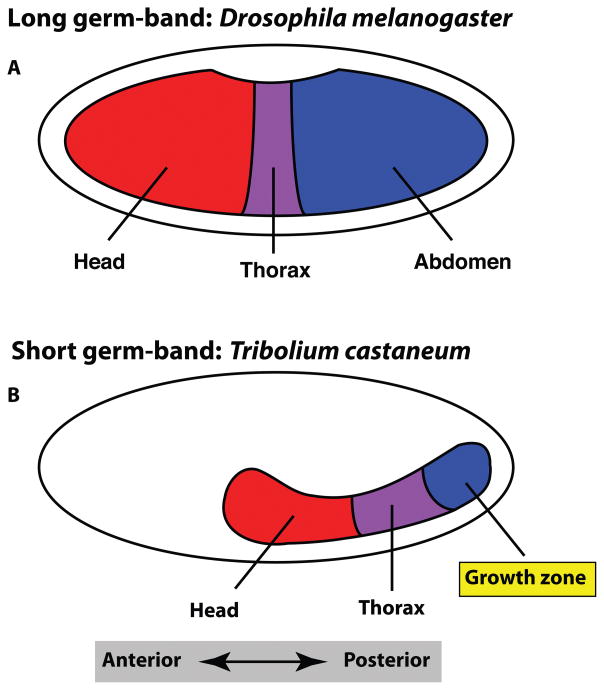
Figure 1. Germ size difference between Drosophila and Tribolium.
In long germ-band insects such as Drosophila, the embryonic germ anlagen occupies the majority of the egg (A), whereas in the short germ-band Tribolium, the anlage is only a fraction of the egg (B). In long germ-band insects the entire AP body axis is specified by the end of the blastoderm stage. In short germ-band insects, only the anterior body is specified, and the rest of the posterior body forms through a process of posterior growth in the growth zone, which will eventually form the abdomen.
Long germ-band insects: Drosophila melanogaster
Establishing the AP axis is an essential step in the development of all bilaterian animals, yet the mechanisms by which this occurs can differ significantly between animal clades. Drosophila is an excellent example of how AP specification can be established, as it utilizes both highly conserved and divergent mechanisms during this process.
Oogenesis establishes AP polarity
During insect development, axis polarity is established in the Drosophila egg well before it is fertilized, during the process of oogenesis2. This includes the establishment of both AP and dorsal-ventral (DV) polarity, which are easily separable both spatially in the egg and embryo and in the downstream factors that regulate these two different axes3. This is in contrast to vertebrate development, which we discuss later, where the establishment of AP and DV axes are extensively intertwined during early development. The initiation of Drosophila AP axis specification occurs during oogenesis when the oocyte moves to the posterior of the egg chamber. The localization of gurken (grk) mRNA (which encodes a TGFα ligand) at one end of the oocyte causes the asymmetric secretion of Grk protein4. Grk signals through the epidermal growth factor (EGF) receptor Torso in the neighboring follicle cells, which induces them to become posterior follicle cells rather than adopt the default anterior state. The posterior follicle cells then signal back to the oocyte, which causes a polarization of the microtubules4. This polarization ultimately results in the localization of mRNA in the anterior and posterior of the oocyte, which will later establish the anterior and posterior structures within the embryo.
Anterior specification in Drosophila
The derived nature of Drosophila AP patterning is exemplified by the anterior specification mechanism, which utilizes a protein called Bicoid (Bcd; Sidebar 2)5. The bcd gene, which encodes a homeodomain transcription factor, is found only in higher dipterans6–9. During Drosophila oogenesis, bcd mRNA is deposited maternally and is localized to the anterior pole of the egg10, 11 (Figure 2). After fertilization, bcd mRNA is translated and the protein diffuses away from the anterior pole, creating a morphogenetic gradient in which Bcd affects gene transcription in a concentration dependent manner12, 13. A key aspect of Drosophila development that allows for a transcription factor to act as morphogen is the fact that the early embryo is a syncytium of nuclei within a common cytoplasm, allowing Bcd, as well as other factors, to freely diffuse throughout the embryo.
Sidebar 2. The importance of morphogenetic gradients in AP patterning.
The Drosophila Bicoid protein was the first factor identified to act as a true morphogen. The level of Bicoid acts in a concentration dependent manner to specify progressive cell fates in the anterior end of the embryo. Although we now know that bicoid is a derived gene found only in dipteran flies, the use of morphogenetic gradients is a conserved mechanism for generating AP pattern. While insects rely on transcription factors such as Bicoid, Nanos, Hunchback, and Orthodenticle to act as morphogens to establish AP pattern, vertebrate embryos utilize secreted signaling molecules such as Wnts and BMPs to establish cell fates across the AP axis.
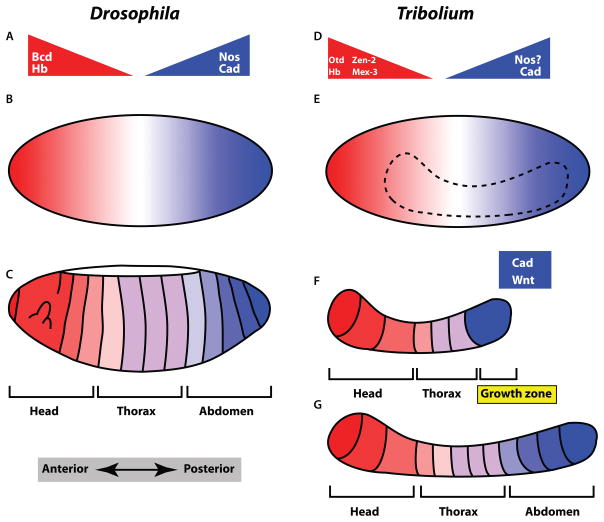
Figure 2. Comparison of Drosophila and Tribolium AP patterning.
A) Protein gradients establish the AP axis in Drosophila. Several of the factors in AP specification, including Bcd, Hb, and Cad are transcription factors and act as morphogens. B) The syncytial blastoderm is essential for allowing transcription factors to act as diffusible morphogens. C) After cellularization, the entire AP axis has been specified. D) Tribolium also utilizes protein gradients to establish the anterior body. Notable differences in Tribolium are the lack of Bcd and the unknown function of Nos, as well as the anterior specifying role of Otd. E) A syncytial blastoderm is also essential for the morphogenetic patterning of the anterior body of Tribolium. The position where nuclei will converge to form the embryo is shown as a dashed line. F) After cellularization, only the anterior body is specified. The posterior end consists of a growth zone that require Wnt and Cad function for posterior body formation. G) During the posterior growth phase the posterior body is formed sequentially.
Bcd affects gene transcription in conjunction with Hunchback (Hb)14, 15. hb mRNA is also deposited maternally within the egg, but is not localized to a particular region. A gradient of Hb protein eventually forms across the AP axis of the embryo, as zygotic hb is transcriptionally regulated by Bcd, while the maternal hb mRNA is translationally repressed by the posterior specific protein Nanos (Nos)16–19, resulting in high levels of Hb anteriorly and low levels posteriorly (Figure 2). Together, the anterior to posterior gradient of Bcd and Hb turn on anterior specific genes that specify anterior body structures15. Drosophila embryos that lack Bcd function have severe defects in anterior specification, including a failure to develop the head, thorax, and some abdominal segments20. Conversely, over-expression of Bcd can induce ectopic anterior structures, demonstrating that Bcd is both necessary and sufficient for anterior specification12. Loss of both maternal and zygotic Hb causes defects in all Bcd regulated processes, indicating that it is likely a co-factor for Bcd15. Like Bcd, Hb also acts as a classic morphogen, where different concentrations of the transcription factor create discrete effects on the transcription of downstream gap genes, which will later refine AP body patterning.
Posterior specification in Drosophila
As with anterior specification, Drosophila embryos also employ maternal factors to instruct posterior specification. As one might expect, the anterior and posterior specification factors interact to create a graded AP response across the embryo. This is particularly evident when Bcd is absent during development. In the absence of Bcd, regions that should be specified as anterior become mis-specified as posterior20 due to the posteriorizing activity of Caudal (Cad). cad mRNA is deposited ubiquitously throughout the developing oocyte, but its translation is repressed in the anterior regions by the anteriorly localized Bcd binding to the cad 3′ UTR, creating a gradient of Cad protein, which is highest in the posterior end of the embryo (Figure 2). Cad is a homeobox transcription factor that regulates posterior specific gene expression in Drosophila21, as in many other bilaterians.
In addition to Cad, another key posteriorizing factor called nanos (nos) is deposited maternally in the egg. nos mRNA is localized at the posterior pole22, 23, and is translated after fertilization. This creates a Nos protein gradient, with highest levels at the posterior pole of the embryo24. Nos functions as a translational inhibitor, and specifically inhibits the translation of Hb, which keeps this anterior specific transcription factor out of the posterior end of the embryo17. The combined actions of Bcd and Hb in the anterior end of the embryo and Cad and Nos in the posterior end create a robust AP pattern across the embryo25. The downstream genes that are regulated by these factors, which further refine AP pattern and create the precise segmental structure of the embryo are very well studied with clear genetic mechanisms26, 27. These downstream processes are beyond the scope of this review and will not be discussed further.
Short germ-band insects: Tribolium castaneum
Tribolium castaneum, commonly referred to as the flour beetle, has been widely used in recent years to examine the evolution of developmental mechanisms, particularly within the insects as a direct comparison to Drosophila28, 29. Tribolium is well suited for evolutionary studies, as it undergoes the short germ-band mode of development, which is considered the basal mode within the insect clade1. In fact, as we will discuss later, short germ-band development has many features in common with vertebrate body formation.
Although not as well studied as in Drosophila, recent work indicates that Tribolium uses a similar mechanism as Drosophila to initially establish the AP axis during oogenesis30. Short germ-band insects initially form a syncytial blastoderm as in Drosophila, but unlike Drosophila the syncytial embryo fills only part of the egg and encompasses just the head and thorax (Figure 1B). Cellularization occurs before the posterior growth that generates the rest of the embryo begins1. This fact, along with the necessity to specify AP regionalization progressively during posterior growth, prevents short germ-band insects from relying entirely on direct transcription factor morphogen gradient mechanisms to specify the entire AP axis at once as in Drosophila.
Anterior specification in Tribolium
As mentioned earlier, the bcd gene, which is the master anterior specification factor in Drosophila, is a derived gene acquired in the higher dipterans. Given that short germ-band insects lack bcd, they must have a basal alternative mechanism of early AP axis establishment. In Drosophila, Bcd transcriptionally activates zygotic hb expression (which is also supplied maternally), as well as the anterior specific zygotic gene orthodenticle (otd)16, 31. Loss of either zygotic Hb or Otd in Drosophila results in mild anterior defects, as opposed to the severe defects when maternal Bcd is lost6, 25. Although the Tribolium genome lacks bcd, it contains both hb and otd, whose protein products are localized to the anterior end of the embryo (Figure 2). Like Drosophila, Tribolium hb is supplied both maternally and zygotically32. Interestingly, otd, which is only zygotically transcribed in Drosophila under the control of Bcd, is deposited as a maternal mRNA in Tribolium33. Loss of Hb or Otd alone leads to partial anterior defects, whereas loss of both has a synergistic effect leading to severe anterior defects reminiscent of loss of Bcd in Drosophila33. These results indicate that Hb and Otd are likely the basal evolutionary mechanism for anterior specification in insects, while Drosophila has added the upstream regulatory gene bcd to their anterior specification repertoire. Further evidence that Otd is part of an ancient developmental mechanism of anterior embryonic specification is the fact that it is also utilized in vertebrates for the specification of anterior neural tissue34.
Posterior specification in Tribolium
Similar to anterior specification, Tribolium posterior specification and patterning utilizes some of the same key factors involved in Drosophila posterior development. Cad, which specifies posterior fates in Drosophila, has a very similar function in Tribolium, with loss of maternal Cad causing severe posterior defects35. Differences appear in creation of the Cad protein gradient. Unlike in Drosophila where the anteriorly localized Bcd represses Cad translation in anterior regions, in Tribolium, two proteins, Mex-3 and Zen-2 translationally inhibit cad mRNA36 (Figure 2). Zen-2 is derived from the same gene as the dipteran Bcd (Zen-2 is a duplication of Zen in Tribolium, while Bcd is an independent duplication of Zen in Drosophila), whereas Mex-3 represents an ancient anteriorizing factor, whose function has been replaced in dipterans by Bcd36.
In Drosophila, Nos is a posteriorly localized translational inhibitor that establishes posterior identity through the inhibition of anterior factors (such as Hb). While this has never been shown in Tribolium, there is potentially at least a partially conserved mechanism involved. The Tribolium otd and hb transcripts contain Nos response elements, which are present in inhibitory targets of Nos25, 33. This suggests that Nos might function in Tribolium to establish a protein gradient of Otd and Hb necessary for proper AP patterning (Figure 2).
Posterior growth: a conserved process
As will be evident later in this review, vertebrate AP patterning differs significantly from the early insect AP patterning mechanisms. The syncytial nature of early development in the majority of insect species (holometabolous insects) is unlike the cellularized early vertebrate embryo. This prevents vertebrate embryos from utilizing the same AP patterning system used in Drosophila and early Tribolium embryos. In Drosophila, once cellularization occurs, the entire AP axis has been established and all of the body segments have been formed1. On the other hand, only anterior segments have formed once Tribolium embryos cellularize. The posterior end of the embryo is specified to undergo a process of posterior growth, in which the remaining body segments form gradually in an anterior to posterior progression1. During this growth regional AP identity is formed progressively as cells exit the posterior growth zone28. This process is highly reminiscent of vertebrate embryonic development, both on a gross morphological level, but also at the molecular level37. Both Tribolium and vertebrates express cad and Wnt ligands (called Wingless in insects) in the posterior growth zone. In vertebrates, cad (called cdx) is a direct target of Wnt signaling and loss of either causes a disruption in posterior growth and a severe loss of the posterior body37. While the direct interaction of Wnt and Cad has not been demonstrated in Tribolium, both are required for posterior growth and formation of the posterior body35, 38 (Figure 2). Based on this striking conservation, it is tempting to speculate that there is an ancestral AP patterning mechanism common to all bilaterians. Further study of other non-model animals will reveal additional aspects of this mechanism and provide a more detailed evolutionary framework for the establishement of AP polarity.
Creating AP identity from AP polarity
In all bilaterian animals, once the AP axis has been created, transcription factors called Hox proteins (also called Homeotic proteins in Drosophila) translate the initial AP polarity into proper tissue specification along the axis39. Hox proteins specifiy particular cell fates within a segment, such as legs or wings, and when a Hox protein is mutant, the segment will adopt the identity of the neighboring segment (specified by a different Hox protein). In Drosophila, Hox gene expression is initiated from a cascade of transciptional responses downstream of the initial AP specification proteins. In Tribolium, despite the difference in segment formation, Hox genes display highly conserved expression pattterns compared to their Drosphila orthologues40. Expression is initiated before posterior segments form from a similar trasciptional cascade utilized in Drosophila, but then are maintained and expanded as segments form during posterior growth40, 41. Due to the highly conserved expression of Hox genes between Drosophila and Tribolium, current models suggest that variation in body form is achieved at least in part through differential regulation of Hox target genes between the species40. While Hox genes are also conserved and function in a similar manner in vertebrate embryos, the regulation of expression is quite different as will be discussed below. Unlike the transcription factor cascades utilized in invertebrates, the regulation of Hox genes in vertebrates is more complex and less well understood.
VERTEBRATES
Zebrafish
Initiation versus Elaboration
AP patterning in the vertebrate embryo can be roughly divided into two major phases: an initiation phase, in which the embryo is generally divided into the head and the body, and an elaboration phase, in which the body progressively forms toward the posterior end, forming the trunk and tail (since this process involves the formation of blocks of muscle tissue called somites we will refer to this as the somitogenesis stages). In zebrafish, the initiation phase occurs prior to the start of gastrulation, such that by the start of gastrulation, the different territories of the final body plan can be roughly mapped onto the embryo (Figure 3). The mesoderm of the head, which comprises part of a very important signaling center called the Organizer, is first specified near the equator on what is defined as the dorsal side of the embryo42. These cells migrate toward the animal pole during gastrulation, where the brain forms. In contrast, the major mesodermal derivative of the body (the fast muscle) and the spinal cord, are at the gastrula stage oriented with their AP axes along what is termed the dorsal-ventral axis (Figure 3). The most posterior cells will migrate during gastrulation toward the vegetal pole where they form a structure called the tailbud, such that by the end of gastrulation, the AP axis will align with the animal-vegetal axis43. As in other vertebrates, and unlike the invertebrates discussed above, the AP axis is not established during oogenesis and instead is created by the multi-cellular environment during the blastula and gastrula stages concomitant with the establishment of the DV axis.
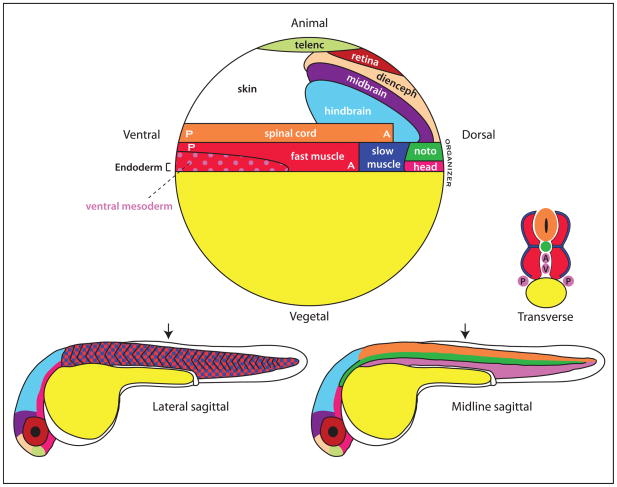
Figure 3. Fate map of the zebrafish embryo.
At top is shown a fate map of the zebrafish embryo at the start of gastrulation (called the shield stage). The organizer is at the equator, on the dorsal side of the embryo. The most posterior cells of the body are at the ventral pole. The 31 hour post-fertilization (hpf) embryos shown at bottom demonstrate a more lateral view at left, showing the muscle, and a midline view at right showing the spinal cord and notochord. Note that slow muscle (dark blue; only a portion is shown in the 31 hpf embryo), ends up in a more lateral position in the body than the fast muscle (see the transverse section).
Because there is so much cell movement within the embryo during gastrulation, it is important not only to understand the factors that regulate the formation of the AP axis, but also to understand when they function. Recent studies have made it very clear that the role of the signaling pathways that regulate formation of the AP axis changes dramatically between the initiation phase of the early gastrula stage and the elaboration phase from mid-gastrulation to the end of somitogenesis. We begin first by considering patterning of the mesoderm. Three major signaling pathways control AP axis formation in the mesoderm during the initiation phase: Wnt/β-catenin, Nodal, and Bmp44, 45. Fgf also has a role in this process by down-regulating bmp expression on the dorsal side of the embryo46.
The role of the Organizer in initiating the AP axis
Local stabilization of maternal β-catenin on one side of the zebrafish embryo, perhaps due to a maternal Wnt signal, creates a new axis of asymmetry45, initiating the first step in formation of the AP axis (Figure 4). Exactly when β-catenin functions in zebrafish is not known, but studies in Xenopus, which uses a similar system of axis determination, demonstrate that β-catenin acts in the very early cleavage stages to modify the histone methylation state of organizer genes48. Nodal signaling, in a vegetal-to-animal gradient, is essential both for establishing the organizer as well as for inducing the mesoderm of the embryo44, 45. The overlap of these two signaling pathways establishes the region of the embryo that will become the organizer, which itself becomes a rich source of signaling molecules.
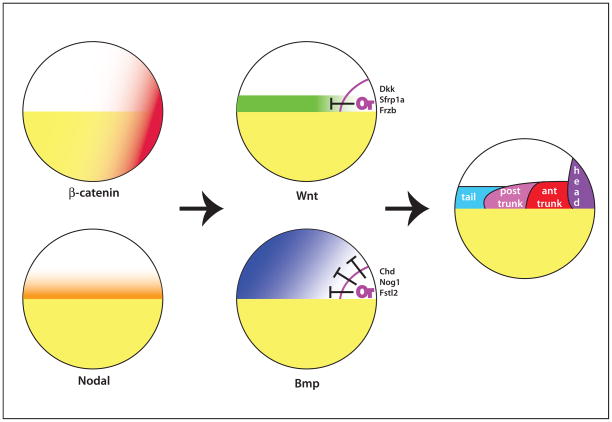
Figure 4. Initial patterning of the zebrafish embryo.
Maternal β-catenin is stabilized on one side of the embryo. Together with Nodal signaling at the equator, the Organizer (Or) is established. The organizer secretes a variety of Bmp and Wnt inhibitors that keep these signals from functioning in the region of the embryo that will form the head. Bmp and Wnts, together with Nodals, pattern the rest of the mesoderm. The region that will form the brain (see Figure 3) expands over time toward the animal pole due to movement of the inhibitors toward the animal pole during gastrulation.
Surprisingly, the Organizer-derived signals are predominantly inhibitors of the Wnt and Bmp pathways44, 49. Bmp and Wnt are expressed in the non-organizer regions of the embryo in partially overlapping expression domains (Figure 4), where they act to antagonize the formation of anterior structures. Thus, anterior structures form precisely because Organizer-derived inhibitors prevent Bmps and Wnts from suppressing anterior structures on one side of the embryo.
Bmp also has an additional important role in inducing the formation of the tail, the most posterior part of the body44, 49–51. In the absence of Bmp signaling, not only do the anterior structures expand to take over a larger part of the embryo (called “dorsalization” in the literature because the anterior structures form on the initial dorsal side of the embryo), but the cells that would form the tail instead develop as trunk tissue. Conversely, removal of the Bmp inhibitors causes the posterior structures to expand at the expense of anterior structures (“ventralization”). Together with Nodal signaling, the interaction of Bmp and Wnt signals with their inhibitors subdivides the embryo into four major regions along the AP axis, the head, anterior trunk, posterior trunk and tail50 (Figure 4; see also 52 for a somewhat different view).
Because the Wnt and Bmp signaling pathways play such critical roles in regulating the early AP axis, they are under very precise control. Many extracellular factors are involved in establishing precise gradients of these signals in the early embryo44, 53. Intriguingly, the Bmp and Wnt pathways interact in the early embryo, both through cross-regulation of their intracellular pathways as well as through downstream transcription factors54, 55. This complexity is necessary not only to correctly specify the AP fate of different cells within the embryo, but also to account for the fact that cells within the early embryo are motile during the period of signaling, and thus can traverse different positions along the Bmp and Wnt gradients.
The importance of timing
The roles of the signaling factors changes dramatically during the course of early development, which is often overlooked in studies of AP patterning. Alterations in a signaling pathway using a mutant or various knockdown or overexpression approaches can produce a complex phenotype because they alter the signaling pathway throughout development. Studies using temporal gain and loss of function are increasingly being used because they separate the various functions of a signal. For example, as described above, the Wnt signaling pathway acts in the very early embryo to specify the anterior end of the embryo, then switches in the late-blastula stage to limiting the size of the anterior end of the embryo, allowing the head to form. From mid-gastrulation onward, as discussed below, it acquires an additional role in maintaining the expression of the posterior progenitors that will form the posterior end of the embryo. Bmp signaling plays a critical role in the late blastula/early gastrula embryo in limiting the size of the organizer and inducing tail formation, but after that it has no role in AP patterning despite continued expression in the most posterior end of the embryo56, 57. Nodal signaling is essential for establishing the organizer at mid-blastula stages, but has no function in AP patterning beyond the late blastula stages58.
Development of the posterior body from a progenitor population
Starting with gastrulation, the posterior body begins to form during the elaboration phase of AP axis formation, in a process that continues throughout somitogenesis. This process involves the continual recruitment of both mesodermal and neural cells from a progenitor population located at the most posterior end of the embryo (Figure 5). Whether or not this population contains multipotent cells or a mixture of cells with more limited fates has been a source of ongoing debate in studies of vertebrate model systems59, with recent studies in the mouse indicating the presence of a bipotential neuromesodermal cell60.
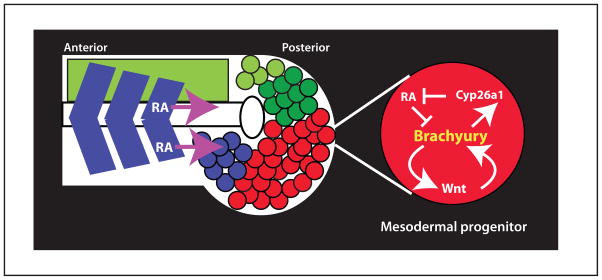
Figure 5. Maintenance of the mesodermal progenitors.
The mesodermal progenitors (red) are located at the most posterior end of the embryo, and they move anteriorly as they differentiate (blue color) and join the somites. Neural progenitors are also located in this region (green), and they differentiate (light green) to join the neural tube. Brachyury works in the mesodermal progenitors to maintain wnt transcription, and to induce transcription of cyp26a1, which degrades the somite-produced retinoic acid (RA), that would otherwise inhibit brachyury transcription. Shown is the most posterior end of a somitogenesis stage embryo.
Among the mesodermal progenitors an essential protein is the transcription factor Brachyury, first identified as a mouse mutant that truncates all but the most anterior somites61. Brachury sustains the mesodermal progenitor population in two important ways: it maintains the transcription of Wnt genes, which are first induced in these cells at the late blastula stage, and it activates the expression of Cyp26a1, a protein that degrades retinoic acid (RA), thus protecting the progenitors from somite-produced RA that would otherwise inhibit brachyury expression (Figure 5)62. Despite its essential role in forming the posterior body, Brachyury is not required in any individual mesodermal progenitor cell for its maintenance and differentiation. Instead, Brachyury acts to regulate transcription within each of the mesodermal progenitor cells to create a niche of high Wnt and low RA that is necessary for the progenitors to remain in the progenitor state62. Thus, any one progenitor cell does not require Brachyury function as long as the surrounding progenitor cells are providing the niche function.
Because the progenitors are continually being depleted as they differentiate, the body will continue to elongate during somitogenesis only as long as there are progenitors to sustain this process. This suggests that the AP length of the embryonic body is determined by the number of initial progenitors, the rate of proliferation of the progenitors, and the rate of depletion. The number of somites is determined by all these factors, as well as the rate of the clock that determines how fast the boundary between somites forms63. It is tempting to speculate that the mechanism of progressive posterior growth used by vertebrates was evolutionarily very useful, since tweaking of a few variables allows great diversity in body length as well as in the number of somites.
Neural AP patterning: Anteriorizing and Posteriorizing factors
A large body of evidence has demonstrated the importance of the mesoderm for AP patterning of the neural ectoderm, although the zebrafish head exhibits a remarkable degree of AP patterning even in the absence of organizer mesoderm64. Most of what is known about AP neural patterning derives from other model systems, particularly studies in amphibians49. In one of the most famous experiments in AP patterning, Otto Mangold showed in newts that anterior mesoderm grafted into an early gastrula host embryo induced an ectopic head, whereas posterior mesoderm grafted the same way induced a tail65. This lead him to speculate that the mesoderm was subdivided into different discrete organizing centers that pattern the AP axis of the neurectoderm. In a more recent view, the entire mesoderm of the early embryo is proposed to function as a continuum of organizing activity from the dorsal (anterior) to the ventral (posterior) side that imparts AP patterning to the overlying ectoderm52, although the data is also consistent with the mesoderm being divided into four territories as shown in Figure 4.
An alternative view came from the work of Nieuwkoop in frogs who proposed that the neural ectoderm is first induced with an anterior character (“activation”) and gradually transformed by later signals from the mesoderm into a more posterior character (“transformation”)66, 67. Stimulated by both Mangold and Nieuwkoop’s work, many authors have strived to identify the activating and transforming factors in recent years, with RA, Wnt, Bmp, Fgf, and their inhibitors, as favorite candidates for one or both of these activities45, 63, 68, 69. Whereas loss of Bmp, Wnt and Nodal signaling by Organizer-derived inhibitors results in anterior neural fates52, 68, 69, consistent with the activation step, clear identification of the transforming factor(s) has proved more difficult. Nonetheless, it is clear from a large body of work in many vertebrate systems that Fgf, Wnt and RA have roles as posteriorizing factors. While particular authors have sometimes championed one specific factor, there is good evidence for all these factors playing a role in posteriorization47, 49, 67, 70. The devil, however, is in the details, with data and models that are sometimes contradictory and not easily summarized. What is clear from all this work is that these posteriorizing factors affect at least some extent of AP patterning of the anterior neural tissues but exactly how they work and what genes they directly affect is still quite unclear49, 67. There are a number of reasons for these complications. First, a number of studies have been done using overexpression analyses, which are very valuable for showing what a specific factor is capable of doing, but do not necessarily show what the factor actually does in the embryo. Second, the factors are likely to work in various combinations, acting in synergistic, antagonistic or linear pathways71. Third, as emphasized above, the role of the factors often change dramatically over short periods of time. Thus even in loss of function studies using a mutant, dominant-negative or other gene knockdown approach, the final phenotype may be the summation of multiple effects due to a loss of the factor. Fourth, since the mesoderm provides patterning to the neural ectoderm, it can be difficult to distinguish what effects are direct on the neurectoderm and what effects are due to changes in the mesoderm that then signals to the neuroectoderm. Thus, understanding how the posteriorizing factors work will require a combination of approaches utilizing temporal and tissue specific regulation of different signaling pathways, including both gain and loss of function experiments. Using cell autonomous pathway regulators as opposed to commonly used secreted activators and inhibitors will also be very desirable, since it allows discrimination between direct effects on a particular cell type and indirect effects due to downstream signaling events. These approaches should help clarify what has been a complex aspect of AP patterning.
Regulation of the Hox genes in posterior patterning
As discussed earlier, the Hox proteins are critical regulators of AP patterning in metazoans. In vertebrates, the Hox proteins are expressed in all but the most anterior regions of the embryo. Within the posterior body, the Hox genes provide the major positional information along the AP axis. The Hox genes are in linear clusters in the genome, with seven sets in zebrafish compared to four in birds and mammals72. Within each cluster, the Hox genes are progressively activated within the progenitor population over developmental time from the 3′ end of the cluster toward the 5′ end73. Thus, as the embryo elongates during gastrulation and somitogenesis, this temporal sequence is converted into spatial information such that the anterior border of the most 3′ Hox gene is most anterior and the anterior border of the most 5′ Hox gene is most posterior73. While this is well documented, what is much less clear is how the Hox genes are activated in this progressive manner. One possibility is that the levels of some factor, perhaps one of the posteriorizing factors, increases in the progenitor population over time such that higher levels activate more posterior Hox genes (Figure 6A). While a number of studies in different systems have shown that the posteriorizing factors are in a posterior-anterior gradient at the most posterior end of the embryo, there is no evidence that the actual concentration of the factors increases over developmental time at the posterior end of the embryo.
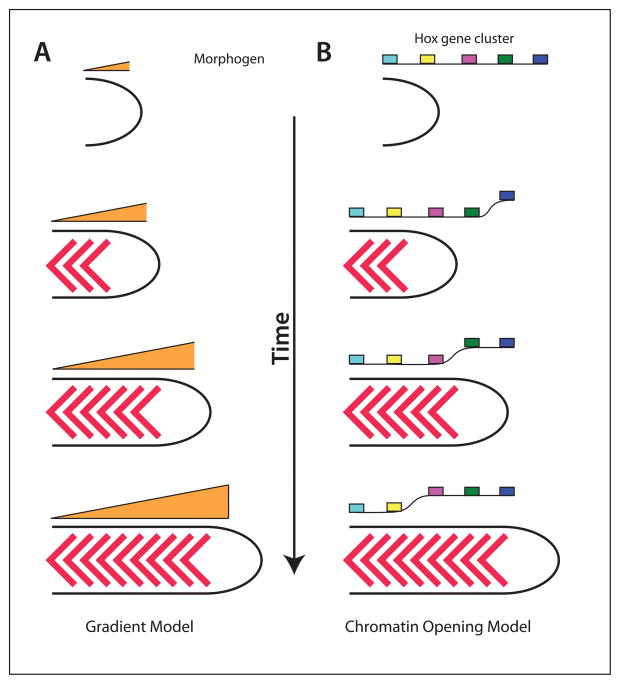
Figure 6. Models for Hox gene regulation.
Left, gradient model. As the embryo extends, the concentration of a secreted factor decreases, which provides a signal for the expression of more posterior Hox genes. It is also possible that a signal increases as the embryo extends. Right, Chromatin model. As the embryo extends, the chromatin opens up progressively in a 3′ to 5′ direction, allowing more posterior Hox genes to be expressed.
As an alternative mechanism, the regulation of the Hox genes could depend on a cell autonomous molecular clock within the progenitor population, thus the longer a cell stays in the progenitor pool, the more 5′ Hox gene it would activate. Such a clock could work by regulating the chromatin structure of the Hox genes, causing them to open up progressively in a 3′ to 5′ direction (Figure 6B)73, 74. Such a model would fit well with studies on Bmps in fish embryos in which pre-gastrula Bmp signaling promotes tail fates by causing progenitor cells to remain held in the progenitor population until it is time for the tail somites to form50. Thus, Bmp might create the tail fates by delaying the exit of cells from the progenitor population, allowing the Hox gene chromatin to continue to open, such that only posterior Hox genes are expressed in cells that saw Bmp at the pre-gastrula stage. Although the clock model is attractive and has received much support, it is hard to reconcile with a recent study in chick embryos (where such experiments are feasible) demonstrating that transplantation of small numbers of progenitors expressing a posterior Hox gene to the progenitor population of a younger embryo causes the posterior Hox gene to be extinguished and instead the cells that leave the progenitor zone express a more anterior Hox gene as do their neighbors75. Even if Hox genes are normally activated by a cell autonomous clock, this result indicates cells in the progenitor zone can communicate with each other to ensure that their clocks are in the correct state. How this might work is not at all clear, but does raise the possibility that normal Hox gene expression might involve a combination of cell autonomous changes in DNA transcription combined with non-autonomous cues from neighboring cells. Since all of the posteriorizing factors, RA, Fgf and Wnt, have been shown to modulate Hox gene expression73, 74, it is possible that they could act together with a cell autonomous clock such as chromatin opening, to synchronize the timing of expression.
Comparison with amphibians
As noted above, much of what is known about zebrafish AP patterning came from studies of the frog Xenopus laevis. Although there are some differences in how genes are used between fish and frogs, much of the mechanism for generating the AP axis seems very well conserved76. One major difference is that Notch signaling, acting through the transcription factor Xhox3 (an ortholog of the mammalian Evx genes) is continuously required for posterior growth in Xenopus77, whereas Notch does not have this role in zebrafish. Notch signaling, however, is important for somitogenesis in zebrafish, as in other vertebrates78.
Comparison with amniotes
One of the major differences between amniotes (mammals, birds and reptiles) and anamniotes (fish and amphibians) is the speed of development. Since anmniote eggs are typically laid into water, their embryos need to develop very rapidly so that they can quickly acquire mobility to avoid becoming snack food for predators. In contrast, amniotes are protected within an eggshell or their mother and can therefore develop more slowly, which may account for some important differences in their development of the AP axes. Whereas the cells that will contribute specifically to the most posterior mesoderm and neural tissue (the tail) can be specified in the early gastrula fish and amphibian embryo as discussed above, the cells that will produce the tail in the amniotes can not be fate mapped at this stage. It has been argued that this is not simply a failure to produce accurate fate maps despite extensive work, and instead reflects the fact that the cells that will give rise to the posterior tissues have not been born at this time67. Similarly, while there is extensive evidence that Bmp signaling at the early gastrula stage patterns the posterior cells of fish and amphibian embryos44, 49, there is no evidence for a similar process occurring in amniotes, consistent with a difference in the means by which the most posterior tissue is specified79. Thus in amniotes, the most anterior tissues are specified early, and the more posterior cells become specified as gastrulation and somitogenesis occur. Nonetheless, with the exception of the role of Bmp in tail patterning, and the presence of the Nodal/Bmp/Wnt inhibitor Cerberus that first showed up with the tetrapods80, the same general factors are used in AP patterning in the amniotes47, 49, 79, 81.
A second difference involves the mechanisms that first break polarity to establish the axis. Whereas in fish (and frogs) microtubules are used to transport maternal factors to one side of the embryo to break symmetry82, in chicks the rotation of the egg in the oviduct asymmetrically distributes one or more maternal components67. In mouse the situation is unclear; AP related asymmetry in gene expression can be detected as early as E3.5 but it is not clear if this is caused by asymmetric distribution of a maternal factor or self-organization based on zygotic gene expression83.
A third difference involves the use of extraembryonic tissues to regulate the AP axis. In both chick and mouse, extraembryonic tissue (the hypoblast in chick and the anterior visceral endoderm [AVE] in mouse) secretes the inhibitors Cerberus and Lefty. Whereas Cerberus inhibits Nodal, Wnt and Bmp80, Lefty has been primarily considered a Nodal inhibitor although it can also inhibit Wnt signaling84. In chick, the hypoblast secretes these factors and acts to prevent premature formation of the organizer67. In mouse, the AVE, which also secretes the Wnt inhibitor Dkk1, acts to prevent posterior gene expression in anterior regions, thus permitting normal anterior development79, 81.
A final possible difference involves the progenitors. Studies in both mouse and chick have demonstrated that the progenitor zone contains stem-like cells that contribute to neural and mesodermal fates59. Intriguingly, these cells can be repeatedly transferred from older to younger embryos and they continue to act as progenitors, releasing differentiated cells. Since the same experiments can not be done in the fish, at least so far, it is difficult to know if this represents a real difference between amniotes and anamniotes or not.
Conclusion
We have illustrated AP axis formation focusing primarily on three species to provide a framework for understanding this long-storied aspect of developmental biology. Although there are major differences between the vertebrates and the insects, particularly in the presence or absence of a syncytial blastoderm and the consequences this has for morphogen gradients, the similarities of posterior growth between the short germ-band insects and vertebrates are intriguing, particularly as a conserved signaling factor is involved37, 85–87. Since posterior growth has been proposed to be ancestral in the bilaterians88, it will be of great interest to learn which aspects of initial AP specification and subsequent posterior growth have been retained among modern species, and which have been modified to suit the requirements of different embryos.
Acknowledgments
We gratefully appreciate thoughtful comments from Claudio Stern and Patrick Tam, and comments on the manuscript from Cort Bouldin, Sarah Malmquist and Richard Row.
Contributor Information
David Kimelman, Department of Biochemistry, University of Washington.
Benjamin L. Martin, Department of Biochemistry, University of Washington
References
- 1.Liu PZ, Kaufman TC. Short and long germ segmentation: unanswered questions in the evolution of a developmental mode. Evol Dev. 2005;7:629–646. doi: 10.1111/j.1525-142X.2005.05066.x. [DOI] [PubMed] [Google Scholar]
- 2.Riechmann V, Ephrussi A. Axis formation during Drosophila oogenesis. Curr Opin Genet Dev. 2001;11:374–383. doi: 10.1016/s0959-437x(00)00207-0. [DOI] [PubMed] [Google Scholar]
- 3.van Eeden F, St Johnston D. The polarisation of the anterior-posterior and dorsal-ventral axes during Drosophila oogenesis. Curr Opin Genet Dev. 1999;9:396–404. doi: 10.1016/S0959-437X(99)80060-4. [DOI] [PubMed] [Google Scholar]
- 4.Roth S, Lynch JA. Symmetry breaking during Drosophila oogenesis. Cold Spring Harb Perspect Biol. 2009;1:a001891. doi: 10.1101/cshperspect.a001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porcher A, Dostatni N. The bicoid morphogen system. Curr Biol. 2010;20:R249–254. doi: 10.1016/j.cub.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 6.Lynch J, Desplan C. Evolution of development: beyond bicoid. Curr Biol. 2003;13:R557–559. doi: 10.1016/s0960-9822(03)00472-x. [DOI] [PubMed] [Google Scholar]
- 7.McGregor AP. How to get ahead: the origin, evolution and function of bicoid. Bioessays. 2005;27:904–913. doi: 10.1002/bies.20285. [DOI] [PubMed] [Google Scholar]
- 8.Sommer R, Tautz D. Segmentation gene expression in the housefly Musca domestica. Development. 1991;113:419–430. doi: 10.1242/dev.113.2.419. [DOI] [PubMed] [Google Scholar]
- 9.Stauber M, Jackle H, Schmidt-Ott U. The anterior determinant bicoid of Drosophila is a derived Hox class 3 gene. Proc Natl Acad Sci U S A. 1999;96:3786–3789. doi: 10.1073/pnas.96.7.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.St Johnston D, Driever W, Berleth T, Richstein S, Nusslein-Volhard C. Multiple steps in the localization of bicoid RNA to the anterior pole of the Drosophila oocyte. Development. 1989;107(Suppl):13–19. doi: 10.1242/dev.107.Supplement.13. [DOI] [PubMed] [Google Scholar]
- 11.Berleth T, Burri M, Thoma G, Bopp D, Richstein S, Frigerio G, Noll M, Nusslein-Volhard C. The role of localization of bicoid RNA in organizing the anterior pattern of the Drosophila embryo. Embo J. 1988;7:1749–1756. doi: 10.1002/j.1460-2075.1988.tb03004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Driever W, Nusslein-Volhard C. The bicoid protein determines position in the Drosophila embryo in a concentration-dependent manner. Cell. 1988;54:95–104. doi: 10.1016/0092-8674(88)90183-3. [DOI] [PubMed] [Google Scholar]
- 13.Driever W, Nusslein-Volhard C. A gradient of bicoid protein in Drosophila embryos. Cell. 1988;54:83–93. doi: 10.1016/0092-8674(88)90182-1. [DOI] [PubMed] [Google Scholar]
- 14.Ma X, Yuan D, Diepold K, Scarborough T, Ma J. The Drosophila morphogenetic protein Bicoid binds DNA cooperatively. Development. 1996;122:1195–1206. doi: 10.1242/dev.122.4.1195. [DOI] [PubMed] [Google Scholar]
- 15.Simpson-Brose M, Treisman J, Desplan C. Synergy between the hunchback and bicoid morphogens is required for anterior patterning in Drosophila. Cell. 1994;78:855–865. doi: 10.1016/s0092-8674(94)90622-x. [DOI] [PubMed] [Google Scholar]
- 16.Driever W, Nusslein-Volhard C. The bicoid protein is a positive regulator of hunchback transcription in the early Drosophila embryo. Nature. 1989;337:138–143. doi: 10.1038/337138a0. [DOI] [PubMed] [Google Scholar]
- 17.Irish V, Lehmann R, Akam M. The Drosophila posterior-group gene nanos functions by repressing hunchback activity. Nature. 1989;338:646–648. doi: 10.1038/338646a0. [DOI] [PubMed] [Google Scholar]
- 18.Lehmann R, Nusslein-Volhard C. hunchback, a gene required for segmentation of an anterior and posterior region of the Drosophila embryo. Dev Biol. 1987;119:402–417. doi: 10.1016/0012-1606(87)90045-5. [DOI] [PubMed] [Google Scholar]
- 19.Tautz D. Regulation of the Drosophila segmentation gene hunchback by two maternal morphogenetic centres. Nature. 1988;332:281–284. doi: 10.1038/332281a0. [DOI] [PubMed] [Google Scholar]
- 20.Frohnhofer HG, Nusslein-Volhard C. The organization of anterior pattern in the Drosophila embryo by the maternal gene bicoid. Nature. 1986;324:120–125. [Google Scholar]
- 21.Macdonald PM, Struhl G. A molecular gradient in early Drosophila embryos and its role in specifying the body pattern. Nature. 1986;324:537–545. doi: 10.1038/324537a0. [DOI] [PubMed] [Google Scholar]
- 22.Nusslein-Volhard C, Frohnhofer HG, Lehmann R. Determination of anteroposterior polarity in Drosophila. Science. 1987;238:1675–1681. doi: 10.1126/science.3686007. [DOI] [PubMed] [Google Scholar]
- 23.Gavis ER, Lehmann R. Localization of nanos RNA controls embryonic polarity. Cell. 1992;71:301–313. doi: 10.1016/0092-8674(92)90358-j. [DOI] [PubMed] [Google Scholar]
- 24.Wang C, Dickinson LK, Lehmann R. Genetics of nanos localization in Drosophila. Dev Dyn. 1994;199:103–115. doi: 10.1002/aja.1001990204. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg MI, Lynch JA, Desplan C. Heads and tails: evolution of antero-posterior patterning in insects. Biochim Biophys Acta. 2009;1789:333–342. doi: 10.1016/j.bbagrm.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akam M. The molecular basis for metameric pattern in the Drosophila embryo. Development. 1987;101:1–22. [PubMed] [Google Scholar]
- 27.Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 28.Schroder R, Beermann A, Wittkopp N, Lutz R. From development to biodiversity--Tribolium castaneum, an insect model organism for short germband development. Dev Genes Evol. 2008;218:119–126. doi: 10.1007/s00427-008-0214-3. [DOI] [PubMed] [Google Scholar]
- 29.Sulston IA, Anderson KV. Embryonic patterning mutants of Tribolium castaneum. Development. 1996;122:805–814. doi: 10.1242/dev.122.3.805. [DOI] [PubMed] [Google Scholar]
- 30.Lynch JA, Peel AD, Drechsler A, Averof M, Roth S. EGF signaling and the origin of axial polarity among the insects. Curr Biol. 2010;20:1042–1047. doi: 10.1016/j.cub.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finkelstein R, Perrimon N. The orthodenticle gene is regulated by bicoid and torso and specifies Drosophila head development. Nature. 1990;346:485–488. doi: 10.1038/346485a0. [DOI] [PubMed] [Google Scholar]
- 32.Wolff C, Sommer R, Schroder R, Glaser G, Tautz D. Conserved and divergent expression aspects of the Drosophila segmentation gene hunchback in the short germ band embryo of the flour beetle Tribolium. Development. 1995;121:4227–4236. doi: 10.1242/dev.121.12.4227. [DOI] [PubMed] [Google Scholar]
- 33.Schroder R. The genes orthodenticle and hunchback substitute for bicoid in the beetle Tribolium. Nature. 2003;422:621–625. doi: 10.1038/nature01536. [DOI] [PubMed] [Google Scholar]
- 34.Acampora D, Boyl PP, Martinez-Barbera JP, Annino A, Signore M, Simeone A. Otx genes in evolution: are they involved in instructing the vertebrate brain morphology? J Anat. 2001;199:53–62. doi: 10.1046/j.1469-7580.2001.19910053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Copf T, Schroder R, Averof M. Ancestral role of caudal genes in axis elongation and segmentation. Proc Natl Acad Sci U S A. 2004;101:17711–17715. doi: 10.1073/pnas.0407327102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schoppmeier M, Fischer S, Schmitt-Engel C, Lohr U, Klingler M. An ancient anterior patterning system promotes caudal repression and head formation in ecdysozoa. Curr Biol. 2009;19:1811–1815. doi: 10.1016/j.cub.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 37.Martin BL, Kimelman D. Wnt signaling and the evolution of embryonic posterior development. Curr Biol. 2009;19:R215–219. doi: 10.1016/j.cub.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bolognesi R, Farzana L, Fischer TD, Brown SJ. Multiple Wnt genes are required for segmentation in the short-germ embryo of Tribolium castaneum. Curr Biol. 2008;18:1624–1629. doi: 10.1016/j.cub.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veraksa A, Del Campo M, McGinnis W. Developmental patterning genes and their conserved functions: from model organisms to humans. Molecular genetics and metabolism. 2000;69:85–100. doi: 10.1006/mgme.2000.2963. [DOI] [PubMed] [Google Scholar]
- 40.Denell R. Establishment of tribolium as a genetic model system and its early contributions to evo-devo. Genetics. 2008;180:1779–1786. doi: 10.1534/genetics.104.98673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cerny AC, Bucher G, Schroder R, Klingler M. Breakdown of abdominal patterning in the Tribolium Kruppel mutant jaws. Development. 2005;132:5353–5363. doi: 10.1242/dev.02154. [DOI] [PubMed] [Google Scholar]
- 42.Saude L, Woolley K, Martin P, Driever W, Stemple DL. Axis-inducing activities and cell fates of the zebrafish organizer. Development. 2000;127:3407–3417. doi: 10.1242/dev.127.16.3407. [DOI] [PubMed] [Google Scholar]
- 43.Myers DC, Sepich DS, Solnica-Krezel L. Convergence and extension in vertebrate gastrulae: cell movements according to or in search of identity? Trends Genet. 2002;18:447–455. doi: 10.1016/s0168-9525(02)02725-7. [DOI] [PubMed] [Google Scholar]
- 44.Schier AF, Talbot WS. Molecular genetics of axis formation in zebrafish. Annu Rev Genet. 2005;39:561–613. doi: 10.1146/annurev.genet.37.110801.143752. [DOI] [PubMed] [Google Scholar]
- 45.Kimelman D. Mesoderm induction: from caps to chips. Nat Rev Genet. 2006;7:360–372. doi: 10.1038/nrg1837. [DOI] [PubMed] [Google Scholar]
- 46.Furthauer M, Van Celst J, Thisse C, Thisse B. Fgf signalling controls the dorsoventral patterning of the zebrafish embryo. Development. 2004;131:2853–2864. doi: 10.1242/dev.01156. [DOI] [PubMed] [Google Scholar]
- 47.Dorey K, Amaya E. FGF signalling: diverse roles during early vertebrate embryogenesis. Development. 2010;137:3731–3742. doi: 10.1242/dev.037689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blythe SA, Cha SW, Tadjuidje E, Heasman J, Klein PS. beta-Catenin primes organizer gene expression by recruiting a histone H3 arginine 8 methyltransferase, Prmt2. Dev Cell. 2010;19:220–231. doi: 10.1016/j.devcel.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Niehrs C. Regionally specific induction by the Spemann-Mangold organizer. Nat Rev Genet. 2004;5:425–434. doi: 10.1038/nrg1347. [DOI] [PubMed] [Google Scholar]
- 50.Szeto DP, Kimelman D. The regulation of mesodermal progenitor cell commitment to somitogenesis subdivides the zebrafish body musculature into distinct domains. Genes Dev. 2006;20:1923–1932. doi: 10.1101/gad.1435306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agathon A, Thisse C, Thisse B. The molecular nature of the zebrafish tail organizer. Nature. 2003;424:448–452. doi: 10.1038/nature01822. [DOI] [PubMed] [Google Scholar]
- 52.Fauny JD, Thisse B, Thisse C. The entire zebrafish blastula-gastrula margin acts as an organizer dependent on the ratio of Nodal to BMP activity. Development. 2009;136:3811–3819. doi: 10.1242/dev.039693. [DOI] [PubMed] [Google Scholar]
- 53.Zakin L, De Robertis EM. Extracellular regulation of BMP signaling. Curr Biol. 2010;20:R89–92. doi: 10.1016/j.cub.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fuentealba LC, Eivers E, Ikeda A, Hurtado C, Kuroda H, Pera EM, De Robertis EM. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell. 2007;131:980–993. doi: 10.1016/j.cell.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramel MC, Lekven AC. Repression of the vertebrate organizer by Wnt8 is mediated by Vent and Vox. Development. 2004;131:3991–4000. doi: 10.1242/dev.01277. [DOI] [PubMed] [Google Scholar]
- 56.Pyati UJ, Cooper MS, Davidson AJ, Nechiporuk A, Kimelman D. Sustained Bmp signaling is essential for cloaca development in zebrafish. Development. 2006;133:2275–2284. doi: 10.1242/dev.02388. [DOI] [PubMed] [Google Scholar]
- 57.Tucker JA, Mintzer KA, Mullins MC. The BMP signaling gradient patterns dorsoventral tissues in a temporally progressive manner along the anteroposterior axis. Dev Cell. 2008;14:108–119. doi: 10.1016/j.devcel.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hagos EG, Dougan ST. Time-dependent patterning of the mesoderm and endoderm by Nodal signals in zebrafish. BMC Dev Biol. 2007;7:22. doi: 10.1186/1471-213X-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson V, Olivera-Martinez I, Storey KG. Stem cells, signals and vertebrate body axis extension. Development. 2009;136:1591–1604. doi: 10.1242/dev.021246. [DOI] [PubMed] [Google Scholar]
- 60.Tzouanacou E, Wegener A, Wymeersch FJ, Wilson V, Nicolas JF. Redefining the progression of lineage segregations during mammalian embryogenesis by clonal analysis. Dev Cell. 2009;17:365–376. doi: 10.1016/j.devcel.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 61.Chesley P. Development of the short-tailed mutant in the house mouse. J Exp Zool. 1935;70:429–435. [Google Scholar]
- 62.Martin BL, Kimelman D. Brachyury establishes the embryonic mesodermal progenitor niche. Genes Dev. 2010;24:2778–2783. doi: 10.1101/gad.1962910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gomez C, Ozbudak EM, Wunderlich J, Baumann D, Lewis J, Pourquie O. Control of segment number in vertebrate embryos. Nature. 2008;454:335–339. doi: 10.1038/nature07020. [DOI] [PubMed] [Google Scholar]
- 64.Gritsman K, Zhang J, Cheng S, Heckscher E, Talbot WS, Schier AF. The EGF-CFC protein one-eyed pinhead is essential for nodal signaling. Cell. 1999;97:121–132. doi: 10.1016/s0092-8674(00)80720-5. [DOI] [PubMed] [Google Scholar]
- 65.Mangold O. Über die induktionsfahigkeit der verschiedenen bezirke der neurula von urodelen. Naturwissenchaften. 1933;21:761–766. [Google Scholar]
- 66.Nieuwkoop PD, Nigtevecht GV. Neural activation and transformation in explants of competent ectoderm under the influence of fragments of anterior notochord in urodeles. J Embryol Exp Morphol. 1954;2:175–193. [Google Scholar]
- 67.Stern CD, Charite J, Deschamps J, Duboule D, Durston AJ, Kmita M, Nicolas JF, Palmeirim I, Smith JC, Wolpert L. Head-tail patterning of the vertebrate embryo: one, two or many unresolved problems? Int J Dev Biol. 2006;50:3–15. doi: 10.1387/ijdb.052095cs. [DOI] [PubMed] [Google Scholar]
- 68.Glinka A, Wu W, Onichtchouk D, Blumenstock C, Niehrs C. Head induction by simultaneous repression of Bmp and Wnt signalling in Xenopus. Nature. 1997;389:517–519. doi: 10.1038/39092. [DOI] [PubMed] [Google Scholar]
- 69.Piccolo S, Agius E, Leyns L, Bhattacharyya S, Grunz H, Bouwmeester T, De Robertis EM. The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature. 1999;397:707–710. doi: 10.1038/17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blumberg B, Bolado J, Jr, Moreno TA, Kintner C, Evans RM, Papalopulu N. An essential role for retinoid signaling in anteroposterior neural patterning. Development. 1997;124:373–379. doi: 10.1242/dev.124.2.373. [DOI] [PubMed] [Google Scholar]
- 71.Kudoh T, Wilson SW, Dawid IB. Distinct roles for Fgf, Wnt and retinoic acid in posteriorizing the neural ectoderm. Development. 2002;129:4335–4346. doi: 10.1242/dev.129.18.4335. [DOI] [PubMed] [Google Scholar]
- 72.Duboule D. The rise and fall of Hox gene clusters. Development. 2007;134:2549–2560. doi: 10.1242/dev.001065. [DOI] [PubMed] [Google Scholar]
- 73.Iimura T, Denans N, Pourquie O. Establishment of Hox vertebral identities in the embryonic spine precursors. Curr Top Dev Biol. 2009;88:201–234. doi: 10.1016/S0070-2153(09)88007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deschamps J, van Nes J. Developmental regulation of the Hox genes during axial morphogenesis in the mouse. Development. 2005;132:2931–2942. doi: 10.1242/dev.01897. [DOI] [PubMed] [Google Scholar]
- 75.McGrew MJ, Sherman A, Lillico SG, Ellard FM, Radcliffe PA, Gilhooley HJ, Mitrophanous KA, Cambray N, Wilson V, Sang H. Localised axial progenitor cell populations in the avian tail bud are not committed to a posterior Hox identity. Development. 2008;135:2289–2299. doi: 10.1242/dev.022020. [DOI] [PubMed] [Google Scholar]
- 76.Heasman J. Patterning the early Xenopus embryo. Development. 2006;133:1205–1217. doi: 10.1242/dev.02304. [DOI] [PubMed] [Google Scholar]
- 77.Beck CW, Slack JM. Notch is required for outgrowth of the Xenopus tail bud. Int J Dev Biol. 2002;46:255–258. doi: 10.1387/ijdb.011489. [DOI] [PubMed] [Google Scholar]
- 78.Holley SA. The genetics and embryology of zebrafish metamerism. Dev Dyn. 2007;236:1422–1449. doi: 10.1002/dvdy.21162. [DOI] [PubMed] [Google Scholar]
- 79.Robb L, Tam PP. Gastrula organiser and embryonic patterning in the mouse. Semin Cell Dev Biol. 2004;15:543–554. doi: 10.1016/j.semcdb.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 80.Bouwmeester T, Kim S, Sasai Y, Lu B, De Robertis EM. Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann’s organizer. Nature. 1996;382:595–601. doi: 10.1038/382595a0. [DOI] [PubMed] [Google Scholar]
- 81.Arnold SJ, Robertson EJ. Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat Rev Mol Cell Biol. 2009;10:91–103. doi: 10.1038/nrm2618. [DOI] [PubMed] [Google Scholar]
- 82.Weaver C, Kimelman D. Move it or lose it: axis specification in Xenopus. Development. 2004;131:3491–3499. doi: 10.1242/dev.01284. [DOI] [PubMed] [Google Scholar]
- 83.Takaoka K, Yamamoto M, Hamada H. Origin of body axes in the mouse embryo. Curr Opin Genet Dev. 2007;17:344–350. doi: 10.1016/j.gde.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 84.Branford WW, Yost HJ. Lefty-dependent inhibition of Nodal- and Wnt-responsive organizer gene expression is essential for normal gastrulation. Curr Biol. 2002;12:2136–2141. doi: 10.1016/s0960-9822(02)01360-x. [DOI] [PubMed] [Google Scholar]
- 85.Mito T, Nakamura T, Noji S. Evolution of insect development: to the hemimetabolous paradigm. Curr Opin Genet Dev. 2010;20:355–361. doi: 10.1016/j.gde.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 86.Niehrs C. On growth and form: a Cartesian coordinate system of Wnt and BMP signaling specifies bilaterian body axes. Development. 2010;137:845–857. doi: 10.1242/dev.039651. [DOI] [PubMed] [Google Scholar]
- 87.Petersen CP, Reddien PW. Wnt signaling and the polarity of the primary body axis. Cell. 2009;139:1056–1068. doi: 10.1016/j.cell.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 88.Jacobs DK, Hughes NC, Fitz-Gibbon ST, Winchell CJ. Terminal addition, the Cambrian radiation and the Phanerozoic evolution of bilaterian form. Evol Dev. 2005;7:498–514. doi: 10.1111/j.1525-142X.2005.05055.x. [DOI] [PubMed] [Google Scholar]
Further Reading/Resources
- Aulehla A, Pourquie O. Signaling gradients during paraxial mesoderm development. Cold Spring Harbor Perspectives in Biology. 2010;2:a000869. doi: 10.1101/cshperspect.a000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SJ, Robertson EJ. Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat Rev Mol Cell Biol. 2009;10:91–103. doi: 10.1038/nrm2618. [DOI] [PubMed] [Google Scholar]
- Brown FD, Prendergast A, Swalla BJ. Man is but a worm: chordate origins. Genesis. 2008;46:605–613. doi: 10.1002/dvg.20471. [DOI] [PubMed] [Google Scholar]
- Heasman J. Patterning the early Xenopus embryo. Development. 2006;133:1205–1217. doi: 10.1242/dev.02304. [DOI] [PubMed] [Google Scholar]
- Jacobs DK, Hughes NC, Fitz-Gibbon ST, Winchell CJ. Terminal addition, the Cambrian radiation and the Phanerozoic evolution of bilaterian form. Evolution & development. 2005;7:498–514. doi: 10.1111/j.1525-142X.2005.05055.x. [DOI] [PubMed] [Google Scholar]
- Mito T, Nakamura T, Noji S. Evolution of insect development: to the hemimetabolous paradigm. Curr Opin Genet Dev. 2010;20:355–361. doi: 10.1016/j.gde.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Niehrs C. On growth and form: a Cartesian coordinate system of Wnt and BMP signaling specifies bilaterian body axes. Development. 2010;137:845–857. doi: 10.1242/dev.039651. [DOI] [PubMed] [Google Scholar]
- Petersen CP, Reddien PW. Wnt signaling and the polarity of the primary body axis. Cell. 2009;139:1056–1068. doi: 10.1016/j.cell.2009.11.035. [DOI] [PubMed] [Google Scholar]
- Rosenberg MI, Lynch JA, Desplan C. Heads and tails: evolution of antero-posterior patterning in insects. Biochimica et biophysica acta. 2009;1789:333–342. doi: 10.1016/j.bbagrm.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CD, et al. Head-tail patterning of the vertebrate embryo: one, two or many unresolved problems? Int J Dev Biol. 2006;50:3–15. doi: 10.1387/ijdb.052095cs. [DOI] [PubMed] [Google Scholar]
- Takaoka K, Yamamoto M, Hamada H. Origin of body axes in the mouse embryo. Curr Opin Genet Dev. 2007;17:344–350. doi: 10.1016/j.gde.2007.06.001. [DOI] [PubMed] [Google Scholar]